Abstract
Muscular dystrophies are genetic conditions leading to muscle degeneration and often, impaired regeneration. Duchenne Muscular Dystrophy is a prototypical form of muscular dystrophy, and like other forms of genetically inherited muscle diseases, pathological progression is variable. Variability in muscular dystrophy can arise from differences in the manner in which the primary mutation impacts the affected protein’s function; however, clinical heterogeneity also derives from secondary mutations in other genes that can enhance or reduce pathogenic features of disease. These genes, called genetic modifiers, regulate the pathophysiological context of dystrophic degeneration and regeneration. Understanding the mechanistic links between genetic modifiers and dystrophic progression sheds light on pathologic remodeling, and provides novel avenues to therapeutically intervene to reduce muscle degeneration. Based on targeted genetic approaches and unbiased genomewide screens, several modifiers have been identified for muscular dystrophy, including extracellular agonists of signaling cascades. This review will focus on identification and possible mechanisms of recently identified modifiers for muscular dystrophy, including osteopontin, latent TGFβ binding protein 4 (LTBP4) and Jagged1. Moreover, we will review the investigational approaches that aim to target modifier pathways and thereby counteract dystrophic muscle wasting.
Keywords: Duchenne muscular dystrophy, genetic modifiers, SPP1, osteopontin, LTBP4, Jagged1, TGFβ, Notch, myostatin, novel drugs, investigational medicinal products, monoclonal antibodies
Introduction: muscular dystrophy and genetic modifiers
Muscular dystrophies are inherited conditions leading to progressive wasting of striated muscle. The most common form in children is Duchenne muscular dystrophy (DMD). DMD occurs in approximately 1:3500 births; in populations with access to carrier screening and prenatal diagnosis, the incidence is 1:5000 – 1:10000 [1]. DMD is caused by mutations that disrupt the DMD gene, which encodes for dystrophin. In myofibers, dystrophin links the actin cytoskeleton to the muscle membrane, the sarcolemma, which helps to maintain a complex of proteins called the dystrophin glycoprotein complex (DGC). The DGC is linked to laminin in the extracellular side of the sarcolemma. Disrupting mutations in dystrophin result in loss of membrane integrity and continuous injury and necrosis of myofibers, which are progressively substituted by fibrofatty tissue. Detrimental remodeling impairs muscle functionality and eventually leads to cardiac and respiratory insufficiency [2]. Heterogeneity in DMD mutations is mirrored by variability in severity and characteristics of disease progression. The clinical phenotype can range from severe, with loss of ambulation in early childhood, to mild, as in the case of Becker muscular dystrophy, where DMD mutations result in a hypofunctional, but not completely dysfunctional, or absent protein [3, 4]. However, phenotypic variability may occur in patients with the same primary DMD mutation, in part explained by partial transcripts produced even in the presence of deletions or frame-shift mutations. Exceptions to the DMD reading frame rule are often explained by mutations that may disrupt exon splicing or generate alternative start codons [5]. In addition, a wide-range of clinical manifestations has been reported even in those patients completely lacking dystrophin, suggesting that genetic modifiers can impart an additive effect on dystrophic disease severity [6, 7].
The existence of modifiers of dystrophinopathy has been substantiated by studies in murine models of DMD. The most widely studied genetic model of DMD is the mdx mouse, originally identified by means of elevated creatine kinase levels in the circulation [8]. Mdx mice bear a premature stop codon in exon 23 of the X-linked dystrophin gene [9]. In mice, phenotypic variability of the same mdx mutation ranges from very severe in the DBA/2J strain [10], to intermediate in the C57/BL10 strain, and to very mild in the 129T2/SvEmsJ genetic background [11]. Thus, DMD progression is modified by secondary mutations and polymorphisms that account for inter-individual variability in patients and differences among strains in laboratory mice. The genes affected by secondary variations are called genetic modifiers, as they significantly modify the pathophysiological context of muscle remodeling, and hence the clinical severity of the primary mutation. Identification of genetic modifiers is useful to predict prognosis and unveil pathways that can be therapeutically targeted [12]. Genetic modifiers can be identified with either targeted, or unbiased approaches. Targeted approaches generally assess the effects of genetic manipulation of candidate genes in muscle homeostasis. For example, downregulation of the transforming growth factor β (TGFβ) pathway was shown to mitigate features of muscular dystrophy in mice using a transgene to express a dominant negative TGFβ receptor [13]. Similarly, fibrosis was reduced in the mdx mouse by ablating Spp1, which encodes osteopontin [14]. Candidate gene approaches have been very useful to identify pathways that alter disease outcomes.
Conversely, genomewide approaches are fundamental to discover unknown candidates since these approaches are largely unbiased. Unbiased studies rely on qualitative or quantitative discrimination of pathological heterogeneity. An example of unbiased approach based on qualitative discrimination has been recently conducted on dystrophic dogs. Within a colony of dogs bearing the same spontaneous mutation in dystrophin, two exceptional cases stood out because they showed mild dystrophic progression and had a normal lifespan. These two “escaper” animals were then compared to control diseased animals by overlaying whole-genome sequencing data with differences in muscle transcriptional profiles [15]. This study identified Jagged1 as beneficial modifier of dystrophic pathology.
Another example of an unbiased approach to identifying modifiers, based on quantitative phenotyping, was conducted on a large cohort of dystrophic mice, which shared the same primary mutation on a mixed DBA/2J-129T2/SvEmsJ background. Mice used for this approach were deleted for the Sgcg gene encoding the dystrophin-associated protein γ-sarcoglycan. However, similar pathological remodeling downstream of the defective DGC renders this murine model relevant to DMD modifiers [16]. The pathological phenotype of these mice was quantified according to muscle injury and fibrosis parameters, while their genome was analyzed by means of microarray tiling. Overlay of these two datasets led to correlation of specific genomic loci with significant changes in pathophysiologic traits of muscular dystrophy (quantitative trait loci analysis) [17, 18]. This study identified, among others, latent TGFβ binding protein 4 (LTBP4) as genetic modifier of muscular dystrophy. Osteopontin, Jagged1, and LTBP4 act as extracellular mediators of signaling cascades, and in this review, we will detail their action on muscular dystrophy from the outside in.
Osteopontin: a multi-faceted modifier
The Spp1 gene encodes osteopontin (also known as secreted phosphoprotein 1), a secreted glycoprotein that signals through integrin and CD44 receptors. In dystrophic human and mouse muscle, osteopontin mRNA is highly upregulated [14, 19–22]. Comparative expression profiling of skeletal muscle from different dystrophic mouse models showed that Spp1 is upregulated in both mildly and severely affected dystrophic murine models, as compared to wildtype controls [23]. Intriguingly, these results align with another study where SPP1 was found as the most upregulated transcript, when comparing the skeletal muscle profiles of dystrophic versus wildtype Golden Retriever dogs at 6 months of age [24]. At the protein level, osteopontin is elevated more than 6 fold in DMD, and between the milder BMD and severe DMD, there is a 2.6 fold difference in protein expression as determined by immunoblotting [25]. A comparison of SPP1 mRNA expression in DMD patient muscle also demonstrated a 2.7 fold increase in samples from diagnostic biopsies of individuals who had a particularly severe clinical course, as compared to biopsies from individuals with a relatively milder course [26].
A polymorphism in the human SPP1 genomic locus, rs28357094, has been shown to correlate with outcomes in DMD. The SNP rs28357094T>G significantly correlated with a more rapid progression of disease, earlier loss of ambulation, and reduction of grip strength in a cohort of DMD patients [26]. This study overlaid mRNA profiling of severe versus mild DMD patients with genome-wide association studies (GWAS) on healthy volunteers [26]. In a subsequent longitudinal study on ethnically restricted DMD patients from Italy, ambulatory DMD patients with the T allele (T/T) were compared to those bearing the G allele (G/T and G/G). In this study, the G allele significantly correlated with faster deterioration of muscle performance using both the North Star ambulatory assessment and 6-minute walk test. This correlation was considered suggestive of a dominant model of action for the G allele [27]. Furthermore, the SPP1 SNP associated with response to glucocorticoid steroids in DMD patients. In a multiethnic cohort, the G allele associated with a 1.2 year earlier median loss of ambulation, and this difference significantly increased to 1.9 years when considering only steroid-treated patients. The same effect size, a 1.9-year difference in loss of ambulation between T/T and G/T-G/G genotypes, was confirmed in a sub-cohort including only patients of European or European-American ancestry [28]. However, a multi-center analysis of DMD cohorts across Europe, in which there was no stratification by ethnicity, did not confirm a significant association between the rs28357094T>G SNP and age of ambulation loss. The study relied on a multivariate analysis, taking into account haplotype, steroid regimen and cohort as covariates [29].
In line with the notion that osteopontin promotes dystrophic remodeling and fibrosis, genetic ablation of osteopontin in mdx mice resulted in dramatic reduction of fibrosis and concomitant improvement of strength and pathophysiology of dystrophic muscle [14]. Macrophages have emerged as mediators of some of the effects exerted by osteopontin, or its loss, in dystrophic muscles. Spp1 ablation skewed muscle macrophages from a pro-inflammatory to a pro-regenerative profile, promoting upregulation of insulin-like factor 1 (IGF1), leukemia inhibitory factor (LIF) and urokinase-type plasminogen activator (uPA) [30].
In addition to the role in inflammation, excess soluble osteopontin was found to increase proliferation but decrease fusion and migration of myoblasts [31], consistent with a direct effect on myoblasts to inhibit features needed for efficient regeneration. However, the role of osteopontin in muscle regeneration in vivo, and specifically after recovery from injury is more complex. In a study assessing degeneration/regeneration using whole muscle autografting as a model of acute injury, Spp1-null grafts had a delay in inflammatory infiltration and regeneration, as compared to wildtype control grafts [32]. These data are distinct from those in [14], and differences between these results may relate to the chronic injury that typifies DMD and/or cell type-specific effects that cannot be assessed by a constitutively deleted allele of Spp1 in mice.
Data from DMD patients, mdx mice and dystrophic dog models suggests that higher levels of osteopontin correlate with promotion of DMD disease [14, 23, 24]. Curiously, the SNP associated with enhanced disease in DMD patients, rs28357094T>G in the human SPP1 promoter, correlated with weaker promoter activity in vitro using luciferase reporter assays [33]. Thus, the association of the G allele (hence, putatively lower SPP1 expression) with increased disease severity is in apparent contrast with not only the reports of SPP1 upregulation in muscle of murine models and patients of DMD, as compared to healthy controls [23, 24], but also the traditional view of osteopontin as a pro-inflammatory cytokine [34]. It must be noted that a direct assessment of SPP1 muscle expression comparing T/T versus T/G or G/G patients did not reveal quantitative changes in SPP1 mRNA levels in some studies [35],
One reason for this apparent lack of parity may relate to the cell types utilized to interrogate the effect of SPP1 SNPs through reporter assays. It is critical to discriminate between the cellular source and the cellular target of osteopontin signaling. Therefore, the use of surrogate cell types for some of the SPP1 gene expression studies may not adequately mirror gene expression in the context of diseased human tissue, especially one as complex as the DMD striated muscle. In addition, steroid hormones may play a role in SPP1 regulation. The rs28357094T haplotype reduces the responsiveness of SPP1 promoter to estrogen-driven transcriptional activation [36]. Moreover, a study performed in malignant astrocytoma cell lines demonstrated binding of the proximal promoter element (surrounding the rs28357094 site) by the glucocorticoid receptor [37]. A potential role of glucocorticoid steroids in the effect size of the rs28357094 SNP might explain disparate results on this modifier; the SNP effect appears to be greater in steroid-treated patients [28] and less detectable in DMD populations where steroid dosing was low [29]. To reconcile these results likely requires examination of gene expression in more relevant cell models in order to more understand the impact of steroid hormones and glucocorticoid regimens on SPP1 regulation in DMD males.
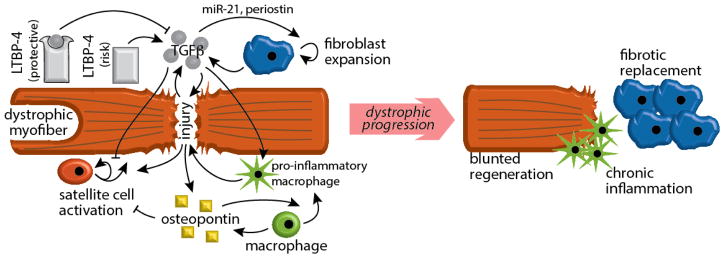
Another emerging hypothesis on the role of osteopontin as a genetic modifier of muscular dystrophy is the still poorly investigated link between osteopontin and TGFβ signaling. Spp1 deletion results in decreased levels of intra-muscular TGFβ in mdx mice [14]. Accordingly, osteopontin drives TGFβ1 upregulation, although this was documented in non-muscle mesenchymal cells [38]. Conversely, the Spp1 promoter is responsive to TGFβ signaling [39], and TGFβ1 is able to increase Spp1 levels [40]. Moreover, although the mechanisms are still unknown, a polymorphism in the gene encoding for the TGFβ receptor 2 (TGFBR2) appeared as a strong predictor of SPP1 mRNA levels in DMD muscle biopsies [35]. Thus, osteopontin is a multi-faceted modifier of muscular dystrophy via regulation of macrophage polarization and regenerative potential in muscle (Figure 1). Scattered evidence points at direct crosstalk between osteopontin and TGFβ pathways; however, such regulatory circuitry in dystrophic myofibers still awaits a more comprehensive evaluation.
Figure 1. Osteopontin and LTBP4 modify dystrophic progression.
Upon chronic myofiber injury, both osteopontin and LTBP4 have the potential to direct dystrophic remodeling via regulation of susceptibility to injury, fibrosis, satellite cell potential and inflammation.
SPP1 is not the only genetic modifier impacting immune cell modulation. Recently, a hypothesis-driven exome screening on DMD patients of European or European-American ancestry identified the rs1883832C>T as modifier of age of loss of ambulation, with the C haplotype associating with earlier age [41]. This SNP falls in the 5′-UTR of CD40, which modulates T cell activation and can be found also on the surface of myofibers. Intriguingly, the same group reported a similar effect of the minor SNP haplotype (T) on loss of ambulation in other three independent cohorts of DMD patients [41]. Although the molecular mechanisms and the cell context of CD40-mediated effects on dystrophic degeneration must still be elucidated, these results reinforce the focus on the link between DMD genetic modifiers and immune system modulation. More specifically, these data trigger the question of whether SPP1 and CD40 haplotypes significantly synergize to shift immune cell regulation in response to dystrophic muscle degeneration.
LTBP4 modifies availability of TGFβ and myostatin
Latent TGFβ binding protein 4 (LTBP4) was first identified in 1997 as novel binding protein for TGFβ in the extra-cellular matrix [42]. The role of LTBP4 as genetic modifier of muscular dystrophy was first discovered from an unbiased, genomewide quantitative trait loci analysis in a large cohort of dystrophic mice. Specifically, mice on the 129T2/SvEmsJ background were found to show a milder phenotype than those on the DBA/2J background. The 129T2/SvEmsJ background carries a protective LTBP4 allele featuring the insertion of 12 amino acids in the proline-rich hinge region of the LTBP4 protein. In contrast, the DBA/2J background and a minority of laboratory mouse strains have a deletion of 12 amino acids in this hinge region. The insertion of 12 residues into the hinge region reduces proteolytic cleavage and latent TGFβ release in the muscle, providing the mechanism by which the “protective” allele acts as compared to the “risk” allele. The protective allele significantly correlated with decreased fibrosis and TGFβ levels in dystrophic murine muscles with a mixed DBA/2J-129T2/SvEmsJ genetic background [17].
In humans, four SNPs (rs2303729, rs1131620, rs1051303 and rs10880) create non-synonymous polymorphisms (V194I, T787A, T820A, and T1140M) in the coding region of the human LTBP4 gene, discriminating two different haplotypes, the VTTT allele (risk) and the IAAM allele (protective). In non-ambulatory DMD patients, homozygous carriers of the IAAM protective LTBP4 allele lost ambulation significantly later than the VTTT risk allele carriers, following a recessive model for the protective allele. Importantly, when restricting the analysis to patients on a glucocorticoid steroid regimen, the age gap in ambulation loss between the two haplotypes increased to almost two years [43]. The association between the protective allele and prolonged ambulation was confirmed in a multivariate analysis on a multi-center DMD patient cohort [29]. The beneficial association was also reported in the European/European-American sub-cohort from another multi-center DMD natural history study [28].
Recently, LTBP4 has also been suggested to be a genetic modifier of dilated cardiomyopathy in DMD patients. A multi-center, longitudinal study was conducted in DMD patients, where left ventricular ejection fraction and end diastolic volume were reported as cardiac parameters. The T/T allele for SNP rs10880 (specifying a methionine at position 1140; part of the protective haplotype) associated with a protective trend and a later onset of DCM in steroid-treated patients [44]. In addition, a retrospective study on DCM risk was conducted on patient groups stratified according to self-identified ethnicity. This study found that the VTTT risk allele associated with increased DCM risk in European Americans, but not African Americans [45]. The two different human alleles of LTBP4 bind TGFβ with different avidity. Specifically, the IAAM protein bound more latent TGFβ than the VTTT allele [45], which would effectively limit the levels of free TGFβ in injured muscle. In addition, LTBP4 can be considered a multi-TGFβ family ligand binding protein, as it also binds and sequesters the latent forms of myostatin and GDF11, a protein highly related to myostatin [46], further enhancing its anti-wasting role in dystrophic muscle.
The role of LTBP4 in tuning a “hyper-TGFβ” state is particularly intriguing, as this pathway regulates both degeneration and regeneration of striated muscle. Unbiased transcriptional profiling of DMD muscle biopsies in presymptomatic and symptomatic individuals revealed a strong induction of the TGFβ pathway in dystrophic muscle [47]. Accordingly, transcriptional profiling of regenerating muscle revealed TGFβ1 among the top differentially expressed ligands during muscle regeneration [48, 49]. The TGFβ pathway promotes expansion of fibroblasts and myofiber replacement by fibrotic tissue through a feed-forward cycle that relies on, among others, miR-21 [50] and periostin [51] upregulation. Furthermore, it is known that TGFβ upregulation is highly detrimental for activation and regenerative potential of resident myoblasts [52]. However, little is known about the effects of LTBP4 on muscle regeneration.
Thus, LTBP4 modifies progression of dystrophic disease by regulating the availability of latent TGFβ around injured myofibers, thereby controlling fibrosis and regeneration, two key features of dystrophic muscles (Figure 1). The multi-ligand binding property of LTBP4 will require more detailed investigation to elucidate the different roles of these different sites in the heterogeneous field of muscle disease. In addition, additional study is necessary to understand whether LTBP4 plays a role in also other conditions related to muscle wasting and atrophy, such as cachexia.
Jagged1: a novel genetic modifier
Jagged1 encodes the trans-membrane ligand of Notch receptors. Jagged1-Notch signaling can be either cell-extrinsic or cell-intrinsic, as both ligand and receptor are transmembrane and the Notch receptor is activated upon physical interaction with its ligands. Activation of the Notch receptor by its ligands, including Jagged1, results in receptor cleavage and migration of its intracellular domain to the nucleus, where it exerts transcriptional regulation in combination with tissue-specific binding partners [53].
Jagged1 has recently been implicated as a genetic modifier of muscular dystrophy by means of whole genome sequencing of dystrophic dogs with variable outcomes. The study compared two “escaper” animals that carried the same loss-of-function dystrophin mutation as other dystrophic dogs. However, the escaper animals had normal lifespan and mild muscle degeneration compared to related dystrophic dogs derived from the same colony. Moreover, progeny from the escaper animals demonstrated transmission of the protective effect to subsequent generations consistent with a genetically-mediated protective effect. Whole genome sequencing data were integrated with muscle transcriptional profiling to uncover a spontaneously occurring mutation in the promoter of Jagged1, just upstream of a CpG island. The mutation introduced a myogenin-responsive element, thereby increasing Jagged1 expression levels within the muscle of the escaper dogs. Furthermore, overexpression of Jagged1 in a zebrafish model of dystrophin deficiency resulted in rescue of the phenotype [15].
Jagged1 upregulation in muscle may ameliorate dystrophic progression through several means. First, muscle regenerative cells from escaper dogs showed greater proliferative capacity [15]. Interestingly, Jagged1 is not expressed in quiescent satellite cells, but is rapidly activated upon cell activation [54]. Accordingly, Notch signaling is a potent regulator of muscle regeneration and is gradually lost in aging muscles [55]. In addition, Notch signaling regulation finely controls not only quiescence and activation of satellite cells [56], but also myogenic ability and engraftment of other stem cells, such as mesenchymal stem cells [57] and resident pericytes [58]. However, the precise signaling pathway linking Jagged1 to its effects on resident myoblasts is still not fully known. Moreover, the question of when Jagged1 upregulation must occur to appropriately expand the stem cell pool, e.g. during fetal development versus after birth, is still open.
However, Jagged1 may exert its effects on myofibers and myofibroblasts as well. In agreement with this hypothesis, Notch and TGFβ pathways are probably linked by complex crosstalk dynamics. The Jagged1-Notch pathway was found sufficient to inhibit TGFβ signaling and fibrotic potential of cardiac fibroblasts [59]. In addition, Notch activation blocks TGFβ signaling via Smad7 upregulation in epithelial stem cells [60]. However, how these pathways intertwine in dystrophic muscles must still be comprehensively assessed. Moreover, it will be important to identify which mechanisms are cell-intrinsic or –extrinsic, and in the latter case, to discriminate the cellular compartments associated with ligand presentation and signaling response.
Genetic modifiers in the context of novel drug development
Identification of genetic modifiers can be useful to identify novel pharmacological targets or pathways to counteract dystrophic progression. However, the basic-to-translational path generally hinges on articulated knowledge of mechanisms and the context in which these target modifiers act. Development of novel medicinal products specifically targeting those pathways in dystrophic muscles will require a greater understanding of the upstream and downstream cascades in each of these pathways with focus on intersecting points in their regulatory pathways. Overall, the genetic data support that upregulating Jagged1 and downregulating osteopontin may be beneficial to the dystrophic muscle, although timing and extent of a putative induced regulation must still be thoroughly addressed as therapeutic strategies for DMD. In the case of Jagged1, this upregulation would ideally be muscle targeted to avoid toxic effects from potentially engaging this pathway in extra-muscle tissues. In the case of osteopontin, a body-wide, constitutive deletion of osteopontin was effective in the mdx mouse [14], suggesting that cell types beyond muscle may be critical for mediating its effect.
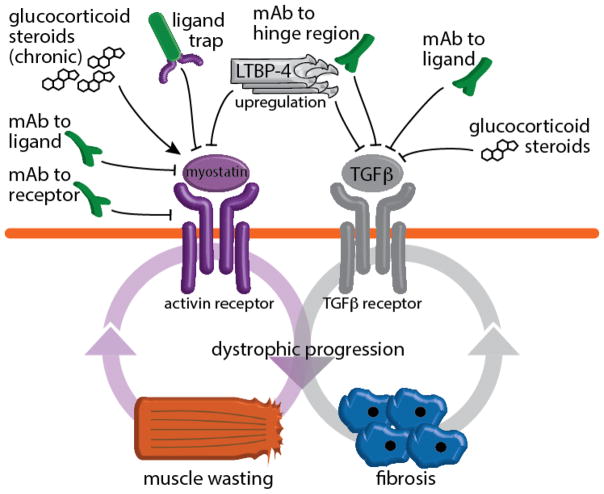
Investigational approaches to neutralizing TGFβ and myostatin (Figure 2) signaling have captured significant attention for their translational potential [61]. Several approaches are currently being considered as avenues to diminish TGFβ signaling in muscular dystrophy and other muscle wasting disorders. In addition, indirect approaches rely, among others, on modulation of LTBP4. Upregulation of the protective LTBP4 allele in dystrophic myofibers improved performance and partially corrected histopathology of dystrophin-deficient murine muscles [46]. Although indirect, LTBP4-involving strategies have the advantage of encompassing the latent forms of all TGFβ ligand isoforms and potentially myostatin. However, whether this holds true in DMD patient muscles must still be appropriately addressed.
Figure 2. Pharmacological strategies to reduce muscle wasting and fibrosis in dystrophic muscles.
TGFβ and myostatin cascades and their intersection with muscle wasting and fibrosis. A number of investigational drugs are currently being tested for reducing both signaling pathways. Glucocorticoid steroids, currently used in DMD treatment, have opposite effects on TGFβ and myostatin activation.
More direct strategies include monoclonal antibodies targeting TGFβ ligands and the inhibitor of the TGFβ receptor kinase, a major effector of the activated TGFβ receptor. Short-term injection of anti-TGFβ monoclonal antibody in the mdx model effectively reduced fibrosis in the diaphragm muscle, a major muscle of respiration and one muscle that shows profound histopathological findings in the mdx mouse [62]. However, a significant increase in CD4+ lymphocytes was concomitantly observed in antibody injected mdx mice [62]. A clinical-grade anti-TGFβ antibody, fresolimumab, is being evaluated in clinical trials for pulmonary fibrosis, systemic sclerosis, and cancer with promising results [63]. TGFβ receptor kinase inhibitor Ki26894 is a small molecule, suitable for oral administration, and partially restores weakness and regenerative potential in a murine model of muscle wasting [64]. However, both strategies presently lack clinical studies designed to target and assess skeletal muscle remodeling. Furthermore, the long-term effects of interfering with TGFβ on normal immune tolerance, especially on the homeostasis of regulatory lymphocytes, must be assessed.
Among novel therapeutic strategies targeting TGFβ family ligands, targeting myostatin is arguably the most advanced for muscle disease. Myostatin is a TGFβ family member that negatively regulates muscle mass, and genetic ablation of myostatin attenuates dystrophic progression in mdx mice [65]. A monoclonal antibody against the mature ligand form was shown to have beneficial effects on muscle mass and strength of dystrophic mice [66]. However, the role of myostatin in dystrophic degeneration is probably nuanced, as a recent study in dystrophic dogs found that heterozygous ablation of myostatin resulted in disproportionate effects on muscle size and, ultimately, in worsening of the condition [67]. Furthermore, genetic ablation of myostatin in non-dystrophic mice resulted in smaller tendons, which presented a decrease in both fibroblast density and expression of type I collagen, as likely results of decreased p38-Smad2/3 cascade activation [68]. These results warrant caution on strategies for unbalanced and profound myostatin inhibition in dystrophic muscles.
Clinical-grade anti-myostatin monoclonal antibodies are currently being tested in clinical trials. Specifically, Regeneron (Tarrytown, NY) is developing a fully humanized antibody (REGN1033; study #NCT01963598), which has been tested in a randomized, double-blind, placebo-controlled, multicenter phase-II study as a subcutaneous formulation in patients with sarcopenia, although results are still pending. Eli Lilly (Indianapolis, IN) has completed phase-II studies with a humanized antibody (LY2495655) in cancer patients with cachexia (#NCT01505530), also with yet undisclosed results. Intriguingly, a previous phase-II study testing the same antibody in elderly subjects with muscle wasting showed a significant increase in lean muscle mass and partial restoration of muscle power [69]. In addition, Pfizer (New York City, NY) is testing a humanized antibody (PF-06252616; study #NCT02310763) and is currently recruiting DMD patients for a phase-II study. Finally, Scholar Rock (Cambridge, MA) is conducting preclinical evaluation of a monoclonal inhibitory antibody targeting the latent form of myostatin (SRK-015), although its specifications and indications are still undisclosed.
An alternative approach relies on blocking the interaction between myostatin and its receptor, particularly the Activin receptor type IIb (ActRIIb). One such strategy is the ligand trap, namely a soluble, immunoglobulin-hybrid of the extracellular portion of the receptor. The ligand trap competitively sequesters myostatin, reducing its downstream signaling in myofibers. Systemic delivery of anti-myostatin ligand trap induced functional improvement and injury reduction in mdx skeletal muscle [70]. Acceleron Pharma (Cambridge, MA) is developing the clinical-grade formulation of a soluble myostatin receptor (ACE-083), currently assessing its safety and tolerability in a phase-I study (#NCT02257489). The same company is also in preclinical development of a multi-GDF ligand trap (ACE-2494), although its indications and plans for further implementation are not yet disclosed.
Inhibitory monoclonal antibodies against the ActRIIb receptor represent a related strategy. Anti-receptor antibodies proved efficacious in slowing muscle mass loss in mice and patients with cachexia [71]. The clinical-grade formulation of anti-ActRIIb antibody (BYM338), developed by Novartis (Basel, Switzerland), has proven effective in increasing lean muscle mass and strength in a randomized, placebo-controlled trial on a limited number (14) of patients with sporadic inclusion body myositis [72]. However, it was subsequently revealed that the phase 2b/3 clinical trial of BYM388 in patients with sporadic inclusion body myositis did not meet its primary endpoint [73]. Nonetheless, the antibody is still being tested on aging-associated sarcopenia in a multi-center study (#NCT01601600), but results are still pending.
Furthermore, an increasing body of evidence links the glucocorticoid steroids to the TGFβ-myostatin circuitry. Glucocorticoid steroids effectively delay loss of ambulation in DMD patients [74–79]. This effect is further increased in the presence of protective SPP1 and LTBP4 polymorphisms. Although the primary focus of the study of Bello and colleagues was to investigate the effect size of SPP1 and LTBP4 polymorphisms on DMD progression, Cox regression analysis indicated that steroids associated with delayed ambulation loss of up to 0.7 years in addition to the protective effect of SPP1 rs28357094 SNP (T/T), and up to 1.0 year in addition to the protective LTBP4 haplotype [28]. Similarly, the van den Bergen study showed that steroid use associated with a significant delay in ambulation loss regardless of genotype association study [29]. With respect to SPP1 and LTBP4 polymorphisms, steroids showed no significant interaction with the protective SPP1 SNP, while the additive effect was significant in patients with the protective LTBP4 haplotype. Furthermore, glucocorticoid steroids seemingly play a role in delaying or alleviating dilated cardiomyopathy (DCM) in DMD patients [80–83]. However, this notion is debated, as studies in mouse models suggest a detrimental role of chronic steroid administration in dystrophic hearts [84–86]. The question of whether genetic modifiers play a role in these divergent effects is still open. In a genetic association study focused on DCM in DMD patients, treatment with steroids did not have a significant independent effect on DCM onset. However, only in steroid-treated patients did the LTBP4 rs10880 SNP (T/T) reach statistical significance in protecting DMD patients from DCM onset [44].
With regards to mechanistic effects on TGFβ/myostatin signaling, glucocorticoid steroids are known to reduce TGFβ levels in mdx skeletal muscle [87]. However, chronic dosing of these steroids has a wide array of negative effects, including myostatin activation and consequently muscle atrophy [88]. Considering that glucocorticoid steroids are presently standard of care for DMD patients, it might be possible to combine myostatin inhibition with steroid regimens in order to harness the beneficial effects of glucocorticoid treatment, while hampering the loss in muscle mass. Furthermore, glucocorticoid regimens may synergize with those genetic modifiers that decrease the TGFβ-myostatin cascades, as with the protective LTBP4 haplotype [29]. However, a deeper understanding of the mechanisms linking glucocorticoid steroids to TGFβ-myostatin cascades in dystrophic myofibers is required to substantiate these hypotheses, to refine current steroid regimens for DMD patients, and to integrate glucocorticoid steroids with pharmacological TGFβ reduction.
Conclusions and future perspectives: breaking the loop of dystrophic progression
In summary, a growing body of evidence is delineating genetic modifiers that regulate the feed-forward loop of muscle wasting and fibrosis that are hallmarks of DMD pathological progression. TGFβ and myostatin cascades mechanistically converge in the promotion of fibrosis and loss of muscle mass, while simultaneously impairing repair and regeneration. It is important to address the question of how osteopontin contributes to the TGFβ-promoted degenerative loop to potentially indicate yet another valuable avenue for pharmacological treatment of dystrophic remodeling. Hypothetically, osteopontin and TGFβ may reinforce each other thereby accelerating the injury-inflammation-fibrosis loop. Analogously, the compelling results obtained from genomic screening of dystrophic dogs corroborate mechanistic studies on the role of Jagged1 in dystrophic myofiber damage.
These pathways provide unique opportunities for development and testing of novel medicinal products to combat degeneration and fibrosis. Detailed knowledge of genetic modifiers in a wide variety of muscle conditions undoubtedly moves the translational field forward, as is the case for the TGFβ family ligands. Currently, post-hoc analyses according to modifying haplotypes are already recommended by FDA guidelines for drug clinical trials in DMD [89]. Careful assessment of numerous clinical parameters in current clinical trials will yield valuable results to validate or further tailor investigational products for DMD treatment. Importantly, comprehensive strategies integrating diverse approaches and effects will most likely produce the highest curative value in clinical settings, particularly in light of the high genetic and clinical heterogeneity of dystrophic patients.
In conclusion, seminal identification and mechanistic understanding of genetic modifiers are re-shaping our knowledge of muscular dystrophy and significantly priming the therapeutic quest for this yet incurable disease.
Highlights.
Genetic modifiers change the course of Duchenne Muscular Dystrophy (DMD)
Genetic modifiers were identified in humans and mice with muscular dystrophy
LTBP4 modifies muscular dystrophy in mice and humans with muscular dystrophy
Osteopontin modifies muscular dystrophy
Jagged1 changes the course of muscular dystrophy
Acknowledgments
Supported by NIH AR052646 (Wellstone Center for Muscular Dystrophy Research), NIH HL61322, NIH NS027072 and the Parent Project Muscular Dystrophy Foundation.
Footnotes
Conflicts of Interest: EMM has provided consulting services for Novartis, Invitae, Mitobridge, Summitplc, AstraZeneca, and Pfizer and served as a data safety monitor for Eli Lilly and Fibrogen, and has patent application 13/957,100 “Mitigating tissue damage and fibrosis via latent TGFβ protein (LTBP4).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Norwood FL, Harling C, Chinnery PF, Eagle M, Bushby K, Straub V. Prevalence of genetic muscle disease in Northern England: in-depth analysis of a muscle clinic population. Brain. 2009;132:3175–3186. doi: 10.1093/brain/awp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emery AE. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 3.Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2:90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 4.Mercier S, Toutain A, Toussaint A, Raynaud M, de Barace C, Marcorelles P, Pasquier L, Blayau M, Espil C, Parent P, Journel H, Lazaro L, Andoni Urtizberea J, Moerman A, Faivre L, Eymard B, Maincent K, Gherardi R, Chaigne D, Ben Yaou R, Leturcq F, Chelly J, Desguerre I. Genetic and clinical specificity of 26 symptomatic carriers for dystrophinopathies at pediatric age. Eur J Hum Genet. 2013;21:855–863. doi: 10.1038/ejhg.2012.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanigan KM, Dunn DM, von Niederhausern A, Soltanzadeh P, Gappmaier E, Howard MT, Sampson JB, Mendell JR, Wall C, King WM, Pestronk A, Florence JM, Connolly AM, Mathews KD, Stephan CM, Laubenthal KS, Wong BL, Morehart PJ, Meyer A, Finkel RS, Bonnemann CG, Medne L, Day JW, Dalton JC, Margolis MK, Hinton VJ, Weiss RB C. United Dystrophinopathy Project. Mutational spectrum of DMD mutations in dystrophinopathy patients: application of modern diagnostic techniques to a large cohort. Hum Mutat. 2009;30:1657–1666. doi: 10.1002/humu.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humbertclaude V, Hamroun D, Bezzou K, Berard C, Boespflug-Tanguy O, Bommelaer C, Campana-Salort E, Cances C, Chabrol B, Commare MC, Cuisset JM, de Lattre C, Desnuelle C, Echenne B, Halbert C, Jonquet O, Labarre-Vila A, N’Guyen-Morel MA, Pages M, Pepin JL, Petitjean T, Pouget J, Ollagnon-Roman E, Richelme C, Rivier F, Sacconi S, Tiffreau V, Vuillerot C, Picot MC, Claustres M, Beroud C, Tuffery-Giraud S. Motor and respiratory heterogeneity in Duchenne patients: implication for clinical trials. Eur J Paediatr Neurol. 2012;16:149–160. doi: 10.1016/j.ejpn.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Zatz M, Pavanello RC, Lazar M, Yamamoto GL, Lourenco NC, Cerqueira A, Nogueira L, Vainzof M. Milder course in Duchenne patients with nonsense mutations and no muscle dystrophin. Neuromuscular disorders: NMD. 2014;24:986–989. doi: 10.1016/j.nmd.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 10.Coley WD, Bogdanik L, Vila MC, Yu Q, Van Der Meulen JH, Rayavarapu S, Novak JS, Nearing M, Quinn JL, Saunders A, Dolan C, Andrews W, Lammert C, Austin A, Partridge TA, Cox GA, Lutz C, Nagaraju K. Effect of genetic background on the dystrophic phenotype in mdx mice. Human molecular genetics. 2016;25:130–145. doi: 10.1093/hmg/ddv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calyjur PC, de Almeida CF, Ayub-Guerrieri D, Ribeiro AF, Jr, de Fernandes SA, Ishiba R, Santos AL, Onofre-Oliveira P, Vainzof M. The mdx Mutation in the 129/Sv Background Results in a Milder Phenotype: Transcriptome Comparative Analysis Searching for the Protective Factors. PloS one. 2016;11:e0150748. doi: 10.1371/journal.pone.0150748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vo AH, McNally EM. Modifier genes and their effect on Duchenne muscular dystrophy. Current opinion in neurology. 2015;28:528–534. doi: 10.1097/WCO.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Accornero F, Kanisicak O, Tjondrokoesoemo A, Attia AC, McNally EM, Molkentin JD. Myofiber-specific inhibition of TGFbeta signaling protects skeletal muscle from injury and dystrophic disease in mice. Human molecular genetics. 2014;23:6903–6915. doi: 10.1093/hmg/ddu413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vetrone SA, Montecino-Rodriguez E, Kudryashova E, Kramerova I, Hoffman EP, Liu SD, Miceli MC, Spencer MJ. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. The Journal of clinical investigation. 2009;119:1583–1594. doi: 10.1172/JCI37662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vieira NM, Elvers I, Alexander MS, Moreira YB, Eran A, Gomes JP, Marshall JL, Karlsson EK, Verjovski-Almeida S, Lindblad-Toh K, Kunkel LM, Zatz M. Jagged 1 Rescues the Duchenne Muscular Dystrophy Phenotype. Cell. 2015;163:1204–1213. doi: 10.1016/j.cell.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hack AA, Ly CT, Jiang F, Clendenin CJ, Sigrist KS, Wollmann RL, McNally EM. Gamma-sarcoglycan deficiency leads to muscle membrane defects and apoptosis independent of dystrophin. The Journal of cell biology. 1998;142:1279–1287. doi: 10.1083/jcb.142.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heydemann A, Ceco E, Lim JE, Hadhazy M, Ryder P, Moran JL, Beier DR, Palmer AA, McNally EM. Latent TGF-beta-binding protein 4 modifies muscular dystrophy in mice. The Journal of clinical investigation. 2009;119:3703–3712. doi: 10.1172/JCI39845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swaggart KA, Demonbreun AR, Vo AH, Swanson KE, Kim EY, Fahrenbach JP, Holley-Cuthrell J, Eskin A, Chen Z, Squire K, Heydemann A, Palmer AA, Nelson SF, McNally EM. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6004–6009. doi: 10.1073/pnas.1324242111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YW, Zhao P, Borup R, Hoffman EP. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. The Journal of cell biology. 2000;151:1321–1336. doi: 10.1083/jcb.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haslett JN, Sanoudou D, Kho AT, Bennett RR, Greenberg SA, Kohane IS, Beggs AH, Kunkel LM. Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15000–15005. doi: 10.1073/pnas.192571199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tkatchenko AV, Le Cam G, Leger JJ, Dechesne CA. Large-scale analysis of differential gene expression in the hindlimb muscles and diaphragm of mdx mouse. Biochimica et biophysica acta. 2000;1500:17–30. doi: 10.1016/s0925-4439(99)00084-8. [DOI] [PubMed] [Google Scholar]
- 22.Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, Leahy P, Li J, Guo W, Andrade FH. A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Human molecular genetics. 2002;11:263–272. doi: 10.1093/hmg/11.3.263. [DOI] [PubMed] [Google Scholar]
- 23.Turk R, Sterrenburg E, van der Wees CG, de Meijer EJ, de Menezes RX, Groh S, Campbell KP, Noguchi S, van Ommen GJ, den Dunnen JT, t Hoen PA. Common pathological mechanisms in mouse models for muscular dystrophies. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20:127–129. doi: 10.1096/fj.05-4678fje. [DOI] [PubMed] [Google Scholar]
- 24.Galindo CL, Soslow JH, Brinkmeyer-Langford CL, Gupte M, Smith HM, Sengsayadeth S, Sawyer DB, Benson DW, Kornegay JN, Markham LW. Translating golden retriever muscular dystrophy microarray findings to novel biomarkers for cardiac/skeletal muscle function in Duchenne muscular dystrophy. Pediatric research. 2016;79:629–636. doi: 10.1038/pr.2015.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanotti S, Gibertini S, Di Blasi C, Cappelletti C, Bernasconi P, Mantegazza R, Morandi L, Mora M. Osteopontin is highly expressed in severely dystrophic muscle and seems to play a role in muscle regeneration and fibrosis. Histopathology. 2011;59:1215–1228. doi: 10.1111/j.1365-2559.2011.04051.x. [DOI] [PubMed] [Google Scholar]
- 26.Pegoraro E, Hoffman EP, Piva L, Gavassini BF, Cagnin S, Ermani M, Bello L, Soraru G, Pacchioni B, Bonifati MD, Lanfranchi G, Angelini C, Kesari A, Lee I, Gordish-Dressman H, Devaney JM, McDonald CM. SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy. Neurology. 2011;76:219–226. doi: 10.1212/WNL.0b013e318207afeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bello L, Piva L, Barp A, Taglia A, Picillo E, Vasco G, Pane M, Previtali SC, Torrente Y, Gazzerro E, Motta MC, Grieco GS, Napolitano S, Magri F, D’Amico A, Astrea G, Messina S, Sframeli M, Vita GL, Boffi P, Mongini T, Ferlini A, Gualandi F, Soraru G, Ermani M, Vita G, Battini R, Bertini E, Comi GP, Berardinelli A, Minetti C, Bruno C, Mercuri E, Politano L, Angelini C, Hoffman EP, Pegoraro E. Importance of SPP1 genotype as a covariate in clinical trials in Duchenne muscular dystrophy. Neurology. 2012;79:159–162. doi: 10.1212/WNL.0b013e31825f04ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bello L, Kesari A, Gordish-Dressman H, Cnaan A, Morgenroth LP, Punetha J, Duong T, Henricson EK, Pegoraro E, McDonald CM, Hoffman EP. Genetic modifiers of ambulation in the Cooperative International Neuromuscular Research Group Duchenne Natural History Study. Annals of neurology. 2015;77:684–696. doi: 10.1002/ana.24370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Bergen JC, Hiller M, Bohringer S, Vijfhuizen L, Ginjaar HB, Chaouch A, Bushby K, Straub V, Scoto M, Cirak S, Humbertclaude V, Claustres M, Scotton C, Passarelli C, Lochmuller H, Muntoni F, Tuffery-Giraud S, Ferlini A, Aartsma-Rus AM, Verschuuren JJ, t Hoen PA, Spitali P. Validation of genetic modifiers for Duchenne muscular dystrophy: a multicentre study assessing SPP1 and LTBP4 variants. Journal of neurology, neurosurgery and psychiatry. 2015;86:1060–1065. doi: 10.1136/jnnp-2014-308409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capote J, Kramerova I, Martinez L, Vetrone S, Barton ER, Sweeney HL, Miceli MC, Spencer MJ. Osteopontin ablation ameliorates muscular dystrophy by shifting macrophages to a pro-regenerative phenotype. The Journal of cell biology. 2016;213:275–288. doi: 10.1083/jcb.201510086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uaesoontrachoon K, Yoo HJ, Tudor EM, Pike RN, Mackie EJ, Pagel CN. Osteopontin and skeletal muscle myoblasts: association with muscle regeneration and regulation of myoblast function in vitro. The international journal of biochemistry & cell biology. 2008;40:2303–2314. doi: 10.1016/j.biocel.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Uaesoontrachoon K, Wasgewatte Wijesinghe DK, Mackie EJ, Pagel CN. Osteopontin deficiency delays inflammatory infiltration and the onset of muscle regeneration in a mouse model of muscle injury. Disease models & mechanisms. 2013;6:197–205. doi: 10.1242/dmm.009993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giacopelli F, Marciano R, Pistorio A, Catarsi P, Canini S, Karsenty G, Ravazzolo R. Polymorphisms in the osteopontin promoter affect its transcriptional activity. Physiological genomics. 2004;20:87–96. doi: 10.1152/physiolgenomics.00138.2004. [DOI] [PubMed] [Google Scholar]
- 34.Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix biology: journal of the International Society for Matrix Biology. 2000;19:615–622. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- 35.Piva L, Gavassini BF, Bello L, Fanin M, Soraru G, Barp A, Ermani M, Angelini C, Hoffman EP, Pegoraro E. TGFBR2 but not SPP1 genotype modulates osteopontin expression in Duchenne muscular dystrophy muscle. The Journal of pathology. 2012;228:251–259. doi: 10.1002/path.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barfield WL, Uaesoontrachoon K, Wu CS, Lin S, Chen Y, Wang PC, Kanaan Y, Bond V, Hoffman EP. Eccentric muscle challenge shows osteopontin polymorphism modulation of muscle damage. Human molecular genetics. 2014;23:4043–4050. doi: 10.1093/hmg/ddu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang D, Yamamoto S, Hijiya N, Benveniste EN, Gladson CL. Transcriptional regulation of the human osteopontin promoter: functional analysis and DNA-protein interactions. Oncogene. 2000;19:5801–5809. doi: 10.1038/sj.onc.1203917. [DOI] [PubMed] [Google Scholar]
- 38.Weber CE, Kothari AN, Wai PY, Li NY, Driver J, Zapf MA, Franzen CA, Gupta GN, Osipo C, Zlobin A, Syn WK, Zhang J, Kuo PC, Mi Z. Osteopontin mediates an MZF1-TGF-beta1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene. 2015;34:4821–4833. doi: 10.1038/onc.2014.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hullinger TG, Pan Q, Viswanathan HL, Somerman MJ. TGFbeta and BMP-2 activation of the OPN promoter: roles of smad- and hox-binding elements. Experimental cell research. 2001;262:69–74. doi: 10.1006/excr.2000.5074. [DOI] [PubMed] [Google Scholar]
- 40.Kubota T, Zhang Q, Wrana JL, Ber R, Aubin JE, Butler WT, Sodek J. Multiple forms of SppI (secreted phosphoprotein, osteopontin) synthesized by normal and transformed rat bone cell populations: regulation by TGF-beta. Biochemical and biophysical research communications. 1989;162:1453–1459. doi: 10.1016/0006-291x(89)90837-1. [DOI] [PubMed] [Google Scholar]
- 41.Bello L, Flanigan KM, Weiss RB, Spitali P, Aartsma-Rus A, Muntoni F, Zaharieva I, Ferlini A, Mercuri E, Tuffery-Giraud S, Claustres M, Straub V, Lochmuller H, Barp A, Vianello S, Pegoraro E, Punetha J, Gordish-Dressman H, Giri M, McDonald CM, Hoffman EP United Dystrophinopathy P G. Cooperative International Neuromuscular Research. Association Study of Exon Variants in the NF-kappaB and TGFbeta Pathways Identifies CD40 as a Modifier of Duchenne Muscular Dystrophy. American journal of human genetics. 2016;99:1163–1171. doi: 10.1016/j.ajhg.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giltay R, Kostka G, Timpl R. Sequence and expression of a novel member (LTBP-4) of the family of latent transforming growth factor-beta binding proteins. FEBS letters. 1997;411:164–168. doi: 10.1016/s0014-5793(97)00685-6. [DOI] [PubMed] [Google Scholar]
- 43.Flanigan KM, Ceco E, Lamar KM, Kaminoh Y, Dunn DM, Mendell JR, King WM, Pestronk A, Florence JM, Mathews KD, Finkel RS, Swoboda KJ, Gappmaier E, Howard MT, Day JW, McDonald C, McNally EM, Weiss RB. LTBP4 genotype predicts age of ambulatory loss in Duchenne muscular dystrophy. Annals of neurology. 2013;73:481–488. doi: 10.1002/ana.23819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barp A, Bello L, Politano L, Melacini P, Calore C, Polo A, Vianello S, Soraru G, Semplicini C, Pantic B, Taglia A, Picillo E, Magri F, Gorni K, Messina S, Vita GL, Vita G, Comi GP, Ermani M, Calvo V, Angelini C, Hoffman EP, Pegoraro E. Genetic Modifiers of Duchenne Muscular Dystrophy and Dilated Cardiomyopathy. PloS one. 2015;10:e0141240. doi: 10.1371/journal.pone.0141240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamar KM, Miller T, Dellefave-Castillo L, McNally EM. Genotype-Specific Interaction of Latent TGFbeta Binding Protein 4 with TGFbeta. PloS one. 2016;11:e0150358. doi: 10.1371/journal.pone.0150358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamar KM, Bogdanovich S, Gardner BB, Gao QQ, Miller T, Earley JU, Hadhazy M, Vo AH, Wren L, Molkentin JD, McNally EM. Overexpression of Latent TGFbeta Binding Protein 4 in Muscle Ameliorates Muscular Dystrophy through Myostatin and TGFbeta. PLoS genetics. 2016;12:e1006019. doi: 10.1371/journal.pgen.1006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YW, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R, Hoffman EP. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology. 2005;65:826–834. doi: 10.1212/01.wnl.0000173836.09176.c4. [DOI] [PubMed] [Google Scholar]
- 48.Goetsch SC, Hawke TJ, Gallardo TD, Richardson JA, Garry DJ. Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiological genomics. 2003;14:261–271. doi: 10.1152/physiolgenomics.00056.2003. [DOI] [PubMed] [Google Scholar]
- 49.Dadgar S, Wang Z, Johnston H, Kesari A, Nagaraju K, Chen YW, Hill DA, Partridge TA, Giri M, Freishtat RJ, Nazarian J, Xuan J, Wang Y, Hoffman EP. Asynchronous remodeling is a driver of failed regeneration in Duchenne muscular dystrophy. The Journal of cell biology. 2014;207:139–158. doi: 10.1083/jcb.201402079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ardite E, Perdiguero E, Vidal B, Gutarra S, Serrano AL, Munoz-Canoves P. PAI-1-regulated miR-21 defines a novel age-associated fibrogenic pathway in muscular dystrophy. The Journal of cell biology. 2012;196:163–175. doi: 10.1083/jcb.201105013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lorts A, Schwanekamp JA, Baudino TA, McNally EM, Molkentin JD. Deletion of periostin reduces muscular dystrophy and fibrosis in mice by modulating the transforming growth factor-beta pathway. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10978–10983. doi: 10.1073/pnas.1204708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allen RE, Boxhorn LK. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. Journal of cellular physiology. 1987;133:567–572. doi: 10.1002/jcp.1041330319. [DOI] [PubMed] [Google Scholar]
- 53.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gnocchi VF, White RB, Ono Y, Ellis JA, Zammit PS. Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PloS one. 2009;4:e5205. doi: 10.1371/journal.pone.0005205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 56.Mourikis P, Tajbakhsh S. Distinct contextual roles for Notch signalling in skeletal muscle stem cells. BMC Dev Biol. 2014;14:2. doi: 10.1186/1471-213X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 58.Quattrocelli M, Costamagna D, Giacomazzi G, Camps J, Sampaolesi M. Notch signaling regulates myogenic regenerative capacity of murine and human mesoangioblasts. Cell death & disease. 2014;5:e1448. doi: 10.1038/cddis.2014.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sassoli C, Chellini F, Pini A, Tani A, Nistri S, Nosi D, Zecchi-Orlandini S, Bani D, Formigli L. Relaxin prevents cardiac fibroblast-myofibroblast transition via notch-1-mediated inhibition of TGF-beta/Smad3 signaling. PloS one. 2013;8:e63896. doi: 10.1371/journal.pone.0063896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsai TH, Sun MH, Ho TC, Ma HI, Liu MY, Tsao YP. Notch prevents transforming growth factor-beta-assisted epithelial-mesenchymal transition in cultured limbal progenitor cells through the induction of Smad7. Mol Vis. 2014;20:522–534. [PMC free article] [PubMed] [Google Scholar]
- 61.Garber K. No longer going to waste. Nat Biotechnol. 2016;34:458–461. doi: 10.1038/nbt.3557. [DOI] [PubMed] [Google Scholar]
- 62.Andreetta F, Bernasconi P, Baggi F, Ferro P, Oliva L, Arnoldi E, Cornelio F, Mantegazza R, Confalonieri P. Immunomodulation of TGF-beta 1 in mdx mouse inhibits connective tissue proliferation in diaphragm but increases inflammatory response: implications for antifibrotic therapy. Journal of neuroimmunology. 2006;175:77–86. doi: 10.1016/j.jneuroim.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, Nakerakanti S, York M, Farina G, Whitfield ML, Spiera RF, Christmann RB, Gordon JK, Weinberg J, Simms RW, Lafyatis R. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. The Journal of clinical investigation. 2015;125:2795–2807. doi: 10.1172/JCI77958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohsawa Y, Okada T, Nishimatsu S, Ishizaki M, Suga T, Fujino M, Murakami T, Uchino M, Tsuchida K, Noji S, Hinohara A, Shimizu T, Shimizu K, Sunada Y. An inhibitor of transforming growth factor beta type I receptor ameliorates muscle atrophy in a mouse model of caveolin 3-deficient muscular dystrophy. Laboratory investigation; a journal of technical methods and pathology. 2012;92:1100–1114. doi: 10.1038/labinvest.2012.78. [DOI] [PubMed] [Google Scholar]
- 65.Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Annals of neurology. 2002;52:832–836. doi: 10.1002/ana.10385. [DOI] [PubMed] [Google Scholar]
- 66.Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 67.Kornegay JN, Bogan DJ, Bogan JR, Dow JL, Wang J, Fan Z, Liu N, Warsing LC, Grange RW, Ahn M, Balog-Alvarez CJ, Cotten SW, Willis MS, Brinkmeyer-Langford C, Zhu H, Palandra J, Morris CA, Styner MA, Wagner KR. Dystrophin-deficient dogs with reduced myostatin have unequal muscle growth and greater joint contractures. Skeletal muscle. 2016;6:14. doi: 10.1186/s13395-016-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mendias CL, Bakhurin KI, Faulkner JA. Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:388–393. doi: 10.1073/pnas.0707069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Becker C, Lord SR, Studenski SA, Warden SJ, Fielding RA, Recknor CP, Hochberg MC, Ferrari SL, Blain H, Binder EF, Rolland Y, Poiraudeau S, Benson CT, Myers SL, Hu L, Ahmad QI, Pacuch KR, Gomez EV, Benichou O, Group S. Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015;3:948–957. doi: 10.1016/S2213-8587(15)00298-3. [DOI] [PubMed] [Google Scholar]
- 70.Pistilli EE, Bogdanovich S, Goncalves MD, Ahima RS, Lachey J, Seehra J, Khurana T. Targeting the activin type IIB receptor to improve muscle mass and function in the mdx mouse model of Duchenne muscular dystrophy. The American journal of pathology. 2011;178:1287–1297. doi: 10.1016/j.ajpath.2010.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hatakeyama S, Summermatter S, Jourdain M, Melly S, Minetti GC, Lach-Trifilieff E. ActRII blockade protects mice from cancer cachexia and prolongs survival in the presence of anti-cancer treatments. Skeletal muscle. 2016;6:26. doi: 10.1186/s13395-016-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amato AA, Sivakumar K, Goyal N, David WS, Salajegheh M, Praestgaard J, Lach-Trifilieff E, Trendelenburg AU, Laurent D, Glass DJ, Roubenoff R, Tseng BS, Greenberg SA. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology. 2014;83:2239–2246. doi: 10.1212/WNL.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MorphoSysAG. MorphoSys Provides Update on Results From Partner’s Phase 2b/3 RESILIENT Study of Bimagrumab. 2016 https://www.morphosys.com/media-investors/media-center/morphosys-ag-morphosys-provides-update-on-results-from-partners-phase.
- 74.Bello L, Gordish-Dressman H, Morgenroth LP, Henricson EK, Duong T, Hoffman EP, Cnaan A, McDonald CM Investigators C. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study. Neurology. 2015;85:1048–1055. doi: 10.1212/WNL.0000000000001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ricotti V, Ridout DA, Scott E, Quinlivan R, Robb SA, Manzur AY, Muntoni FN. NorthStar Clinical, Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. Journal of neurology, neurosurgery and psychiatry. 2013;84:698–705. doi: 10.1136/jnnp-2012-303902. [DOI] [PubMed] [Google Scholar]
- 76.Henricson EK, Abresch RT, Cnaan A, Hu F, Duong T, Arrieta A, Han J, Escolar DM, Florence JM, Clemens PR, Hoffman EP, McDonald CM Investigators C. The cooperative international neuromuscular research group Duchenne natural history study: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle & nerve. 2013;48:55–67. doi: 10.1002/mus.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Biggar WD, Gingras M, Fehlings DL, Harris VA, Steele CA. Deflazacort treatment of Duchenne muscular dystrophy. J Pediatr. 2001;138:45–50. doi: 10.1067/mpd.2001.109601. [DOI] [PubMed] [Google Scholar]
- 78.Angelini C, Pegoraro E, Turella E, Intino MT, Pini A, Costa C. Deflazacort in Duchenne dystrophy: study of long-term effect. Muscle & nerve. 1994;17:386–391. doi: 10.1002/mus.880170405. [DOI] [PubMed] [Google Scholar]
- 79.Fenichel GM, Florence JM, Pestronk A, Mendell JR, Moxley RT, 3rd, Griggs RC, Brooke MH, Miller JP, Robison J, King W, et al. Long-term benefit from prednisone therapy in Duchenne muscular dystrophy. Neurology. 1991;41:1874–1877. doi: 10.1212/wnl.41.12.1874. [DOI] [PubMed] [Google Scholar]
- 80.Barber BJ, Andrews JG, Lu Z, West NA, Meaney FJ, Price ET, Gray A, Sheehan DW, Pandya S, Yang M, Cunniff C. Oral corticosteroids and onset of cardiomyopathy in Duchenne muscular dystrophy. J Pediatr. 2013;163:1080–1084 e1081. doi: 10.1016/j.jpeds.2013.05.060. [DOI] [PubMed] [Google Scholar]
- 81.Markham LW, Kinnett K, Wong BL, Woodrow Benson D, Cripe LH. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscular disorders: NMD. 2008;18:365–370. doi: 10.1016/j.nmd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Silversides CK, Webb GD, Harris VA, Biggar DW. Effects of deflazacort on left ventricular function in patients with Duchenne muscular dystrophy. Am J Cardiol. 2003;91:769–772. doi: 10.1016/s0002-9149(02)03429-x. [DOI] [PubMed] [Google Scholar]
- 83.Hussein G, Mansour L, Ghafar HA, Mostafa FA, Fawaz L. Short-term effects of corticosteroid therapy on cardiac and skeletal muscles in muscular dystrophies. J Investig Med. 2014;62:875–879. doi: 10.1097/01.JIM.0000446835.98223.ce. [DOI] [PubMed] [Google Scholar]
- 84.Janssen PM, Murray JD, Schill KE, Rastogi N, Schultz EJ, Tran T, Raman SV, Rafael-Fortney JA. Prednisolone attenuates improvement of cardiac and skeletal contractile function and histopathology by lisinopril and spironolactone in the mdx mouse model of Duchenne muscular dystrophy. PloS one. 2014;9:e88360. doi: 10.1371/journal.pone.0088360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guerron AD, Rawat R, Sali A, Spurney CF, Pistilli E, Cha HJ, Pandey GS, Gernapudi R, Francia D, Farajian V, Escolar DM, Bossi L, Becker M, Zerr P, de la Porte S, Gordish-Dressman H, Partridge T, Hoffman EP, Nagaraju K. Functional and molecular effects of arginine butyrate and prednisone on muscle and heart in the mdx mouse model of Duchenne Muscular Dystrophy. PloS one. 2010;5:e11220. doi: 10.1371/journal.pone.0011220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uaesoontrachoon K, Quinn JL, Tatem KS, Van Der Meulen JH, Yu Q, Phadke A, Miller BK, Gordish-Dressman H, Ongini E, Miglietta D, Nagaraju K. Long-term treatment with naproxcinod significantly improves skeletal and cardiac disease phenotype in the mdx mouse model of dystrophy. Human molecular genetics. 2014;23:3239–3249. doi: 10.1093/hmg/ddu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hartel JV, Granchelli JA, Hudecki MS, Pollina CM, Gosselin LE. Impact of prednisone on TGF-beta1 and collagen in diaphragm muscle from mdx mice. Muscle & nerve. 2001;24:428–432. doi: 10.1002/1097-4598(200103)24:3<428::aid-mus1018>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 88.Wang R, Jiao H, Zhao J, Wang X, Lin H. Glucocorticoids Enhance Muscle Proteolysis through a Myostatin-Dependent Pathway at the Early Stage. PloS one. 2016;11:e0156225. doi: 10.1371/journal.pone.0156225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.P.P.M.D. Guidance for Industry: Duchenne Muscular Dystrophy Developing Drugs for Treatment over the Spectrum of Disease. http://www.parentprojectmd.org/site/DocServer/Guidance_Document_Submission_-_Duchenne_Muscular_Dystrop.pdf?docID=15283.