Abstract
The ventricular-subventricular zone (V-SVZ), which lies in the walls of the lateral ventricles (LV), is the largest neurogenic niche within the adult brain. Whether radiographic contact with the LV influences survival in glioblastoma (GBM) patients remains unclear. We assimilated and analyzed published data comparing survival in GBM patients with (LV+GBM) and without (LV−GBM) radiographic LV contact. PubMed, EMBASE, and Cochrane electronic databases were searched. Fifteen studies with survival data on LV+GBM and LV−GBM patients were identified. Their Kaplan–Meier survival curves were digitized and pooled for generation of median overall (OS) and progression free (PFS) survivals and log-rank hazard ratios (HRs). The log-rank and reported multivariate HRs after accounting for the common predictors of GBM survival were analyzed separately by meta-analyses. The calculated median survivals (months) from pooled data were 12.95 and 16.58 (OS), and 4.54 and 6.25 (PFS) for LV+GBMs and LV−GBMs, respectively, with an overall log-rank HRs of 1.335 [1.204–1.513] (OS) and 1.387 [1.225–1.602] (PFS). Meta-analysis of log-rank HRs resulted in summary HRs of 1.58 [1.35–1.85] (OS, 10 studies) and 1.41 [1.22–1.64] (PFS, 5 studies). Meta-analysis of multivariate HRs resulted in summary HRs of 1.35 [1.14–1.58] (OS, 6 studies) and 1.64 [0.88–3.05] (PFS, 3 studies). Patients with GBM contacting the LV have lower survival. This effect may be independent of the common predictors of GBM survival, suggesting a clinical influence of V-SVZ contact on GBM biology.
Keywords: Ventricular-subventricular zone, V-SVZ, Subventricular zone, SVZ, Glioblastoma, Glioma, Lateral ventricle
Introduction
Glioblastomas (GBMs) are the most common primary adult brain tumor and remain highly therapy-resistant, with a median survival of 16 months despite standard of care treatment [1, 2]. These tumors are comprised of multiple distinct cell populations, including cancer cells with tumor-propagating potential often characterized as glioma stem cells, which have self-renewal properties and are thought to be highly resistant to therapy [3, 4].
Growing evidence suggests a direct relationship between GBM malignancy and tumor proximity to the lateral ventricles (LV) and the ventricular-subventricular zone (V-SVZ). The V-SVZ, which lies in the lateral wall of the LVs, harbors neural stem cells during development and persists into postnatal life as the largest neurogenic niche within the adult brain [5, 6]. In early postnatal human brain, the V-SVZ supports the generation of many highly migratory immature neuroblasts, and this niche retains latent proliferative capacity into adulthood [5–8].
The direct and indirect roles of the V-SVZ in gliomagenesis continue to be widely investigated, including testing of the hypothesis that GBMs with radiographic contact with the LV (LV+GBM), and therefore the V-SVZ, have reduced survival. This was first explored by Lim and collaborators in 2007, who reported that LV+GBMs were more likely to be multifocal and have distal recurrences when compared to those without LV contact (LV−GBM) [9]. Thereafter, the influence on the overall survival of this increased malignancy associated with LV contact has been explored by numerous studies with variable results. This meta-analysis aims to comprehensively review published survival data comparing LV+GBM and LV−GBM and analyze the influence of observed radiographic LV contact on patient survival.
Materials and methods
Search strategy and selection criteria
The common evidence medicine framework PICO was used to formulate the research question: Do patients with GBM (population) contacting the LVs (indicator) compared those without LV contact (comparator) have a shorter overall survival (outcome) with standard-of-care treatment? This meta-analysis was performed by referencing the PRISMA guideline [10]. No review protocol existed at the time of this study; hence, a systematic electronic search of titles and abstracts of published journal articles was conducted (by A.M.M.) using the following terms “(glioma OR gbm OR glioblastoma) AND (ependyma OR ependymal OR ventricle OR ventricles OR subependymal OR “peri-ventricular” OR periventricular OR ventricular OR subventricular OR SVZ OR “lateral ventricle” OR “lateral ventricles”)” in the NCBI/NLM PubMed (from 1966 to Feb 7, 2016), OVID EMBASE (from 1980 to Feb 7, 2016), and Cochrane databases (to Feb 7, 2016) without language restriction. Unpublished studies and conference abstracts were excluded. References obtained from these searches (909 from PubMed 745 from OVID EMBASE, and 6 from Cochrane) were imported into the reference manager EndNote X7 (Thompson Reuters, Philadelphia, PA), during which duplicate references were removed. Titles and abstracts of journal articles were then screened for studies comparing survival data between LV+GBM and LV−GBM patients. Studies of patients treated with SVZ-specific therapies or radiation were excluded due to potential masking of the sought outcome. Six such studies were identified and excluded. The entire screening process was repeated one month later (by A.M.M.) to capture any missing studies. Fifteen final studies [11–25], all with retrospective observational data, were included after full text review of studies identified through the screening process (see Supplemental Figure S1 for detailed flow diagram). The bibliographies of these studies were also searched for additional references but no new articles were included. Where possible, authors were contacted regarding availability of additional non-published data as well as confirmation of published data.
Data extraction
Data were extracted independently by two reviewers (A.M.M. and A.T.H). Numbers of LV+GBM and LV−GBM patients and survival statistics [median or mean overall (OS) and progression free survival (PFS) times and hazard ratios (HRs)] were extracted. Further, when available, HRs were noted if they were obtained from multivariate analyses accounting for common predictors of survival in GBM patients aside from chemo- and radiation therapies—e.g., age, KPS (Karnofsky Performance Scale), and extent of resection. When multiple multivariate analyses were performed, HR resulting from inclusion of the maximum number of variables were recorded. For missing survival data in studies, relevant corresponding authors were contacted. When interpretation of published results differed between A.M.M. and A.T.H, the results were interpreted by R.A.I. for resolution.
Reconstruction of individual patient data
To calculate summary survival statistics (median OS and PFS times and HR by log-rank analysis) in the absence of original individual patient data, published Kaplan–Meier survival curves comparing LV+GBM and LV−GBM patients were used to recreate individual patient data after digitization using WebPlotDigitizer (Version 3.8, May 2015; http://arohatgi.info/WebPlotDigitizer), an accepted methodology for meta-analysis of time-to-event data [26]. Survival time and percent values obtained were used to recreate individual patient survival data, which were in turn used to reconstruct published Kaplan–Meier curves and generate median survival times and HRs by log-rank test using GraphPad Prism version 6.00 for Windows (GraphPad Software, San Diego, CA, USA). Reconstructed curves were then compared with published data to assess accuracy of reconstructed data. When data were categorized according to the group I–IV GBM classification scheme described in Lim et al. [9], data were combined for groups I and II (representing LV+GBMs) and groups III and IV (LV−GBMs). Compared to the published data, near accurate reconstructed Kaplan–Meier curves (Supplementary Figure S2) and summary survival statistics (Supplementary Tables S1 and S2) were generated in all studies except one [19], which was the only study included in the analysis that did not plot censored values on its Kaplan–Meier curves. Therefore, an assumption of no censored values was made when reconstructing individual patient data, as suggested in published methodologies [27], resulting in an error of 7 % in LV+GBM and 3 % in LV−GBM median survival values. Derived individual patient survival data were collated to generate cumulative, representative overall and progression free Kaplan–Meier survival curves as well as calculate summary median survival times and HRs by log-rank test.
Meta-analyses
Meta-analyses were performed by referencing the Cochrane Handbook for Systematic Reviews of Interventions [28]. Survival meta-analyses to generate a summary HR were conducted with the HRs from log-rank and reported multivariate cox proportional analyses using the Comprehensive Meta-Analysis software Version 2.2 (Biostat Inc., Englewood, NJ, USA) for Windows. Survival confidence intervals (CIs) are commonly reported as asymmetric, being calculated from the standard error using an asymmetrical method [29]. In these instances, the reported 95 % CIs were converted to standard error using [ln CIhigher end—ln HR]/1.96. The use of standard errors resulted in generation of symmetric 95 % confidence intervals around the ln HRs. Inverse variance, random effects model was used to perform the meta-analyses and test for statistical significance of the resulting summary HR. Statistical significance was set at p < 0.05. Heterogeneity was assessed using the Cochran Q (Chi2), I2, and Tau2 statistics. A p value <0.1 from the Chi2 test, I2 > 75 %, and Tau2 > 1 indicated considerable heterogeneity [28]. Sensitivity analysis consisted of excluding one study at a time from the meta-analysis to assess significant change in the summary effect size. Potential publication bias was evaluated by generating a funnel plot plotting the HRs against variance. In addition to an asymmetric plot, publication bias was evaluated by Begg and Mazumdar’s test and Egger’s test. A two-tailed p value < 0.05 indicated publication bias. To compensate for the effects of publication bias, the trim and fill method was used to adjust the summary HR by theoretical incorporation of non-existent studies. Sensitivity and publication bias analyses were not performed for cases of meta-analysis of less than 5 studies.
Results
Study characteristics
We identified fifteen studies [11–25] with retrospective observational survival data on a cumulative 2311 patients with GBMs categorized as either LV+GBM or LV−GBM. In all studies, LV+GBM was defined by contact of the post-contrast enhancement of the GBM with the LV in a T1-weighted MRI. No clear tendencies for the incidences of LV+GBM or LV−GBM was noted in these studies: The proportion of LV+GBM to LV−GBM patients ranged from 36 to 225 % (mean 100.8 %, median 104.8 %) with a cumulative 1010 LV+GBM and 1301 LV−GBM patients (Table 1).
Table 1.
List of studies comparing survival between GBMs with and without lateral ventricle contact (LV+ and LV−)
| Study [#] | LV+/LV−GBM (n) | Survival (log-rank analysis)
|
Multivariate analysis
|
Confounder analysis
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median PFS (months) |
PFS HR | Median OS (months) |
OS HR | PFS HR | OS HR | Age | KPS | EOR | Tumor Size | ||
| Chaichana [11] | 26/26 | – | – | 8/11 | 1.843 [1.196–3.859] | – | Sc | ✓ | ✓ | ✓ | ✓ |
| Chaichana [12] | 104/289 | – | – | – | – | – | 1.321 [1.016–1.700] | ✓ | ✓ | ✓ | – |
| Kappadakunnel [13] | 25/22 | – | – | 11.9/21.8 | 1.753 [0.967–3.315] | – | – | – | – | – | – |
| Young [14] | 16/23 | 5.14/7.61 | 2.180 [1.438–6.226] | 13.5/20.6 | 2.299 [1.383–6.441] | 4.24 [1.47–12.2] | 3.70 [1.41–9.73] | ✓ | ✓ | ✓ | ✓ |
| Tejada-Solis[15] | 45/20 | – | – | 15/33 | 2.940 [1.282–5.177] | – | 2.137 [0.774–5.901] | ✓ | ✓ | – | |
| Chaichana [16] | 105/229 | – | – | – | – | – | 1.241 [1.136–1.456] | ✓ | ✓ | ✓ | |
| Ho [17] | 27/59 | 5.7/8.9 | S | 17.5/23.3 | NS | NS | NS | ✓ | – | ✓ | ✓ |
| Jafri [18] | 49/42 | 5.55/10.94 | 1.898 [1.270–3.059] | 15.4/22.8 | 2.015 [1.306–3.264] | S | S | – | – | ✓ | ✓ |
| Adeberg [19] | 309/225 | 4.8/6.9 | 1.350 [1.144–1 608 | 12.3/16.3 | 1.341 [1.145–1.636] | NSd | NSd | – | – | ✓ | – |
| Sonoda [20] | 102/121 | – | – | 16/22 | 1.442 [1.115–2.014] | – | – | – | – | – | – |
| Tomita [21] | 22/21 | – | – | 15.9a/15.8a | NS | – | – | – | – | – | – |
| Han [22] | 27/16 | 4.9/8.7 | 1.038 [0.562–1.925] | 11.6/16.0 | 1.213 [0.664–2.232] | 1.141 [0.542–2.400] | 1.464 [0.687–3.123] | ✓ | ✓ | ✓ | ✓ |
| Liang [23] | 57/51 | 12.6/15.1 | – | 21/26.9 | 1.367 [0.899–2.130]b | 1.35 [0.85–2.13] | 1.38 [0.86–2.23] | ✓ | ✓ | ✓ | ✓ |
| Nestler [24] | 43/40 | – | – | 16.7a/19.6a | NS | – | – | – | – | – | – |
| Pina Batista [25] | 53/117 | 2.96/4.48 | 1.582 [1.216–2.506] | 5.23/10.5 | 1.923 [1.569–3.373] | – | – | ✓ | ✓ | ✓ | – |
Italicized data are data derived from reconstructed Kaplan–Meier curves
PFS progress free survival, OS overall survival, HR hazard ratio with 95 % Confidence Intervals, KPS preoperative karnofsky performance scale, EOR extent of resection, mo months, S statistically significant, NS statistically non-significant,
Reference number
Variable accounted in survival analysis. Median OS and PFS values are reported as LV+/LV−
Mean values, instead of medians reported—data not available
Unpublished Kaplan–Meier curve obtained from study author
Case-controlled analysis
Conflicting interpretations resolved by a third reviewer
Patient treatment in these studies consisted of any combination of surgery (biopsy, subtotal, or gross total resection), postoperative temozolomide, and/or radiation therapy. No study reported significant differences in the overall treatment regimen of LV+GBM and LV−GBM patients. In a few studies some patients received additional non-conventional therapies. In the Adeberg et al. study [19], 37 patients received either bevacizumab, cetuximab, cilengitide, imatinib, or temsirolimus. All 91 patients in Jafri et al. [18] received either erlotinib, cis-retinoic acid, or enzastaurin. In the three studies by Chaichana et al. [11, 12, 16], a subset of patients received polifeprosan with carmustine wafer implantation at the surgeon’s discretion (14/52 in the 2008 study). Twenty-six patients in the Tejada-Solis et al. study [15] participated in a dendritic cell vaccination trial and irinotecan-bevazucimab was commonly a second line treatment. In the Sonoda et al. study [20], some patients received nitrosourea instead of temozolomide. Exact numbers were not always reported.
Survival difference between LV+GBM and LV−GBM patients
Individual patient survival data derived from digitized Kaplan–Meier curves of OS, available in ten studies [11, 13–15, 18–20, 22, 23, 25] (LV+GBM n = 709; LV−GBM n = 663), and PFS, available in five studies [14, 18, 19, 22, 25] (LV+GBM n = 454; LV−GBM n = 423), were pooled together for generation of cumulative, representative OS and PFS Kaplan–Meier curves of patients included in our meta-analyses (Fig. 1). These representative curves allowed for calculations of overall medians of 12.95 and 16.58 months for OS and 4.54 and 6.25 months for PFS for LV+GBM and LV−GBM patients, respectively. HRs for OS and PFS were 1.335 [1.204–1.513] and 1.387 [1.225–1.602], respectively, with p < 0.0001 for both (log-rank test), indicating a significant difference in survival between LV+GBM and LV−GBM patients. A meta-analysis conducted with log-rank HRs with 95 % CIs resulted in a summary OS HR of 1.58 ([1.35–1.85], p < 0.00001) and PFS HR of 1.41 ([1.22–1.64], p < 0.00001; Fig. 2a, c). We found a lack of heterogeneity among the studies by Chi2, Tau2, and I2 analyses. Sensitivity analysis by exclusion of any one study did not alter the significance of OS or PFS HR. Funnel plots as well as tests for publication bias for the OS and PFS meta-analyses suggested a possibility for publication bias (Kendall’s tau from Begg and Mazumdar’s correlation and Egger’s regression intercept for OS: 0.27, p = 0.28 and 1.49, p = 0.04; PFS: = 0.40, p = 0.81 and 0.69, p = 0.44, respectively). Adjusting for publication bias did not alter the significance of the summary HRs (Fig. 2b, d).
Fig. 1.
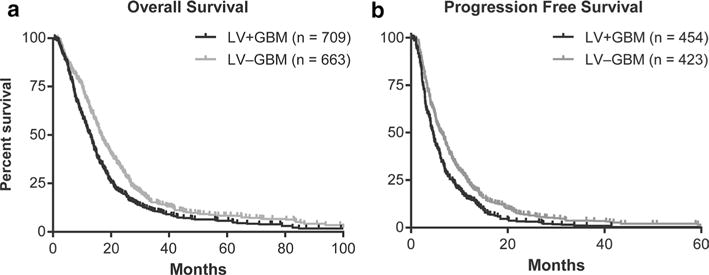
Cumulative Kaplan–Meier overall (a) and progression free (b) survival curves
Fig. 2.
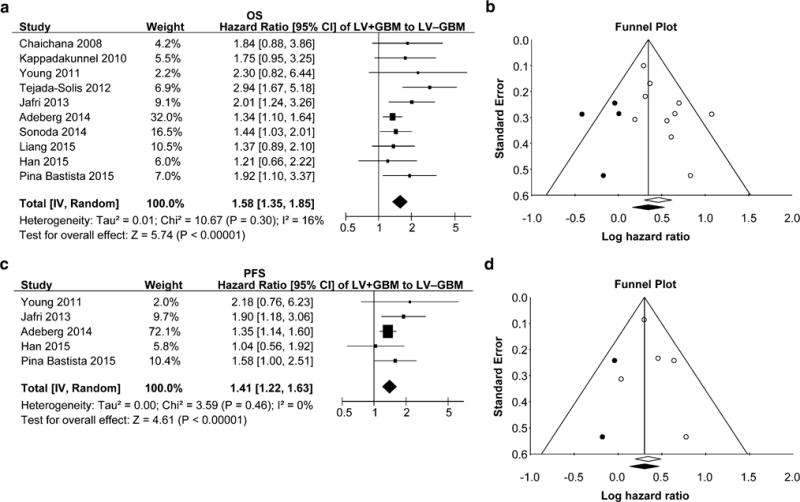
Meta-analyses of log-rank HRs comparing overall (a) and progression free (c) survival in LV+GBM and LV−GBM patients. b, d Funnel plots demonstrating publication bias for a and c meta-analyses, respectively. Lines represent 95 % confidence interval obtained after trim and fill analysis. Each open circle represents a study included in the meta-analysis. Closed, dark circles represent non-existent studies compensating for publication bias with the trim and fill method. The resulting summary HR and confidence interval is represented by a closed, dark diamond
Confounder analyses
In addition to chemoradiation, all studies variably accounted for age, pre-operative KPS, extent of resection, and tumor size in their survival analyses (Table 1). Other confounding variables accounted in these studies included pre-operative motor deficits and intraoperative polifeprosan with carmustine wafer implantation [11, 12, 16], tumor multifocality [14], vaccine administration [15], and MGMT promoter methylation or IDH1 mutation status [22].
Six and three studies reported a multivariate cox proportional OS HR and PFS HR for patients with LV+GBMs and LV−GBMs, respectively. To further analyze the effect of GBM contact with the LV on survival, we performed a meta-analysis using only the multivariate OS and PFS HRs obtained after accounting for the common confounding variables and predictors of survival in GBM patients. The summary OS and PFS HRs obtained were 1.35 ([1.14–1.58], p = 0.0003; LV+GBM n = 354; LV−GBM n = 628) and 1.64 ([0.88–3.05], p = 0.12; LV+GBM n = 100; LV−GBM n = 90), respectively, (Fig. 3a, c). We found a lack of heterogeneity among the studies by Chi2, Tau2, and I2 analyses. Sensitivity analysis by exclusion of any one study did not alter the significance of OS HR. Funnel plots as well as tests for publication bias for OS meta-analysis depicted publication bias (Kendall’s tau from Begg and Mazumdar’s correlation and Egger’s regression intercept for OS: 0.80, p = 0.02 and 1.39, p = 0.04). Theoretical compensation for publication bias did not alter the significance of the result (Fig. 3b). Funnel plot for the PFS meta-analysis is depicted in Fig. 3d. With inclusion of only three studies, there would be low reliability for further analyses and therefore these were not performed.
Fig. 3.
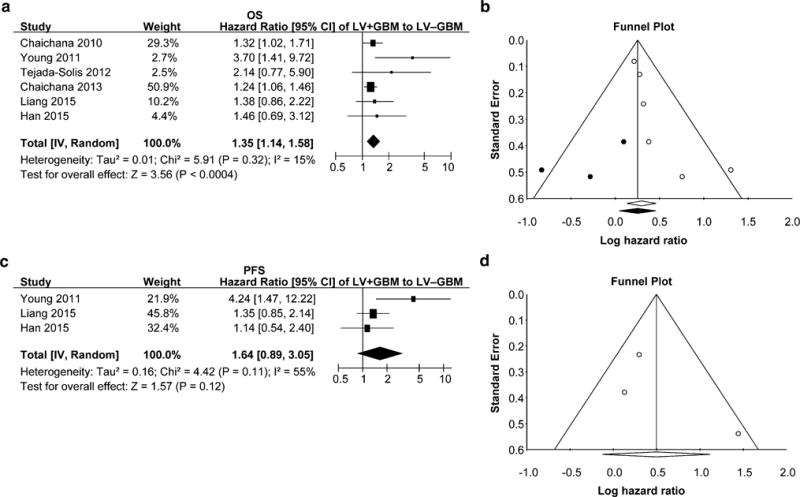
Meta-analyses of published multivariate HRs comparing overall (a) and progression free (c) survival in LV+GBM and LV−GBM patients. b, d Funnel plots demonstrating publication bias for a and c meta-analyses, respectively. Lines represent 95 % confidence interval obtained after trim and fill analysis. Each open circle represents a study included in the meta-analysis. Closed, dark circles represent non-existent studies compensating for publication bias with the trim and fill method and resulting summary HR and confidence interval is represented as a closed, dark diamond. These analyses were not performed in d
Three studies [11, 18, 19] which assessed OS significance for at least one confounding variable but without multivariate cox proportional analyses were excluded from the meta-analyses. Two of these three studies [11, 18] noted independent influence of LV contact of GBM on OS. PFS was analyzed in two of these studies [18, 19], and noted to be independently significant in one [18].
Discussion
Identification of prognostic indicators of survival in patients with GBM is important in tailoring treatment to reduce burden and improve quality of life, as well as to guide the development of novel therapies. In this study, we analyzed the published literature and demonstrate a significant reduction in survival in patients with LV+GBM compared to LV−GBM. We further propose this effect may be independent of the common predictors of GBM survival—age, KPS, and extent of resection, chemotherapy, and radiation therapy—implying a distinct pathobiology of GBMs with and without LV contact.
The discovery of neural stem cells in the V-SVZ has led to the emergence of scientific hypotheses bridging developmental biology with neuro-oncology, suggesting that stem or precursor cells may be likely cells of origin for malignant brain tumors [30–33]. However, in the mouse some experimental models have also generated gliomas from mature progenitors, arguing that the V-SVZ may not be an obligate site of origin [34–36]. Computer models of glioma growth based on MRI sequences suggest that sites of origins of GBMs are widely distributed throughout the brain and not limited to the V-SVZ [37]. However, the decreased survival noted in patients with LV+GBM suggests increased tumor malignancy with V-SVZ contact. The molecular basis of this clinical phenomenon remains unknown. The tumor cell properties or cellular composition of V-SVZ-contacting GBMs may be intrinsically different, or the uniquely supportive, growth factor-rich environment of the V-SVZ may enhance the malignant potential of cancer cells regardless of their origin. The proximity of the V-SVZ to, and its extensive contact with, the CSF may also allow for widespread dissemination of tumor cells, potentially affecting survival.
Several studies have aimed to test these hypotheses. However, no significant difference in the expression of several stem cell genes between GBMs with and without radiographic LV or V-SVZ contact has been uncovered to date [13, 21, 22]. Two studies explored the molecular signature of sub-classifications [9] of GBMs based on ventricular contact. One found presence of an IDH1 mutation and expression of YKL40, a mesenchymal marker, were associated with GBMs without V-SVZ or cortical contact, or GBMs that were multifocal or multicentric [25]. Another demonstrated that markers of V-SVZ cells were highly expressed in GBMs with both V-SVZ and cortical contacts [38]. Other studies have suggested a “seed-and-soil” relationship between tumor cells and V-SVZ. Orthotopic xenograft studies have demonstrated that GBM tumor cells exhibit a tropism for the fertile, growth-promoting environment of the V-SVZ of adult brain [39–41]. Conversely, it has been demonstrated that normal neural stem cells can also exhibit tropism for neoplastic environments [42–45].
Tumor seeding through CSF dissemination is also a potential contributing factor to multifocality or poor clinical outcome. At present, conflicting observations have been reported regarding the correlation between V-SVZ contact and multifocality at presentation of GBM. In our review, two studies [9, 19] have observed this correlation, while another two [13, 14] reported no significant correlation. While entering the ventricle during tumor resection might be expected to lead to widespread seeding of tumor cells through the CSF or leakage of the CSF itself, and consequently decreased survival, of the studies included in this report, two noted that surgical disruption of the ventricular wall did not correlate with survival [19, 24].
While molecular or cellular signatures that identify GBMs with V-SVZ contact remain elusive, we present clinical evidence suggesting their increased malignancy and associated decrease in patient survival. However, the data herein must be interpreted with some caveats. First, without individual patient data, we cannot definitively conclude that reduced survival is independent of the other well-known predictors of GBM survival. Studies varied in their overall treatment regimen and sometimes, a minority of patients received non-conventional therapies. The number of confounders and methods used to account for them also varied. Additional, larger studies (>100 patients total) are needed to increase the confidence of our meta-analysis and the conclusion that GBM contact with the LV is an independent predictor of survival. We look forward to a recent report which may confirm this conclusion: in a multivariate analysis of 647 GBM patients, adjusting for age, gender, KPS, extent of resection, post-operative treatment, and tumor volume, V-SVZ contact was found to be an independent predictor of survival (Multivariate OS HR: 1.58 [1.27–1.95]) [46]. Second, most published studies did not adjust survival for known molecular prognostic indicators in GBM—MGMT promoter methylation or IDH1 mutation status. Only a few studies have reported promoter MGMT methylation or IDH1 mutation status of LV+GBMs and LV−GBMs [19, 22, 25, 47], and taking them collectively, presently no clear association of GBM molecular status with contact to the LV is discernable.
In summary, this meta-analysis demonstrates potential clinical relevance for the contact of glioblastoma with the V-SVZ, implied by observed radiographic contact with the LV. Observing the negative influence on survival of V-SVZ tumor contact, some treatment centers have exploited this niche as a therapeutic target by including it in the irradiated region, and have largely reported promising preliminary results [48, 49], although conflicting reports exist regarding the effects and dose used [50]. To better understand the role of V-SVZ in glioma biology and develop novel therapies, in addition to larger clinical studies, molecular and cellular investigations to decipher the basis for the decreased survival associated with GBM contact with the V-SVZ are warranted.
Supplementary Material
Acknowledgments
We thank Dr. Tseng for providing unpublished Kaplan–Meier survival curves of LV+GBM and LV−GBM patient data analyzed in the study Liang et al. [22].
Funding This work was by supported a grant from the Institute for Clinical and Translational Research, Vanderbilt University, part of the National Center for Advancing Translational Sciences, CTSA award No. UL1TR000445 (A.M.M.) and Discovery Grants from the Vanderbilt-Ingram Cancer Center (R.A.I.).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ, Jr, Mehta MP. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 3.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 6.Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, Rowitch DH, Alvarez-Buylla A. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 8.Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 9.Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, Berger MS. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro-oncol. 2007;9:424–429. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol. 2008;89:219–224. doi: 10.1007/s11060-008-9609-2. [DOI] [PubMed] [Google Scholar]
- 12.Chaichana K, Parker S, Olivi A, Quinones-Hinojosa A. A proposed classification system that projects outcomes based on preoperative variables for adult patients with glioblastoma multiforme. J Neurosurg. 2010;112:997–1004. doi: 10.3171/2009.9.JNS09805. [DOI] [PubMed] [Google Scholar]
- 13.Kappadakunnel M, Eskin A, Dong J, Nelson SF, Mischel PS, Liau LM, Ngheimphu P, Lai A, Cloughesy TF, Goldin J, Pope WB. Stem cell associated gene expression in glioblastoma multiforme: relationship to survival and the subventricular zone. J Neurooncol. 2010;96:359–367. doi: 10.1007/s11060-009-9983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young GS, Macklin EA, Setayesh K, Lawson JD, Wen PY, Norden AD, Drappatz J, Kesari S. Longitudinal MRI evidence for decreased survival among periventricular glioblastoma. J Neurooncol. 2011;104:261–269. doi: 10.1007/s11060-010-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tejada-Solis S, Aldave-Orzaiz G, Pay-Valverde E, Marigil-Sanchez M, Idoate-Gastearena MA, Diez-Valle R. Prognostic value of ventricular wall fluorescence during 5-aminolevulinic-guided surgery for glioblastoma. Acta neurochirurgica. 2012;154:1997–2002. doi: 10.1007/s00701-012-1475-1. doi: 10.1007/s00701-012-1475-1 (discussion 2002) [DOI] [PubMed] [Google Scholar]
- 16.Chaichana KL, Pendleton C, Chambless L, Camara-Quintana J, Nathan JK, Hassam-Malani L, Li G, Harsh GRt, Thompson RC, Lim M, Quinones-Hinojosa A. Multi-institutional validation of a preoperative scoring system which predicts survival for patients with glioblastoma. J Clin Neurosci. 2013;20:1422–1426. doi: 10.1016/j.jocn.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho J, Ondos J, Ning H, Smith S, Kreisl T, Iwamoto F, Sul J, Kim L, McNeil K, Krauze A, Shankavaram U, Fine HA, Camphausen K. Chemoirradiation for glioblastoma multiforme: the national cancer institute experience. PloS One. 2013;8:e70745. doi: 10.1371/journal.pone.0070745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jafri NF, Clarke JL, Weinberg V, Barani IJ, Cha S. Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro-oncol. 2013;15:91–96. doi: 10.1093/neuonc/nos268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adeberg S, Konig L, Bostel T, Harrabi S, Welzel T, Debus J, Combs SE. Glioblastoma recurrence patterns after radiation therapy with regard to the subventricular zone. Int J Radiat Oncol Biol Phys. 2014;90:886–893. doi: 10.1016/j.ijrobp.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Sonoda Y, Saito R, Kanamori M, Kumabe T, Uenohara H, Tominaga T. The association of subventricular zone involvement at recurrence with survival after repeat surgery in patients with recurrent glioblastoma. Neurologia Medico-Chirurgica. 2014;54:302–309. doi: 10.2176/nmc.oa.2013-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomita T, Akimoto J, Haraoka J, Kudo M. Clinicopathological significance of expression of nestin, a neural stem/progenitor cell marker, in human glioma tissue. Brain Tumor Pathol. 2014;31:162–171. doi: 10.1007/s10014-013-0169-6. [DOI] [PubMed] [Google Scholar]
- 22.Han S, Li X, Qiu B, Jiang T, Wu A. Can lateral ventricle contact predict the ontogeny and prognosis of glioblastoma? J Neurooncol. 2015;124:45–55. doi: 10.1007/s11060-015-1818-x. [DOI] [PubMed] [Google Scholar]
- 23.Liang TH, Kuo SH, Wang CW, Chen WY, Hsu CY, Lai SF, Tseng HM, You SL, Chen CM, Tseng WI. Adverse prognosis and distinct progression patterns after concurrent chemoradiotherapy for glioblastoma with synchronous subventricular zone and corpus callosum invasion. Radiother Oncol J Eur Soc Ther Radiol Int Soc Cell. 2015 doi: 10.1016/j.radonc.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Nestler U, Lutz K, Pichlmeier U, Stummer W, Franz K, Reulen HJ, Bink A, Group ALAGS Anatomic features of glioblastoma and their potential impact on survival. Acta neurochirurgica. 2015;157:179–186. doi: 10.1007/s00701-014-2271-x. [DOI] [PubMed] [Google Scholar]
- 25.Pina Batista KM, Vega IF, de Eulate-Beramendi SA, Morales J, Kurbanov A, Asnel D, Meilan A, Astudillo A. Prognostic significance of the markers IDH1 and YKL40 related to the subventricular zone. Folia Neuropathol. 2015;53:52–59. doi: 10.5114/fn.2015.49974. [DOI] [PubMed] [Google Scholar]
- 26.Wan X, Peng L, Li Y. A review and comparison of methods for recreating individual patient data from published Kaplan– Meier survival curves for economic evaluations: a simulation study. PloS One. 2015;10:e0121353. doi: 10.1371/journal.pone.0121353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration Wiley-Blackwell; Chichester: 2011. [Google Scholar]
- 29.Machin D, Cheung YB, Parmar M. Survival analysis: A practical approach. Wiley; 2006. [Google Scholar]
- 30.Galvao RP, Kasina A, McNeill RS, Harbin JE, Foreman O, Verhaak RG, Nishiyama A, Miller CR, Zong H. Transformation of quiescent adult oligodendrocyte precursor cells into malignant glioma through a multistep reactivation process. Proc Natl Acad Sci USA. 2014;111:E4214–E4223. doi: 10.1073/pnas.1414389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alcantara Llaguno SR, Wang Z, Sun D, Chen J, Xu J, Kim E, Hatanpaa KJ, Raisanen JM, Burns DK, Johnson JE, Parada LF. Adult lineage-restricted CNS progenitors specify distinct glioblastoma subtypes. Cancer Cell. 2015;28:429–440. doi: 10.1016/j.ccell.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H, Foreman O, Bronson RT, Nishiyama A, Luo L, Zong H. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146:209–221. doi: 10.1016/j.cell.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zong H, Parada LF, Baker SJ. Cell of origin for malignant gliomas and its implication in therapeutic development. Cold Spring Harbor Perspectives Biol. 2015;7 doi: 10.1101/cshperspect.a020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kusne Y, Sanai N. The SVZ and its relationship to stem cell based neuro-oncogenesis. Adv Exp Med Biol. 2015;853:23–32. doi: 10.1007/978-3-319-16537-0_2. [DOI] [PubMed] [Google Scholar]
- 35.Marumoto T, Tashiro A, Friedmann-Morvinski D, Scadeng M, Soda Y, Gage FH, Verma IM. Development of a novel mouse glioma model using lentiviral vectors. Nat Med. 2009;15:110–116. doi: 10.1038/nm.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, Ellisman MH, Verma IM. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 2012;338:1080–1084. doi: 10.1126/science.1226929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohman LE, Swanson KR, Moore JL, Rockne R, Mandigo C, Hankinson T, Assanah M, Canoll P, Bruce JN. Magnetic resonance imaging characteristics of glioblastoma multiforme: implications for understanding glioma ontogeny. Neurosurgery. 2010;67:1319–1327. doi: 10.1227/NEU.0b013e3181f556ab. doi: 10.1227/NEU.0b013e3181f556ab (discussion 1327–1318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haskins WE, Zablotsky BL, Foret MR, Ihrie RA, Alvarez-Buylla A, Eisenman RN, Berger MS, Lin CH. Molecular Characteristics in MRI-Classified Group 1 Glioblastoma Multiforme. Front Oncol. 2013;3:182. doi: 10.3389/fonc.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroonen J, Nassen J, Boulanger YG, Provenzano F, Capraro V, Bours V, Martin D, Deprez M, Robe P, Rogister B. Human glioblastoma-initiating cells invade specifically the subventricular zones and olfactory bulbs of mice after striatal injection. Int J Cancer. 2011;129:574–585. doi: 10.1002/ijc.25709. [DOI] [PubMed] [Google Scholar]
- 40.Sadahiro H, Yoshikawa K, Ideguchi M, Kajiwara K, Ishii A, Ikeda E, Owada Y, Yasumoto Y, Suzuki M. Pathological features of highly invasive glioma stem cells in a mouse xenograft model. Brain Tumor Pathol. 2014;31:77–84. doi: 10.1007/s10014-013-0149-x. [DOI] [PubMed] [Google Scholar]
- 41.Goffart N, Kroonen J, Di Valentin E, Dedobbeleer M, Denne A, Martinive P, Rogister B. Adult mouse subventricular zones stimulate glioblastoma stem cells specific invasion through CXCL12/CXCR4 signaling. Neuro-Oncol. 2015;17:81–94. doi: 10.1093/neuonc/nou144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziu M, Schmidt NO, Cargioli TG, Aboody KS, Black PM, Carroll RS. Glioma-produced extracellular matrix influences brain tumor tropism of human neural stem cells. J Neurooncol. 2006;79:125–133. doi: 10.1007/s11060-006-9121-5. [DOI] [PubMed] [Google Scholar]
- 44.Zhao D, Najbauer J, Garcia E, Metz MZ, Gutova M, Glackin CA, Kim SU, Aboody KS. Neural stem cell tropism to glioma: critical role of tumor hypoxia. Mol Cancer Res: MCR. 2008;6:1819–1829. doi: 10.1158/1541-7786.mcr-08-0146. [DOI] [PubMed] [Google Scholar]
- 45.Capilla-Gonzalez V, Lavell E, Quinones-Hinojosa A, Guerrero-Cazares H. Regulation of subventricular zone-derived cells migration in the adult brain. Adv Exp Med Biol. 2015;853:1–21. doi: 10.1007/978-3-319-16537-0_1. [DOI] [PubMed] [Google Scholar]
- 46.Berendsen S, Schoysman L, Kroonen J, Hendrikse J, Seute T, Spliet WGM, Poulet C, Bours V, Robe PA. Abstracts from the 20th Annual Scientific Meeting of the Society for Neuro-Oncology. Oxford University Press; San Antonio: 2015. Glioblastoma involving the subventricular zone: correlations to patient survival and tumor biology; p. v79. [Google Scholar]
- 47.Fahrendorf D, Hesselmann V, Schwindt W, Wolfer J, Jeibmann A, Kooijman H, Kugel H, Heindel W, Bink A. Variations of ITSS-morphology and their relationship to location and tumor volume in patients with Glioblastoma. J Neuroimaging. 2015 doi: 10.1111/jon.12228. [DOI] [PubMed] [Google Scholar]
- 48.Chen L, Guerrero-Cazares H, Ye X, Ford E, McNutt T, Kleinberg L, Lim M, Chaichana K, Quinones-Hinojosa A, Redmond K. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int J Radiat Oncol Biol Phys. 2013;86:616–622. doi: 10.1016/j.ijrobp.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee P, Eppinga W, Lagerwaard F, Cloughesy T, Slotman B, Nghiemphu PL, Wang PC, Kupelian P, Agazaryan N, Demarco J, Selch MT, Steinberg M, Kang JJ. Evaluation of high ipsilateral subventricular zone radiation therapy dose in glioblastoma: a pooled analysis. Int J Radiat Oncol Biol Phys. 2013;86:609–615. doi: 10.1016/j.ijrobp.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 50.Elicin O, Inac E, Uzel EK, Karacam S, Uzel OE. Relationship between survival and increased radiation dose to subventricular zone in glioblastoma is controversial. J Neurooncol. 2014;118:413–419. doi: 10.1007/s11060-014-1424-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


