Abstract
We posit that feasible reversal of the growth of atmospheric CH4 and other trace gases would provide a vital contribution toward averting dangerous anthropogenic interference with global climate. Such trace gas reductions may allow stabilization of atmospheric CO2 at an achievable level of anthropogenic CO2 emissions, even if the added global warming constituting dangerous anthropogenic interference is as small as 1°C. A 1°C limit on global warming, with canonical climate sensitivity, requires peak CO2 ≈ 440 ppm if further non-CO2 forcing is +0.5 W/m2, but peak CO2 ≈ 520 ppm if further non-CO2 forcing is -0.5 W/m2. The practical result is that a decline of non-CO2 forcings allows climate forcing to be stabilized with a significantly higher transient level of CO2 emissions. Increased “natural” emissions of CO2, N2O, and CH4 are expected in response to global warming. These emissions, an indirect effect of all climate forcings, are small compared with human-made climate forcing and occur on a time scale of a few centuries, but they tend to aggravate the task of stabilizing atmospheric composition.
Keywords: climate change, carbon dioxide, methane, fossil fuels
Global surface temperature increased in the past century by >0.5°C (1, 2). This warming is, at least in part, a result of anthropogenic climate-forcing agents (3).
A climate forcing is an imposed, natural or anthropogenic, perturbation of the Earth's energy balance with space (4, 5). Increasing anthropogenic greenhouse gases (GHGs) cause the largest positive (warming) forcing. Aerosols are also a major climate forcing, but they are not well measured. Aerosols are increasing in some regions but decreasing in others (3, 6), so continued growth of GHGs is likely to provide the predominant global anthropogenic climate forcing in the 21st century.
Climate sensitivity to forcings is defined as the equilibrium global temperature change per unit forcing. Empirical data from the Earth's history, as well as climate models, indicate that climate sensitivity is ≈0.75 ± 0.25°C per W/m2 (7). Climate model studies suggest that the simple concept of a global mean response to forcings works well provided that an “efficacy” factor is included for forcings that are inhomogeneously distributed, such as aerosols, ozone, and surface albedo changes (4, 5, 8).
The thermal inertia of the climate system, primarily due to the ocean, partially delays the global response to forcings. One consequence of this inertia is additional global warming “in the pipeline” due to gases already in the atmosphere (9). Based on climate models (7, 9) and the observed rate of ocean heat storage (10), the climate system is presently out of energy balance by 0.5-1 W/m2, i.e., solar energy absorbed by the Earth exceeds outgoing thermal radiation by that amount.
The United Nations Framework Convention on Climate Change has the goal of stabilizing atmospheric composition at a level that avoids “dangerous anthropogenic interference” (DAI) with the climate system. One of us argues (11) that the level of DAI is set by the need to preserve global coastlines, and this goal implies that additional global warming should not exceed ≈1°C. Others using the sea level criterion suggest that up to 2°C additional warming may be acceptable (12). Taking account of the Earth's energy imbalance and the transient climate response to forcings, a 1°C limit on further warming requires a limit on added climate forcing ΔF ≈1.5 W/m2 in the 21st century. Scenarios considered by the Intergovernmental Panel on Climate Change (IPCC) (3) have ΔF in the range ≈3-7 W/m2.
Here we examine observed growth rates of GHG climate forcings. Current trends provide indications about prospects for future GHG changes and changes in emissions that would be needed to keep global warming within specified bounds.
Climate Forcing Due to Observed GHG Changes
We calculate climate forcings as a function of GHG changes by using analytic relationships (table 1 in ref. 13). These forcings are similar to those of IPCC (3), although our sensitivity for CO2 is ≈10% larger (4.1 vs. 3.7 W/m2 for doubled CO2).
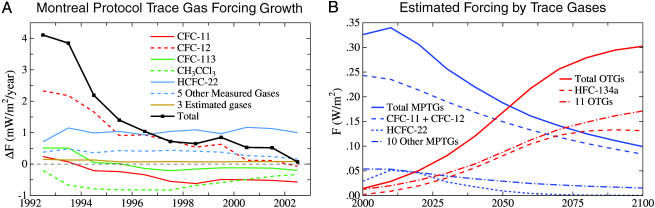
Montreal Protocol Trace Gases (MPTGs) and Other Trace Gases (OTGs). Fig. 1A shows the growth rate of climate forcing due to gases covered by the Montreal Protocol. Production of MPTGs is being phased out because they contain chlorine or bromine that has a destructive effect on stratospheric ozone. Measured growth rates for 10 of the 13 MPTGs that cause a significant greenhouse effect are available at ftp.cmdl.noaa.gov. We use IPCC projections (3) for the other 3 of these 13 MPTGs, which is probably an upper limit because measured amounts of the 10 measured MPTGs have fallen below IPCC projections.
Fig. 1.
Growth rates of climate forcing by trace gases. (A) Growth rate of climate forcings for 13 gases controlled by the Montreal Protocol (CFC-11, CFC-12, CFC-113, CFC-114, CFC-115, CCl4,CH3CCl3, HCFC-22, HCFC-141b, HCFC-142b, HCFC-123, CF2BrCl, and CF3Br). CFC, chlorofluorocarbon; HCFC, hydrochlorofluorocarbon; HFC, hydrofluorocarbon. Measured abundances of 10 of these gases are reported by the National Oceanic and Atmospheric Administration Climate Monitoring and Diagnostics Laboratory (ftp://ftp.cmdl.noaa.gov). Abundances of CFC-114, CFC-115, and HCFC-123 are from IPCC (3) projections. (B) Climate forcing by trace gases for the 21st century baseline scenario (A1B) of IPCC (3).
The net annual change of climate forcing by these 13 MPTGs declined to near zero in 2003 (Fig. 1A). A watershed is thus just being passed, because the future net change should be negative. This negative growth of climate forcing is mainly due to the declining abundances of CFC (chlorofluorocarbon)-11, CH3CCl3, and CFC-113, and the fact that CFC-12 is just changing from positive to negative growth. These declines balance continuing increases of some of the 13 gases, with positive growth almost entirely due to hydrochlorofluorocarbon (HCFC)-22. HCFC-22, with lifetime ≈12 years, has been a temporary substitute for longer-lived CFCs for refrigeration applications, but it also is being phased out, and its abundance should begin to decline within about a decade.
The total climate forcing by the MPTGs, now >0.3 W/m2 (Fig. 1B), should decline to ≈0.1 W/m2 by 2100 as the production of these gases is phased out and the longer-lived constituents are slowly photolyzed in the stratosphere. This reduced climate forcing could be more than offset by increases of OTGs that are not controlled by the Montreal Protocol. A midrange scenario (A1B) of IPCC (3) has the forcing of 12 OTGs (CF4, C2F6, SF6, HFC-23, HFC-32, HFC-125, HFC-134a, HFC-143a, HFC-152a, HFC-227ea, HFC-245ca, and HFC-43-10mee) increasing to ≈0.3 W/m2 by 2100, with almost half of the growth due to HFC-134a (Fig. 1B). Other IPCC scenarios have this 12-gas OTG forcing ranging from 0.15 W/m2 (scenario B1) to 0.34 W/m2 (scenario A2p).
There are additional minor OTGs besides the 12 mentioned above. No additional OTG with forcing as great as 0.01 W/m2 has been identified, but new ones may be developed. We conclude only that the likely change in MPTG + OTG forcing between 2000 and 2100 is in the range -0.25 to +0.25 W/m2. A negative change probably requires phasing out gases that contribute most to OTG forcing, such as HFC-134a.
We argue below that prospects for avoiding DAI depend on control of the OTGs and that the OTG climate forcing even influences the rate at which the major atmospheric GHGs are sequestered. First, however, we look at the trends of the major GHGs.
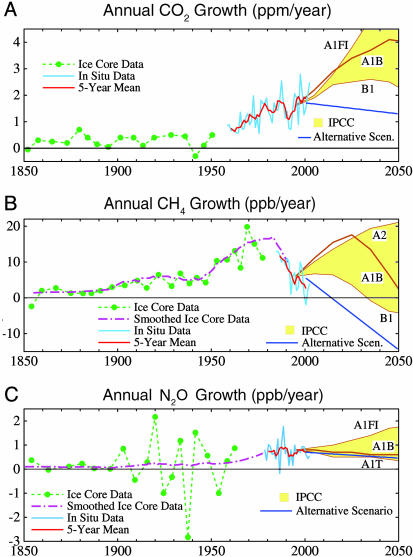
CO2, CH4, and N2O. Fig. 2 shows observed growth rates of global CO2, CH4 and N2O. Methods used to obtain global means from measurements at specific locations are described in Supporting Text (which is published as supporting information on the PNAS web site).
Fig. 2.
Growth rates of atmospheric CO2 (ppm/year) (A), CH4 (parts per billion per year) (B), and N2O (parts per billion per year) (C) based on ice core and in situ observations (see Supporting Text). A1B is the midrange baseline IPCC (3) scenario. ppb, parts per billion.
The annual increase of global atmospheric CO2 (Fig. 2A) doubled between 1958, when annual in situ measurements began, and the 1970s. The annual increment continued to increase, on average, over the past three decades. We show below that the decadal trends of the atmospheric CO2 growth rate are an accurate reflection of the CO2 emission rate from fossil fuel burning. Year-to-year fluctuations of atmospheric CO2 growth must reflect fluctuations of the land and ocean sinks for CO2 and the biomass-burning source.
Annual CH4 growth (Fig. 2B) accelerated after World War II, in a manner similar to the growth of CO2. However, since 1980 the CH4 growth rate has decreased by at least two-thirds. This slowdown could result from a leveling off or decrease of one or more of the CH4 sources, such as leakage during the mining, production, and distribution of fossil fuels (coal, oil, and gas) and emissions from landfills, waste-management lagoons, and rice farming (14). It is also possible that the CH4 lifetime changed because of changes in the abundance of atmospheric OH, the primary sink for CH4 (14).
Annual N2O growth (Fig. 2C) shows practically zero trend (constant growth rate) during the period (since 1978) of annual in situ data. Principal anthropogenic sources responsible for the N2O growth are believed to be agricultural soils, cattle and feedlots, industry, and biomass burning, but there are large uncertainties in the magnitudes of each of these sources (14). The greatest potential for source reduction is perhaps more carefully measured application of nitrogen fertilizers.
The “alternative” scenario for future CO2,CH4, and N2O growth rates in Fig. 2 is defined so as to keep added GHG climate forcing in the period 2000-2050 at ≈1 W/m2 (13). In the alternative scenario, it is assumed that the added forcing by MPTGs and OTGs is ≈0. A1B is a midrange IPCC scenario. The colored range for IPCC scenarios in Fig. 2 is defined by the scenarios that yield the extreme IPCC climate forcings in 2100.
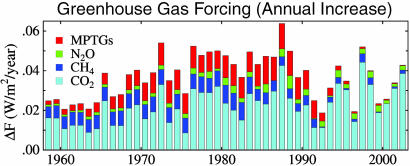
GHG Climate Forcings. Fig. 3 shows the annual growth of climate forcing due to well mixed GHGs (O3 and stratospheric H2O, which are not well mixed or well measured, are not included). The highest growth rates occurred in the late 1970s and the 1980s, when the MPTGs and CH4 made large contributions. (See also Fig. 8, which is published as supporting information on the PNAS web site.)
Fig. 3.
Annual growth rate of climate forcing by well mixed GHGs.
CO2 has accounted for 90% or more of the increased GHG climate forcing in recent years. In 2003, the portions of added forcing were CO2 (90%), N2O (5%), CH4 (4%), and MPTGs and OTGs (1%). Recent changes of CO2, CH4, and N2O growth rates are affected by short-term fluctuations of sinks as well as sources and are not necessarily indicative of future trends.
CO2 increases are the main cause of the increasing anthropogenic greenhouse effect, so efforts to mitigate global warming must focus on CO2. However, it would be a mistake to infer that non-CO2 forcings are unimportant relative to CO2. Future non-CO2 gas changes can be positive or negative, thus adding to or subtracting from the CO2 forcing. Given the difficulty of halting near-term CO2 growth, the only practical way to avoid DAI with climate may be simultaneous efforts to reverse the growth of some non-CO2 gases while slowing and eventually halting the growth of CO2.
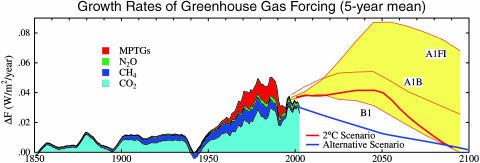
Fig. 4 shows the growth rate of GHG climate forcing in the industrial era, including scenarios for the 21st century. Histories of the gases and non-IPCC scenarios are tabulated, respectively, in Tables 1 and 2, which are published as supporting information on the PNAS web site.
Fig. 4.
Five-year mean of the growth rate of climate forcing by well mixed GHGs (CO2,CH4,N2O, MPTGs, and OTGs). O3 and stratospheric H2O, which are neither well mixed nor well measured, are not included.
The slowdown in the growth rate of GHG climate forcing from the peak in the 1980s is due mainly to the phase-out of CFC production. If the 10% per year exponential growth of CFC production that existed until the 1970s had continued for several more years, the MPTG climate forcing (mostly from CFC-11 and CFC-12) now would exceed that of CO2 (15).
The reason for the slowdown of CH4 growth is not well understood, but it may reflect a leveling off of anthropogenic emissions (14). The slowdown in CH4 growth could be temporary. A warming climate itself may add to CH4 growth, as discussed below.
We have suggested (13) that a concerted effort to reduce CH4 emissions could yield a negative forcing, which would be amplified ≈40% by the indirect effects of CH4 on stratospheric H2O and tropospheric O3.CH4 by itself could yield a forcing change of -0.25 W/m2 if it were reduced from today's 1,755 ppb to 1,215 ppb, which would require reducing anthropogenic CH4 emissions by 40-50% (ref. 14 and Drew Shindell, personal communication). Conversely, CH4 could provide large positive forcing if emissions grow, e.g., CH4 increases to 3,140 ppb in 2100 in the IPCC (3) IS92a scenario, yielding +0.5 W/m2 forcing.
The alternative scenario (Fig. 4) limits added GHG forcing to 1 W/m2 during 2000-2050 (13). The extension of that scenario to 2100 and the 2°C scenario are designed to limit global warming to 1°C and 2°C, respectively, above the temperature in 2000 assuming climate sensitivity 0.75°C per W/m2 (11).
Growth rates of climate forcings in the past several years have fallen below all IPCC (2001) scenarios (Fig. 4). Forcings for observations and scenarios are calculated with the same equations (table 1 in ref. 13), so the gap between the IPCC scenarios and observations is not an artifact of calculation uncertainties. Scenarios and observations include CO2,CH4,N2O, MPTGs, and OTGs, but they do not include tropospheric O3 and stratospheric H2O. If the latter gases were included, the gap between the alternative and IPCC scenarios would widen, because these gases decrease in the alternative scenario (due to decreasing CH4) but increase in IPCC scenarios.
Bonuses for Non-CO2 Forcing Reductions
If the global warming that constitutes DAI is as small as 1°C (11), or even 2°C (12), aggressive efforts to reduce emissions of both CO2 and non-CO2 gases will be needed. We point out here several factors that warrant attention in strategic planning to limit global warming.
Soaking-up CO2 Bonus. Consider the following gedanken experiment. Case A: CO2 increases by an amount (≈16 ppm) that causes a climate forcing of +0.25 W/m2, whereas CH4 decreases by an amount (≈0.5 ppm) that causes a climate forcing of -0.25 W/m2. Case B: CO2 decreases so as to cause a forcing of -0.25 W/m2, whereas CH4 increases to cause a forcing of +0.25 W/m2. Cases A and B both yield no net forcing and thus no tendency for a climate change. However, case A has practical advantages.
One advantage of case A is that CO2 is removed from the atmosphere at a faster rate. Stated differently, the climate is in equilibrium (no warming) with a larger anthropogenic CO2 source. The larger amount of CO2 in the air in case A causes the ocean and biosphere to remove CO2 at a higher rate. Atmospheric CO2 is greater in case A than in case B, but climate forcings are identical.
The magnitude of this effect can be estimated from the assumption that the net uptake of CO2 from the air is proportional to “excess” atmospheric CO2, i.e., the difference (ΔCO2) between ambient CO2 and the equilibrium CO2 that would exist for the current climate without any anthropogenic CO2 emissions. As a first approximation, let us take this equilibrium CO2 as 285 ppm (the amount in 1850) and today's CO2 as 370 ppm. Then case A has ΔCO2 = 101 ppm, and case B has ΔCO2 = 69 ppm.
Today, with CO2 = 370 ppm, the rate at which CO2 is taken up by the ocean plus land is ≈2.8 gigatons of C per year (40% of the 7 gigatons of C fossil fuel emissions). In case A, the flux of CO2 out of the atmosphere (removed by the ocean and continents) is ≈3.3 gigatons of C per year, whereas in case B it is 2.3 gigatons of C per year, assuming that 40% of excess CO2 continues to be taken up. Cases A and B have the same climate forcing, but in case A the flux of CO2 out of the atmosphere is 47% larger than in case B. Cases A and B have the same climate, but case A has fossil fuel emissions that are larger by 1 gigaton of C per year.
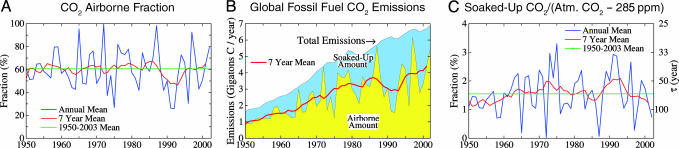
Let us check the assumption that the earth absorbs CO2 in proportion to excess atmospheric CO2. Fig. 5A shows the CO2”airborne fraction,” the ratio of the annual increase in atmospheric CO2 to annual fossil fuel CO2 emissions. Despite large year-to-year fluctuations, the airborne fraction has been remarkably constant at ≈60% of emissions during the post World War II period of high fossil-fuel emissions. Interpretation of the 60% airborne fraction as the fraction of total emissions requires an assumption that imbalance between any CO2 deforestation source and compensating biomass regrowth is small compared with the fossil-fuel source.
Fig. 5.
Measures of atmospheric CO2 growth rate. (A) CO2 airborne fraction, i.e., the ratio of observed atmospheric CO2 increase to fossil fuel CO2 emissions. (B) Global fossil fuel CO2 emissions and division into portions that remain airborne and are soaked up by the ocean and continents. (C) Ratio of soaked-up CO2 to the excess CO2 in the air (atmospheric CO2 - 285 ppm). Atm., atmospheric.
Fossil-fuel emissions (Fig. 5B) increased by >4-fold in the past half-century (16). There are large year-to-year fluctuations in the apportionment of emissions into airborne and “soaked-up” portions. However, averaged over several years, the behavior has been consistent. Fig. 5C shows that, whereas emissions increased a factor of 4, soaked-up CO2 remained nearly proportional to “excess” atmospheric CO2. The time constant for removal of excess atmospheric CO2 (right scale) is ≈65 years. If land-use change has been a net CO2 source, the time constant for CO2 uptake should be reduced accordingly, and the soaked-up fraction is >40%. Recent analysis (17) suggests that over the industrial era, the terrestrial biosphere has been a net source of CO2 16 ± 11% as large as the fossil fuel source.
Fig. 5C implies that the earth (ocean plus land, including terrestrial biosphere and soil), for at least a half-century, has been taking up CO2 consistently at a rate of ≈40 megatons of C per excess ppm of CO2 in the air. This rate need not continue; indeed, we discuss below a concern that the Earth may become a less efficient sink for CO2 as global warming proceeds. Further, there are limits on the ability of the Earth to absorb fossil CO2. CO2 entering the ocean exerts a back-pressure on the atmosphere (18) such that of the order of 10% of the fossil fuel emissions will remain in the atmosphere even after thousands of years (18, 19) and larger amounts on shorter time scales. However, it is for this reason that reduction of non-CO2 forcings, and collection of associated bonuses, is important.
Existence of the soaking-up CO2 bonus requires only that the CO2 airborne fraction is <100%. Some modeling studies (20) suggest that global warming might induce the terrestrial biosphere to release large amounts of CO2, yet the airborne fraction in those studies remained <100%. We anticipate that if CO2 emissions leveled out and began to decline in the next few decades, as in the alternative scenario, the airborne fraction would stay well below 100%.
Our emphasis is on the next 25-50 years. Thus, empirical data from the past half-century are relevant, as are empirical data from paleoclimate variations of the past 400,000 years, during which time the global mean temperature did not exceed the present level by more than ≈1°C. The next 25-50 years are important because the world's energy infrastructure makes it difficult, if not impossible, to reverse growth rates of CO2 emissions in a brief period.
The soaking-up CO2 bonus is especially valuable if we are close to the level of DAI (11). The permitted level of continuing CO2 emissions depends significantly on the magnitude of non-CO2 climate forcings.
GHG Indirect Forcings. Paleoclimate data indicate that the atmosphere contains more CO2, CH4, and N2O when the Earth is warmer (21-23). Cause and effect work both ways, i.e., warming releases GHGs and GHGs cause warming, but the temperature changes tend to lead, indicating that at least in part the GHG change is a feedback on climate change.
The climate-GHG feedbacks should continue to operate today, even though GHG amounts now are controlled primarily by human emissions and land-use changes. The GHG changes resulting from global warming should be book-kept as an indirect climate forcing apportioned among all forcings that give rise to the global warming. Thus, for example, MPTGs, OTGs, O3, and black carbon (BC) aerosols may deserve a fraction of the “blame” for global warming that normally is associated with CO2, CH4, and N2O. As we shall see, however, this climate-GHG feedback is small compared with anthropogenic GHG changes, and much of the indirect effect is probably still in the pipeline.
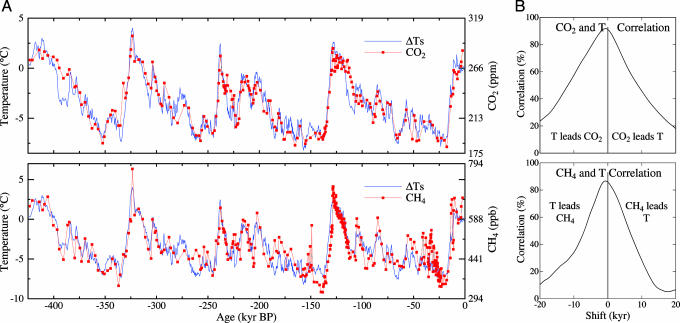
Fig. 6A is time series of temperature, CO2 and CH4 from the Vostok ice core (ref. 21; more information on the data is in Supporting Text). The vertical placement of gas amount relative to temperature is such that their means coincide. The amplitude of the temperature and gas amount scales is chosen so that the root-mean-square variations are equal. The time scale has been adjusted for the ice-gas age difference associated with the time for air bubbles to be sealed (21), which introduces an uncertainty in relative timing of several hundred years, and corrected for variations of climate in the water vapor source regions (24).
Fig. 6.
Antarctic ice core records. (A) Temperature CO2 (Upper) and CH4 (Lower) time series (thousands of years before present) from the Vostok Antarctic ice core (21, 24). (B) Correlation of the temperature in A with the gas amounts as a function of a temporal shift of the curves.
Fig. 6B is the correlation of the temperature and gas amounts as a function of temporal displacement of the curves. The time of peak correlation and the shape of the correlation curves indicate that the temperature tends to lead the gas change, as inferred by others (21-23). The peak correlation has both CO2 and CH4 changes lagging the temperature change by several hundred years. Such a lag is not inconsistent with the ocean-overturning time and perhaps a comparable time for changes of continental vegetation distributions, factors that could be involved in the response of the carbon cycle to climate change.
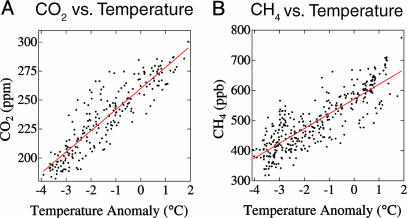
Fig. 7 is scatter plots of the correlation of CO2 and CH4 with temperature based on the Vostok ice core data (21). We approximate global temperature change as half that in Antarctica, a typical polar enhancement of global temperature change suggested by climate models (3) as well as empirical paleoclimate data. We infer from Fig. 7 levels of 18 ppm CO2 per °C global warming and 49 ppb CH4 per °C. N2O, which has both soil and ocean upwelling sources, has shown rapid (century time scale) response to regional and global temperature change (25) with the glacial-interglacial change suggesting a positive feedback of 15 ppb per °C.
Fig. 7.
Scatter plots of CO2 (A) and CH4 (B) in the Vostok ice core (21, 24) as a function of global temperature change, approximated as half of the polar temperature change.
The eventual GHG changes that might be associated with the observed 0.7°C warming of the past century are, thus, ≈13 ppm CO2, 34 ppb CH4, and 10 ppb N2O. These correspond to 14%, 4%, and 25% of the observed 1850-2003 increases of CO2, CH4, and N2O, respectively. Only some fraction of these increases, perhaps less than half, would be realized to date, with the remainder in the pipeline. Thus, it appears that small portions of the CO2 and N2O increases could be feedbacks due to global warming, which should be attributed as indirect effects of the forcings that caused global warming. The eventual climate forcing associated with gas changes induced by 0.7°C warming is ≈0.4 W/m2, with CO2,CH4, and N2O responsible for 85%, 5%, and 10% of this forcing, respectively.
This eventual indirect GHG climate forcing is ≈25% of either the 1850-2000 CO2 forcing or the net 1850-2000 anthropogenic forcing that we have estimated (11). However, only an uncertain fraction of this forcing has been realized, so we cannot assign a quantitative indirect forcing for 1850-2000 among the primary forcing mechanisms. Nevertheless, it is worth bearing in mind that other positive forcings, especially O3 and BC aerosols, probably are responsible for a small fraction of the observed CO2, N2O, and CH4 increases.
Anthropogenic emissions are directly responsible for most of the observed GHG increases. Other anthropogenic effects, such as land use changes, may exceed the magnitude of the “natural” feedback effect (indirect forcing) that we are discussing. However, the fact that this climate feedback on atmospheric composition is exceeded by other effects provides no reason to believe that it does not continue to operate in the present climate system.
Fig. 7 also allows us to address the concern that global warming may unleash nonlinear biogeochemical feedbacks that greatly accelerate climate change, e.g., release of methane from permafrost or methane clathrates. The data in Fig. 7 reveal no indication of nonlinear sensitivity for the available range of global temperature, which is up to 1°C warmer than today. This finding suggests that the alternative scenario, with a maximum global warming of 1°C, would not run the risk of a large nonlinear positive climate feedback.
Other Bonuses. Reduction of non-CO2 positive climate forcings has practical benefits. Reducing CH4 tends to reduce tropospheric O3 and stratospheric H2O (3, 13), thus further reducing global warming and ameliorating O3 impacts on human health and agricultural productivity (26). Reduction of BC aerosols has positive effects on human health, agriculture, and environmental aesthetics (26).
Summary and Implications
CO2 Growth Rate. Annual growth of atmospheric CO2 fluctuates widely from year to year. It is sometimes said that mean CO2 growth in recent decades has been reasonably stable at ≈1.5 or 1.6 ppm/yr. However, the long-term near constancy of the CO2 airborne fraction at ≈60% (Fig. 5A) indicates that the more steadily changing fossil-fuel emissions provide a good measure of the underlying CO2 growth rate. Fossil-fuel emissions have increased 50% since 1973 to ≈7 gigatons/yr. Thus, the underlying atmospheric CO2 growth rate is now ≈7 gigatons/yr × 60% × 0.453 ppm/gigaton = 1.9 ppm/yr. One consequence is that, unless CO2 emissions begin to level off soon and then decline, it will become impractical to limit additional global warming to 1°C (13).
GHG Feedback and Indirect Forcings. Global warming itself probably causes the major GHGs (CO2, N2O, and CH4) to increase, with a full response time that may be as long as hundreds of years. On the basis of paleoclimate evidence, this climate feedback is unlikely to cause a large nonlinear effect if additional global warming is held to ≈1°C or less.
CO2 Emissions. Stabilization of atmospheric composition requires CO2 emissions to be reduced to match the CO2 absorbed by the ocean and biosphere. We point out that the permitted level for the atmospheric CO2 amount depends sensitively on the magnitude of non-CO2 forcings. The corresponding CO2 emissions depend on the time scale considered, because of the limited ability of the ocean and biospheric sinks to absorb more CO2.
For example, if non-CO2 climate forcing decreases this century by 0.5 W/m2, rather than increasing by 0.5 W/m2, the allowed CO2 emissions are higher and more plausibly attainable. Say, as in the alternative scenario, that the aim is to keep added forcing <1.5 W/m2. If non-CO2 forcings increase 0.5 W/m2, the remaining 1 W/m2 allowed for CO2 corresponds to ΔCO2 = 69 ppm or CO2 = 439 ppm. Conversely, if non-CO2 forcings decrease 0.5 W/m2, the 2 W/m2 allowed for CO2 correspond to ΔCO2 = 150 ppm or CO2 = 520 ppm (≈540 ppm for IPCC CO2 sensitivity). A different choice for the maximum forcing would not alter the large impact of non-CO2 forcings on the permissible CO2 amount.
The near-term permitted CO2 emissions in these two cases also differ. If 1.6% of excess atmospheric CO2 continues to be taken up, as in the past half century (Fig. 5C), the case with CO2 = 439 ppm would have atmospheric CO2 being soaked up at a rate of 5.3 gigatons of C per yr (75% of today's fossil fuel emission rate), whereas the case with CO2 = 520 ppm would have CO2 being taken up at a rate of 8.0 gigatons of C per yr (115% of today's emissions).
In reality, the ability of the ocean and terrestrial biosphere to take up CO2 is expected to decline as anthropogenic emissions continue. CO2 emissions consistent with stable atmospheric composition will be less than the above percentages and will decline with time. The aim of our gedanken experiment is not to quantify allowed emissions but, rather, to show that non-CO2 forcings have a large impact on allowed CO2 emissions. If non-CO2 forcings are reduced, acceptable CO2 emissions in coming decades may be greater than commonly assumed.
Practical Implications. The success of the Montreal Protocol in reversing the growth of MPTGs, at moderate cost, suggests a similar approach for OTGs. The machinery in place for MPTGs could be used, e.g., with the World Bank supporting the phase-out of gases such as HFC-134a. Indeed, some of the OTGs are a consequence of the phase-out of MPTGs, so it is not difficult to rationalize that approach.
CH4 deserves special attention in efforts to stem global warming, for reasons given in this paper. We suggest that a reward approach for emission reductions, analogous to that used for MPTGs, could achieve much reduced CH4 emissions at low cost.
N2O and BC aerosols are other significant climate forcings that have received inadequate research support and regulatory attention, given their potential to impact the level of constraints that will be needed for CO2. BC reductions are needed to counterbalance expected reductions of reflective aerosols and would have numerous other benefits (26).
Supplementary Material
Acknowledgments
We thank Jack Kaye, Don Anderson, Phil DeCola, and Tsengdar Lee of the National Aeronautics and Space Administration headquarters for support of our research; the members of the National Oceanic and Atmospheric Administration Climate Monitoring and Diagnostics Laboratory for access to current measurements of GHGs; Jean Jouzel and Francoise Vimeux for the Vostok ice core data; Inez Fung, Jos Lelieveld, Michael Prather, and Gavin Schmidt for criticisms of the manuscript; and Darnell Cain for technical assistance.
Abbreviations: GHG, greenhouse gas; BC, black carbon; CFC, chlorofluorocarbon; HCFC, hydrochlorofluorocarbon; HFC, hydrofluorocarbon; DAI, dangerous anthropogenic interference; IPCC, Intergovernmental Panel on Climate Change; MPTG, Montreal Protocol trace gas; OTG, other trace gas; ppb, parts per billion.
References
- 1.Hansen, J., Ruedy, R., Sato, M., Imhoff, M., Lawrence, W., Easterling, D., Peterson, T. & Karl, T. (2001) J. Geophys. Res. 106, 23947-23963. [Google Scholar]
- 2.Jones, P. D. & Moberg, A. (2003) J. Climate 16, 206-223. [Google Scholar]
- 3.Houghton J. T., Ding, Y., Griggs, D. J., Noguer, M., van der Linden, P. J., Dai, X., Maskell, K. & Johnson, C. A., eds. (2001) Climate Change 2001: The Scientific Basis: Contributions of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge Univ. Press, Cambridge, U.K.).
- 4.Hansen, J., Sato, M. & Ruedy, R. (1997) J. Geophys. Res. 102, 6831-6864. [Google Scholar]
- 5.Ramaswamy, V. (2001) in Climate Change 2001: The Scientific Basis: Contributions of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change, eds. Houghton J. T., Ding, Y., Griggs, D. J., Noguer, M., van der Linden, P. J., Dai, X., Maskell, K. & Johnson, C. A. (Cambridge Univ. Press, Cambridge, U.K.), pp. 349-416.
- 6.Ramanathan, V. Crutzen, P. J., Kiehl, J. T. & Rosenfeld, D. (2001) Science, 294, 2119-2124. [DOI] [PubMed] [Google Scholar]
- 7.Hansen, J., Lacis, A., Ruedy, R., Sato, M. & Wilson, H. (1993) Natl. Geogr. Res. Explor. 9, 142-158. [Google Scholar]
- 8.Hansen, J. & Nazarenko, L. (2004) Proc. Natl. Acad. Sci. USA 101, 423-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen, J., Lacis, A., Rind, D., Russell, G., Stone, P., Fung, I., Ruedy, R. & Lerner, J. (1984) Geophys. Monogr. 29, 130-163. [Google Scholar]
- 10.Levitus, S., Antonov, J. I., Boyer, T. P. & Stephens, C. (2000) Science 287, 2225-2229. [Google Scholar]
- 11.Hansen, J. (2004) Sci. Am. 290 (3), 68-77. [DOI] [PubMed] [Google Scholar]
- 12.O'Neill, B. C. & Oppenheimer, M. (2002) Science 296, 1971-1972. [DOI] [PubMed] [Google Scholar]
- 13.Hansen, J. E., Sato, M., Ruedy, R., Lacis, A. & Oinas, V. (2000) Proc. Natl. Acad. Sci. USA 97, 9875-9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prather, M. & Ehhalt, D. (2001) in Climate Change 2001: The Scientific Basis: Contributions of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change, eds. Houghton J. T., Ding, Y., Griggs, D. J., Noguer, M., van der Linden, P. J., Dai, X., Maskell, K. & Johnson, C. A. (Cambridge Univ. Press, Cambridge, U.K.), pp. 239-287.
- 15.Hansen, J., Lacis, A. & Prather, M. (1989) J. Geophys. Res., 94, 16417-16421. [Google Scholar]
- 16.Marland, G. & Boden, T. (2004) CO2 Information Center (Oak Ridge Natl. Lab., Oak Ridge, TN).
- 17.Sabine, C. L., Feely, R. A., Gruber, N., Key, R. M., Lee, K., Bullister, J. L., Wanninkhof, R., Wong, C. S., Wallace, D. W. R., Tilbrook, B., et al. (2004) Science 305, 367-371. [DOI] [PubMed] [Google Scholar]
- 18.Broecker, W. S., Takahashi, T., Simpson, H. J. & Peng, T. H. (1979) Science 206, 409-418. [DOI] [PubMed] [Google Scholar]
- 19.Archer, D. E., Kheshgi, H. & Maier-Reimer, E. (1998) Global Biogeochem. Cycles 12, 259-276. [Google Scholar]
- 20.Friedlingstein, P., Defresne, J. L., Cox, P. M. & Rayner, P. (2003) Tellus 55B, 692-700. [Google Scholar]
- 21.Petit, J. R., Jouzel, J., Raynaud, D., Barkov, N. I., Barnola, J. M., Basile, I., Bender, M., Chappellaz, J., Davis, M., Delaygue, G., et al. (1999) Nature 399, 429-436. [Google Scholar]
- 22.Fischer, H., Wahlen, M., Smith, J., Mastroianni, D. & Deck, B. (1999) Science 283, 1712-1714. [DOI] [PubMed] [Google Scholar]
- 23.Cuffey, K. M. & Vimeux, F. (2001) Nature 412, 523-527. [DOI] [PubMed] [Google Scholar]
- 24.Vimeux, F., Cuffey, K. M. & Jouzel, J. (2002) Earth Planet Sci. Lett. 203, 829-843. [Google Scholar]
- 25.Fluckiger, J., Blunier, T., Stauffer, B., Chappellaz, J., Spahni, R., Kawamura, K., Schwander, J., Stocker, T. F. & Dahl-Jensen, D. (2004) Science 285, 227-230. [Google Scholar]
- 26.Hansen, J. E., ed. (2002) Conference Proceedings: Air Pollution as a Climate Forcing (NASA Goddard Institute for Space Studies, New York).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.