Abstract
Background and Purpose
Muse cells are endogenous non-tumorigenic stem cells with pluripotency harvestable as pluripotent marker SSEA-3+ cells from the bone marrow (BM) from cultured BM-mesenchymal stem cells (MSCs). After transplantation into neurological disease models, Muse cells exert repair effects, but the exact mechanism remains inconclusive.
Methods
We conducted mechanism-based experiments by transplanting serum/xeno-free cultured-human BM-Muse cells into the peri-lesion brain at two weeks after lacunar infarction in immunodeficient mice.
Results
Approximately 28% of initially transplanted Muse cells remained in the host brain at 8 weeks, spontaneously differentiated into cells expressing NeuN (~62%), MAP2 (~30%), and GST-pi (~12%). Dextran tracing revealed connections between host neurons and Muse cells at the lesioned motor cortex and the anterior horn. Muse cells extended neurites through the ipsilateral pyramidal tract, crossed to contralateral side and reached to the pyramidal tract in the dorsal funiculus of spinal cord. Muse-transplanted stroke mice displayed significant recovery in cylinder tests, which was reverted by the human-selective diphtheria toxin. At 10 months post-transplantation, human specific Alu sequence was detected only in the brain but not in other organs, with no evidence of tumor formation.
Conclusions
Transplantation at the delayed subacute phase showed Muse cells differentiated into neural cells, facilitated neural reconstruction, improved functions, and displayed solid safety outcomes over prolonged graft maturation period, indicating their therapeutic potential for lacunar stroke.
Keywords: cerebral infarction, lacunar stroke, cerebral ischemia, stem cells, axonal regeneration
Introduction
Lacunar infarcts account for approximately 25% of all ischemic stroke1. Patients with lacunar infarcts normally present with a good vital prognosis because of small lesion size. However, when the pyramidal tract is involved in the lesion, the functional outcome of lacunar infarct patients is always unfavorable, irrespective of the size of the lesion, and often associated with long-lasting motor disabilities2,3. While tissue plasminogen activator (tPA) is reported to confer functional recovery in acute phase (within 4.5 hour after onset) ischemic stroke4, rehabilitation is primarily the only option beyond this narrow therapeutic window of tPA post-stroke. Such unmet clinical need has warranted novel approaches for ischemic stroke, with stem cell therapy emerging as an experimental stroke therapeutic in recent years. Mesenchymal stem cells (MSCs) are considered pertinent to clinical use since they are non-tumorigenic, easily accessible from donor tissue sources, such as banked bone marrow (BM), do not involve ethical problems and are expandable to clinical scale. The postulated mechanism of action involves trophic factor secretion, with replenishment of new functional cells not well documented, owing in part to poor homing rate of MSCs into damaged tissue after transplantation5. Preclinical and clinical studies have reported mixed outcomes in grafted MSCs, with mediocre transplant survival in the host tissue and inconsistent efficacy in the long-term6–9. To this end, it is desirous to cater stem cell therapy towards affording ‘cell replenishment’ to the injured host brain, where transplanted cells may integrate with the stroke brain in affording robust and stable functional recovery10. In an effort to test this hypothesis, we posit that the lacunar infarcts serve as a good disease platform for assessment of stem cell graft-mediated cell replenishment mechanism, since the lesion is highly confined to the white matter whose structure is homogenously composed of neurons and myelinating oligodendrocytes.
Multilineage-differentiating stress-enduring (Muse) cells are a novel type of endogenous stem cells that are able to self-renew, display pluripotency, and differentiate into cells representative of all three germ layers from a single cell and tolerate stresses. They reside in the non-tumorigenic mesenchymal tissues such as the BM, adipose tissue, and dermis11, expressing the pluripotent surface marker, stage specific embryonic antigen (SSEA)-311–13. The proportion of Muse cells in the BM-mononucleated cells is ~0.03%, so that ~30 ml bone marrow aspirate yields approximately 1 million Muse cells by ~3 days11. Intravenously injected naive Muse cells migrate to and integrate into damaged sites and spontaneously differentiate into functional cells in injury models of the liver, muscle and skin11,14. Grafted Muse cells contribute to tissue reconstruction in skin ulcers of a diabetes mellitus model by replenishing new dermal and epidermal cells; similarly human skin fibroblast-derived Muse cells rebuild pyramidal and sensory tracts by replacing new functional neuronal cells in the cortex that could extend neurites into the contralateral spinal cord and physiologically evoke firing potentials15–17. Unlike embryonic stem (ES) and induced pluripotent stem (iPS) cells, naive Muse cells require neither introduction of exogenous genes for re-programming cells nor implementation of cytokine induction protocol to make them lineage-committed cells, including their spontaneous differentiation into functional neuronal cells after homing into the damaged brain, indicating their suitability as donor cells for transplantation in neurological disorders, such as stroke11–13,17.
In this study, with the goal of translating human BM-Muse cells for clinical use, we employed serum- and xeno-free cell culture system in preparing Muse cells for transplantation into the peri-lesion brain of immunodeficient mice at subacute phase of lacunar infarction. Initially, we performed fluorescence-activated cell sorter (FACS), which can purify green fluorescent protein (GFP)-labeled Muse cells, thereby allowing us to accurately ascribe functional and histological outcomes of transplantation to the Muse cells. We also prepared magnetic-activated cell sorter (MACS)-isolated Muse cells; while MACS neither achieves 100% purity nor allows collecting GFP(+)-Muse cells, cytotoxicity is moderate and final collection efficiency is generally higher than FACS. Furthermore, MACS-sorted cells have already been applied in a clinical study18. Since cells other than the targeted Muse cells may contaminate the positive fraction, we realized that data generated from MACS-sorted cells may not be an outcome of pure Muse cells. The individual drawbacks by FACS and MACS therefore guided us to use FACS-isolated Muse cells for mechanism-based investigations, whereas for translational application, we assessed efficiency and safety MACS-sorted Muse cells.
Materials and Methods
Animal Model
All animals (male severe combined immunodeficiency (SCID; CB17/Icr-Prkdc<scid>/CrlCrlj at 8–10w) were treated in accordance with the Code of Ethics of the World Medical Association as well as Tohoku University guidelines based on the International Guiding Principles for Biomedical Research Involving Animals, and the animal protocols were approved by Tohoku University’s Administrative Panel on Laboratory Animal Care. The same size of lacunar infarction was induced using previously reported method19 (Figure 1A).
Figure 1. Generation of lacunar infarct model and preparation of Muse cells sorted by MACS.
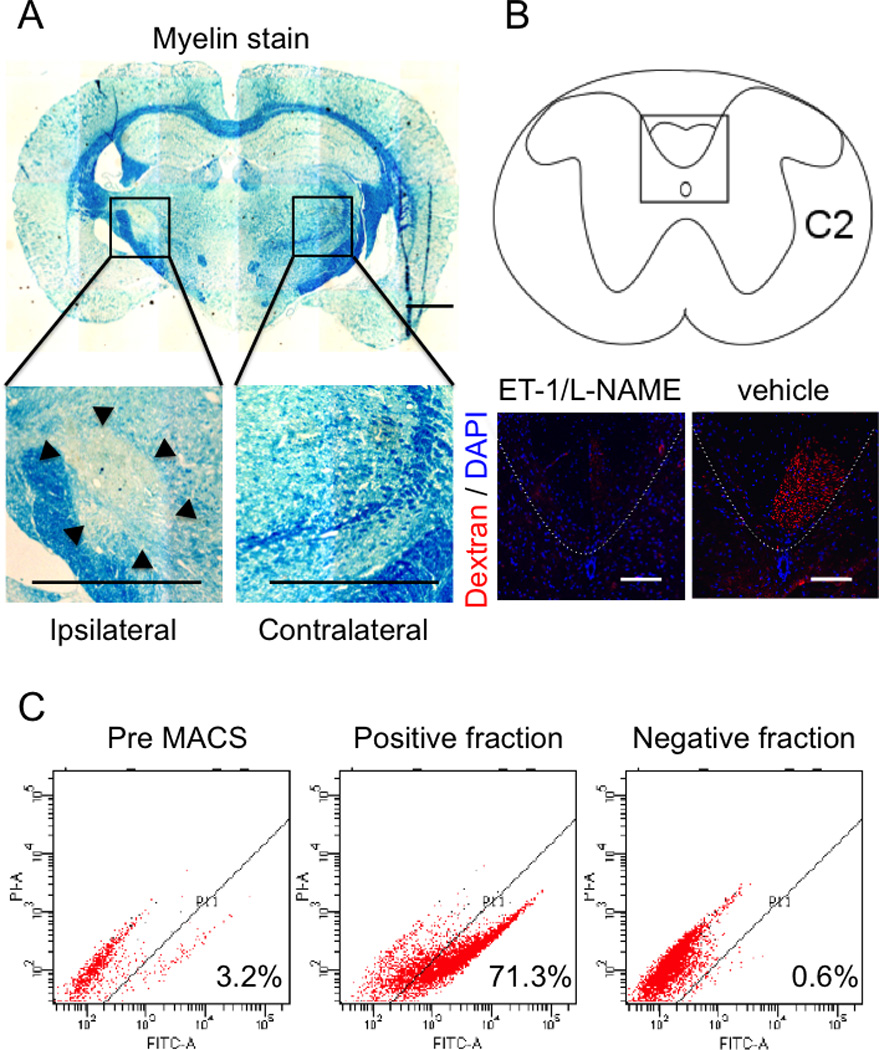
Lacunar infarcts were made by administration of two vasoconstrictive peptides. 8 weeks after, demyelination (arrow heads) in ipsilateral side was reconfirmed by luxol fast blue staining (A) and the axonal interruption was also reconfirmed by dextran tracing at cervical spinal cord C1–2 level (red signal) (B). Analysis of SSEA-3 positive cells before and after MACS sorting was shown in (C). The positive fraction containing more than 70% Muse cells was used for transplantation as Muse-rich cells. The negative fraction contained Muse cells at ~0.6%. Scale bar in A = 1000 µm, B = 100 µm.
Preparation of Human Muse Cells
Human Muse cells were separated from human BM-MSCs as previously described11,13, and processed either by FACS isolation20 or MACS-sorted. Non-muse SSEA-3(−) cells served as controls.
Cell Transplantation
Two weeks after induction of lacunar infarction by ET-1/L-NAME19, 100,000 FACS isolated GFP-labeled Muse cells, MACS-sorted Muse-rich cells or serum/xeno-free MSCs as ‘MSC’ were stereotaxically transplanted into the peri-lesion site. Infusion of the vehicle PBS with the same volume served as control groups. For behavior analysis, we prepared 7 animals per group for of the MACS-sorted Muse-rich, MSC and vehicle groups. For histological analysis which included anterograde tracing experiment, we prepared 6 and 5 animals for the FACS-sorted Muse and MACS-sorted Muse-rich groups, respectively. Of note, employing different cell selection methods for multiple experiments may induce a risk for methodological and qualitative biases, which should be addressed by incorporating proper controls as pursued in this study.
Statistics
Data were expressed mean ± standard deviation (SD). Statistical analysis was performed with GraphPad Prism 5 (MDF, Japan), with behavioral, histological, and Q-PCR data assessed using repeated measures ANOVA, followed by Bonferroni posthoc tests.
Defined methods were mentioned in Supplemental Materials I.
Results
Differentiation Ability of Muse Cells into Neural Lineage Cells
Muse cells when grown in suspension formed characteristic clusters from a single cell. These clusters resembled embryonic stem cell-derived embryoid bodies. On the other hand, none of such clusters were formed from non-Muse cells grown in suspension (Supplemental Figures IA)11–13,21. These Muse cells spontaneously differentiated into triploblastic lineages: neurofilament (ectodermal, 2.6 ± 1.1%), cytokelatin7 (endodermal, 2.1 ± 1.5%) and smooth muscle actin (mesodermal, 16.8 ± 2.4%) (Supplemental Figure IB). Under neural stem cell induction medium, Muse cells formed neural spheres that contained cells positive for neural stem cell markers NeuroD1, Sox2, Nestin and Musashi-1 (Supplemental Figure IC). These results indicated that the basic property of Muse cells was not altered by serum/xeno-free culture system11–13,21, and that single cell-derived clusters reflect triploblastic and neural differentiation potentials of Muse cells.
MSCs, which originally contained 3.2 ± 0.79% of SSEA-3+ Muse cells, yielded the positive fraction with 71.3 ± 0.65% of Muse cells after MACS separation (Figure 1C). In negative fraction, the proportion of Muse cell was 0.6 ± 0.08% (Figure 1C). MACS-sorted Muse-rich cells generated cluster formation from a single cell in suspension, reminiscent of triploblastic differentiation of the single cell-derived cluster, as described above (not shown). Furthermore, under neurosphere induction, Muse-rich cells formed neurosphere-like clusters positive for NeuroD1, Nestin, Sox2 and Musashi-1, as described above (not shown). These results indicated that the phenotype of Muse cells contained in the Muse-rich cells after MACS sorting did not differ from FACS-isolated Muse cells.
Distribution of Transplanted Muse Cells within the Host Infarcted Brain
At 8 weeks after transplantation, few MSCs were detected in the brain (not shown), while many FACS isolated GFP-labeled Muse cells populated the region between −1 mm and −3 mm from the bregma. Mean numbers of Muse cells per slice were 21 ± 2.2, 82.3 ± 13.7, and 23.3 ± 5.2 cells at −1 mm, −2 mm, and −3 mm, respectively (Figure 2A), indicating that most transplanted Muse cells remained proximal to the lesion site (Figure 2B upper). A similar graft distribution was detected in transplanted MACS-sorted Muse-rich cells (Figure 2B lower). The total number of grafted cells that integrated into the host brain was ~28% of initially injected Muse cells.
Figure 2. Distribution of transplanted Muse cells in the lacunar infarct brain.
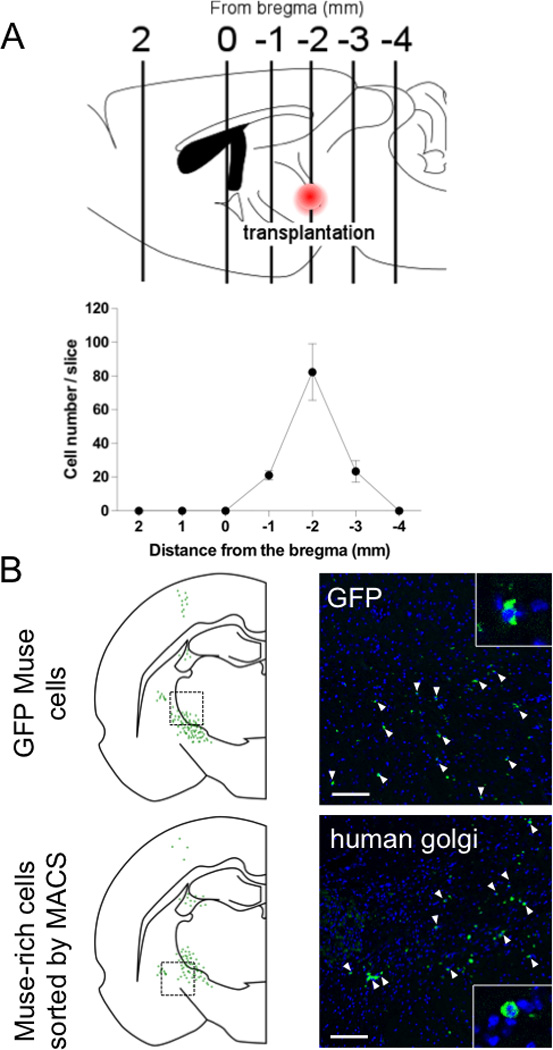
Engrafted Muse cells were counted in each section from the bregma (schema of brain sagittal section) 8 weeks after transplantation (A). At the sections from −2 mm from the bregma, FACS-isolated GFP-Muse cells (white arrow heads) were detected near the lesion site and but were not recognized in contralateral side (B). Similar distribution was observed in the section transplanted MACS sorted serum/xeno free Muse-rich cells (B). MACS sorted Muse-rich cells were stained with anti-human golgi antibody (white arrow heads). Scale bar = 100 µm.
Neural Differentiation of Transplanted Muse Cells
Whereas FACS-sorted GFP-Muse cells could not be detected in contralateral part of the brain (not shown), several Muse cells were observed within 400 εm area from the ipsilateral transplanted site (Figure 2A) expressing NeuN (neuronal marker), MAP2 (neuronal marker) and GST-pi (oligodendrocytic marker) (Figure 3A). Positivity for NeuN was 62.2 ± 2.4% of GFP-positive Muse cells, MAP2 30.6 ± 3.1% and GST-pi 12.1 ± 1.1% (Figure 3B). GFAP (astrocytic marker), Iba-1 (microglial marker) and Ki-67 (marker for proliferating cells) could not be detected in GFP-Muse cells (Figure 3B). GFP-labeled Muse cells were further shown to be positive for human golgi complex and human mitochondria, suggesting their human origin (Figure 3C). In the brain sections of MACS-sorted Muse-rich cells, human golgi complex-positive Muse cells expressed NeuN, suggesting their neuronal differentiation ability (Figure 3D).
Figure 3. Differentiation of FACS-isolated GFP-Muse cells after engraftment (8 weeks).
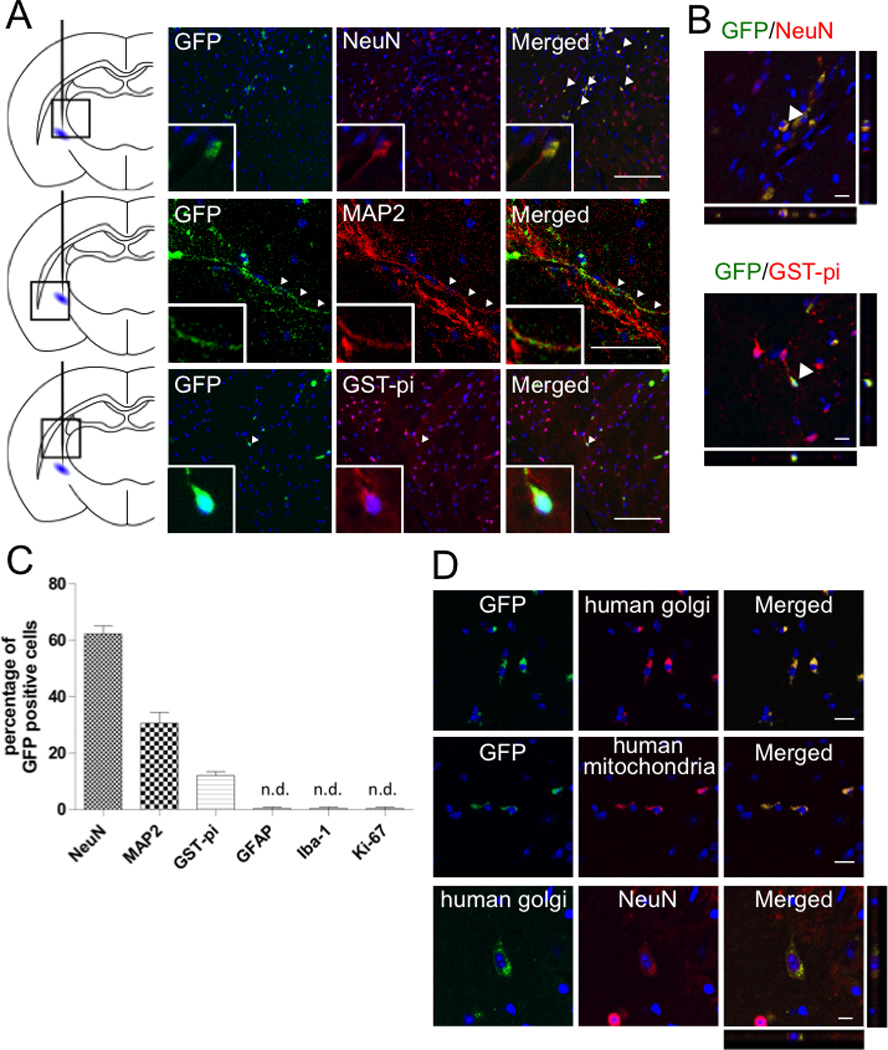
GFP-Muse cells, which were positive for NeuN (red), MAP2 (red) and GST-pi (red), were detected near the lesion site (arrow heads) (A, left). Z-axis images strained with NeuN (red) and GST-pi (red) were shown in (A, right). The percentage of each marker among GFP-Muse cells (B). GFP-labeled Muse cells were both positive for human golgi (red) and mitochondria (red) (C). MACS-sorted Muse-rich cells, which were positive for human golgi (green), differentiated into NeuN (red) positive neurons (D). Scale bars, A (left) = 100 µm; A (right), C and D = 10 µm.
Transplanted Muse Cells Aid in Pyramidal Tract Reconstruction
The anterograde tracer, dextran, when injected into the motor cortex labeled the host pyramidal tract. Focusing on the region close to the lacunar infarct lesion, the stump of dextran-labeled pyramidal tract displayed synaptophysin positivity and was detected adjacent to GFP-Muse cells, suggesting proximal juxtaposition between neurites of motor cortex neurons and Muse cells transplanted at the perilesion site (Figure 4A). On the other hand, when injected into the perilesion site, dextran incorporated into the transplanted Muse cells and was transported through the ipsilateral pyramidal tract (Figure 4B1), crossed to the contralateral side at the level of medulla (Figure 4B2), then transported through the contralateral pyramidal tract in the dorsal funiculus of spinal cord, specifically recognized at the level of upper cervical spinal cord (C1–2) (Figure 4B3). Since majority of neuritis in the same region was shown to be interrupted in C2 level spinal cord at 8 weeks (see Figure 1B), the dextran-positive signals (recognized in Figure 4B3) likely represented newly regenerated nerve fibers. Furthermore, VGluT (glutamatergic neuronal marker)-positive Muse cells, which positively stained with dextran and synaptophysin, were observed in the anterior horn of cervical spinal cord (Figure 4B4).
Figure 4. Involvement of Muse cells into reconstruction of pyramidal tract.
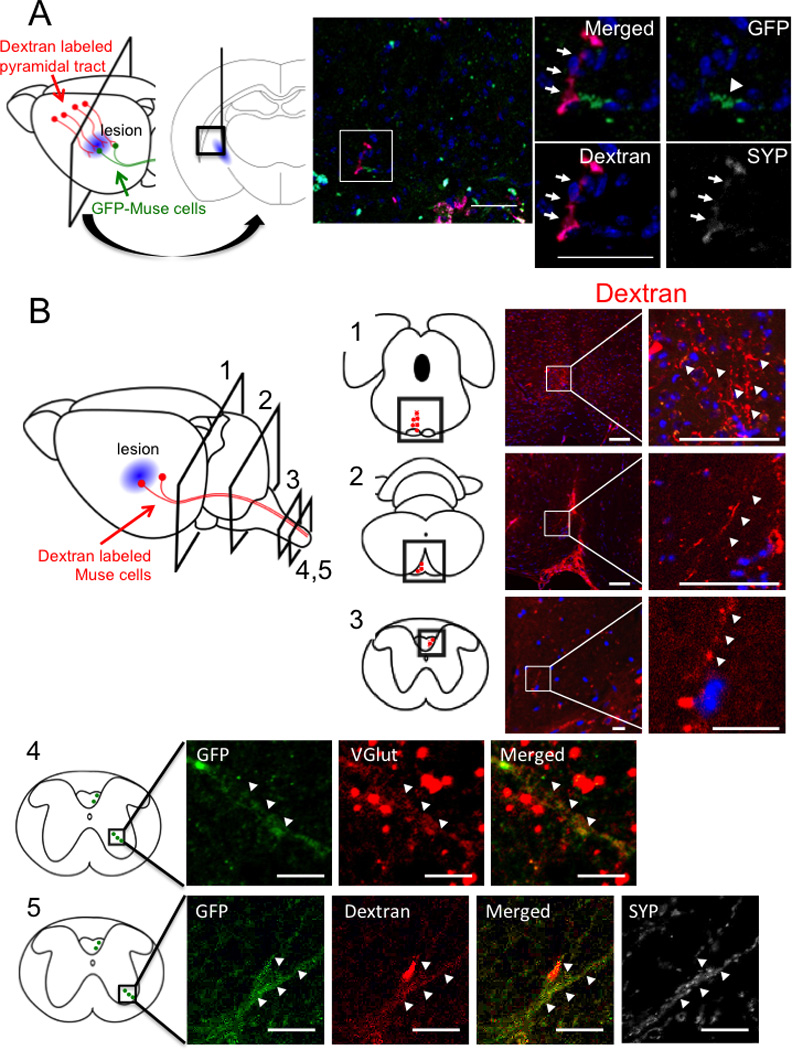
(A) Eight weeks after engraftment of FACS-isolated GFP-Muse cells. Motor cortical neurons were anterogradely labeled with dextran. Interruption of nerve axons in pyramidal tract was confirmed in Fig. 1B. FACS-isolated GFP-Muse cells (arrowhead) transplanted nearby the lesion was observed to connect to dextran-labeled motor neuron axons (red, arrows) which merged with synaptophysin (SYP, arrows), suggesting that Muse cells connected with motor cortex neurons and participated in the restoration of pyramidal tract. (B) Eight weeks after engraftment of MACS-sorted Muse-rich cells (data from level 1–3) and FACS-isolated GFP-Muse cells (data from level 4, 5). Dextran labeled axons (red, arrowheads) were detected at level 1: midbrain and level 2: medulla in the ipsilateral side, and in cervical spinal cord at level 3–5 in the contralateral side. Level 4 and 5 show the area of anterior horn in the spinal cord where pyramidal tract axons formed synapses with motor neurons. GFP+ neurite were positive for VGlut, suggesting differentiation of Muse cells into glutamatergic neuron (Level 4). Dextran (red)-labeled GFP (green) positive Muse cells were shown to be positive for synaptophysin (white) in upper cervical spinal cord (Level 5). Scale bars, A = 100 µm; B-Level 1 and 2 = 50 µm; Level 3–5 = 10 µm.
Transplanted Muse Cells Improve Behavioral Score
Corner turn test at 6 and 8 weeks demonstrated significant recovery in the Muse-rich group compared to the MSC (P* < 0.05) and vehicle groups (P* < 0.05) (Figure 5A). Cylinder test at 4, 6 (both for MSC and vehicle groups; P* < 0.05) and 8 (both at P** < 0.01) weeks revealed significant improvement of the neurological performance in the Muse-rich group compared to the MSC and vehicle groups (Figure 5B). Treatment with diphtheria toxin (DT), known to selectively ablate human cells in rodent model, deteriorated behavioral recovery, with the functional deficit resembling that of the vehicle group (P*** < 0.001; Figure 5C).
Figure 5. Behavioral analysis and loss of function study.
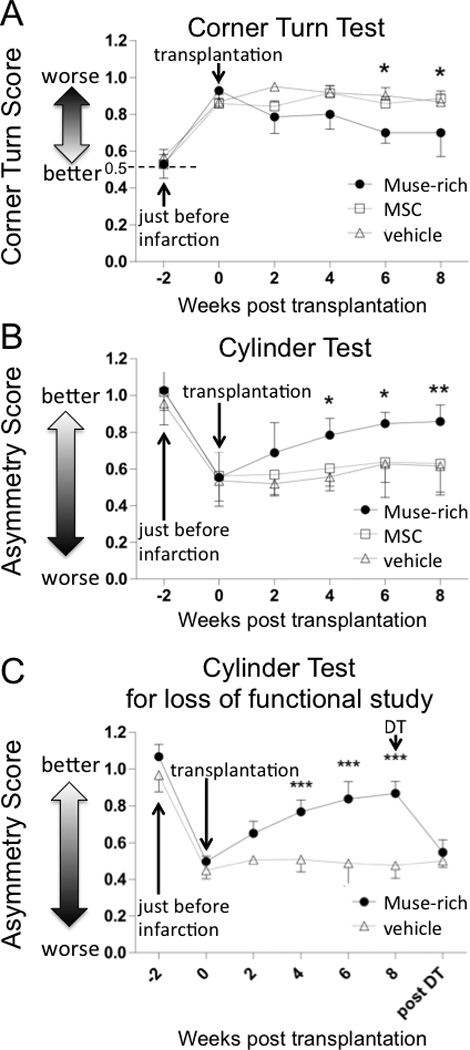
The number of animals in each group was 7. Transplantation of Muse-rich cells resulted in functional recovery in corner turn (A, 0.5: no deficit, scores higher or lower than 0.5: severe deficit) and cylinder (B, 1: no deficit, 0: severe deficit) test with statistical differences to the MSC and vehicle groups. The functional recovery was ablated by the administration of DT (C, 1: no deficit, 0: severe deficit). P* < 0.05, P** < 0.01, P*** < 0.001.
Safety Assessment of Muse Cell Grafts
Human specific Alu sequence was detected only in the brain, which was more pronounced at 6 months compared to 2 months post-transplantation of Muse-rich cells in stroke animals, but was not found in any organ at 10 months post-transplantation in intact mice (Figure 6A). Tumor formation was not evident in any organ investigated (Figure 6B).
Figure 6. Safety evaluation.
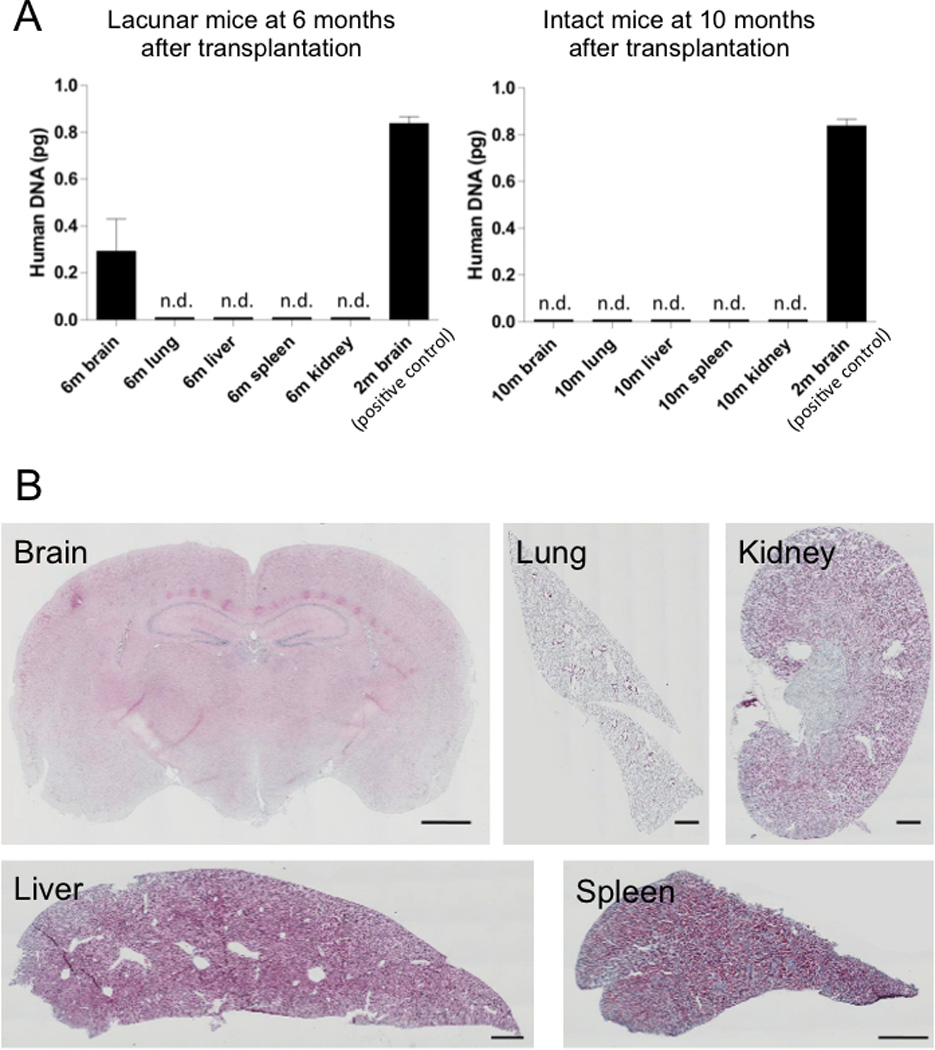
Q-PCR for human specific Alu sequence was acquired from lacunar infarcts model 2 and 6 months after Muse-rich group and from intact model 10 months after Muse-rich cell transplantation (A). Human specific Alu signal was detected only in the brain of lacunar infarcts model 2 and 6 months after transplantation. Histologically, tumor formation was not recognized in any organ (the brain, lung, kidney liver and spleen) at 6 months after transplantation (B). Scale bar = 1000 µm.
Discussion
The present study used the subacute phase (i.e., 2 weeks post-injury) lacunar infarct model to reveal the effectiveness and safety of locally injected human Muse cells in SCID mice. In addition to FACS-isolated Muse cells cultured in serum/xeno-free system which is free from potential cross-species contaminants, we also examined MACS-sorted Muse-rich cells under the same culture system. Our present data demonstrated that both FACS-isolated and MACS-sorted Muse cells retained their neuronal differentiation ability and afforded robust and stable therapeutic benefits against stroke.
Clinical trials of neural stem cell transplantation were initially implemented to replace degenerated or dead neuronal cells with viable neuronal cells22, but to date only scarce reports support the occurrence of neuronal circuitry reconstruction, with safety concerns of tumorigenic potential23. Such adverse event of tumor formation is circumvented by MSCs since these cells do not persist in the host brain after transplantation in stroke animal models24–28. Despite lack of graft persistence, MSCs exert neuroprotection via anti-inflammatory and cytokine secretory effects in laboratory studies6, 7, 24–28, but clinically relevant outcomes in stroke patients treated with MSCs warrant further investigations8, 9, 22. Sub-acute (as in the present study of two weeks post-stroke) and the chronic (1 month post-stroke) both represent delayed stages of stroke that preclude stem cell therapy at this late time point as likely abrogating the acute cell death processes of stroke. Interestingly, delayed transplantation of MSCs still produced robust behavioral recovery despite modest graft survival, suggesting that rather than graft persistence, the by-stander effects such as endogenous neurogenesis and neuroplasticity, as seen in preclinical studies27, 28 and alluded to in a clinical study29, appear to largely mediate MSC functional benefits. That delayed MSC transplantation induces a cell replacement mechanism remains elusive. Along the same vein, MSCs may confer limited effects when transplanted at the delayed two weeks or one month post-stroke, but even with this delayed transplantation, MSCs remain resilient in inducing behavioral recovery and it is the graft persistence (albeit cell replacement) that becomes highly restricted. To this end, the effectiveness of MSCs especially in delayed transplantation regimen is largely attributed to the by-stander effects and not on cell replacement. Conversely, and here forth advances the main tenet of our study, the beneficial effects of Muse cells entail the cell replacement mechanism even when transplanted at the late phase of stroke. This novel observation of cell replacement at the delayed stage of stroke suggests that Muse cells differ from MSCs in that they persist in the host tissue, replenish neuronal cells, and participate in structural regeneration, in particular neuronal circuitry reconstruction that renders sustainable functional recovery in the stroke brain. However, whether Muse cells equally deliver robust by-stander effects as seen in MSCs was not examined in this study. Accordingly, MSCs and Muse cells may possess unique therapeutic mechanisms in attenuating stroke deficits9, 16, 17, 27–29, indicating perhaps a combination of MSCs and Muse cells in the proper proportion may synergistically enhance the functional outcomes of cell therapy.
Eight weeks after transplantation, the majority of engrafted Muse cells differentiated spontaneously into NeuN- (62.2 ± 2.4% of GFP positive cells) and MAP2- (30.6 ± 3.1%) positive cells which engrafted close to the lesion site. Muse cells did not disperse widely throughout the brain probably because the damaged site was focal. Furthermore, they differentiated into GST-pi-positive (12.1 ± 1.1%) cells, suggesting their commitment was not confined to neuronal cells but also included an oligodendrocyte phenotype. Interestingly, Muse cells did not differentiate into astrocyte and microglia, likely because of microenvironmental cues. Damaged cells in the lesion area comprised primarily of neuronal cells and oligodendrocytes, with only minimal proportion of astrocytes, which likely influenced the low differentiation capacity of Muse cells towards the astrocytic phenotype.
Neuron tracing results showed that transplanted Muse cells extended their neurites into the contralateral site, crossing at the medulla, and reached at least the upper cervical spinal cord (C1–2). Neurites of Muse cells in the spinal cord expressed VGluT and synaptophysin, markers for glutamatergic neurons and presynapses. Transplantation of Muse-rich cells significantly improved the neurological performance compared to MSC and vehicle treatments. In addition, human-selective toxin DT abrogated the functional recovery produced by Muse-rich cell transplantation, suggesting that successful integration of Muse cells into host neural network by connecting to motor neurons likely mediated the behavioral outcome.
Functional recovery may be related to ~71% purity of the Muse cell, which was achieved here via serum/xeno-free system and the clinically accepted MACS set-up18. Of note, animals that received MSC transplantation, which contained a small fraction of Muse cells, did not demonstrate significant better recovery compared to vehicle-treated animals, which is consistent with a previous report demonstrating that MSC transplantation was not effective when initiated in the late phase of stroke5. Here, we provide evidence that Muse cells, as opposed to a general MSC phenotype, may facilitate improved cell graft survival, integration into the host brain, and differentiation into neuronal cells, which stand as key components for initiating neuronal circuitry reconstruction even in the delayed subacute phase of stroke.
MSCs as donor transplantable cells are non-tumorigenic, thereby garnering a solid safety record in the clinic29,30, but the surgical procedure of cell transplantation may generally have potential detrimental effects such as infusion toxicity, infection and formation of tumor30. We showed here that Muse cells were also safe, with no tumor formation detected up to at least 6 months post-transplantation. In lacunar infarcted mice which received MACS-sorted Muse-rich cells, human specific Alu sequence was detected only in the brain, indicating that intracerebral local injections of Muse cells did not migrate to peripheral organs. Neither ectopic tissue formation nor unregulated Ki67 proliferation of Muse cells was observed in the brain and other organs examined. That intact mice transplanted with MACS-sorted Muse-rich cells exhibited no human specific Alu in organ including the brain even though Muse-rich cells were directly injected into the brain, suggests that Muse cells engrafted only into appropriate injured site (i.e., damaged area), but not when ‘vacant seat’ (loss of cells) was not provided by the microenvironment. Nevertheless, potential detrimental effects need to be validated in higher mammals such as non-human primates in future study.
Transplantation of human Muse cells in the subacute phase of lacunar infarcts in SCID mice produced favorable neurological recovery, accompanied by grafted Muse cells differentiating into neurons and oligodendrocytes, and participating in the reconstruction of pyramidal tract. Combined with the observed safety outcomes in the long-term graft maturation period, the present study advances the use of Muse cells for transplant therapy in stroke.
Supplementary Material
Supplemental Figure I: Characterization of Serum-/xeno-free Muse Cells in Vitro
Supplemental Material I: Detailed Materials and Methods
Acknowledgments
Source of Funding
This study was supported by a Grant-in-Aid from the New Energy and Industrial Technology Development Organization (NEDO), and Grant-in-Aid from Japan Agency for Medical Research and Development (AMED). CVB is supported by National Institutes of Health, National Institute of Neurological Disorders and Stroke 1R01NS071956, 1R01NS090962, and 1R21NS089851, Department of Defense W81XWH-11-1-0634, SanBio Inc., KM Pharmaceuticals, NeuralStem Inc., International Stem Cell Corp., and Karyopharm Inc.
Disclosures
Hiroki Uchida, Kuniyasu Niizuma, and Teiji Tominaga Department of Neurosurgery, Tohoku University Graduate School of Medicine, and Yoshihiro Kushida, Shohei Wakao, and Mari Dezawa of Department of Stem Cell Biology and Histology, Tohoku University Graduate School of Medicine, which are parties to a co-development agreement concluded with Clio, Inc. under subsidy from the New Energy and Industrial Technology Development Organization and Japan Agency for Medical Research and Development.
References
- 1.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28:491–499. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 2.Jackson C, Sudlow C. Comparing risks of death and recurrent vascular events between lacunar and non-lacunar infarction. Brain. 2005;128:2507–2517. doi: 10.1093/brain/awh636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norrving B. Long-term prognosis after lacunar infarction. Lancet Neurol. 2003;2:238–245. doi: 10.1016/s1474-4422(03)00352-1. [DOI] [PubMed] [Google Scholar]
- 4.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 5.Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiol. Dis. 2010;37:275–283. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ. Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 8.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 9.Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe K, Yamashita T, Takizawa S, Kuroda S, Kinouchi H, Kawahara N. Stem cell therapy for cerebral ischemia: from basic science to clinical applications. J. Cereb. Blood Flow Metab. 2012;32:1317–1331. doi: 10.1038/jcbfm.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc. Natl. Acad. Sci. U. S. A. [Internet] 2010;107:8639–8643. doi: 10.1073/pnas.0911647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakao S, Kitada M, Kuroda Y, Shigemoto T, Matsuse D, Akashi H, et al. Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9875–9880. doi: 10.1073/pnas.1100816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M, Dezawa M. Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat. Protoc. 2013;8:1391–1415. doi: 10.1038/nprot.2013.076. [DOI] [PubMed] [Google Scholar]
- 14.Katagiri H, Kushida Y, Nojima M, Kuroda Y, Wakao S, Ishida K, et al. A Distinct Subpopulation of Bone Marrow Mesenchymal Stem Cells, Muse Cells, Directly Commit to the Replacement of Liver Components. Am. J. Transplant. 2016;16:468–483. doi: 10.1111/ajt.13537. [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita K, Kuno S, Ishimine H, Aoi N, Mineda K, Kato H, et al. Therapeutic Potential of Adipose-Derived SSEA-3-Positive Muse Cells for Treating Diabetic Skin Ulcers. Stem Cells Transl. Med. 2015;4:146–155. doi: 10.5966/sctm.2014-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamauchi T, Kuroda Y, Morita T, Shichinohe H, Houkin K, Dezawa M, et al. Therapeutic effects of human multilineage-differentiating stress enduring (MUSE) cell transplantation into infarct brain of mice. PLoS One. 2015;10:e0116009. doi: 10.1371/journal.pone.0116009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchida H, Morita T, Niizuma K, Kushida Y, Kuroda Y, Wakao S, et al. Transplantation of Unique Subpopulation of Fibroblasts, Muse Cells, Ameliorates Experimental Stroke Possibly via Robust Neuronal Differentiation. Stem Cells. 2016;34:160–173. doi: 10.1002/stem.2206. [DOI] [PubMed] [Google Scholar]
- 18.Richel DJ, Johnsen HE, Canon J, Guillaume T, Schaafsma MR, Schenkeveld C, et al. Highly purified CD34+ cells isolated using magnetically activated cell selection provide rapid engraftment following high-dose chemotherapy in breast cancer patients. Bone Marrow Transplant. 2000;25:243–249. doi: 10.1038/sj.bmt.1702136. [DOI] [PubMed] [Google Scholar]
- 19.Uchida H, Sakata H, Fujimura M, Niizuma K, Kushida Y, Dezawa M, et al. Experimental model of small subcortical infarcts in mice with long-lasting functional disabilities. Brain Res. 2015;1629:318–328. doi: 10.1016/j.brainres.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Hayase M, Kitada M, Wakao S, Itokazu Y, Nozaki K, Hashimoto N, et al. Committed neural progenitor cells derived from genetically modified bone marrow stromal cells ameliorate deficits in a rat model of stroke. J. Cereb. Blood Flow Metab. 2009;29:1409–1420. doi: 10.1038/jcbfm.2009.62. [DOI] [PubMed] [Google Scholar]
- 21.Ogura F, Wakao S, Kuroda Y, Tsuchiyama K, Bagheri M, Heneidi S, et al. Human adipose tissue possesses a unique population of pluripotent stem cells with nontumorigenic and low telomerase activities: potential implications in regenerative medicine. Stem Cells Dev. 2014;23:717–728. doi: 10.1089/scd.2013.0473. [DOI] [PubMed] [Google Scholar]
- 22.Bersano A, Ballabio E, Lanfranconi S, Boncoraglio GB, Corti S, Locatelli F, et al. Clinical studies in stem cells transplantation for stroke: a review. Curr. Vasc. Pharmacol. 2010;8:29–34. doi: 10.2174/157016110790226570. [DOI] [PubMed] [Google Scholar]
- 23.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acosta SA, Tajiri N, Hoover J, Kaneko Y, Borlongan CV. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke. 2015;46:2616–2627. doi: 10.1161/STROKEAHA.115.009854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janowski M, Wagner D-C, Boltze J. Stem cell-based tissue replacement after stroke: Factual necessity or notorious fiction? Stroke. 2015;46:2354–2363. doi: 10.1161/STROKEAHA.114.007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heo JS, Choi S-M, Kim EH, You J, Park T, Kim E, et al. Neural transdifferentiation of human bone marrow mesenchymal stem cells on hydrophobic polymer-modified surface and therapeutic effects in an animal model of ischemic stroke. Neuroscience. 2013;238:305–318. doi: 10.1016/j.neuroscience.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Yasuhara T, Matsukawa N, Hara K, Maki M, Ali MM, Yu SJ, et al. Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem Cells Dev. 2009;18:1501–1514. doi: 10.1089/scd.2009.0011. [DOI] [PubMed] [Google Scholar]
- 28.Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 29.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 30.Battistella V, de Freitas GR, da Fonseca LMB, Mercante D, Gutfilen B, Goldenberg RCS, et al. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen. Med. 2011;6:45–52. doi: 10.2217/rme.10.97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure I: Characterization of Serum-/xeno-free Muse Cells in Vitro
Supplemental Material I: Detailed Materials and Methods


