Abstract
Background
Frail individuals are at increased risk for poor outcomes, including adverse drug events. Kidney function is often compromised in frailty and is a key consideration in medication choice and dosing; however, creatinine-based measures of kidney function may be biased in frail individuals.
Study Design
Observational study.
Setting & Participants
4,987 community-dwelling older men and women with complete data who participated in visit 5 of the Atherosclerosis Risk in Communities (ARIC) Study (2011–2013).
Predictor
Kidney measures included glomerular filtration rate (GFR) estimated using serum creatinine (eGFRcr) and serum cystatin C (eGFRcys) and urine albumin-creatinine ratio (ACR).
Outcomes
Frailty, defined using established criteria of 3 or more frailty characteristics (weight loss, slowness, exhaustion, weakness, and low physical activity).
Results
In total, 341 participants (7%) were classified as frail, 1475 (30%) had eGFRcr <60 ml/min/1.73 m2, 2480 (50%) had eGFRcys <60 ml/min/1.73 m2, and 1006 (20%) had albuminuria ≥30 mg/g. Among frail participants, prevalences of eGFRcr and eGFRcys <60 ml/min/1.73 m2 were 45% and 77%, respectively. Adjusted for covariates, frailty showed a moderate association with eGFRcr and a strong association with eGFRcys and ACR. Frail individuals with eGFRcr 60–<75 ml/min/1.73 m2 were frequently reclassified to lower eGFR categories using eGFRcys (49% to 45–<60, 32% to 30–<45, and 3% to <30 ml/min/1.73 m2). Hyperpolypharmacy (taking ≥10 classes of medications) was more common in frail individuals (54% vs 38% of non-frail), including classes requiring kidney clearance (e.g., digoxin) and associated with falls and subsequent complications (e.g., hypnotic/sedatives, anticoagulants).
Limitations
Cross-sectional study design.
Conclusions
Frail individuals had a high prevalence of reduce kidney function, with large discrepancies when reduced kidney function was classified by eGFRcys versus eGFRcr. Given the substantial medication burden and uncertainty in CKD classification, confirmation of kidney function with alternative biomarkers may be warranted to ensure careful prescribing practices in this vulnerable population.
Keywords: frailty, frail, prefrail, geriatric, older adults, chronic kidney disease (CKD), estimated glomerular filtration rate (eGFR), reduced kidney function, serum creatinine, serum cystatin C, urine albumin, albuminuria, biomarker, polypharmacy
Frailty is a phenotype of multisystem dysregulation.1 National data from the United States suggest that 15% of community-dwelling persons aged 65 years or older are frail and 45% are prefrail.2 The high prevalence of frailty is a significant public health problem. The older adult population is growing, and frailty is associated with increased risk of disability, hospitalization, and mortality.1–4 Frail individuals are more susceptible to adverse outcomes, including adverse drug effects such as delirium and falls. These risks may be aggravated by inappropriate use and dosing of individual drugs as well as polypharmacy, a common issue in frail individuals.5 A recent study estimated that 39% of adults aged 65 years and older were taking 5 or more medications.6
Chronic kidney disease (CKD) is another public health problem that disproportionately affects older adults, has significant consequences for drug dosing, and has been associated with a higher prevalence of frailty.7–17 However, previous studies have relied on serum creatinine for estimating kidney function, which is problematic in frailty since estimation of glomerular filtration rate (GFR) from serum creatinine assumes a similar muscle mass across all individuals of the same age, sex, and race; frail individuals often suffer from sarcopenia. Few studies have examined the relationship between frailty and kidney function measured by cystatin C, an alternative kidney filtration marker less influenced by muscle mass and diet.9, 16 In addition, little has been done to investigate the independent association between frailty and albuminuria, a key component of the new CKD classification system.18 Thus, the relationship between frailty and kidney function assessed with different filtration markers could have significant public health impact in the prescription of medications requiring adjustment in dosing or monitoring in kidney disease.
We investigated the cross-sectional association of frailty with kidney function as measured by both established and novel biomarkers (creatinine, cystatin C, and urine albumin) in a biracial population-based cohort of men and women aged 66 years or older, comparing the strength of associations between the alternative biomarkers. We then investigated the clinical implications of accurate assessment of kidney function in frail individuals by quantifying medication use and, in particular, medications that are cleared by the kidney.
Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) study cohort was established in 1987 as a prospective cohort of 15,792 men and women, aged 45–64 years, from the following four communities in the United States: suburban Minneapolis, Minnesota; Forsyth County, North Carolina; Washington County, Maryland; and Jackson, Mississippi. Extensive physical examinations were performed at baseline and at four subsequent clinic visits. Participants of the ARIC cohort were selected for the present study if they completed the fifth clinical examination (Visit 5, 2011–2013; n=6,538). We excluded participants whose race was not white or black (n=18), black participants from Minnesota (n=9) and Maryland (n=16), individuals who were missing data on other covariates (n=1,410), and individuals missing the frailty components (n=98). Thus, 4,987 participants were included in the final study population. The ARIC Study has been approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board (IRB number: H.34.99.07.02.A1), and all participants provided informed consent.
Frailty Definition
We identified frail individuals at the visit 5 clinical examination using the operational definition developed by Fried et al.1, which includes five components: 1) weight loss, 2) slowness, 3) exhaustion, 4) weakness, and 5) low physical activity.1 The ARIC-specific classification of the presence of the components has been previously described.19 Briefly, weight loss was classified as an unintentional weight loss of 10% between visit 4 (1996–1998) and visit 5 (2011–2013) or a body mass index (BMI) of <18.5 kg/m2 at visit 5. Slowness was classified as the 20th percentile in gender- and height-adjusted gait speed during a 4-m walk test using previous population-based thresholds.1 Exhaustion was classified as responding “some of the time” or “most of the time” to two questions from the 11-item Center for Epidemiologic Studies–Depression (CES-D) scale20, 21: “I felt everything I did was an effort” and “I could not get ‘going’.” Weakness was classified as the 20th percentile in gender- and BMI-specific grip strength based on population-based cut points.1 Low physical activity was classified as the 20th percentile in gender-specific Baecke leisure sport activity index.22 Participants were characterized as frail if 3 or more of the components were present. Using a conservative approach, participants missing information on any of the component characteristics were classified as missing the frailty phenotype, unless three or four frail components were present among the non-missing components. These participants were classified as frail.
Kidney Measures
We estimated GFR from serum creatinine and serum cystatin C using the 2009 CKD-EPI (CKD Epidemiology Collaboration) creatinine equation23 (eGFRcr) and the 2012 CKD-EPI cystatin C equation24 (eGFRcys). Serum creatinine was measured using a creatinase enzymatic method on a Roche Modular P Chemistry Analyzer (Roche Diagnostics, Indianapolis, Indiana) and standardized to isotope-dilution mass spectrometry. Serum cystatin C level measured by a turbidimetric method (Gentian AS, Moss, Norway), calibrated and standardized to International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) reference25.
Albuminuria was defined as the ratio of urine albumin to urine creatinine. Urine creatinine was measured by the modified kinetic Jaffé method. Albumin was measured from urine samples by a nephelometric method on either the Dade Behring BN100 or the Beckman Image Nephelometer.
Measurement of Other Covariates
Trained study personnel and research technicians took all physical measurements and administered all questionnaires following a standardized protocol that included quality control measures during the visit 5 clinical examination. Race was classified as black or white. Education was categorized as less than high school, high school or equivalent, and college/graduate school. Cigarette smoking and alcohol consumption were categorized as current user or never/former user.
The BMI was defined as weight (in kilograms) divided by height (in meters) squared. Diabetes was defined as self-reported history of physician diagnosis, antidiabetic medication use during the past 2 weeks, fasting blood glucose level ≥ 126 mg/dL, or nonfasting blood glucose level ≥ 200 mg/dL. Certified technicians measured blood pressure three times with participants in the sitting position after 5-minute rest, using a validated automatic sphygmomanometer (the Omron HEM-907 XL), and the average of the last two readings was recorded. Anemia was defined using sex-specific cut points for hemoglobin (based on the World Health Organization definition,26 <12 g/dL for non-pregnant women and <13 g/dL for men). C-reactive protein was included as a marker of inflammation. Prevalent cardiovascular disease (including coronary heart disease, peripheral artery disease, heart failure, and stroke) was established by self-report at visit 1 or an adjudicated event between visits 1 and 5. Reduced kidney function was defined as eGFRcr or eGFRcys of <60 ml/min/1.73m2.
All medication use was self-reported by participants and confirmed by staff from the medications brought to the visit. Polypharmacy was defined as use of 5 or more medication classes; hyperpolypharmacy was defined as use of 10 or more medication classes. Each drug class was examined in relation to frailty prevalence.
Statistical Analysis
Crude proportions and mean values were used to describe demographics, health behaviors, and risk factors and comorbidities according to frailty status. Differences between groups in univariate analyses were assessed using chi-square tests for categorical variables and two sample t-tests or analysis of variance for continuous variables as appropriate.
The associations between kidney measures and frailty were assessed using Poisson regression models to estimate prevalence ratios. The eGFR and ACR were analyzed in categories (<30, 30–<45, 45–<60, and ≥60 ml/min/1.73m2, and <30, 30–<300, and ≥300 mg/g, respectively). When included as covariates in other models, eGFR and ACR (log-transformed ACR) were examined continuously. Prevalence of frailty was also examined by kidney function categorized by eGFR and albuminuria stage according to the KDIGO (Kidney Disease: Improving Global Outcomes) CKD guideline (ie, G1 to G5 and A1 to A3).18 Model 1 included age (continuous), sex, race/center, and education. Model 2 included all variables in Model 1 plus smoking status and alcohol consumption. Model 3 included all variables in Model 2 plus relevant comorbidities of BMI, systolic blood pressure, anemia, inflammation, prevalent cardiovascular disease, diabetes, hypertension medication use, and statin medication use. Because diabetes mellitus is one of the most common causes of CKD in older adults and also a strong predictor of frailty,27 we performed stratified analysis by diabetes status to determine differences by categories of both eGFR and ACR.
Unadjusted eGFRcr and eGFRcys categories (<30, 30–<45, 45–<60, 60–<75, 75–<90, and ≥90 ml/min/1.73m2) were cross-tabulated to determine the number of individuals reclassified up or down to different categories when using eGFRcys instead of eGFRcr. We also evaluated the categorical net reclassification improvement (NRI) for categories of GFR (<30, 30–<45, 45–<60, and ≥60 ml/min/1.73m2 and <30, 30–<60, and ≥60 ml/min/1.73m2) with frailty.28
The associations of medication classes and frailty were assessed using crude proportions as well as with unadjusted and demographically-adjusted Poisson regression models.
All P values were 2 sided, and P < 0.05 was considered statistically significant. Statistical analyses were conducted using Stata version 13.0 (StataCorp LP, College Station, TX, USA).
Results
Baseline Characteristics by Frailty Status
Among the 4,987 participants, 341 were classified as frail (Table 1). Those who were frail were older (mean age, 78.0 versus 75.4 years) and more likely to be women (64.8% versus 55.1%) than participants who were not frail. There was no statistically significant difference in the prevalence of frailty by race. Individuals who were classified as frail had lower levels of education, were less likely to be current alcohol users, and had slightly higher BMI than participants who were not frail. There was no significant difference in smoking status or systolic blood pressure levels. Frail individuals were more likely to have diabetes (53.1% versus 37.5%). Among participants with diabetes, frail individuals had similar hemoglobin A1c levels but had a longer duration of disease (mean, 12.5 ± 6.4 [standard deviation] versus 10.7 ±6.3 years; p<0.001). Frail individuals were also more likely to have prevalent cardiovascular disease, anemia, and an elevated inflammatory marker (C-reactive protein).
Table 1.
Characteristics by frailty status.
| Characteristic | Not frail | Frail | p-value |
|---|---|---|---|
| No. | 4,646 (93.2%) | 341 (6.8%) | |
| Age, y | 75.4 (5.1) | 78 (5.6) | <0.001 |
| Female sex | 2,561 (55.1%) | 221 (64.8%) | 0.001 |
| Black race | 1,029 (22.1%) | 84 (24.6%) | 0.3 |
| Education level | <0.001 | ||
| <HS | 611 (13.2%) | 76 (22.3%) | |
| HS, GED, or vocational school | 1,914 (41.2%) | 163 (47.8%) | |
| College, graduate, or professional school | 2,121 (45.7%) | 102 (29.9%) | |
| Smoking status | 0.5 | ||
| Current | 271 (5.8%) | 23 (6.7%) | |
| Former/Never | 4,375 (94.2%) | 318 (93.3%) | |
| Alcohol consumption | <0.001 | ||
| Current | 2,359 (50.8%) | 115 (33.7%) | |
| Former/Never | 2,287 (49.2%) | 226 (66.3%) | |
| BMI, kg/m2 | 28.7 (5.6) | 29.8 (7.1) | <0.001 |
| Systolic blood pressure | 130.6 (17.9) | 132.0 (20.7) | 0.2 |
| eGFRcr | 69.6 (17.1) | 61.8 (19.9) | <0.001 |
| eGFRcys | 61.8 (19.1) | 47.4 (17.4) | <0.001 |
| ACR, log-transformed | 2.3 [1.8–3.1] | 3.0 [2.1–4.0] | <0.001 |
| DM | 1,744 (37.5%) | 181 (53.1%) | <0.001 |
| Hemoglobin A1c* | 6.6 (1.1) | 6.6 (1.2) | 0.6 |
| Duration of DM, y | 10.7 (6.3) | 12.5 (6.4) | <0.001 |
| Prevalent cardiovascular disease | 1,122 (24.1%) | 143 (41.9%) | <0.001 |
| Anemia | 1,200 (25.8%) | 131 (38.4%) | <0.001 |
| C-reactive protein | 3.9 (6.6) | 5.3 (8) | <0.001 |
| Any HTN medication | 3,516 (75.7%) | 298 (87.4%) | <0.001 |
| No. of medications taken | 8.8 (4.6) | 10.5 (5.0) | <0.001 |
Note: Values for categorical variables are given as percentages; values for continuous variables are given as mean ± standard deviation or median [interquartile range].
For participants with DM
ACR, albumin-creatinine ratio; BMI, body mass index; DM, diabetes mellitus; eGFRcr, creatinine-based estimated glomerular filtration rate; eGFRcys, cystatin C–based estimated glomerular filtration rate; GED, General Education Development; HS, high school; HTN, hypertension;
Frailty and CKD
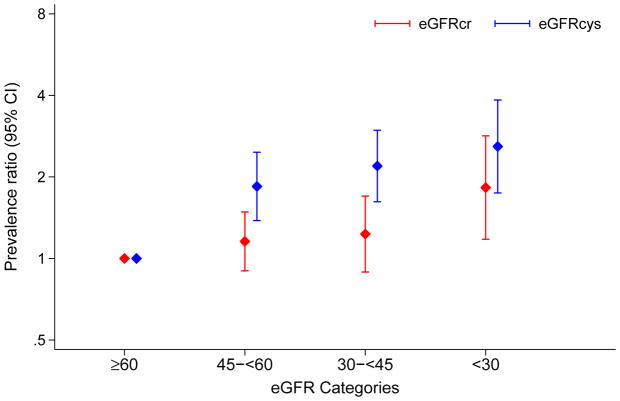
Reduced eGFR (<60 ml/min/1.73 m2) was common in frail individuals, but the proportion with eGFR <60 ml/min/1.73 m2 was much greater when kidney function was estimated using eGFRcys compared to eGFRcr (77% versus 45%). In univariate analyses, both eGFRcr and eGFRcys were inversely associated with frailty. Compared to eGFRcr of ≥60 ml/min/1.73m2, an eGFRcr of <30, 30–<45, and 45–<60 ml/min/1.73m2 were significantly associated with frailty in a demographic-adjusted model (Model 1) (Table 2). This association remained significant when further adjusted for health behaviors (Model 2), but was attenuated in the fully adjusted model (Model 3) and the only significant category was eGFR <30 ml/min/1.73m2 (Figure 1). When kidney function was assessed using eGFRcys, categories of <30, 30–<45, and 45–<60 ml/min/1.73m2 were all significantly associated with frailty in a graded manner; this association was present in all models. Although diabetes was associated with frailty in all analyses (prevalence ratios in fully adjusted models for eGFRcr and eGFRcys of 1.35 [95% CI, 1.08–1.68] and 1.32 [95% CI, 1.06–1.65]), relationships between eGFR and frailty were similar within categories of diabetes and no diabetes and consistently stronger when using eGFRcys (Figure S1, available as online supplementary material).
Table 2.
Prevalence ratios for frailty associated with eGFRcr, eGFRcys, and ACR.
| Unadjusted prevalence: % Frail (No. Frail/Total No.) | PR (95% CI) | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| eGFRcr category | ||||
| ≥60 | 5.3% (186/3,512) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 45–<60 | 8.5% (86/1,012) | 1.35* (1.05, 1.74) | 1.34* (1.04, 1.71) | 1.16 (0.90, 1.49) |
| 30–<45 | 12.7% (47/369) | 1.86*** (1.35, 2.55) | 1.84*** (1.34, 2.52) | 1.23 (0.89, 1.70) |
| <30 | 23.4% (22/94) | 3.40*** (2.21, 5.21) | 3.34*** (2.17, 5.12) | 1.83** (1.18, 2.84) |
| P for trend | p<0.001 | p<0.001 | p=0.02 | |
| eGFRcys category | ||||
| ≥60 | 3.1% (77/2,507) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 45–<60 | 7.9% (110/1,386) | 2.17*** (1.62, 2.90) | 2.10*** (1.57, 2.82) | 1.85*** (1.38, 2.47) |
| 30–<45 | 12.3% (103/838) | 3.08*** (2.29, 4.15) | 2.98*** (2.21, 4.02) | 2.19*** (1.62, 2.97) |
| <30 | 19.9% (51/256) | 4.76*** (3.31, 6.83) | 4.62*** (3.21, 6.63) | 2.59*** (1.75, 3.85) |
| P for trend | p<0.001 | p<0.001 | p<0.001 | |
| ACR | ||||
| <30 | 5.5% (217/3,981) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 30–<300 | 11.6% (100/865) | 1.83*** (1.44, 2.31) | 1.80*** (1.42, 2.27) | 1.53*** (1.21, 1.95) |
| ≥300 | 17.0% (24/141) | 2.75*** (1.82, 4.15) | 2.78*** (1.84, 4.19) | 1.95** (1.28, 2.97) |
| P for trend | p<0.001 | p<0.001 | p<0.001 | |
p≤0.05;
p≤0.01;
p≤0.001
Note: eGFRs expressed in mL/min/1.73 m2 and ACRs in mg/g. Model 1: eGFR or ACR + Demographics (age, sex, race-center, education); Model 2: Model 1 + Health behaviors (smoking status, alcohol consumption); Model 3: Model 2 + Comorbidities (body mass index, systolic blood pressure, anemia [hemoglobin], inflammation [c-reactive protein], cardiovascular disease [history of coronary heart disease, peripheral artery disease, heart failure, stroke], diabetes, hypertension medications use, statin medication use) and either urine ACR (log-transformed) or eGFR.
ACR, albumin-creatinine ratio; CI, confidence interval; eGFRcr, creatinine-based estimated glomerular filtration rate; eGFRcys, cystatin C–based estimated glomerular filtration rate; PR, prevalence ratio
Figure 1. Adjusted prevalence ratios for frailty status by eGFR.
Prevalence ratios adjusted for age, sex, race-center, education, smoking status, alcohol consumption, BMI, systolic blood pressure, anemia [hemoglobin], inflammation [C-reactive protein], cardiovascular disease [history of coronary heart disease, peripheral artery disease, heart failure, stroke], diabetes, hypertension medications use, statin medication use) and either ACR (log-transformed).
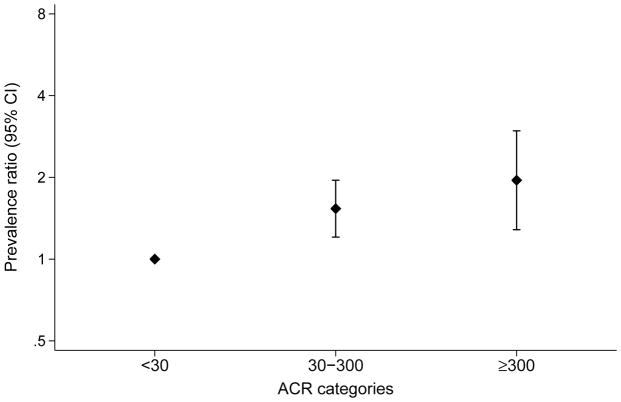
Categories of ACR 30–<300 mg/g and ≥300 mg/g were also strongly associated with frailty and the association remained in the fully adjusted models (Figure 2). Relationships between albuminuria and frailty were similar within categories of diabetes and no diabetes (Figure S2).
Figure 2. Adjusted prevalence ratios for frailty status by ACR.
Prevalence ratios adjusted for age, sex, race-center, education, smoking status, alcohol consumption, BMI, systolic blood pressure, anemia [hemoglobin], inflammation [C-reactive protein], cardiovascular disease [history of coronary heart disease, peripheral artery disease, heart failure, stroke], diabetes, hypertension medications use, statin medication use) and eGFR.
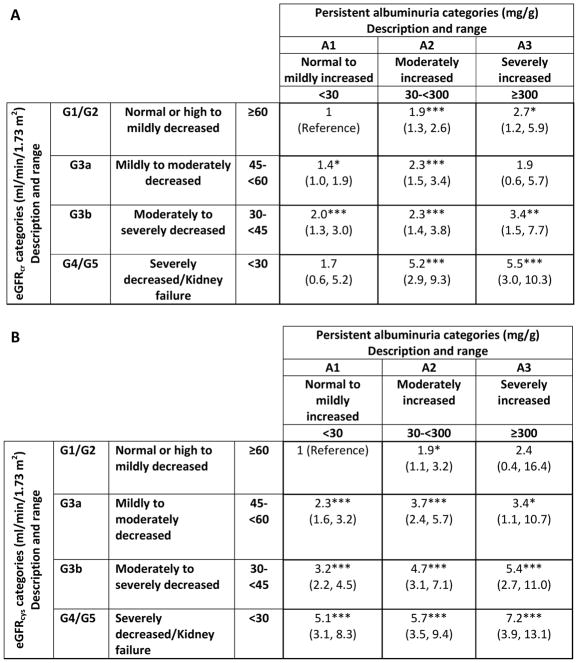
When taking into account both eGFR stage and albuminuria stage, the relationship between eGFR stage and frailty was stronger for eGFRcys compared with eGFRcr (Figure 3 and Figure S3). In demographic-adjusted analyses, prevalence of frailty generally increased with higher stage of eGFRcr and albuminuria (prevalence ratios increasing from 1.4 [95% CI, 1.0–1.9] to 5.5 [95% CI, 3.0–10.3]). The relationship was more pronounced when examining by stage of eGFRcys and albuminuria, with prevalence ratios of frailty increasing from 1.9 (95% CI, 1.1–3.2) to 7.2 (95% CI, 3.9–13.1); reference groups: eGFRcys ≥60 mL/min/1.73 m2 and ACR <30 mg/g).
Figure 3. Demographically-adjusted prevalence ratios (95% CIs) of frailty by eGFRcr (A) or eGFRcys (B) and albuminuria category.
*p≤.05; **p≤.01; ***p≤.001
Implications of Frailty and Reduced Kidney Function: Reclassification Based on Cystatin C
Overall, eGFRcr categorized 30% of this older adult study sample as <60 ml/min/1.73 m2 compared to 50% using eGFRcys (Tables S1, S2, and S3). Of those with an eGFRcr ≥60 ml/min/1.73m2, 33% of those individuals were reclassified as <60 ml/min/1.73m2 using eGFRcys. These differences were more pronounced in the frail than in the non-frail individuals (Table 3). For the individuals with an eGFRcr ≥60 ml/min/1.73m2, eGFRcys reclassified 31.3% to be <60 ml/min/1.73m2 among non-frail individuals and 62.4% to be <60 ml/min/1.73m2 among frail individuals. Similarly, within the narrow range of eGFRcr 60–<75 ml/min/1.73m2, those who were reclassified to eGFRcys 45–<60 ml/min/1.73m2 were more likely to be frail than those who were not reclassified (7.3% frail versus 2.1% frail). The NRI was positive (favoring eGFRcys) when using eGFR categories <30, 30–<45, 45–<60, and ≥60 ml/min/1.73m2 and categories of <30, 30–<60, and ≥60 ml/min/1.73m2 with frailty: estimated at 0.153 (95% CI, 0.070–0.235) and 0.092 (95% CI, 0.020–0.163), respectively.
Table 3.
Reclassification across eGFRcys on eGFRcr in non-frail and frail participants
| eGFRcr category | eGFRcys category
|
||||||
|---|---|---|---|---|---|---|---|
| ≥90 | 75–<90 | 60–<75 | 45–<60 | 30–<45 | <30 | Total | |
|
| |||||||
| Nonfrail | |||||||
| ≥90 | 157 (38.2%) | 134 (32.6%) | 92 (22.4%) | 25 (6.1%) | 3 (0.7%) | 411 | |
| 75–<90 | 198 (13.0%) | 457 (30.0%) | 552 (36.2%) | 270 (17.7%) | 47 (3.1%) | 1,524 | |
| 60–<75 | 33 (2.4%) | 156 (11.2%) | 506 (36.4%) | 560 (40.3%) | 131 (9.4%) | 5 (0.4%) | 1,391 |
|
| |||||||
| 45–<60 | 5 (0.5%) | 21 (1.9%) | 143 (13.1%) | 456 (41.7%) | 431 (39.4%) | 31 (3.4%) | 926 |
| 30–<45 | 1 (0.3%) | 2 (0.6%) | 33 (10.3%) | 185 (57.5%) | 101 (31.4%) | 322 | |
| <30 | 4 (5.6%) | 68 (94.4%) | 72 | ||||
| Total | 394 | 762 | 1,274 | 1,276 | 735 | 205 | |
| Frail | |||||||
| ≥90 | 2 (8.7%) | 5 (21.7%) | 10 (43.5%) | 6 (26.1%) | 23 | ||
| 75–<90 | 1 (1.4%) | 11 (15.1%) | 27 (37.0%) | 27 (37.0%) | 7 (9.6%) | 73 | |
| 60–<75 | 3 (3.3%) | 11 (12.2%) | 44 (48.9%) | 29 (32.2%) | 3 (3.3%) | 90 | |
|
| |||||||
| 45–<60 | 7 (8.1%) | 31 (36.1%) | 45 (52.3%) | 3 (3.5%) | 86 | ||
| 30–<45 | 2 (4.3%) | 22 (46.8%) | 23 (48.9%) | 47 | |||
| <30 | 22 (100%) | 22 | |||||
| Total | 3 | 19 | 55 | 110 | 103 | 51 | |
Note: Values are given as n (row percentage). eGFRcr categories expressed in mL/min/1.73 m2. Italic indicates reclassification to higher eGFR categories using eGFRcys compared to eGFRcr, bold indicates reclassification to lower eGFR categories using eGFRcys compared to eGFRcr.
eGFRcr, creatinine-based estimated glomerular filtration rate; eGFRcys, cystatin C–based estimated glomerular filtration rate;
Implications of Frailty and Reduced Kidney Function: Medication Use
While polypharmacy was seen in the majority of the study population (84%), frail participants took a greater number of medications than participants who were not frail. For example, hyperpolypharmacy was seen in 54.0% of frail individuals versus 38.4% of the non-frail (p<0.001). In addition, several medication classes were used more frequently among frail individuals than non-frail individuals (Table S4). In particular, certain medications requiring precaution or dosing adjustment in CKD were more prevalent in frail individuals, including digoxin (4.4% versus 1.6%), metformin (15.0% versus 11.6%), and anticoagulation (12.0% versus 7.3%), as well as the class of hypnotic/sedatives (10.0% versus 7.2%).
Discussion
In this bi-racial cohort of older men and women, frailty was present in 6.8% of the population and was associated with kidney disease. Lower levels of both eGFRcr and eGFRcys and higher albuminuria were independently associated with frailty, with much stronger associations between frailty and eGFR based on cystatin C compared with creatinine. This suggests there may be uniform overestimation of kidney function among frail individuals, given that eGFR is most commonly estimated using serum creatinine in clinical practice. The implications of overestimation of kidney function in frail individuals could be substantial: we demonstrated that hyperpolypharmacy was common in frail individuals, including many medication classes with clear warnings for use in CKD. Current KDIGO guidelines suggest confirmation of kidney function testing in the diagnosis of CKD; for frail individuals, clinicians may consider repeat testing with an alternative filtration marker or direct measurement of GFR.
Our results expand on the current literature by providing clear contrasts in the associations between frailty and kidney function based on creatinine to those based on cystatin C and presenting detailed data on stage of albuminuria. Serum creatinine may be inaccurately low in persons with low muscle mass, which can accompany the weakness and weight loss that are part of the definition of frailty. Cystatin C may be high in the setting of inflammation, but the associations between frailty and eGFR based on cystatin C remained strong even after adjustment for C-reactive protein, a measure of inflammation. Given the strength of the associations, our results suggest that eGFRcys may be a better marker of kidney function in frail individuals. This could be important both in medication dosing but also in patient counseling. Indeed, eGFRcys has been shown to have stronger associations with prognosis than eGFRcr, even in the general population.29
Previous work on the association of albuminuria and frailty was in a referred population of almost all men (81%) and only examined the presence/absence of microalbuminuria without concomitant evaluation of a range of eGFR categories.16 We demonstrated that albuminuria had independent associations with frailty beyond that of eGFR. These findings highlight the importance of increasing awareness for the frailty phenotype, and perhaps suggest a need for enhanced albuminuria testing in this vulnerable population. Elevated albuminuria has been shown to have a strong association with risk of bone fractures, cardiovascular disease, end-stage renal disease, and mortality in the general population,30–32 and significant absolute risk increases in the older adult population.33
The association between CKD and frailty has a number of possible mechanisms. First, associations may be driven by common causes of CKD rather than CKD itself.34 For example, diabetes is one of the most common etiologies of CKD and has been shown to be associated with increased risk of frailty.27 However, we demonstrate consistent associations between kidney function and frailty in the presence and absence of diabetes. Alternatively, downstream effects of CKD could mediate the relationship. Often CKD is characterized by an inflammatory state, and higher inflammatory burden has been associated with frailty.15 In addition, low GFR is associated with erythropoietin deficiency and shortened erythrocyte survival, and lower hemoglobin levels have been associated with frailty, possibly by limiting participation in physical activities or the development of left ventricular hypertrophy.35, 36 However, our study found that the association between CKD and frailty generally persisted when adjusting for these comorbid diseases, indicating that there may be an independent contribution of CKD to frailty.
Mitigating polypharmacy and adhering to careful medication dosing may be an important aspect in the management of frail individuals. In our study, >50% of frail individuals were taking 10 or more medications, which is consistent with previous research demonstrating higher medication burden among frail individuals.37–39 While frail individuals are more likely to have more comorbid illness warranting the use of prescription medications, these individuals are also at higher risk of adverse events. Frail individuals are at high-risk for fractures and falls, which may be precipitated and/or exacerbated by certain medications such as hypnotic/sedatives, central nervous system altering medications, and glucocorticoids, all of which were more commonly prescribed among frail persons in our study.40–43 In addition, frail individuals in our study were more likely to be taking anticoagulants, which increase bleeding risk after falls.44 They may also be particularly vulnerable to drug accumulation with medications such as digoxin and other renally cleared medications, particularly if kidney function is misclassified using serum creatinine.5 Greater provider awareness of the frailty phenotype and its associated risks may lead to improved prognosis.
In considering these results, there are certain strengths and limitations of our study that should be mentioned. The ARIC Study is a community-based cohort of predominantly white and black individuals; other races and ethnicities were not represented in our analyses. This study is also limited to older adults and results may not be generalizable to younger individuals; however, the ARIC population may be representative of the population with the greatest burden of both CKD and frailty. Urine ACR, eGFRcr, and eGFRcys were measured per protocol, limiting confounding by indication for measurement. There is heterogeneity in the existing frailty instruments, but the frailty variable used in these analyses was rigorously derived to accurately capture the frailty phenotype using component measures that are comparable to other study definitions.45 Although we found strong and statistically significant cross-sectional associations, it is not possible to establish the temporality of the observed associations. We were only able to evaluate relationships in participants attending visit 5 of the ARIC study, who represent a healthier subset of the surviving ARIC population that may have less severe disease. Medications are typically dosed by creatinine clearance rather than clinically used eGFR, although recent Food and Drug Administration issuances have amended drug labeling to incorporate eGFR in drug dosage.46 Finally, for several important measures (e.g., smoking status, alcohol consumption, and physical activity), analyses relied on self-reported information.
In conclusion, this study provides evidence of an independent relationship of reduced kidney function and albuminuria with elevated frailty prevalence in an older bi-racial community-dwelling cohort, with stronger associations observed using the alternative filtration marker cystatin C compared with creatinine. We demonstrate the clear clinical importance of this finding: frail individuals were commonly taking medications requiring caution or dose adjustment in CKD. Further studies are needed to determine whether greater awareness of the frailty phenotype and more careful assessment of kidney function in frail individuals could improve prognosis.
Supplementary Material
Figure S1: Adjusted prevalence ratios for frailty status by eGFR, stratified by diabetes status.
Figure S2: Adjusted prevalence ratios for frailty status by ACR, stratified by diabetes status.
Figure S3: Proportion of frail individuals by eGFRcys or eGFRcr and albuminuria categories.
Table S1: Characteristics by eGFRcr category.
Table S2: Characteristics by eGFRcys category.
Table S3: Reclassification across eGFRcys on eGFRcr for all participants.
Table S4: Unadjusted and demographically adjusted prevalence ratios for medications.
Acknowledgments
The authors thank the staff and participants of the ARIC Study for their important contributions.
Support: The ARIC Study is carried out as a collaborative study supported by National Heart, Lung and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data are collected with support by U01 HL096812,HL096814,HL096899,HL096902, andHL096917, with previous brain magnetic resonance imaging examinations funded by R01-HL70825. Dr. McAdams-DeMarco is supported by National Institute on Aging grant K01AG043501. Dr Selvin was supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants K24DK106414 and 2R01DK089174. Dr Grams is supported by NIH/NIDDK grant K08DK092287. The funders of this study had no role in the study design; collection, analyses, and interpretation of data; writing of the report; or decision to submit the report for publication.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Footnotes
Contributions: Research idea and study design: SHB, JGG, BGW, JC, ES, MEG; data acquisition: BGW, JC, ES; data analysis/interpretation: SHB, YC, NRD, JGG, BGW, MM-D, JC, ES, MEG; statistical analysis: SHB, YC, NRD; supervision or mentorship: JC, ES, MEG. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. SHB takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Peer Review: Evaluated by 3 external peer reviewers, a Statistical Editor, a Co-Editor, and an Acting Editor-in-Chief.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology. Series A, Biological sciences and medical sciences. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in Older Adults: A Nationally Representative Profile in the United States. The journals of gerontology. Series A, Biological sciences and medical sciences. 2015;70(11):1427–1434. doi: 10.1093/gerona/glv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaslavsky O, Cochrane BB, Thompson HJ, Woods NF, Herting JR, LaCroix A. Frailty: a review of the first decade of research. Biological research for nursing. 2013;15(4):422–432. doi: 10.1177/1099800412462866. [DOI] [PubMed] [Google Scholar]
- 4.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing research reviews. 2013;12(2):719–736. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Hubbard RE, O’Mahony MS, Woodhouse KW. Medication prescribing in frail older people. European journal of clinical pharmacology. 2013;69(3):319–326. doi: 10.1007/s00228-012-1387-2. [DOI] [PubMed] [Google Scholar]
- 6.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in Prescription Drug Use Among Adults in the United States From 1999–2012. JAMA. 2015;314(17):1818–1831. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 8.Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17(8):2275–2284. doi: 10.1681/ASN.2005121273. [DOI] [PubMed] [Google Scholar]
- 9.Dalrymple LS, Katz R, Rifkin DE, et al. Kidney function and prevalent and incident frailty. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(12):2091–2099. doi: 10.2215/CJN.02870313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004;43(5):861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 11.Bowling CB, Muntner P. Epidemiology of chronic kidney disease among older adults: a focus on the oldest old. The journals of gerontology. Series A, Biological sciences and medical sciences. 2012;67(12):1379–1386. doi: 10.1093/gerona/gls173. [DOI] [PubMed] [Google Scholar]
- 12.Walker SR, Brar R, Eng F, et al. Frailty and physical function in chronic kidney disease: the CanFIT study. Can J Kidney Health Dis. 2015;2:32. doi: 10.1186/s40697-015-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilhelm-Leen ER, Hall YN, MKT, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. The American journal of medicine. 2009;122(7):664–671. e662. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohm C, Storsley L, Tangri N. The assessment of frailty in older people with chronic kidney disease. Curr Opin Nephrol Hypertens. 2015;24(6):498–504. doi: 10.1097/MNH.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 15.Chang SS, Weiss CO, Xue QL, Fried LP. Association between inflammatory-related disease burden and frailty: results from the Women’s Health and Aging Studies (WHAS) I and II. Archives of gerontology and geriatrics. 2012;54(1):9–15. doi: 10.1016/j.archger.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;60(6):912–921. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reese PP, Cappola AR, Shults J, et al. Physical performance and frailty in chronic kidney disease. American journal of nephrology. 2013;38(4):307–315. doi: 10.1159/000355568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements. 2013;3(1):1–150. doi: 10.1016/j.kisu.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kucharska-Newton A, Palta P, Burgard S, et al. Operationalizing frailty in the Atherosclerosis Risk in Communities (ARIC) Study cohort. The Journals of Gerontology, Series A: Medical Sciences. doi: 10.1093/gerona/glw144. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 21.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5(2):179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 22.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;58(4):682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grubb A, Blirup-Jensen S, Lindstrom V, et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48(11):1619–1621. doi: 10.1515/CCLM.2010.318. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. (WHO/NMH/NHD/MNM/11.1) [Google Scholar]
- 27.Garcia-Esquinas E, Graciani A, Guallar-Castillon P, Lopez-Garcia E, Rodriguez-Manas L, Rodriguez-Artalejo F. Diabetes and risk of frailty and its potential mechanisms: a prospective cohort study of older adults. J Am Med Dir Assoc. 2015;16(9):748–754. doi: 10.1016/j.jamda.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in medicine. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207–112. [DOI] [PubMed] [Google Scholar]
- 29.Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80(1):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daya NR, Voskertchian A, Schneider AL, et al. Kidney Function and Fracture Risk: The Atherosclerosis Risk in Communities (ARIC) Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016;67(2):218–226. doi: 10.1053/j.ajkd.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallan SI, Matsushita K, Sang Y, et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308(22):2349–2360. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker SR, Wagner M, Tangri N. Chronic kidney disease, frailty, and unsuccessful aging: a review. J Ren Nutr. 2014;24(6):364–370. doi: 10.1053/j.jrn.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. The journals of gerontology. Series A, Biological sciences and medical sciences. 2005;60(6):729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- 36.Pires Corona L, Drumond Andrade FC, de Oliveira Duarte YA, Lebrao ML. The Relationship between Anemia, Hemoglobin Concentration and Frailty in Brazilian Older Adults. The journal of nutrition, health & aging. 2015;19(9):935–940. doi: 10.1007/s12603-015-0502-3. [DOI] [PubMed] [Google Scholar]
- 37.Gnjidic D, Hilmer SN, Blyth FM, et al. High-risk prescribing and incidence of frailty among older community-dwelling men. Clinical pharmacology and therapeutics. 2012;91(3):521–528. doi: 10.1038/clpt.2011.258. [DOI] [PubMed] [Google Scholar]
- 38.Bennett A, Gnjidic D, Gillett M, et al. Prevalence and impact of fall-risk-increasing drugs, polypharmacy, and drug-drug interactions in robust versus frail hospitalised falls patients: a prospective cohort study. Drugs & aging. 2014;31(3):225–232. doi: 10.1007/s40266-013-0151-3. [DOI] [PubMed] [Google Scholar]
- 39.Crentsil V, Ricks MO, Xue QL, Fried LP. A pharmacoepidemiologic study of community-dwelling, disabled older women: Factors associated with medication use. Am J Geriatr Pharmacother. 2010;8(3):215–224. doi: 10.1016/j.amjopharm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Jette N, Lix LM, Metge CJ, Prior HJ, McChesney J, Leslie WD. Association of antiepileptic drugs with nontraumatic fractures: a population-based analysis. Arch Neurol. 2011;68(1):107–112. doi: 10.1001/archneurol.2010.341. [DOI] [PubMed] [Google Scholar]
- 41.Vestergaard P, Rejnmark L, Mosekilde L. Anxiolytics, sedatives, antidepressants, neuroleptics and the risk of fracture. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2006;17(6):807–816. doi: 10.1007/s00198-005-0065-y. [DOI] [PubMed] [Google Scholar]
- 42.Ensrud KE, Blackwell T, Mangione CM, et al. Central nervous system active medications and risk for fractures in older women. Archives of internal medicine. 2003;163(8):949–957. doi: 10.1001/archinte.163.8.949. [DOI] [PubMed] [Google Scholar]
- 43.Buehring B, Viswanathan R, Binkley N, Busse W. Glucocorticoid-induced osteoporosis: an update on effects and management. J Allergy Clin Immunol. 2013;132(5):1019–1030. doi: 10.1016/j.jaci.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 44.Ho P, Brooy BL, Hayes L, Lim WK. Direct oral anticoagulants in frail older adults: a geriatric perspective. Seminars in thrombosis and hemostasis. 2015;41(4):389–394. doi: 10.1055/s-0035-1550158. [DOI] [PubMed] [Google Scholar]
- 45.Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing research reviews. 2016;26:53–61. doi: 10.1016/j.arr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.US Food and Drug Administration. [Accessed July 12, 2016];FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. 2016 Apr 8; http://www.fda.gov/Drugs/DrugSafety/ucm493244.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Adjusted prevalence ratios for frailty status by eGFR, stratified by diabetes status.
Figure S2: Adjusted prevalence ratios for frailty status by ACR, stratified by diabetes status.
Figure S3: Proportion of frail individuals by eGFRcys or eGFRcr and albuminuria categories.
Table S1: Characteristics by eGFRcr category.
Table S2: Characteristics by eGFRcys category.
Table S3: Reclassification across eGFRcys on eGFRcr for all participants.
Table S4: Unadjusted and demographically adjusted prevalence ratios for medications.