Abstract
Adult mammalian CNS axons generally do not regenerate, creating an obstacle to effective repair and recovery after neuronal injury. The canonical Wnt/β-catenin signaling pathway is an essential signal transduction cascade that regulates axon growth and neurite extension in the developing mammalian embryo. In this study, we investigated whether a Wnt/β-catenin signaling activator could be repurposed to induce regeneration in the adult CNS after axonal injury. We used a retinal ganglion cell (RGC) axon crush injury model in a transgenic Wnt reporter mouse, and intravitreal injections were used to deliver Wnt3a or saline to the RGC cell bodies within the retina. Our findings demonstrated that Wnt3a induced Wnt signaling in RGCs and resulted in significant axonal regrowth past the lesion site when measured at two and four weeks post-injury. Furthermore, Wnt3a-injected eyes showed increased survival of RGCs and significantly higher PERG amplitudes compared to the control. Additionally, Wnt3a-induced axonal regeneration and RGC survival was associated with elevated activation of the transcription factor Stat3, and reducing expression of Stat3 using a conditional Stat3 knock-out mouse line led to diminished Wnt3a-dependent axonal regeneration and RGC survival. Therefore, these findings reveal a novel role for retinal Wnt signaling in axonal regrowth and RGC survival following axonal injury, which may lead to the development of novel therapies for axonal regeneration.
Keywords: optic nerve regeneration, Wnt signaling, retina, Stat3, optic nerve crush, intravitreal injection
Introduction
The axons of retinal ganglion cells (RGCs) are contained within the optic nerve, which is the essential conduit for transmitting visual information to the brain. Degeneration of RGCs and atrophy of the optic nerve are hallmarks of many ocular diseases and trauma and can result in permanent vision loss (You et al., 2013). Unfortunately, there are currently no treatments to repair or regrow RGC axons and reverse vision loss. Previous studies have reported axonal regrowth after optic nerve injury for various molecular pathways, such as loss of negative regulators of STAT3 (SOCS3) and PTEN and grow-suppressive KLF4 (Moore et al., 2009) (Sun et al., 2011). Although these studies identified compelling candidate pathways, the use of knockout mice does not have a direct translation to therapy. However, axonal regeneration in studies using delivered molecules, which is more relevant to therapies than knock-out mice, has been low (Pernet and Schwab, 2014). Thus, there is a critical need for identifying new regenerative molecular pathways. The purpose of the current study was to investigate whether the Wnt3a ligand activator of the Wnt/β-catenin pathway can induce RGC survival and regeneration of axons after traumatic injury to the optic nerve.
The canonical Wnt/β-catenin pathway (referred to as “Wnt” for simplicity) is an essential signaling cascade in the retina and elsewhere in the CNS that regulates retinal development, stem cell proliferation, neuronal regeneration, and neuroprotection from mutation, oxidative stress, laser and light injuries (Kubo et al., 2003, Hunter et al., 2004, Liu et al., 2007, Mizukami et al., 2009, Seitz et al., 2010, Braunger et al., 2013, Liu et al., 2013, Sanges et al., 2013, Patel et al., 2015b). When Wnt ligands bind to the membrane coreceptors LRP5/6 and Frizzled, levels of the central effector β-catenin accumulate in the cytoplasm, allowing β-catenin to translocate into the nucleus where it binds to TCF/LEF transcription factors and induces Wnt target genes (Nusse, 2005). A separate class of Wnt pathways, known as non-canonical β-catenin-independent Wnt signaling, are mediated by effectors such as Wnt5a ligand and Ryk receptors and function through altered levels of calcium and JNK activation.
Both canonical Wnt/β-catenin signaling and non-canonical Wnt pathways regulate axon growth, pathfinding and synaptic assembly in the developing CNS. In the embryonic optic nerve, brain and spinal cord, Wnt pathways act as critical axon guidance factors and play an active role in axon growth and remodeling (Schmitt et al., 2006). In the developing retina, canonical Wnt ligands Wnt3a, 4 and 7b, binding to Frizzled receptors, attract growing axons and regulate dorsoventral specificity of RGC retinal projections, whereas non-canonical ligands Wnt1 and 5a, binding to Ryk receptors, have the opposite effect and repel axons (Sato et al., 2006, Fuhrmann, 2008, Yam and Charron, 2013). Although canonical Wnt ligands are important for developmental axonal growth throughout the CNS, their role in axonal growth and recovery after adult optic nerve injury is not known.
Optic nerve crush (ONC) injury is a widely used model of axonal damage that causes rapid neuronal degeneration and atrophy, and is often used to investigate signaling pathways that mediate axonal repair and growth in the CNS (Schwartz, 2004, Shum et al., 2016). ONC injury leads to rapid death of RGCs by Wallerian degeneration, with approximately 50% of RGCs lost by 1 week post-injury, and 80–90% lost by 2 weeks post injury (Berkelaar et al., 1994). Experimental therapies that have shown success in the ONC model include manipulating molecular pathways to reduce RGC death and directly targeting regulators of axonal growth. Several molecules promote RGC survival after ONC, such as the pro-survival factors BDNF and CNTF (Mansour-Robaey et al., 1994, Leaver et al., 2006a, Leaver et al., 2006b), and axonal regeneration was observed after overexpression of CNTF by viral delivery (Pernet et al., 2013b). Ideally, a molecular therapy would target both survival pathways and regeneration pathways, but few exogenous molecules have been identified so far that induce both robust RGC survival and axonal regeneration (Shum et al., 2016).
Wnt/β-catenin signaling has several properties that suggest it could be a candidate pro-regeneration molecular pathway for optic nerve axons. Wnt signaling is active in RGCs in the adult mouse retina (Liu et al., 2006, Yi et al., 2007), it induces expression of genes that have pro-regenerative properties, such as STAT3 and CNTF (Seitz et al., 2010, Fragoso et al., 2012) and is suppressed by genes that inhibit regeneration, such as KLF4 and ephrins (Schmitt et al., 2006, Cui et al., 2013). Also, Wnt3a may directly regulate growth cone remodeling by altering microtubule stability through differential binding of its downstream components, APC and Dvl1, to the positive ends of microtubules (Purro et al., 2008). Wnt signaling has also been shown to promote axonal growth within the adult spinal cord and sensory neurons after injury (Liu et al., 2008, Hollis and Zou, 2012) (Yin et al., 2008, David et al., 2010), and Wnt signaling regulates astrogliosis and radial glial neurogenesis during axon regeneration following CNS injury (Duncan et al., 2014, Briona et al., 2015). Wnt signaling is known to regulate inflammatory signaling, which has reparative and pro-regenerative effects (Marchetti and Pluchino, 2013). In contrast, non-canonical Wnt-Ryk signaling at the injury site inhibited spinal cord regeneration (Liu et al., 2008, Miyashita et al., 2009). Furthermore, our group and others demonstrated that Wnt signaling increased survival of RGCs and other retinal neurons after injury (Seitz et al., 2010, Patel et al., 2015b).
In this study, we tested the regenerative and pro-survival properties of Wnt3a after optic nerve injury. We demonstrated that delivery of Wnt3a to the retina led to increased Wnt signaling in RGCs, elevated RGC survival and induced significant axonal regrowth following optic nerve crush. Additionally, we used conditional Stat3 knock-out mice to demonstrate that Stat3 contributed to the effects of Wnt3a on RGCs. Therefore, these results identify Wnt3a as a novel molecular pathway for RGC survival and axonal regrowth after optic nerve crush.
Materials and methods
Experimental animals
All procedures involving mice were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care and Use Committee at the University of Miami. The Tcf/LacZ mouse is a transgenic canonical Wnt reporter line that allows localization of active Wnt/β-catenin signaling (Mohamed et al., 2004). In these mice, binding of endogenous nuclear β-catenin to T-cell factor/lymphoid enhancer-binding factor (TCF/Lef) elements in a Wnt-responsive enhancer/promoter region upstream of the LacZ transgene leads to induction of LacZ/β-galactosidase (β-gal) expression wherever Wnt signaling is active. The Tcf/LacZ strain was previously backcrossed onto the C3H background (Yi et al., 2007); the absence of the photoreceptor degeneration-causing Pde6brd1 mutation commonly found in C3H was confirmed in all animals by genotyping using PCR. Tcf/LacZ mice have normal retinas, indicated by normal scotopic ERG response and visual acuity (data not shown). The inducible conditional Stat3 knock-out mice, Stat3f/f ;C57BL/6J, were purchased from Jackson Laboratory (stock number 016923). For this study, we used a total of 58 mice. For the immunohistochemistry analyses, 4 mice per group were used from each of three groups (uninjected, Wnt, and Saline) for a total of 12 mice. For imaging and electroretinography, 4 mice per group were also used for a total of 12 mice. For the regeneration experiments, 6 mice were used in each group (2 groups, Wnt and Saline for a total of 12 mice) at each time point (2 and 4 weeks). For the Stat3 KO experiments 5 mice were used for each group (Wnt and Saline for a total of 10 mice).
Optic nerve crush and intravitreal injections
Optic nerve crush (ONC) injury was performed as described previously (Park et al., 2008). Tcf/LacZ mice at age 2 months were anesthetized using a ketamine/xylazine cocktail injected intraperitoneally and eyes were locally anesthetized using 0.5% proparacaine hydrochloride. A small incision was made in one eye in the superior posterior area of the conjunctiva, eye muscles were gently moved to reveal the optic nerve behind the globe and the nerve was crushed with extra-fine self-closing forceps at ~1 mm behind the globe for 5 seconds without damaging the retinal vessels or blood supply. Mice of either sex were randomly assigned into Wnt or saline control groups, and were intravitreally injected with 20 ng of recombinant Wnt3a (R&D Systems Inc, Minneapolis, MN) or the equivalent volume of PBS (saline) using a 1.5 cm 33-gauge Hamilton needle (Hamilton Company, Reno, NV) into the same eye that had the optic nerve crush injury. The injection needle was angled to avoid hitting the lens. The intravitreal injections of Wnt3a or saline control were made immediately following axonal injury. Each injected eye was treated topically post-injection with a polymyxin B sulfate ointment. We excluded from further analysis mice that had excessive bleeding, and the structure of the globe and retina were analyzed in each mouse using OCT (Patel and Hackam, 2014a) which allowed us to exclude animals with ocular damage. The investigators were masked to the identity of the injected compound for all phenotype and molecular analyses.
To ablate Stat3 expression in RGCs, Cre recombinase cDNA was inserted downstream of the CMV promoter/β-globin intron enhancer in the plasmid pAAV-MCS (Stratagene, La Jolla, CA, USA), which contains the AAV2 inverted terminal repeats and a human growth hormone polyA signal, as described in(Yungher et al., 2015). The pAAV-RC plasmid (Stratagene), which encodes the AAV2 genes rep and cap, and the helper plasmid (Stratagene), which encodes E2A, E4 and VA, were used for co-transfection in 293T cells to generate recombinant AAV. The AAV-Cre was produced at a titer of 1–4x1013 particles/ml at the University of Miami Viral Vector Core. For Cre expression and Stat3 ablation, Stat3f/f mice were intravitreally injected at age 6 weeks with AAV2-Cre, followed by another injection of AAV2-Cre a week later, as described in (Luo et al., 2016) (Sun et al., 2011). AAV2-Cre injection in RGCs are well characterized (Luo et al.2016, Sun et al., 2011). Our recent publication demonstrated that 2 weeks after AAV2-Cre injection, Cre expression is observed predominantly in the GCL, in morphologies consistent with RGCs, with occasional cells infected with the AAV in the inner nuclear layer. We also note that the titer of AAV2 affects the types of cells in the retina being infected, with AAV2 at higher titer infecting more cells in the inner nuclear layer than AAV at lower titer (Luo et al., 2016). The Wnt or saline intravitreal injections and ONC were then performed a week after the second AAV-Cre injection, as described above.
Optical coherence tomography
Retinas were imaged using a spectral domain optical coherence tomography (SD-OCT) (Bioptigen, Research Triangle Park, NC) that is modified for use in mice, as described previously(Patel et al., 2015a). One hundred b-scans were generated over a volume of 1.3 x 1.3 x 1.56 mm, centered on the optic nerve head. The region of the retina that includes the nerve fiber layer, ganglion cell layer and inner plexiform layer (NFL/GCL/IPL), known as the ganglion cell complex (GCC), was quantified using manual segmentation of 70–80 cross-sectional b-scan images spanning approximately 0.5–0.6 mm from the optic disc. Quantification and segmentation of the GCC layer thickness was assessed by using MATLAB software and programs developed by the Ophthalmic Biophysics department at Bascom Palmer Eye Institute(Ruggeri et al., 2007).
Pattern ERG (PERG)
PERG recordings were performed as described (Porciatti, 2007). Briefly, mice were anesthetized using a ketamine/xylazine cocktail and maintained at a constant body temperature using a temperature controlled heating pad. A ground electrode was inserted under the skin near the tail of the mouse and a reference electrode was inserted under the skin of the head of the animal. Silver wire electrodes were placed on each cornea and the mice were exposed to a visual stimulus of horizontal contrast-reversing stripes (field area, 69.4°×63.4°; mean luminance, 50 cd/m2; spatial frequency, 0.05 cycles/deg contrast, 100%; temporal frequency, 1 Hz). Three PERG responses to 600 contrast reversals were recorded and superimposed to confirm consistency and then averaged. Peaks and troughs were automatically identified, and the peak-to-trough amplitude in a time window 50–300 ms was measured. PERG recording signals were amplified by 10,000 fold and were filtered using 1–30 Hz band pass filter.
Axon quantification
Regenerating RGC axons were anterogradely labeled using cholera toxin β subunit (CTB) with a conjugated Alexafluor 546 (Thermo Fisher Scientific, Waltham, MA) that was intravitreously injected 2 days prior to animal euthanasia. To obtain tissue, the animals were perfused using 4% paraformaldehyde and eyes and optic nerves were dissected and processed through a 5–20% sucrose gradient and then embedded in OCT Compound (Tissue Tek) for cyrosectioning. Longitudinal optic nerve sections were cut at a thickness of 8 μm and mounted onto slides and then imaged using a fluorescent microscope (Zeiss). Axon growth was quantified by counting the number of CTB-positive axons that extended past the crush site every 100 μm division from the end of the injury site, as described in (Park et al., 2008). Three separate locations across the diameter of the nerve were counted and averaged for each animal. The total number of axons in each optic nerve was determined using the following equation: Axon number =π (radius of optic nerve)2 x (average axons/mm)/(thickness of section) (Leon et al., 2000).
Immunohistochemistry
Cryoembedded eyes were sectioned in 10 μm sections, mounted onto slides and immunostained using the procedure described in Patel et al., 2015(Patel and Hackam, 2014a) using anti-β-gal (BD Transduction Laboratories, San Jose, CA), anti-pan Brn3 (Santa Cruz, Dallas, TX), anti-RPBMS (PhosphoSolutions, Aurora, CO), or anti-IBA1 (Wako, Osaka, Japan). Retina sections were counterstained using DAPI to label the nuclei, and imaged using a Zeiss Axiovert 200 fluorescent microscope. Control immunostaining using an irrelevant antibody or omitting the primary antibody was used to confirm antibody specificity. For RGC counts, Brn3-positive or RPBMS-positive and DAPI-positive cells within the GCL were counted.
Western blotting
Whole retina lysates were prepared as in (Yi et al., 2012b). Briefly, freshly isolated retinas were lysed in buffer (50 mM Tris pH7.4, 150 mM NaCl, 1% NP40, 0.05% SDS) containing a proteinase and phosphatase inhibitor cocktail on ice. Twenty micrograms of protein lysate was electrophoresed using 10% sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gels and proteins were transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were probed with anti-phosphorylated Stat3 (1:200 dilution, Cell Signaling, Danvers, MA), anti-total Stat3 (1:200 dilution, Cell Signaling), and anti-β-actin (1:8000 dilution, Sigma, St. Louis, MO), followed by the appropriate secondary antibody that was conjugated to HRP. Signal detection was using the LumiGLO Peroxidase Chemiluminescent Substrate Kit (Kirkegaard & Perry Laboratories, Gaithersburg MD) and imaged with a Fuji Film Luminescent Image Analyzer. The ImageJ software (Abramoff et al, 2004) was used for quantification.
Statistical analysis
Each animal was used in a single experiment and entered as an individual data point. Statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc, La Jolla, CA). Normality tests and variance heterogeneity tests were performed on all datasets. Multiple comparison analyses were conducted using Analysis of Variance (ANOVA) with Tukey’s post-hoc test. P values less than 0.05 were considered statistically significant. Datasets with N≥5 were found to have Gaussian distributions, whereas for the remaining datasets we were not able to verify, or demonstrate the failure of, the assumption for normality because there were too few samples. Because of this limitation, we performed a sensitivity analysis on the datasets with smaller Ns by reanalyzing the data after appropriate transformations. For data involving counts, we performed analysis after square root transforming them (Figures 2B, 3E, 3F, 5C, 5D, 6). For PERG data, OCT and Western blots, we log-transformed the data (Figures 2C, 2D and 4). We note that transformations that correct heterogeneity of variance often also corrects non-normality (Armitage, P. Statistical Methods in Medical Research. 1971. Blackwell Scientific. Oxford and Edinburgh. Page 351). In all cases, the parametric analyses on the transformed data confirmed the original significance tests, leading us to the same conclusions as with the original untransformed data. Untransformed data are shown in all figures, and sample sizes in each experimental group are reported in the figure legends.
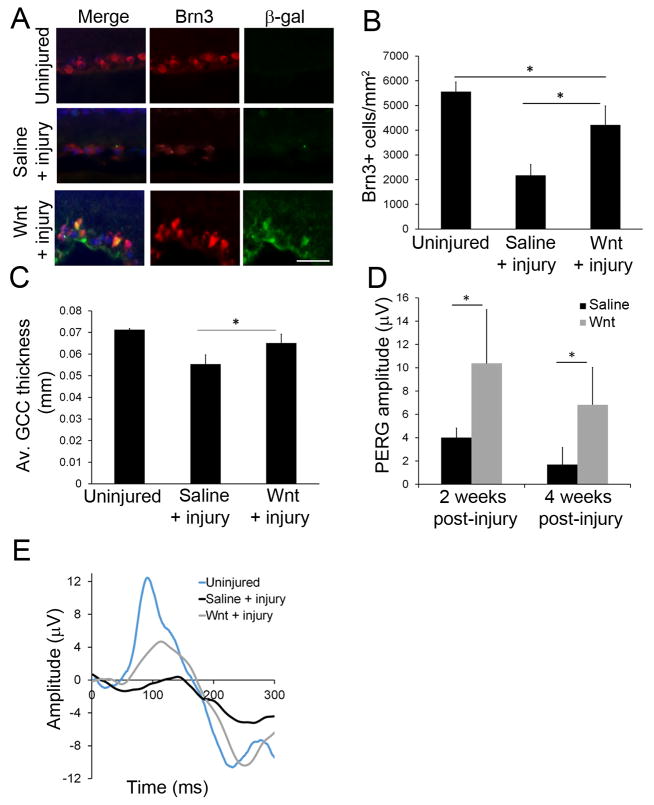
Fig 2. Wnt3a increased RGC survival after axonal injury.
(A) Immunohistochemistry of the GCL region of injected retinas, showing codetection of the RGC marker protein Brn3 and the Wnt reporter β-gal, indicating activated Wnt signaling within RGCs. Additionally, more Brn3-positive cells were observed in the Wnt3a + injury group than the saline + injury group, indicating increased survival of RGCs after Wnt stimulation. Scale bar = 50μm. (B) Quantification of pan-Brn3 immunopositive cells that colocalized with DAPI, showing increased cells (putative RGCs) in Wnt3a-injected mice. n=4 mice per group. *p<0.05. (ANOVA, F = 36.97, df = 11; Tukey’s post-hoc test, Uninjured vs Saline + injury: p = 0.001, Uninjured vs Wnt + injury: p = 0.01952, Saline + injury vs Wnt + injury: p=0.0016.) (C) Quantification of the ganglion cell region (including the NFL, GCL and IPL layers) by OCT imaging indicated a thicker ganglion cell region in the Wnt3a injected mice compared with the saline injected animals. n=3 mice per group. *p<0.05. (ANOVA, F = 14.3, df = 8; Tukey’s post-hoc test, Uninjured vs Saline + injury: p = 0.0033, Uninjured vs Wnt + injury: p = 0.1518, Saline + injury vs Wnt + injury: p = 0.0322.) (D) Quantification of RGC function using PERG recordings at 2 and 4 weeks post-crush, showing higher amplitude in retinas injected with Wnt3a after axonal injury compared with saline. n=4 mice per group, *p<0.05 (t-test, 2 weeks: t = 3.704, df = 6, p = 0.01; 4 weeks: t = 3.576, df = 6, p = 0.0117). (E) Representative RGC-generated PERG waveforms at 2 weeks post-crush responses.
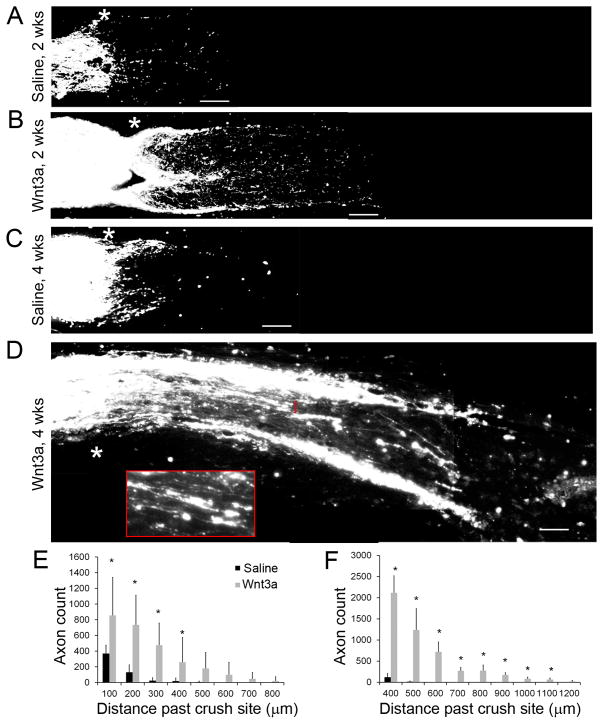
Fig 3. Axonal regeneration after injury following a single intravitreal injection of Wnt3a.
(A–D) CTB-labeled axons showing increased number of axons past the crush region (*) in Wnt3a injected eyes following axonal injury, compared to saline injected animals. (A–B) Optic nerves at 2 weeks following crush. (C–D) Optic nerves at 4 weeks following crush (inset in D shows non-straight morphology and extension past the crush site of regenerating axons from the bracketed region). (E–F) Quantification of axon regeneration at 2 weeks (E) and 4 weeks (F) post-crush showing progressive growth of axons in the Wnt3a-injected mice. n=6 mice for Wnt3a, n=4 for Saline, *p<0.05. ((E) t-test, 100 μm: t = 2.649, df 8, p = 0.0293; 200 μm: t = 4.396, df 8, p = 0.0023; 300 μm: t = 5.125, df 8, p = 0.0009; 400 μm: t = 2.485, df 8, p = 0.0378; 500 μm: t = 2.296, df = 8, p = 0.0508 (ns); 600 – 800 μm: ns. (F) t-test, 400 μm: t = 3.201, df = 10, p = 0.0095; 500 μm: t = 8.043, df = 8, p<0.0001; 600 μm: t = 7.5434, df = 5, p = 3.53169E-05; 700μm: t = 8.5118, df = 5, p = 1.37372E-05; 800 μm: t = 5.1521, df = 5, p = 0.0002; 900 μm: t = 7.8299, df = 5, p = 2.61393E-05; 1000 μm: t = 5.3913, df = 5, p = 0.00022; 1100 μm: t = 4.5836, df = 5, p = 0.00042; 1200 μm: t = 2.0902, df = 5, ns). Scale bar = 100μm.
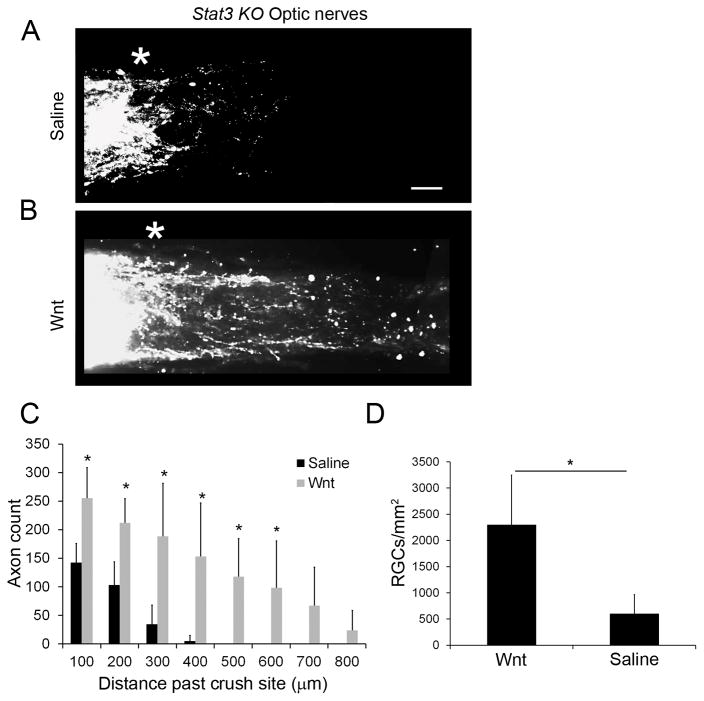
Fig 5. Stat3 signaling is a potential mediator of Wnt3a function.
(A–B) CTB labelled axons in optic nerves of Stat3 KO mice following Wnt or saline intravitreal injections and optic nerve crush injury. Scale bar = 100 μm (C) Quantification of axonal growth two weeks following injury. Although axonal growth was lower than the injected wild type mice (see Fig 3), Wnt3a stimulation resulted in significantly increased number and length of axons in the optic nerve compared to saline injections in the Stat3 KO mice. n=5 mice for Wnt3a, n=4 for saline, *p<0.05 (t-tests, 100 μm: t = 3.716, df = 7, p=0.0075; 200 μm: t = 3.896, df = 7, p = 0.0089; 300 μm: t = 3.647, df = 7, p = 0.0082; 400μm: t = 5.106, df = 7, p = 0.0014; 500 μm: t = 3.9563, df = 4, p = 0.001; 600 μm: t = 2.6726, df = 4, p = 0.0066; 700 and 800 μm, ns). (D) Quantification of RGCs showed higher survival in the Wnt3a-injected mice compared to the saline-injected group, although cell numbers were lower than in the wildtype mice. n=4 mice for saline, n=3 for Wnt3a, *p<0.05 (t-test, t = 3.756, df = 5, p = 0.0132).
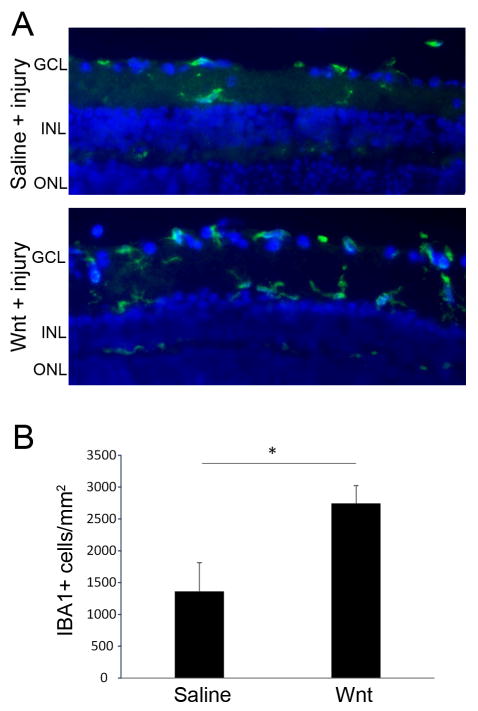
Fig 6. Increased numbers of microglia in Wnt3a-injected retinas.
(A) IBA1-expressing microglia (green) were detected in saline-injected and Wnt3a-injected injured retinas by immunohistochemistry. (B) Quantification of IBA-1 positive microglia in retinas from each group indicates an increased inflammatory response in the Wnt3a-injected mice. n=3 mice per group *p<0.01 (t-test, t = 4.670, df = 4, p = 0.0095).
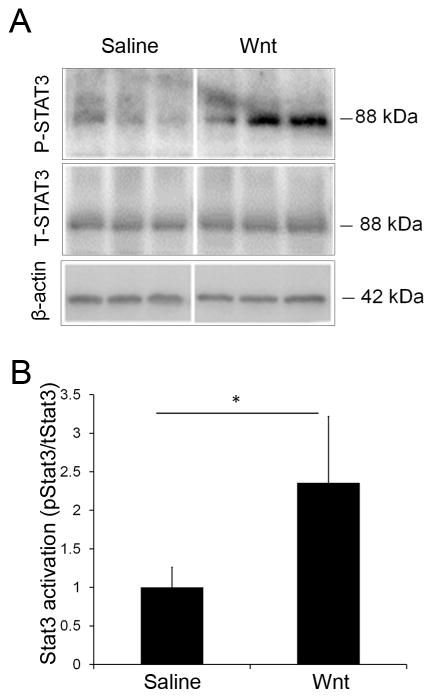
Fig 4. Stat3 signaling is upregulated by Wnt3a.
(A) Representative Western blots of total Stat3 (T-Stat3) and phospho-Stat3 (P-Stat3). Retinas from three animals are shown for each group. (B) Quantification of the Western blots demonstrated that Wnt signaling increased Stat3 activation (ratio of phosphorylated Stat3 to total Stat3 expression) two weeks following optic nerve injury by 2.4 fold. n=4 mice per group, *p<0.05 (t-test, t = 3.293, df = 6, p = 0.0166). Total protein amount was normalized to β-actin levels.
Results
Wnt3a delivery protects RGCs after optic nerve crush
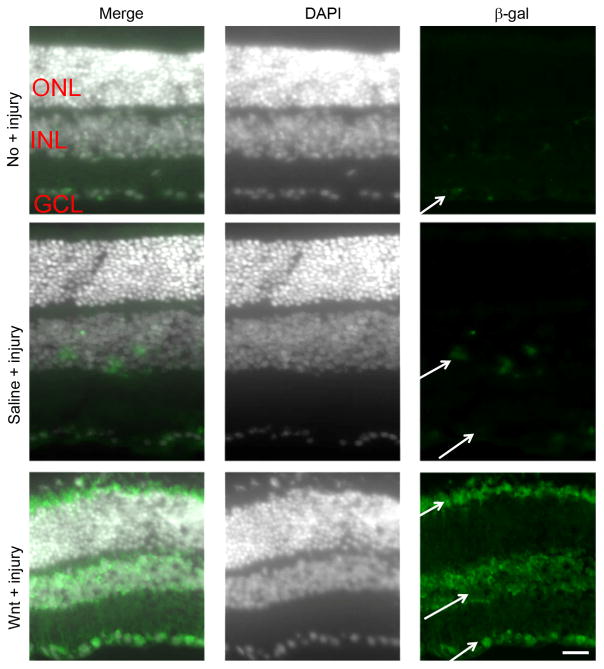
Our experimental approach was to deliver a Wnt signaling activator directly to retinal RGCs using intravitreal injection, instead of delivery at the site of optic nerve damage, in order to avoid a potential proliferative effect of Wnt signaling on the glial scar. The β-gal reporter in Tcf/LacZ mice accurately reflects activation of the Wnt signaling pathway in numerous cell types (Mohamed et al., 2004, Liu et al., 2006, Yi et al., 2007, Usongo and Farookhi, 2012, Edwards et al., 2015). To localize which cells show stimulation of Wnt signaling after Wnt3a injection, we performed immunodetection using an anti-β-gal antibody. As shown in Figure 1, β-gal-positive cells were localized in the INL and GCL in uninjured retinas, indicating basal levels of active Wnt signaling in the retina. These β-gal-positive cells likely correspond to Wnt-responsive cells RGC, microglia and amacrine cells that reside in these layers (Liu et al., 2006, Yi et al., 2007). In the optic nerve injured mice that were injected with saline, β-gal positive cells were present in the INL 1 week after injury. In the injured mice injected with Wnt3a, strongly staining β-gal-positive cells were present in the GCL and in the INL, and immunostaining was also identified in the edge of the ONL, likely in the endfeet of Muller glia that are located in this area (Figure 1). Codetection of β-gal with Brn3 confirmed that the β-gal-positive cells in the GCL are RGCs (Figure 2). The β-gal positive cells that are Brn3 negative in the GCL could be injured RGCs that have downregulated the Brn3 protein, or could be non-RGCs in the GCL that express β-gal, such microglia. Therefore, RGCs are Wnt3a-responsive in the optic nerve injury model.
Fig 1. Localization of Wnt signaling.
The Tcf/LacZ transgenic Wnt reporter mice were used to localize cells that are responsive to Wnt3a stimulation. DAPI-stained nuclei are shown in white to demonstrate the retinal layers. Uninjured eyes had barely detectable basal Wnt signaling in the GCL, indicated by low levels of β-gal detection (green; arrows). However, increased expression of β-gal was detected in injured retinas that received saline injection (saline + injury), notably in the INL and GCL, and Wnt3a injection induced prominent Wnt signaling in the INL and GCL, indicated by increased immunostaining of β-gal (Wnt3a + injury). ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar = 50μm
RGCs were quantified after axonal injury using immunodetection of the RGC-marker protein Brn3. Uninjured retinas had 5562 + 389 (mean + SD) RGCs/mm2 (Figure 2), which is similar to values reported in various mouse strains (Danias et al., 2003). As expected, axonal injury led to a substantial reduction in the number of Brn3-positive RGCs (compare injured eyes injected with saline to uninjured eyes). However, delivery of Wnt3a to the retina at the time of optic nerve injury significantly increased the number of Brn3-positive RGCs, compared with injured eyes injected with saline (Wnt: 4213 + 771 RGCs/mm2, saline: 2169 + 449, Figure 2A–B). Although Brn3 expression decreases in degenerating retinas, we detected significantly higher numbers of Brn3 immunopositive cells (presumptive RGCs) in injured mice injected with Wnt3a compared with injured mice injected with saline.
Next, we used OCT imaging as an additional measure of RGC survival. OCT allows quantification of nerve fiber layer thickness, which has been shown to decrease with increasing RGC death (Camp et al., 2011, Munguba et al., 2014). Because the mouse NFL-GCL region is extremely thin, the ganglion cell complex (GCC), which is comprised of NFL, GCL, and the inner plexiform layer (IPL), was used as a surrogate. OCT imaging indicated significantly thicker GCC regions in injured mice injected with Wnt3a compared with injured mice injected with saline (18% thicker, p<0.05, n=3) at two weeks after injury (Figure 2C). Therefore, increased thickness of the ganglion cell region after Wnt3a injection is consistent with elevated RGC survival measured above.
Pattern electroretinography (PERG) was used to test the functionality of the surviving RGCs in the mice following optic nerve injury. PERG amplitudes in eyes that were not injured were 23 μV, whereas PERG amplitudes in injured mice injected with saline were substantially diminished compared with non-injured eyes when measured at 2 weeks (83% loss compared to uninjured) and 4 weeks (94% loss compared to uninjured) after injury (Figure 2D–E). In contrast, PERG amplitudes in injured mice injected with Wnt3a were significantly enhanced compared with the saline injected eyes, reaching up to 4-fold higher amplitudes (Figure 2). Therefore, retinal Wnt3a signaling increased the functional recovery of RGCs following axonal injury.
Wnt3a induces axonal growth after optic nerve injury
To determine the effect of Wnt3a on axonal growth, we quantified the number and length of CTB-labeled optic nerve axons that extended past the crush injury site. As shown in Figure 3, in the optic nerves of injured eyes injected with Wnt3a there were significantly more axons growing past the lesion site and significantly longer axons, compared with injured eyes injected with saline. In the saline injected group, at 2 weeks post-injury there was almost no axon growth past 300 μm from the injury site (Figure 3). In comparison, in the Wnt3a injected group, an average of 475 axons grew to 300 μm past the injury site with the longest axons reaching up to 800 μm past injury site. Furthermore, at 4 weeks post-injury, the differences between the saline and Wnt3a injected groups were more striking. In the saline injected group, there was no additional regrowth of the optic nerves compared with the 2 week timepoint. In contrast, Wnt3a stimulation led to axonal regrowth at 4 weeks, with an average of 1236 axons reaching 500 μm past the injury site and the axons extended up to 1.2 mm past the injury site. Therefore, these results show that a single intravitreal injection of Wnt3a ligand activated retinal Wnt signaling and led to sustained and significant axon regrowth following axonal injury.
Loss of Stat3 reduced Wnt3a-induced axonal growth
The transcription factor Stat3 plays a role in axonal regrowth (Pellegrino and Habecker, 2013, Qin et al., 2013) and Stat3 is induced by Wnt3a and contributes to Wnt3a-mediated survival of multiple retinal cell types (Fragoso et al., 2012, Patel and Hackam, 2012, 2014b, Patel et al., 2015b). To determine whether the Stat3 pathway contributes to Wnt3a-induced RGC survival and axonal growth after injury, we first tested whether Wnt3a activates Stat3 signaling in the retina. A commonly used marker of Stat3 activation is detection of phosphorylated Stat3. To measure Stat3 levels after Wnt3a delivery, we used Western blotting to quantify phospho-Stat3 and total Stat3 from retinal extracts of the Wnt3a and saline injected eyes. As shown in Figure 4A–B, there is a 2.4-fold increase in Stat3 activation in retinas from injured eyes injected with Wnt3a compared with saline.
To determine whether Wnt3a-induced axonal regrowth is mediated by Stat3 signaling, we used a virally-delivered Cre-driven conditional Stat3 knock-out mouse model to eliminate Stat3 in adult RGCs (Luo et al., 2016) and injured the optic nerve using the same procedure as in the wildtype mice. Our previous studies demonstrated that expression of Cre is typically in ~90% of GCL cells using this approach (Luo et al., 2016). As shown in Figure 5A, in injured Stat3 KO mice injected with saline there was negligible amount of axonal growth, which is comparable to the low levels of axonal regrowth in the saline injected eyes in the wildtype mice (Figure 3A). Therefore, loss of Stat3 did not significantly change the amount of regrowing axons in the saline injected eyes after injury. In contrast, there were lower numbers of regrown axons in the eyes of Stat3 KO mice injected with Wnt3a, compared to the eyes of wild type mice injected with Wnt3a. In the Wnt3a-injected Stat3 KO mice there was an average of 188 axons reaching 300 μm from the injury site, which is approximately 60% less than the number of axons in the wildtype mice injected with Wnt3a (Figure 5C). Furthermore, the number of axons in the Stat3 KO mice injected with Wnt3a remained significantly higher than saline injected eyes, which suggests that Wnt signaling may also function independently of Stat3.
RGC counts were also performed on the injected Stat3 KO mice. As shown in Figure 5D, overall numbers of surviving RGCs in mice injected with Wnt3a were reduced in the Stat3 KO mice, suggesting that Stat3 contributes to Wnt3a-mediated survival of RGCs (Figure 5). However, RGC survival remained significantly higher in Stat3 KO mice injected with Wnt3a than mice injected with saline, as observed in the wildtype mice. Therefore, loss of Stat3 in RGCs reduced the protective and regenerative function of Wnt3a.
Finally, we examined whether Wnt3a injection alters the number of inflammatory cells in the retina. Accumulating evidence indicates that Wnt signaling regulates inflammation (Marchetti and Pluchino, 2013) and the inflammatory response can promote optic nerve regeneration (Benowitz and Popovich, 2011). Retinal microglia in the injured retina were quantified using immunodetection of IBA1. As shown in Figure 6, microglia were present in both saline-injected and Wnt3a-injected injured eyes, but there was two-fold more IBA-1-positive microglia in the Wnt3a-injected retinas compared with saline-injected. Therefore, microglia activation and infiltration into the retina is also associated with Wnt3a-mediated optic nerve regeneration.
Discussion
The main objective of this study was to determine whether canonical Wnt/β-catenin signaling, which promotes axonal growth during embryonic development, can be repurposed to regenerate axons after RGC injury in adults. RGCs, similar to other neurons of the CNS, are characterized by limited regenerative abilities following axonal injury (Moore and Goldberg, 2010, Fischer and Leibinger, 2012). Our findings demonstrated that stimulation of the canonical Wnt pathway within the retina protected RGCs and induced axonal regrowth following traumatic injury to the axon. To our knowledge, this is the first study showing that Wnt signaling promotes both neuronal survival and regeneration of CNS axons following an axonal injury. Our findings are consistent with previous studies that showed a neuroprotective role for Wnt signaling in the RGCs following neuronal insults, such as excitotoxicity (Seitz et al., 2010), and an axonal growth role for Wnt within the adult spinal cord and sensory neurons after injury (Liu et al., 2008, Hollis and Zou, 2012) (Yin et al., 2008, David et al., 2010).
The use of the Wnt reporter transgenic mouse allowed us to identify the main cellular targets of Wnt3a, which were the RGCs themselves, as well as in cells with a distribution consistent with Muller glia and amacrine cells. In addition to Wnt signaling inducing survival and regeneration signals within RGCs, Muller glia are possible mediators of enhanced RGC survival and axon outgrowth. Muller glia are generally protective to RGCs (Chong and Martin, 2015), induce RGC neuritogenesis in vitro (Garcia et al., 2002), and viral delivery of CNTF to Muller glia promoted optic nerve axonal extension and sprouting (Pernet et al., 2013a). Therefore, it is possible that Muller glia play a supportive role to RGCs following Wnt stimulation that aids in survival and promotes regeneration, similar to its function in astroglia following spinal cord injury (Duncan et al., 2014, Briona et al., 2015). In contrast, amacrine cells are generally inhibitory to RGC regeneration (Goldberg et al., 2002, Chong and Martin, 2015). Future studies will examine the roles that different Wnt3a-responsive cell types play in RGC axonal regeneration.
RGC cell bodies are in the retina and their axons extend through the optic nerve. The effect of Wnt signaling at the axon and lesion site may differ from its effect at the cell body. In our study, we showed that stimulation of Wnt/β-catenin signaling by Wnt3a at the cell body induces axonal outgrowth and elongation, possibly because it avoids the glial proliferative effect of Wnt at the site of injury. Previous studies have shown that Wnt signaling ligands can act as attractive or repulsive axonal guidance cues for ascending and descending axons in the corticospinal tract during development (Zou, 2004). Several studies have shown that repulsive Wnt ligands are upregulated in the CNS following axonal injury. Wnt signaling ligands Wnt1 and Wnt5a, which have repulsive properties, were increased in corticospinal gray matter following spinal cord injury, suggesting that non-canonical Wnt signaling inhibits axonal regeneration (Liu et al., 2008). Also, a study by Rodriguez et al. demonstrated that activated β-catenin over-expressed specifically within oligodendrocytes and astrocytes at the optic nerve crush site led to increased glial proliferation, scar formation and reduced regeneration of RGC axons (Rodriguez et al., 2014). We speculate that our approach of using an intravitreal injection delivery method for Wnt stimulation does not penetrate to the optic nerve or reach the injury site, and avoids over-activation of the Wnt pathway in glia at the scar.
Regarding mechanisms, Wnt3a is known to regulate APC levels (Kikuchi et al., 2011), and the amount of APC bound to growth cones of extending DRG neurites correlates with increased neurite growth (Zhou et al., 2004). Therefore, Wnt3a may directly regulate growth cone remodeling by altering microtubule stability through binding of its downstream components, APC and Dvl1, to the positive ends of microtubules (Purro et al., 2008) and possibly allow RGCs to be more receptive to axon growth cues. Furthermore, phospho-β-catenin levels, which are reduced by Wnt3a, regulate microtubule reorganization (Ligon et al., 2001, Huang et al., 2007) and may be involved in neurite stabilization and growth.
Wnt3a may also indirectly promote regeneration by inducing expression of its target genes such as pro-regeneration molecules STAT3, CNTF and neurotrophins (Fragoso et al., 2012, Yi et al., 2012a, Patel et al., 2015b). Furthermore, Wnt signaling could be enhanced through endogenous regulation of Wnt antagonists, such as through microRNA-431-mediated targeting of the Wnt inhibitor Kremen1, as observed with axon regeneration of sensory neurons (Wu and Murashov, 2013). Previous findings indicate that the Stat3 pathway can be activated by Wnt signaling within the retina, and reduction of Stat3 eliminated the neuroprotective properties of Wnt3a in photoreceptors and cell lines (Fragoso et al., 2012, Patel et al., 2015b). We tested Stat3 as a potential mechanism of Wnt3a-dependent axonal growth because several lines of evidence indicate that Stat3 is an important part of the intrinsic regulation of axonal regeneration (Pellegrino and Habecker, 2013, Qin et al., 2013). Several studies showed that knockdown of the endogenous Stat3 inhibitor SOCS3 led to robust axonal outgrowth and elongation (Smith et al., 2009, Sun et al., 2011), and direct activation of Stat3 through cytokine stimulation or overexpression induced axonal regrowth (Pellegrino and Habecker, 2013). We demonstrated that axonal regeneration after Wnt3a stimulation was diminished after ablating Stat3 expression within RGCs, indicating a role for Stat3 in Wnt-induced axonal regrowth. However, because axonal regeneration was not eliminated entirely, it is possible that Wnt signaling induces axonal regeneration through Stat3-independent pathways and/or RGC-independent Wnt/Stat3 signaling. Additionally, although we demonstrated that Stat3 is upregulated at the 2 week time point after injury, it is not yet known whether Wnt3a upregulates Stat3 acutely after injury. Previous work demonstrated that Stat3 is upregulated after ONC at 3 days post-injury (Kirsch et al., 2010), and Wnt3a induces Stat3 in cell lines within 5 hours (Fragoso et al., 2012), suggesting the possibility the there would be a similar acute Stat3 activation in response to Wnt3a after ONC. Finally, we cannot at this stage distinguish whether Stat3 is important for both Wnt3a-dependent survival and regeneration, or for only survival and that the surviving RGCs were able to respond to Wnt3a-induced axonal growth cues.
Wnt3a-induced RGC survival was lower in the Stat3 KO mice but still higher than the saline treated mice. There are several possible explanations for this observation. Wnt3a may have stimulated Stat3-independent pro-survival pathways, such as BDNF or anti-apoptotic pathways (Leaver et al., 2006a, Yi et al., 2007, Seitz et al., 2010, Yi et al., 2012a, Sanchez-Migallon et al., 2016). It is also possible that the surviving RGCs are a subtype of cell that do not need Stat3 for viability. Also, we cannot exclude the possibility that these RGCs were in the small proportion of cells that did not receive Cre and retained Stat3 expression and responsiveness to Wnt protective pathways because the surviving RGCs were negative for Cre.
Wnt signaling may promote axonal regeneration through its direct and indirect effects on the inflammatory response. Numerous lines of evidence indicate that Wnt/β-catenin signaling has positive and negative regulatory effects on local inflammatory signaling pathways after brain injury due to trauma, stroke or disease (reviewed in (Marchetti and Pluchino, 2013)). Furthermore, stimulation of retinal inflammation by intravitreal injection has been reported to lead to robust axonal regeneration in the optic nerve (Yin et al., 2009). It has been hypothesized that cytokines released during mild inflammation can stimulate the regeneration process and augment the ability of trophic factors to induce corticospinal tract sprouting (Benowitz and Popovich, 2011). Our results suggest an increased inflammatory response associated with Wnt signaling in response to injury because the increased numbers of IBA1-positive inflammatory cells in the Wnt3a-injected retinas are consistent with the known role of inflammation in promoting optic nerve regeneration, therefore suggesting a possible inflammatory mediated mechanism through which Wnt signaling regulates axonal regeneration.
In conclusion, we demonstrated that a single injection of Wnt3a is sufficient to stimulate axonal regeneration in the optic nerve crush model. Further studies will test whether Wnt3a promotes survival and regeneration at later timepoints, and whether it is effective in other models of optic nerve injury.
Highlights.
Activation of the canonical Wnt/β-catenin signaling pathway led to axonal regeneration in a mouse optic nerve injury model
Wnt signaling was active in retinal ganglion cells (RGCs)
Wnt3a also led to increased RGC survival after injury
The transcription factor Stat3 contributes to Wnt3a-induced axonal regeneration and RGC survival
These findings demonstrate a novel role for retinal Wnt signaling in axonal regrowth
Acknowledgments
Support for this study was from the Karl Kirchgessner Foundation (ASH) and NEI 1R01EY022961 (KKP), and institutional support to BPEI was from a Research to Prevent Blindness Unrestricted Grant and an NEI Center Core Grant P30 EY014801. Assistance with OCT analysis techniques was provided by Dr. Marco Ruggeri and the Ophthalmic Biophysics Center of University of Miami, AAV2-Cre virus was provided by Ben Yungher, PERG expertise was provided by Tsung-Han Chou, Paola Blanco assisted with immunohistochemistry, and Yuan Liu assisted with sample collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benowitz LI, Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol. 2011;24:577–583. doi: 10.1097/WCO.0b013e32834c208d. [DOI] [PubMed] [Google Scholar]
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunger BM, Ohlmann A, Koch M, Tanimoto N, Volz C, Yang Y, Bosl MR, Cvekl A, Jagle H, Seeliger MW, Tamm ER. Constitutive overexpression of Norrin activates Wnt/beta-catenin and endothelin-2 signaling to protect photoreceptors from light damage. Neurobiol Dis. 2013;50:1–12. doi: 10.1016/j.nbd.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Briona LK, Poulain FE, Mosimann C, Dorsky RI. Wnt/ss-catenin signaling is required for radial glial neurogenesis following spinal cord injury. Dev Biol. 2015;403:15–21. doi: 10.1016/j.ydbio.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp AS, Ruggeri M, Munguba GC, Tapia ML, John SW, Bhattacharya SK, Lee RK. Structural correlation between the nerve fiber layer and retinal ganglion cell loss in mice with targeted disruption of the Brn3b gene. Invest Ophthalmol Vis Sci. 2011;52:5226–5232. doi: 10.1167/iovs.10-6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong RS, Martin KR. Glial cell interactions and glaucoma. Curr Opin Ophthalmol. 2015;26:73–77. doi: 10.1097/ICU.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Shi M, Quan M, Xie K. Regulation of EMT by KLF4 in gastrointestinal cancer. Curr Cancer Drug Targets. 2013;13:986–995. doi: 10.2174/15680096113136660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danias J, Lee KC, Zamora MF, Chen B, Shen F, Filippopoulos T, Su Y, Goldblum D, Podos SM, Mittag T. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: comparison with RGC loss in aging C57/BL6 mice. Invest Ophthalmol Vis Sci. 2003;44:5151–5162. doi: 10.1167/iovs.02-1101. [DOI] [PubMed] [Google Scholar]
- David MD, Canti C, Herreros J. Wnt-3a and Wnt-3 differently stimulate proliferation and neurogenesis of spinal neural precursors and promote neurite outgrowth by canonical signaling. J Neurosci Res. 2010;88:3011–3023. doi: 10.1002/jnr.22464. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Assinck P, Hilton BJ. Canonical Wnt signalling in PDGFRalpha-expressing cells is a critical regulator of astrogliosis and axon regeneration following CNS injury. J Neurosci. 2014;34:16163–16165. doi: 10.1523/JNEUROSCI.4052-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards N, Farookhi R, Clarke HJ. Identification of a beta-galactosidase transgene that provides a live-cell marker of transcriptional activity in growing oocytes and embryos. Mol Hum Reprod. 2015;21:583–593. doi: 10.1093/molehr/gav020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Leibinger M. Promoting optic nerve regeneration. Prog Retin Eye Res. 2012;31:688–701. doi: 10.1016/j.preteyeres.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Fragoso MA, Patel AK, Nakamura RE, Yi H, Surapaneni K, Hackam AS. The Wnt/beta-catenin pathway cross-talks with STAT3 signaling to regulate survival of retinal pigment epithelium cells. PLoS One. 2012;7:e46892. doi: 10.1371/journal.pone.0046892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S. Wnt Signaling in Eye Organogenesis. Organogenesis. 2008;4:60–67. doi: 10.4161/org.4.2.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Forster V, Hicks D, Vecino E. Effects of muller glia on cell survival and neuritogenesis in adult porcine retina in vitro. Invest Ophthalmol Vis Sci. 2002;43:3735–3743. [PubMed] [Google Scholar]
- Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Zou Y. Reinduced Wnt signaling limits regenerative potential of sensory axons in the spinal cord following conditioning lesion. Proc Natl Acad Sci U S A. 2012;109:14663–14668. doi: 10.1073/pnas.1206218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Senga T, Hamaguchi M. A novel role of phospho-beta-catenin in microtubule regrowth at centrosome. Oncogene. 2007;26:4357–4371. doi: 10.1038/sj.onc.1210217. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Zhang M, Ferguson JW, Koch M, Brunken WJ. The extracellular matrix component WIF-1 is expressed during, and can modulate, retinal development. Mol Cell Neurosci. 2004;27:477–488. doi: 10.1016/j.mcn.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A, Matsumoto S. New insights into the mechanism of Wnt signaling pathway activation. Int Rev Cell Mol Biol. 2011;291:21–71. doi: 10.1016/B978-0-12-386035-4.00002-1. [DOI] [PubMed] [Google Scholar]
- Kirsch M, Trautmann N, Ernst M, Hofmann HD. Involvement of gp130-associated cytokine signaling in Muller cell activation following optic nerve lesion. Glia. 2010;58:768–779. doi: 10.1002/glia.20961. [DOI] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- Leaver SG, Cui Q, Bernard O, Harvey AR. Cooperative effects of bcl-2 and AAV-mediated expression of CNTF on retinal ganglion cell survival and axonal regeneration in adult transgenic mice. Eur J Neurosci. 2006a;24:3323–3332. doi: 10.1111/j.1460-9568.2006.05230.x. [DOI] [PubMed] [Google Scholar]
- Leaver SG, Cui Q, Plant GW, Arulpragasam A, Hisheh S, Verhaagen J, Harvey AR. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006b;13:1328–1341. doi: 10.1038/sj.gt.3302791. [DOI] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon LA, Karki S, Tokito M, Holzbaur EL. Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol. 2001;3:913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- Liu B, Hunter DJ, Rooker S, Chan A, Paulus YM, Leucht P, Nusse Y, Nomoto H, Helms JA. Wnt signaling promotes Muller cell proliferation and survival after injury. Invest Ophthalmol Vis Sci. 2013;54:444–453. doi: 10.1167/iovs.12-10774. [DOI] [PubMed] [Google Scholar]
- Liu H, Thurig S, Mohamed O, Dufort D, Wallace VA. Mapping canonical Wnt signaling in the developing and adult retina. Invest Ophthalmol Vis Sci. 2006;47:5088–5097. doi: 10.1167/iovs.06-0403. [DOI] [PubMed] [Google Scholar]
- Liu H, Xu S, Wang Y, Mazerolle C, Thurig S, Coles BL, Ren JC, Taketo MM, van der Kooy D, Wallace VA. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev Biol. 2007;308:54–67. doi: 10.1016/j.ydbio.2007.04.052. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang X, Lu CC, Kerman R, Steward O, Xu XM, Zou Y. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci. 2008;28:8376–8382. doi: 10.1523/JNEUROSCI.1939-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Ribeiro M, Bray ER, Lee DH, Yungher BJ, Mehta ST, Thakor KA, Diaz F, Lee JK, Moraes CT, Bixby JL, Lemmon VP, Park KK. Enhanced transcriptional activity and mitochondrial localization of STAT3 co-induce axon regrowth in the adult central nervous system. Cell Rep. 2016;15:398–410. doi: 10.1016/j.celrep.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti B, Pluchino S. Wnt your brain be inflamed? Yes, it Wnt! Trends Mol Med. 2013;19:144–156. doi: 10.1016/j.molmed.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Koda M, Kitajo K, Yamazaki M, Takahashi K, Kikuchi A, Yamashita T. Wnt-Ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J Neurotrauma. 2009;26:955–964. doi: 10.1089/neu.2008.0776. [DOI] [PubMed] [Google Scholar]
- Mizukami M, Souchelnytskyi N, Kiuchi Y, Kanamoto T. Wnt14 inhibits death of retinal precursor cells. Exp Eye Res. 2009;89:462–468. doi: 10.1016/j.exer.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Dufort D, Clarke HJ. Expression and estradiol regulation of Wnt genes in the mouse blastocyst identify a candidate pathway for embryo-maternal signaling at implantation. Biol Reprod. 2004;71:417–424. doi: 10.1095/biolreprod.103.025692. [DOI] [PubMed] [Google Scholar]
- Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DL, Goldberg JL. Four steps to optic nerve regeneration. J Neuroophthalmol. 2010;30:347–360. doi: 10.1097/WNO.0b013e3181e755af. [DOI] [PubMed] [Google Scholar]
- Munguba GC, Galeb S, Liu Y, Landy DC, Lam D, Camp A, Samad S, Tapia ML, Lee RK. Nerve fiber layer thinning lags retinal ganglion cell density following crush axonopathy. Invest Ophthalmol Vis Sci. 2014;55:6505–6513. doi: 10.1167/iovs.14-14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Hackam A. A novel protective role for the innate immunity Toll-Like Receptor 3 (TLR3) in the retina via Stat3. Mol Cell Neurosci. 2014a;68C:38–48. doi: 10.1016/j.mcn.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AK, Akinsoji E, Hackam AS. Defining the Relationships Among Retinal Function, Layer Thickness and Visual Behavior During Oxidative Stress-Induced Retinal Degeneration. Curr Eye Res. 2015a:977–986. doi: 10.3109/02713683.2015.1083588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AK, Hackam AS. Toll-like receptor 3 (TLR3) protects retinal pigmented epithelium (RPE) cells from oxidative stress through a STAT3-dependent mechanism. Mol Immunol. 2012;54:122–131. doi: 10.1016/j.molimm.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AK, Hackam AS. A novel protective role for the innate immunity Toll-Like Receptor 3 (TLR3) in the retina via Stat3. Mol Cell Neurosci. 2014b;63:38–48. doi: 10.1016/j.mcn.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AK, Surapaneni K, Yi H, Nakamura RE, Karli SZ, Syeda S, Lee T, Hackam AS. Activation of Wnt/beta-catenin signaling in Muller glia protects photoreceptors in a mouse model of inherited retinal degeneration. Neuropharmacology. 2015b;91:1–12. doi: 10.1016/j.neuropharm.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino MJ, Habecker BA. STAT3 integrates cytokine and neurotrophin signals to promote sympathetic axon regeneration. Mol Cell Neurosci. 2013;56:272–282. doi: 10.1016/j.mcn.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet V, Joly S, Dalkara D, Jordi N, Schwarz O, Christ F, Schaffer DV, Flannery JG, Schwab ME. Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol Dis. 2013a;51:202–213. doi: 10.1016/j.nbd.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Pernet V, Joly S, Jordi N, Dalkara D, Guzik-Kornacka A, Flannery JG, Schwab ME. Misguidance and modulation of axonal regeneration by Stat3 and Rho/ROCK signaling in the transparent optic nerve. Cell Death Dis. 2013b;4:e734. doi: 10.1038/cddis.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet V, Schwab ME. Lost in the jungle: new hurdles for optic nerve axon regeneration. Trends Neurosci. 2014;37:381–387. doi: 10.1016/j.tins.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Porciatti V. The mouse pattern electroretinogram. Doc Ophthalmol. 2007;115:145–153. doi: 10.1007/s10633-007-9059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purro SA, Ciani L, Hoyos-Flight M, Stamatakou E, Siomou E, Salinas PC. Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli. J Neurosci. 2008;28:8644–8654. doi: 10.1523/JNEUROSCI.2320-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Zou Y, Zhang CL. Cross-talk between KLF4 and STAT3 regulates axon regeneration. Nat Commun. 2013;4:2633–2636. doi: 10.1038/ncomms3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JP, Coulter M, Miotke J, Meyer RL, Takemaru K, Levine JM. Abrogation of beta-Catenin signaling in oligodendrocyte precursor cells reduces glial scarring and promotes axon regeneration after CNS injury. J Neurosci. 2014;34:10285–10297. doi: 10.1523/JNEUROSCI.4915-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri M, Wehbe H, Jiao S, Gregori G, Jockovich ME, Hackam A, Duan Y, Puliafito CA. In vivo three-dimensional high-resolution imaging of rodent retina with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:1808–1814. doi: 10.1167/iovs.06-0815. [DOI] [PubMed] [Google Scholar]
- Sanchez-Migallon MC, Valiente-Soriano FJ, Nadal-Nicolas FM, Vidal-Sanz M, Agudo-Barriuso M. Apoptotic retinal ganglion cell death after optic nerve transection or crush in mice: delayed RGC loss with BDNF or a caspase 3 inhibitor. Invest Ophthalmol Vis Sci. 2016;57:81–93. doi: 10.1167/iovs.15-17841. [DOI] [PubMed] [Google Scholar]
- Sanges D, Romo N, Simonte G, Di Vicino U, Tahoces AD, Fernandez E, Cosma MP. Wnt/beta-catenin signaling triggers neuron reprogramming and regeneration in the mouse retina. Cell Rep. 2013;4:271–286. doi: 10.1016/j.celrep.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Sato M, Umetsu D, Murakami S, Yasugi T, Tabata T. DWnt4 regulates the dorsoventral specificity of retinal projections in the Drosophila melanogaster visual system. Nat Neurosci. 2006;9:67–75. doi: 10.1038/nn1604. [DOI] [PubMed] [Google Scholar]
- Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Optic nerve crush: protection and regeneration. Brain Res Bull. 2004;62:467–471. doi: 10.1016/S0361-9230(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Seitz R, Hackl S, Seibuchner T, Tamm ER, Ohlmann A. Norrin mediates neuroprotective effects on retinal ganglion cells via activation of the Wnt/beta-catenin signaling pathway and the induction of neuroprotective growth factors in Muller cells. J Neurosci. 2010;30:5998–6010. doi: 10.1523/JNEUROSCI.0730-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum JW, Liu K, So KF. The progress in optic nerve regeneration, where are we? Neural Regen Res. 2016;11:32–36. doi: 10.4103/1673-5374.175038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, He Z. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–375. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usongo M, Farookhi R. beta-catenin/Tcf-signaling appears to establish the murine ovarian surface epithelium (OSE) and remains active in selected postnatal OSE cells. BMC Dev Biol. 2012;12:1–15. doi: 10.1186/1471-213X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Murashov AK. MicroRNA-431 regulates axon regeneration in mature sensory neurons by targeting the Wnt antagonist Kremen1. Front Mol Neurosci. 2013;6:1–13. doi: 10.3389/fnmol.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam PT, Charron F. Signaling mechanisms of non-conventional axon guidance cues: the Shh, BMP and Wnt morphogens. Curr Opin Neurobiol. 2013;23:965–973. doi: 10.1016/j.conb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Yi H, Hu J, Qian J, Hackam AS. Expression of brain-derived neurotrophic factor is regulated by the Wnt signaling pathway. Neuroreport Vol. 2012a;23:189–194. doi: 10.1097/WNR.0b013e32834fab06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Nakamura RE, Mohamed O, Dufort D, Hackam AS. Characterization of Wnt signaling during photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2007;48:5733–5741. doi: 10.1167/iovs.07-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Patel AK, Sodhi C, Hackam DJ, Hackam AS. Novel role for the innate immune receptor Toll-like receptor 4 (TLR4) in the regulation of the Wnt signaling pathway and photoreceptor apoptosis. PLoS One. 2012b;7(5):e36560 1–15. doi: 10.1371/journal.pone.0036560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Gilbert HY, Yang Y, Yang Z, Berlinicke C, Li Z, Zaverucha-do-Valle C, He H, Petkova V, Zack DJ, Benowitz LI. Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci U S A. 2009;106:19587–19592. doi: 10.1073/pnas.0907085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin ZS, Zu B, Chang J, Zhang H. Repair effect of Wnt3a protein on the contused adult rat spinal cord. Neurol Res. 2008;30:480–486. doi: 10.1179/174313208X284133. [DOI] [PubMed] [Google Scholar]
- You Y, Gupta VK, Li JC, Klistorner A, Graham SL. Optic neuropathies: characteristic features and mechanisms of retinal ganglion cell loss. Rev Neurosci. 2013;24:301–321. doi: 10.1515/revneuro-2013-0003. [DOI] [PubMed] [Google Scholar]
- Yungher BJ, Luo X, Salgueiro Y, Blackmore MG, Park KK. Viral vector based improvement of optic nerve regeneration: characterization of individual axons’ growth patterns and synaptogenesis in a visual target. Gene Ther. 2015;22:811–821. doi: 10.1038/gt.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Zou Y. Wnt signaling in axon guidance. Trends Neurosci. 2004;27:528–532. doi: 10.1016/j.tins.2004.06.015. [DOI] [PubMed] [Google Scholar]