Abstract
Objective
We evaluated the effectiveness of cabotegravir (CAB; GSK1265744 or GSK744) long-acting (LA) as pre-exposure prophylaxis (PrEP) against intravenous SIV challenge in a model that mimics blood transfusions based on the per-act probability of infection.
Design
CAB LA is an InSTI formulated as a 200 mg/mL injectable nanoparticle suspension that is an effective pre-exposure prophylaxis (PrEP) agent against rectal and vaginal SHIV transmission in macaques.
Methods
Three groups of rhesus macaques (n=8/group) were injected intramuscularly with CAB LA and challenged intravenously with 17 AID50 SIVmac251 on week 2. Group 1 was injected with 50 mg/kg on week 0 and 4 to evaluate the protective efficacy of the CAB LA dose used in macaque studies mimicking sexual transmission. Group 2 was injected with 50 mg/kg on week 0 to evaluate the necessity of the second injection of CAB LA for protection against intravenous challenge. Group 3 was injected with 25 mg/kg on week 0 and 50 mg/kg on week 4 to correlate CAB plasma concentrations at the time of challenge with protection. Five additional macaques remained untreated as controls.
Results
CAB LA was highly protective with 21 of the 24 CAB LA-treated macaques remaining aviremic, resulting in 88% protection. The plasma CAB concentration at the time of virus challenge appeared to be more important for protection than sustaining therapeutic plasma concentrations with the second CAB LA injection.
Conclusions
These results support the clinical investigation of CAB LA as PrEP in people who inject drugs.
Keywords: GSK1265744, cabotegravir, long-acting, macaque, pre-exposure prophylaxis
Introduction
It is estimated that 1.7 million (13.1%) of the 12.1 million people who inject drugs worldwide are infected with HIV-1 [1]. Pre-exposure prophylaxis (PrEP) with antiretroviral drugs is a promising biomedical intervention to prevent HIV-1 transmission. A once daily oral tenofovir disoproxil fumarate (TDF) or TDF-emtricitabine (FTC) regimen as PrEP has reduced HIV-1 infections via sexual transmission by 44 to 75% in randomized clinical trials [2–4]. In the Bangkok Tenofovir Study, daily oral TDF reduced HIV-1 transmission by 49% in injection drug users, with the protective efficacy further increased to 74% when restricted to participants with detectable levels of tenofovir [5]. All clinical trials to date, including those that failed to demonstrate TDF or TDF/FTC-based PrEP efficacy [6, 7] have reported a direct correlation between the effectiveness of an agent and adherence to the prescribed regimen. These studies suggest the need for additional methods to improve PrEP adherence, and long-acting PrEP agents are one such possibility.
Cabotegravir (CAB), an analogue of dolutegravir, is an integrase strand transfer inhibitor (InSTI) with physiochemical properties that permit its formulation as an injectable 200 mg/mL nanosuspension. CAB long-acting (LA) is undergoing clinical development for both HIV-1 treatment and prevention [8–11]. Recent data from a phase 2b clinical trial indicated that CAB LA in conjunction with rilpilvirine LA is sufficient to maintain virologic suppression in HIV-1-infected adults when administered every 4 or 8 weeks [12]. In healthy individuals, a phase 2 clinical study suggested that CAB LA is safe and well tolerated, but intramuscular dosing every 12 weeks failed to result in plasma CAB trough concentrations above levels that were highly protective in preclinical studies of sexual transmission in non-human primates [13]. In both studies, participants indicated a preference for the injectable formulation compared with an oral regimen [12, 13]
The utility of CAB LA as PrEP has previously been evaluated extensively in macaque models mimicking HIV-1 sexual transmission [14–16]. In low dose challenge models designed to more closely mimic HIV-1 transmission in humans, macaques administered CAB LA as PrEP were completely protected from repeated intrarectal [14] or intravaginal [15] SHIV162P3 challenges. Additionally, in a female rhesus macaque model utilizing Depo-Provera to thin the cervicovaginal epithelium to increase the probability of infection, CAB LA protected 6 of 8 female macaques from repeated high-dose intravaginal challenges [16]. Interestingly, in this study, a late breakthrough infection was observed in one animal with virus detected 7 weeks after the last challenge suggesting the importance of maintaining levels of CAB not only at challenge but also for weeks thereafter. However, there could the importance of drug levels at the time of and after challenge may differ between mucosal challenge and infection by intravenous exposure. In the current study, we evaluated the protective efficacy of CAB LA administered as PrEP against intravenous challenge in a macaque model mimicking parenteral transmission. In addition, we modified the CAB LA treatment regimen to assess the relative importance of plasma CAB levels at the time of and the contribution of maintaining CAB concentrations subsequent to intravenous challenge.
Methods
Animals
The intravenous challenge study was a 4-arm study that included 24 female Indian rhesus macaques. Six of the macaques were recycled from earlier intravaginal challenge studies [16] after confirmation of lack of SHIV162P3 infection. Five of the macaques utilized in the 25 mg/kg; 50 mg/kg study were recycled into the single 50 mg/kg study 40 weeks after the challenge and confirmation of lack of infection. Assuming that 100% of control macaques would become infected following one challenge, each study had 89% power to detect 75% PrEP effectiveness using log-rank test with a p-value of 0.05. The Institutional Animal Care and Use Committee (IACUC) of Tulane National Primate Research Center (TNPRC) approved all studies. TNPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC # 000594). TNPRC’s OLAW animal welfare assurance number is A4499-01 and USDA registration number is 72-R-0002.
SIVmac251 viral stock
SIVmac251 viral stock was kindly provided by Dr. Ron Desrosiers. The viral stock was previously titrated in rhesus macaques via intravenous inoculation. The SIVmac251 challenge stock also had an end point titer of 5 × 103 TCID50/mL on CEMx174 cells by the method of Reed and Muench [17].
Efficacy of CAB LA in preventing SIV intravenous transmission
The efficacy of CAB LA against intravenous SIV transmission was evaluated in three groups of Indian rhesus macaques (Macaca mulatta) (n=8/group) injected IM with CAB LA and challenged intravenously with 17 AID50 SIVmac251 on week 2. Group 1 was injected with 50 mg/kg CAB LA on week 0 and 4, the same dosing regimen used in previous studies assessing CAB LA prevention efficacy against mucosal transmission [14, 16]. Group 2 was injected with 50 mg/kg of CAB LA on week 0 to understand the relative importance of CAB concentrations at the time of challenge and negating the potential benefit of a second injection that would prevent infection distal to the time of challenge as had been seen in the high-dose challenge experiments in female rhesus macaques [16]. Group 3 was injected with 25 mg/kg CAB LA on week 0 and 50 mg/kg CAB LA on week 4 to determine the importance of CAB concentration at the time of challenge while maintaining the second injection thereby modifying a single variable, peak drug concentrations at the time of intravenous challenge. CAB LA is a 200 mg/mL nanosuspension that was administered based on body weights measured at the time of dosing (5.4 to 11.3 kg) with the dose split into four injections, two per quadriceps. Five additional macaques remained untreated as controls. Systemic infection was monitored weekly for 20 weeks after the last CAB LA administration by detection of SIV RNA in plasma using real-time RT-PCR assay with a sensitivity of 40 SIV RNA copies/mL as previously described [14]. PBMC proviral DNA amplification was performed as previously described [16]. Serology was performed utilizing SIVmac251 gp120-coated plates (ImmuneTech IT-001-156p). CAB plasma concentration analyses were performed as previously described [14]. Integrase sequence analyses from bulk plasma virus was performed as previously described [14, 16].
Construction of single-cycle SIV recombinant virus and determination for susceptibility to CAB
Mutant viruses were constructed and their susceptibility to CAB was evaluated as previously described [16].
Statistical Methods
Fisher’s exact test was used to compare CAB LA-treated and untreated macaques for number of infections. An unpaired two-tailed t-test or one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison post hoc test was performed to assess CAB concentration differences. All statistical analyses were performed using GraphPad Prism software (version 6.0).
Results
PrEP efficacy of CAB LA using doses previously evaluated in macaques
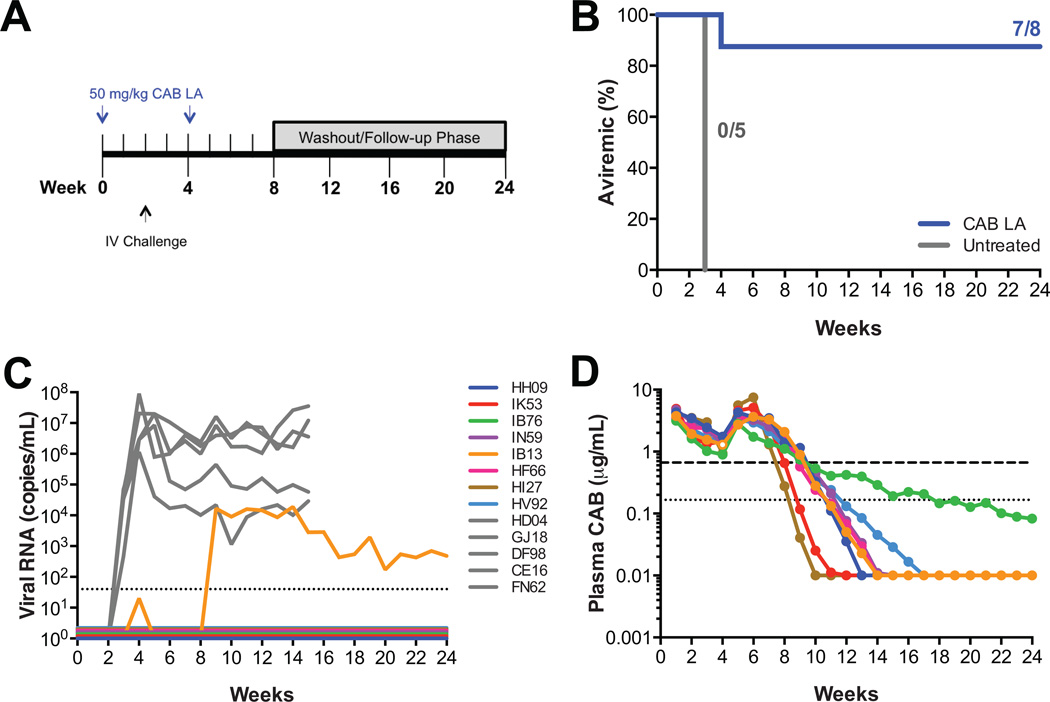
We previously evaluated the pharmacokinetic profile of CAB LA in male rhesus macaques and established that dosing every four weeks with 50 mg/kg maintained plasma concentrations similar to those achieved in a phase 1 study of healthy human volunteers administered 800 mg of CAB LA intramuscularly every three months [14]. This dosing regimen provided drug concentrations that were completely protective against repeated low dose rectal [14] and vaginal [15] challenge. In this study, we wanted to determine if the same dosing regimen of CAB LA that protected macaques from mucosal SHIV transmission, 50 mg/kg CAB LA administered on weeks 0 and 4, could protect macaques from intravenous challenge. Eight macaques were administered 50 mg/kg CAB LA on weeks 0 and 4, with five macaques remaining untreated as controls. All macaques were challenged by intravenous inoculation with 17 AID50 SIVmac251 at week 2 (Fig. 1A). Plasma vRNA was detected in all five untreated macaques one week after challenge (Fig. 1B, 1C). Proviral DNA was also detected in PBMCs one week after challenge and anti-SIV antibodies were detected 3–4 weeks after challenge (data not shown). Seven of the 8 CAB LA-treated macaques remained aviremic through week 24 (p=0.0047; Fisher’s exact test). Plasma vRNA was detected in one CAB LA-treated macaque, IB13, two weeks after challenge (Fig. 1B, 1C). Because this macaque was administered a second dose of CAB LA at week 4, the viral loads were subsequently suppressed and were detectable again at week 9 (Fig. 1C). Proviral DNA was detected in PBMCs from IB13 concurrently with plasma vRNA and anti-SIV antibodies were detected 7 weeks after challenge (data not shown). The seven aviremic macaques did not have detectable proviral DNA or anti-SIV antibodies. IB13 did not have the lowest CAB plasma concentration at the time of challenge. The plasma CAB concentration for IB13 at the time of challenge was 1.93 µg/mL compared with the mean plasma CAB concentration of 2.58 (range: 1.63 to 3.51) µg/mL for the seven aviremic macaques (Fig. 1D). The integrase gene from plasma virus from IB13 collected between weeks 9 to 16 was sequenced, and one mutation, I191M, was identified, compared with the original SIVmac251 inoculum (Table S1). This mutant virus demonstrated susceptibility to CAB similar to wild-type virus with an IC50 in TZM-bl cells of 3.2 nM and 3.1. nM, respectively.
Fig. 1.
Two 50 mg/kg injections of CAB LA protects macaques against intravenous SIV challenge. (A) Study design. Eight macaques were dosed with 50 mg/kg CAB LA on weeks 0 and 4. Five macaques remained untreated. All macaques were challenged intravenously on week 2 with SIVmac251. (B) Kaplan-Meier plot of CAB LA-treated (blue) and untreated (gray) rhesus macaques remaining aviremic following intravenous challenge. (C) Plasma viremia in control rhesus macaques (in gray) and CAB LA-treated rhesus macaques (in color). Dotted line represents the LOQ, >40 SIV RNA copies/mL plasma. Baseline values are offset from each other for clarity. (D) Pharmacokinetic profile of plasma CAB concentrations in individual macaques. Open symbol corresponds with plasma concentration at the time of first vRNA detection for IB13. Dotted and dashed horizontal lines represent 1× and 4× PAIC90, respectively.
PrEP efficacy of single 50 mg/kg CAB LA dose
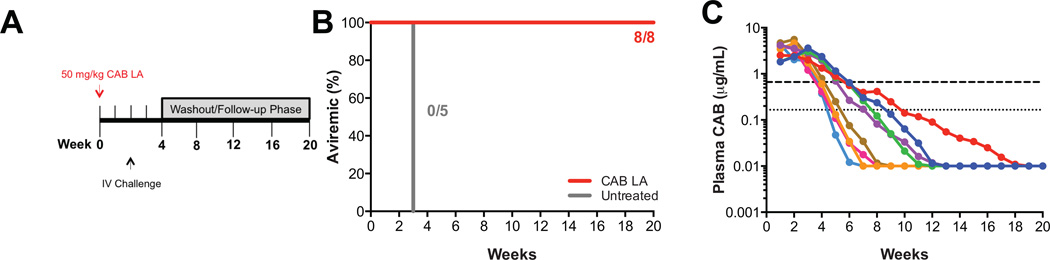
Next, we wanted to determine if the second CAB LA injection was required to maintain protective efficacy against intravenous challenge. In this study, eight macaques were given one 50 mg/kg CAB LA injection on week 0 and challenged intravenously with 17 AID50 of the same stock of SIVmac251 on week 2 (Fig. 2A). All eight macaques given a single 50 mg/kg CAB LA dose remained aviremic (p=0.0008; Fisher’s exact test; Fig. 2B), proviral DNA negative and seronegative through week 20. As expected, individual plasma CAB concentrations from macaques administered a single 50 mg/kg dose on week 0 fell below 4× protein-adjusted IC90 (PAIC90) between weeks 4 and 6 (Fig. 2C), compared with weeks 8 and 10 for macaques administered 50 mg/kg CAB LA on weeks 0 and 4 (Fig. 1D). The mean plasma CAB concentration at the time of challenge was comparable between the two groups administered 50 mg/kg of CAB LA on week 0 irrespective of receiving a second 50 mg/kg CAB LA injection at week 4, 3.31 (range: 2.03 to 5.56) and 2.50 (range: 1.63 to 3.51) µg/mL, respectively (Fig. S1). Of the 16 macaques that were dosed with 50 mg/kg of CAB LA prior to challenge, the mean plasma CAB concentration of the macaques that remained aviremic following challenge was 2.97 µg/mL (n=15) compared with 1.93 µg/mL for IB13, the macaque that became infected.
Fig. 2.
A single dose CAB LA protects macaques against intravenous SIV challenge. (A) Study design. Eight macaques were dosed with 50 mg/kg CAB LA on week 0 and challenged intravenously on week 2 with SIVmac251. (B) Kaplan-Meier plot of CAB LA-treated (red) and untreated (gray) rhesus macaques remaining aviremic following intravenous challenge. (C) Plasma CAB concentrations in individual macaques. Dotted and dashed horizontal lines represent 1× and 4× PAIC90, respectively.
Influence of plasma CAB concentration at time of challenge on PrEP efficacy
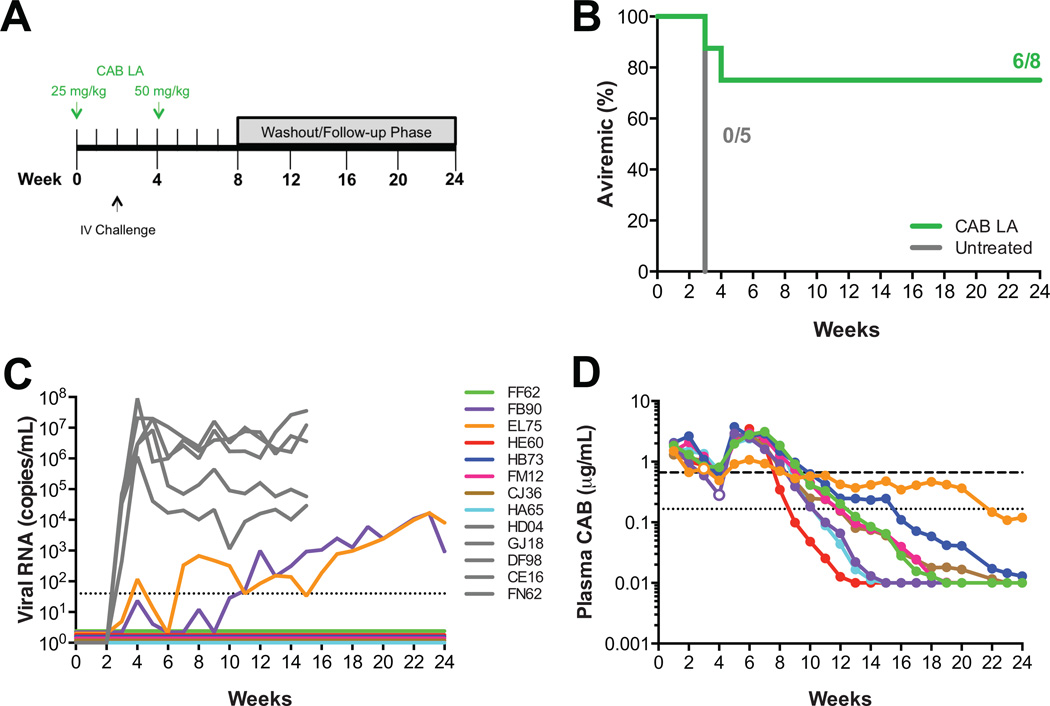
From the previous experiment (Fig. 2), it appeared that CAB concentrations at the time of challenge were critical in providing protection against intravenous challenge. We then went on to challenge animals after dosing with a lower initial dose of CAB to confirm that lower plasma CAB concentrations at the time of challenge could affect protective efficacy. For this experiment, eight macaques were administered 25 mg/kg CAB LA on week 0, challenged intravenously with 17 AID50 SIVmac251 on week 2 and administered 50 mg/kg CAB LA on week 4 (Fig. 3A). The second injection of CAB LA at week 4 was maintained as per the first challenge experiment to avoid changing two variables simultaneously, which could be potentially confounding in interpreting the results of the experiment. We posited that in the absence of the second injection plasma CAB concentrations could decrease below protective levels earlier simply due to lower initial dosing, thereby not allowing us to assess the impact of plasma CAB concentration at time of challenge on protective efficacy. Based on the repeated high-dose vaginal challenge experiment where one macaque had detectable viremia 7 weeks after the last challenge [16], it was expected that plasma concentrations must to be maintained above the protective threshold for a given time to prevent infection. The single 50 mg/kg CAB dose indicates that one 50 mg/kg CAB LA dose provides sufficient CAB concentrations over a given time to protect the macaques. In this experiment, if the animals were only given a single 25 mg/kg CAB LA injection, the concentration at challenge would have decreased as well as the protective duration. Therefore, to only evaluate CAB concentration at the time of challenge, the animals were given 50 mg/kg CAB LA at week 4 to maintain the protective duration. Six of the 8 macaques given a 25 mg/kg dose followed by a 50 mg/kg dose of CAB LA remained aviremic through week 24 (p=0.021; Fisher’s exact test; Fig. 3B). Two CAB LA-treated macaques, EL75 and FB90, became infected with plasma virus detected one or two weeks after challenge, respectively (Fig. 3C). EL75 and FB90 had the lowest plasma CAB concentrations at the time of challenge, 0.67 and 0.91 µg/mL, respectively (Fig. S1), and vRNA was first detected when plasma CAB concentrations were 0.77 and 0.28 µg/mL, respectively (Fig. 3D). Proviral DNA was detected two weeks after challenge for both infected animals, while the aviremic animals remained proviral DNA negative (data not shown). Both infected animals had anti-SIV antibodies detected 7–8 weeks after challenge, while all aviremic animals remained seronegative (data not shown).
Fig. 3.
Lower plasma CAB concentrations at time of intravenous SIV challenge result in infection. (A) Study design. Eight macaques were dosed with 25 mg/kg CAB LA on week 0 and 50 mg/kg CAB LA on week 4. Macaques were challenged intravenously on week 2 with SIVmac251. (B) Kaplan-Meier plot of CAB LA-treated (green) and untreated (gray) rhesus macaques remaining aviremic following intravenous challenge. (C) Plasma viremia in control rhesus macaques (in gray) and CAB LA-treated rhesus macaques (in color). Dotted line represents the LOQ, >40 SIV RNA copies/mL plasma. Baseline values are offset from each other for clarity. (D) Pharmacokinetic profile of plasma CAB concentrations in individual macaques. Open symbols correspond with plasma concentration at the time of first vRNA detection for infected macaques. Dotted and dashed horizontal lines represent 1× and 4× PAIC90, respectively.
The integrase-coding region was sequenced from plasma virus collected from EL75 at weeks 7 and 8 revealing that EL75 was infected with SIVmac251 containing the V110I mutation (Table S1), which is a polymorphic site in the integrase protein. In previous rectal [14] and vaginal [16] challenge studies, the SHIV162P3 viral stock contained an isoleucine at the 110 position, indicating the susceptibility of this mutation to CAB. EL75 exhibited a unique plasma CAB pharmacokinetic profile (Fig. 3D) as demonstrated by the extremely slow decay of CAB from the plasma. Plasma CAB concentrations decreased below 4× PAIC90 at week 9 and it took until week 20 for the plasma concentrations to decrease below 1× PA1C90 (Fig. 3D). During this time two additional mutations (D232N and A248T) accumulated in the integrase protein; however, these mutations are not known to be resistance-conferring mutations (Table S1). Due to the low viral loads observed in FB90, the first time point post infection that was successfully amplified was week 15, which corresponded to wild-type SIVmac251 (Table S1). I210V was identified at week 16, but this mutation was transient (Table S1).
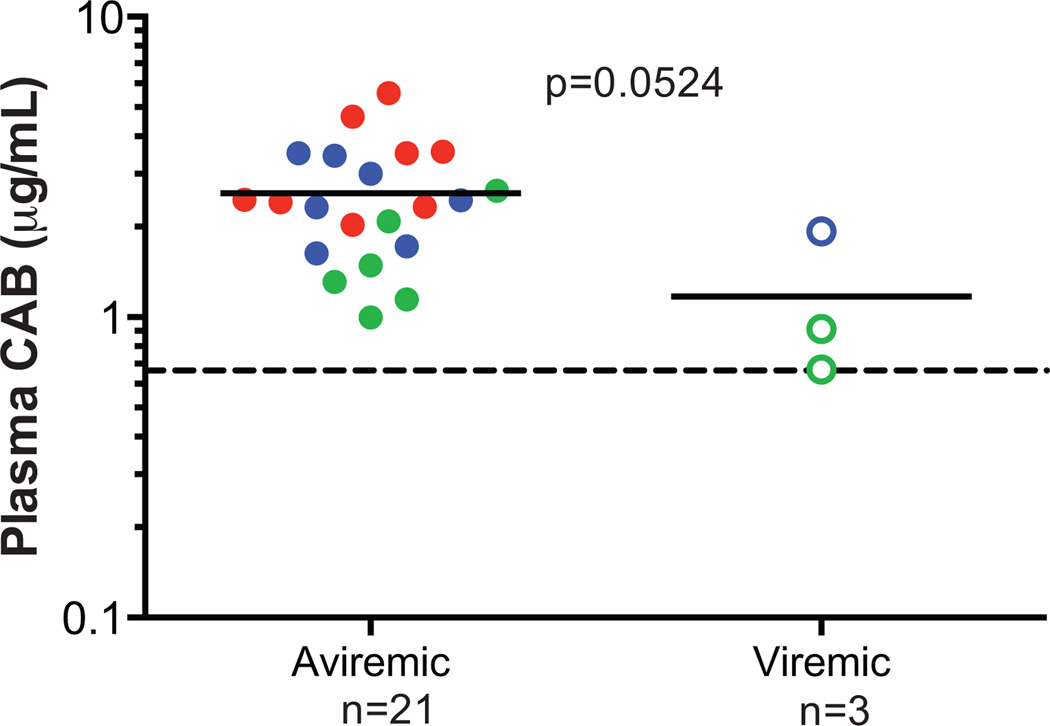
Overall, CAB LA protected 88% (21/24) of macaques from intravenous SIVmac251 challenge (Fig. S2A). The mean plasma pharmacokinetics from each group are as expected based on the dosing regimen (Fig. S2B). The mean plasma CAB concentration at the time of challenge in the macaques remaining aviremic was 2.58 (range 1.00 to 5.56; n=21) µg/mL, compared with 1.17 (range 0.67 to 1.93; n=3) µg/mL for the animals that became infected (p=0.0524; t-test; Fig. 4), and while not significantly different, the number of animals infected was small.
Fig. 4.
Impact of plasma CAB concentration at time of intravenous challenge on prophylactic efficacy. Each data point denotes the CAB concentration an individual macaque dose with 50 mg/kg on week 0 and 4 (blue), 50 mg/kg on week 0 (red) or 25 mg/kg on week 0 and 50 mg/kg on week 4 (green). Closed symbols represent macaques remaining aviremic, and open symbols represent macaques that became viremic. Each symbol represents a different animal, and the solid line represents the mean of each group. The dashed horizontal line represents 4× PAIC90.
Discussion
People who inject drugs are at high risk for HIV-1 infection and account for an estimated 30% of new HIV-1 infections outside of sub-Saharan Africa [18]. Long-acting formulations offer an alternative to daily oral PrEP regimens that may be preferred by some and offer the potential to increase adherence. Here, we demonstrated that CAB LA at concentrations achievable in humans affords protection against intravenous SIV challenge in rhesus macaques. Plasma CAB concentrations at the time of challenge appear to correlate with protective efficacy; however, maintaining therapeutic plasma CAB concentrations after challenge by a second administration of 50 mg/kg CAB LA at week 4 are not required for protection.
Macaque models have been valuable tools in demonstrating the protective efficacy of tenofovir-based PrEP agents prior to clinical evaluation. Early studies demonstrated that PMPA (tenofovir) protected macaques when administered as PrEP 48 hours prior to intravenous challenge with SIVmne [19]. In our study, macaques were challenged with 17 AID50 SIVmac251, a dose determined by in vivo titration in rhesus macaques via intravenous inoculation. As expected, all control macaques (100%) were infected following one challenge. Based on the estimated per-act HIV-1 transmission risk by exposure act, our macaque model more closely mimics blood transfusions where an estimated 9,250 transmissions occur during every 10,000 exposures to contaminated blood products, which is significantly higher than 63 transmissions during every 10,000 exposures to contaminated needles during injection drug use (92.5 and 0.63%, respectively) [20].
Overall, 15 of 16 macaques administered 50 mg/kg CAB LA, which provides plasma concentrations similar to those achieved in humans, were protected from intravenous challenge. No correlation could be made between the plasma CAB concentration of IB13 at the time of challenge and the outcome. This animal is a clear outlier and we are hard pressed to explain this outcome. Viral infection even in the face of drug may be stochastic, or the possibility also exists that systemic virus traveled to a compartment with low CAB concentrations relative to plasma where infection and replication occurred. While the plasma CAB concentrations at the time of challenge were the lowest in the study for the two animals administered 25 mg/kg CAB LA that became infected (0.67 and 0.91 µg/mL), the plasma CAB concentrations were higher than 3× PAIC90 (0.498 µg/mL), the plasma concentration which correlated with 100% protective efficacy in a low dose rectal challenge experiment [14]. In these experiments, macaques were challenged at week 2, after peak plasma CAB concentrations waned; however, the mean plasma concentration of the protected animals at the time of challenge was 2.58 µg/mL. The seemingly higher plasma CAB concentrations required for protection in this study is likely due to the large inoculum used in this macaque challenge model where 100% of controls become infected with one challenge.
Conclusion
CAB LA afforded a high level of protective efficacy in this stringent macaque model demonstrating CAB activity and supporting the clinical investigation of CAB LA as PrEP in people who inject drugs not only to determine efficacy but also to understand tolerability and acceptability of long-acting injections in this high risk group. Preclinical studies with CAB LA have demonstrated complete or high protective efficacy in both mucosal and parenteral transmission in macaques demonstrating its potential utility to reduce infections through multiple routes of infection.
Supplementary Material
Acknowledgments
We thank Dr. Ronald Desrosiers for providing tittered challenge viral stock; Mar Boente-Carrera and Mili R. Gajjar for technical assistance and Yun Lan Yueh for DMPK support. C.D.A., W.R.S., Z.H., D.D.H., and M.M. designed experiments. A.G., K.R.L. and J.B. executed the macaque studies. C.D.A., L.S., A.Y.P., and N.G. performed sample analyses. H.M. designed assays and evaluated drug susceptibility of mutant viruses. C.D.A. and H.M. performed statistical analyses. C.D.A. and M.M. wrote the manuscript. This work was supported by NIH grants R01-AI100724 and the Tulane National Primate Research Center grant 2P51-OD11104-52.
Conflicts of Interest and Source of Funding: W.R.S. is a full time employee of, and holds shares in ViiV Healthcare, Z.H. is a full time employee of, and holds shares in GlaxoSmithKline and serves on the ViiV Healthcare Board. D.D.H. is a paid consultant to GlaxoSmithKline and M.M. receives grants from GlaxoSmithKline. This work was supported by NIH grants R01-AI100724 and the Tulane National Primate Research Center grant 2P51-OD11104-52.
Footnotes
Data presented previously at Conference on Retroviruses and Opportunistic Infections (CROI) in Boston, MA on February 22–26, 2016.
References
- 1.Crime UNOoDa. World Drug Report 2014. 2014 [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 5.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 6.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spreen W, Min S, Ford SL, Chen S, Lou Y, Bomar M, et al. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials. 2013;14:192–203. doi: 10.1310/hct1405-192. [DOI] [PubMed] [Google Scholar]
- 9.Spreen W, Ford SL, Chen S, Wilfret D, Margolis D, Gould E, et al. GSK1265744 pharmacokinetics in plasma and tissue after single-dose long-acting injectable administration in healthy subjects. J Acquir Immune Defic Syndr. 2014;67:481–486. doi: 10.1097/QAI.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 10.Spreen W, Williams P, Margolis D, Ford SL, Crauwels H, Lou Y, et al. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr. 2014;67:487–492. doi: 10.1097/QAI.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 11.Margolis DA, Brinson CC, Smith GH, de Vente J, Hagins DP, Eron JJ, et al. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15:1145–1155. doi: 10.1016/S1473-3099(15)00152-8. [DOI] [PubMed] [Google Scholar]
- 12.Margolis DA, Gonzalez-Garcia J, Stellbrink H-J, Eron JJ, Yazdanpanah Y, Griffith S, et al. Cabotegravir+Rilpivirine as Long-Acting Maintenance Therapy: LATTE-2 Week 32 Results. Conference on Retroviruses and Opportunistic Infections; 2016; Boston, MA. [Google Scholar]
- 13.Markowitz M, Frank I, Grant R, Mayer KH, Margolis DA, Hudson KJ, et al. ÉCLAIR: Phase 2A Safety and PK Study of Cabotegravir LA in HIV-Uninfected Men. Conference on Retroviruses and Opportunistic Infections; 2016; Boston, MA. [Google Scholar]
- 14.Andrews CD, Spreen WR, Mohri H, Moss L, Ford S, Gettie A, et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science. 2014;343:1151–1154. doi: 10.1126/science.1248707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radzio J, Spreen W, Yueh YL, Mitchell J, Jenkins L, Garcia-Lerma JG, et al. The long-acting integrase inhibitor GSK744 protects macaques from repeated intravaginal SHIV challenge. Sci Transl Med. 2015;7:270ra275. doi: 10.1126/scitranslmed.3010297. [DOI] [PubMed] [Google Scholar]
- 16.Andrews CD, Yueh YL, Spreen WR, St Bernard L, Boente-Carrera M, Rodriguez K, et al. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med. 2015;7:270ra274. doi: 10.1126/scitranslmed.3010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. American Journal of Epidemiology. 1938;27:493–497. [Google Scholar]
- 18.UNAIDS. The Gap Report. 2014 [Google Scholar]
- 19.Tsai CC, Follis KE, Sabo A, Beck TW, Grant RF, Bischofberger N, et al. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 20.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28:1509–1519. doi: 10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.