Abstract
Introduction
The impact of selective surgical resection for esophageal cancer patients treated with definitive chemoradiation has not been clearly evaluated long-term.
Methods
NRG Oncology RTOG 0246 was a multi-institutional, single arm, open-label, non-randomized phase II study, which enrolled forty-three patients from Sept 2003 to March 2008 with clinical stage T1-4N0-1M0 squamous cell or adenocarcinoma of the esophagus or gastroesophageal junction (GEJ) from 19 sites. Patients (pts) received induction chemotherapy with 5-FU (650 mg/m2/d), cisplatin (15 mg/m2/d) and paclitaxel (200 mg/m2/d) for 2 cycles followed by concurrent chemoradiation with 50.4 Gy (1.8 Gy/Fx) and daily 5-FU (300 mg/m2/d) with cisplatin (15 mg/m2/d) over the first five days. Following definitive chemoradiation pts were evaluated for residual disease. Selective esophagectomy was considered only for pts with residual disease after chemoradiation (Clin Non-CR) or recurrent disease on surveillance.
Results
This report looks at the long-term outcome of this selective surgical strategy. With a median follow-up of 8.1 yrs (for 12 alive patients, min-max: 7.2 – 9.8 yrs), the estimated 5 and 7-yr survival rates are 36.6% (CI, 22.3%-51.0%) and 31.7% (CI, 18.3%-46.0%). Clin CR was achieved in 15 pts (37%) with 5 and 7-yr survival rates of 53.3% (CI, 26.3%-74.4%) and 46.7% (CI, 21.2%-68.7%). Esophageal resection was not required in 20/41 pts (49%) on this trial.
Conclusions
The long-term results of NRG Oncology RTOG 0246 demonstrate promising efficacy of a selective surgical resection strategy and suggest the need for larger randomized studies to further evaluate this organ preserving approach.
Keywords: esophageal cancer, selective esophagectomy, definitive chemoradiation
Introduction
Radiation Therapy Oncology Group (RTOG) trial RTOG 85-01 demonstrated long-term survival with a predominantly non-operative approach for non-metastatic squamous cell carcinoma of the esophagus treated with cisplatin, 5-FU and 50.4 Gy of concurrent radiation.1 Subsequent attempts by RTOG to decrease the high locoregional relapse rate (40-60%) of this non-operative approach with induction chemotherapy and higher doses of radiation therapy (INT 122, 123) resulted only in increased toxicity without any improvement in survival or locoregional relapse (60%).2,3 NRG Oncology RTOG 0246 was therefore put forward as an esophageal preserving selective surgical strategy to address this high locoregional relapse rate with surgery for clinical non-complete response patients. Although encouraging the preliminary results of NRG Oncology RTOG 0246 (1-yr 71%; CI: 54%, 82%) did not achieve the target goal of 77.5%.4 The long-term results of this organ preserving selective surgical strategy are now the focus of this report.
Materials and Methods
Protocol Population
Patients were eligible for the trial if they had non-metastatic squamous cell or adenocarcinoma of the esophagus or gastroesophageal junction (GEJ) with greater than 2 cm of gastric involvement. Adequate bone marrow, liver and renal functions and the ability to tolerate surgical resection with a Zubrod performance status of 0 or 1 were required for entry. Patients were also required to have greater than a cT1N0 on EUS. Exclusion criteria included celiac adenopathy (>2 cm) or supraclavicular adenopathy unless biopsy proof of “no cancer” was obtained prior to study entry.
Pretreatment Assessment
All patients were assessed by a multi-disciplinary team consisting of a medical, surgical and radiation oncologist prior to study entry. Computed tomography (CT) of the chest and abdomen, endoscopy (EGD) and endoscopic ultrasound (EUS) were required. Bronchoscopy was performed for tumors <25 cms from incisors. CT/PET was optional but strongly encouraged. All institutions obtained institutional review board approval prior to patient recruitment and all patients signed approved informed consents prior to trial enrollment.
Therapy
Induction Chemotherapy
Induction chemotherapy consisted of two cycles of continuous 5-FU (650 mg/m2/day) and intravenous 1 hour infusion cisplatin (15 mg/m2/day) on days 1 to 5 and 29 to 33; and paclitaxel (200 mg/m2/day) as a 2 hour infusion on days 1 and 29. Pegylated G-CSF (6 mg) was administered on days 6 and 34 or G-CSF (300 or 480 μg) was administered on days 6 to 15 and 34-42.
Chemoradiotherapy
Chemoradiotherapy was begun upon completion of induction chemotherapy on day 58 of the protocol. The total radiotherapy dose of 50.4 Gy was delivered in 28 daily fractions (1.8 Gy/Fx) 5 days/week. Simulation utilized CT, EGD and esophagography to determine boundaries of the carcinoma. Three-dimensional conformal radiotherapy was used but intensity-modulated radiotherapy was not allowed. Borders of the field were as previously described. Concurrent outpatient chemotherapy consisted of 5-FU (300 mg/m2/day) as continuous infusion for 5 days per week during radiotherapy; cisplatin was administered at 15 mg/m2/day as 1 hour infusion on day 1 to 5 of radiotherapy; dose modification (only radiation) were allowed as previously described.4
Assessment for Clinical CR
Six to eight weeks following completion of chemoradiation, patients were assessed for clinical complete response (Clin CR) with EGD, EUS, CT of the chest and abdomen and CT/PET (optional but highly encouraged). Patients were also evaluated by a multi-disciplinary team of medical, surgical and radiation oncologists. Clinical CR was defined as no evidence of increased mass or metastases on CT of the chest and abdomen, negative biopsy on repeat EGD and resolution of FDG activity if CT/PET obtained. All patients with suspected residual disease in the esophagus and/or locoregional lymph nodes (Clin Non-CR) and no metastatic disease that were physiologically fit underwent selective esophageal resection of both the esophagus and locoregional lymph nodes at that time.
Patients who were felt to be Clin CR were carefully followed without surgical resection with a history and physical examination, serum chemistry profile, CT scan of the chest and abdomen, endoscopic biopsy, endoscopic ultrasound and PET scan (optional but encouraged) (3 months × 2, 6 months × 3, then yearly). During surveillance selective esophageal resection of both the esophagus and locoregional lymph nodes was considered for patients who developed recurrent disease in the esophagus and/or locoregional lymph nodes who did not have systemic disease and were physiologically fit for surgery.
Statistical Analysis
The primary end point of the study was 1-year overall survival for all patients eligible for analysis (biopsy proven non-metastatic resectable squamous cell or adenocarcinoma of esophagus or gastro-esophageal junction with celiac adenopathy (<2cm) and no supraclavicular adenompathy who were physiologically fit for surgery). Secondary endpoints included disease-free survival (failure includes local, regional, and distant failure, as well as death due to any cause) and feasibility of a selective approach with induction chemotherapy, concurrent chemoradiation and selective surgical resection. On the basis of a 1-year survival rate of 60% from the RTOG esophageal database, it was decided that a one-year survival rate of 77.5% or better was needed for the trial to be deemed promising enough for study in a phase III protocol (≈ hazard reduction of 50% with type I error of 0.05 and type II error of 0.20). Adjusting this figure by 10% to account for patient ineligibility or loss, a total sample size of 42 patients was estimated to be required for this study. The data provided in this report are as of May 15, 2014. The outcome endpoints are calculated from date of trial registration. Time to distant failure (DF) was estimated by the cumulative incidence method.5 Failure for overall survival (OS) was death as a result of any cause. OS rates were estimated univariately with the Kaplan-Meier method.6 All analyses were performed using SAS/STAT® software.
Results
NRG Oncology RTOG 0246 opened September 5, 2003 and closed on March 17, 2006 after accruing a total of 43 patients (pts). Two patients were ineligible for analysis because one patient never had bronchoscopy and the second patient had unverifiable histology. The median follow-up for the 12 pts still alive at the time of this analysis is 8.10 years (min-max: 7.23-9.81 yrs). Pretreatment characteristics have been previously reported.4 At the completion of definitive chemoradiation, 15 pts were classified as Clin CR (15/36 =42%) and 21 pts (21/36= 58%) were classified as Clin Non-CR by a multi-disciplinary group of surgical, medical and radiation oncologists. The majority of patients did not receive CT-PET in their surveillance making interpretation of the benefit of this modality for decision making of residual disease difficult. Clinical factors associated with treatment response are listed in Table 1.
Table 1. Clinical Factors by Treatment Response (n=36).
| Clinical CR (n=15) | Clinical Non-CR (n=21) | Fisher's Exact Test p-value | |
|---|---|---|---|
| Length of Primary (cm) | n=15 | n=20* | |
| Mean | 4.57 | 5.39 | |
| Std. Dev. | 2.16 | 2.12 | |
| Median | 4.1 | 5.0 | |
| Min - Max | 2.0 – 8.0 | 3.0 – 9.0 | |
| Q1 - Q3 | 2.0 – 7.0 | 3.9 – 7.0 | |
| T-Stage (clinical) | |||
| T1/T2 | 7 (47%) | 3 (14%) | 0.06 |
| T3/T4 | 8 (53%) | 18 (86%) | |
| N-Stage (clinical) | |||
| N0 | 6 (40%) | 6 (30%) | 0.50 |
| N1 | 9 (60%) | 15 (71%) | |
| AJCC 6th Edition | |||
| IIA/IIB | 10 (67%) | 7 (33%) | 0.09 |
| III | 5 (33%) | 14 (67%) | |
| Histology | |||
| Adenocarcinoma | 10 (67%) | 16 (76%) | 0.71 |
| Squamous Cell | 5 (33%) | 5 (24%) |
Q1 = first quartile; Q3 = third quartile
One patient did not have their size reported on the case report form.
Long-Term Survival and Morbidity
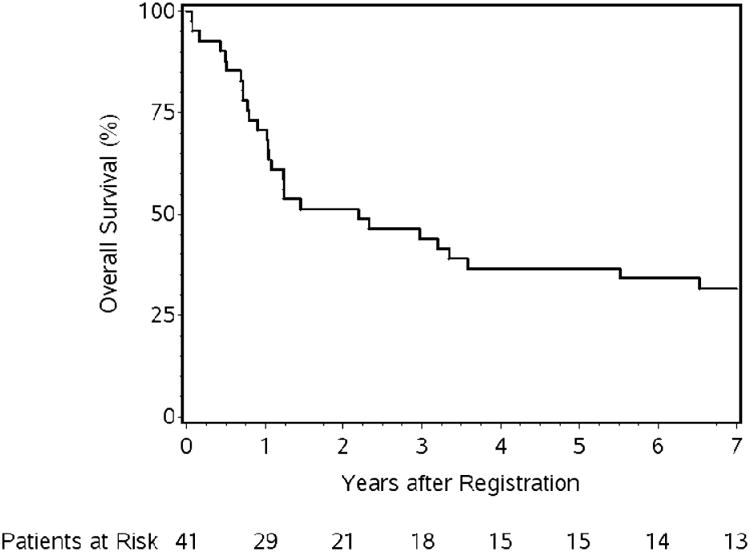
As Figure 1 demonstrates, 41 pts were eligible for analysis. A total of four treatment-related deaths (grade 5s) were noted (9.8%).4 Two patients during induction chemotherapy (1 pneumonia, 1 multi-organ failure), one patient after surgery (esophageal leak), and one patient 179 days after the start of chemoradiation from pneumonitis. Long-term OS of all pts (n=41, 29 deaths) at 5 and 7-year (yr) is 36.6% (95% Confidence Interval (CI), 22.3%-51.0%) and 31.7% (CI, 18.3%-46.0%), respectively (Table 2, Figure 2). The causes of death include the following: due to disease (n=14), complication of protocol treatment (n=4, mentioned above), other cause (n=5, majority being cardiopulmonary arrest), and unknown (n=6). Clin-CR pts (n=15, 41%) demonstrated 5 and 7-yr OS of 53.3% (CI, 26.3%-74.4%) and 46.7% (CI, 21.2%-68.7%). Six Clin-CR pts are still alive and free of disease (4 without esophagectomy, 2 following salvage esophageal resection). Out of the 11 Clin-CR pts who did not have surgery, 7 patients have died with the following causes of death: due to disease (n=2), other cause (n=1, hemorrhaging), and unknown (n=4). Clin-Non-CR pts (n=21, 57%) demonstrated 5 and 7-yr OS of 33.3% (CI, 14.9%-53.1%) and 28.6% (CI, 11.7%-48.2%) (online: Figure A). Seventeen of these pts underwent selective esophagectomy for suspected local residual disease with 5 and 7-yr OS of 41.2% (CI, 18.6%-62.6%) and 35.3% (CI, 14.5%-57.0%). Survival is similar by histology (Table 2). There was no statistically significant association with histology and Clin-CR status (Table 1) after definitive chemoradiation.
Figure 1. NRG Oncology RTOG 0246 CONSORT Diagram.
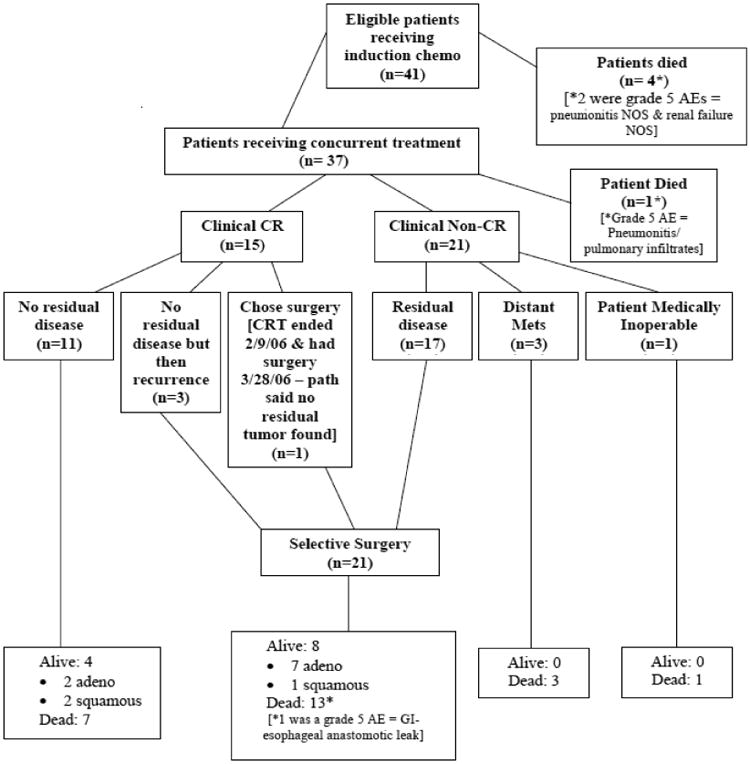
Table 2. Overall Survival by Treatment Response and Histology.
| Years | All Patients | Clinical CR | Clinical Non-CR | Clinical Non-CR Surgery |
Adenocarcinoma | Squamous Cell | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # at risk |
%Alive (95% CI) |
# at risk |
%Alive (95% CI) |
# at risk |
%Alive (95% CI) |
# at risk |
%Alive (95% CI) |
# at risk |
%Alive (95% CI) |
# at risk |
%Alive (95% CI) |
|
| 0 | 41 | 100 | 15 | 100 | 21 | 100 | 17 | 100 | 30 | 100 | 11 | 100 |
| 1 | 29 | 70.7 (54.3, 82.2) | 14 | 93.3 (61.3, 99.0) | 15 | 71.4 (47.2, 86.0) | 15 | 88.2 (60.6, 96.9) | 21 | 70.0 (50.3, 83.1) | 8 | 72.7 (37.1, 90.3) |
| 2 | 21 | 51.2 (35.1, 65.2) | 11 | 73.3 (43.6, 89.1) | 10 | 47.6 (25.7, 66.7) | 10 | 58.8 (32.5, 77.8) | 16 | 53.3 (34.3, 69.1) | 5 | 45.5 (16.7, 70.7) |
| 3 | 18 | 43.9 (28.6, 58.2) | 10 | 66.7 (37.5, 84.6) | 8 | 38.1 (18.3, 57.8) | 8 | 47.1 (23.0, 68.0) | 13 | 43.3 (25.6, 65.2) | 5 | 45.5 (16.7, 70.7) |
| 4 | 15 | 36.6 (22.3, 51.0) | 8 | 53.3 (26.3, 74.4) | 7 | 33.3 (14.9, 53.1) | 7 | 41.2 (18.6, 62.6) | 11 | 36.7 (20.1, 53.4) | 4 | 36.4 (11.2, 62.7) |
| 5 | 15 | 36.6 (22.3, 51.0) | 8 | 53.3 (26.3, 74.4) | 7 | 33.3 (14.9, 53.1) | 7 | 41.2 (18.6, 62.6) | 11 | 36.7 (20.1, 53.4) | 4 | 36.4 (11.2, 62.7) |
| 6 | 14 | 34.1 (20.3, 48.5) | 7 | 46.7 (21.2, 68.7) | 7 | 33.3 (14.9, 53.1) | 7 | 41.2 (18.6, 62.6) | 10 | 33.3 (17.5, 50.0) | 4 | 36.4 (11.2, 62.7) |
| 7 | 13 | 31.7 (18.3, 46.0) | 7 | 46.7 (21.2, 68.7) | 6 | 28.6 (11.7, 48.2) | 6 | 35.3 (14.5, 57.0) | 10 | 33.3 (17.5, 50.0) | 3 | 27.3 (6.5, 53.9) |
| Total dead | 29/41 | 9/15 | 15/21 | 11/17 | 21/30 | 8/11 | ||||||
| MST (95% CI) | 2.18 (1.04, 5.51) | 5.51 (1.24, nc) | 1.24 (0.8, 6.53) | 2.97 (1.04, nc) | 2.25 (1.04, 8.04) | 1.45 (0.49, nc) | ||||||
Abbreviations: nc = not calculated due to right censoring; MST, median survival time
Figure 2. Overall survival for the 41 eligible patients.
Patterns of Failure
Twenty-one pts (57%) demonstrated locoregional relapse following definitive chemoradiation. Three Clin-CR pts developed recurrent locoregional disease on surveillance and underwent selective esophageal resection (5.3 to 14.9 months after completion of definitive chemoradiation). Eighteen Clin-Non CR pts had residual locoregional disease immediately after definitive chemoradiation (17 underwent surgical resection and all had residual local disease in their resected specimen, 1 pt was medically inoperable). Distant failure for all pts at both 5 and 7 yrs was 31.7% (CI, 17.2%-46.2%) (online: Figure B).
Discussion
NRG Oncology RTOG 0246 attempts to determine in a predominantly adenocarcinoma population if selective surgical resection after definitive chemoradiation is feasible. The long-term results of this study (5 and 7-year survival) suggest that this strategy is not only feasible but may also confer benefit over definitive chemoradiation alone with prolonged follow-up. The cause of death in the Clin CR non-surgical patients demonstrated only a minority due to disease progression suggesting observation may be reasonable for this subset of patients. The 5 and 7-year survival achieved in NRG Oncology RTOG 0246 appears greater than the 5 and 7-year survival of RTOG 85-01 (37% and 32% vs. 20% and 14%)1,4 and are similar to recent definitive chemoradiation studies (INT 123, PRODIGES5/ACCORD17, SCOPE1) with 3-year survival rates of 28%, 27%, and 28%, respectively, suggesting that the improved outcomes noted in NRG Oncology RTOG 0246 are not due solely to the more recent time period or improved staging of the current study.4,7,8
Long-term distant failure is similar between NRG Oncology RTOG 0246 and RTOG 85-01 with a 7-year failure rate of 32%.3,4 The possibility therefore exists that the prolonged survival noted in NRG Oncology RTOG 0246 may be due to improved locoregional control achieved through improved chemoradiation strategies. NRG Oncology RTOG 0246 utilized chemotherapy prior to chemoradiation and this strategy has been associated with a nonsignificant trend toward increased pathologic CR (pathCR) in a randomized phase II study.9 The addition of taxanes to cisplatin and 5-flouracil has also demonstrated high pathCR in several studies.10
The use of selective surgery for Clin-Non CR patients may also have contributed to the improved long-term results of NRG Oncology RTOG 0246. Two randomized European studies have been completed comparing chemoradiation and surgery to definitive chemoradiation alone. These studies suggest that surgery does not benefit clinical responders to a large degree. In the Fondation Francaise de Cancerologie Digestive (FFCD) trial 9901, only clinical responders were randomized and there was no benefit in the addition of surgery.11 In the German study, all patients were randomized but only the subset of clinical non-responders appeared to benefit from the addition of surgery (3-yr survival of 18% vs. 9%).12 In the German trial, clinical non-responders in which an R0 resection was able to be achieved were noted to have a 3-year survival of 32%. These observations suggest that selective surgical resection targeting clinical non-responders may allow selective use of surgical resection for the group of patients most in need of locoregional control.
Another important difference between NRG Oncology RTOG 0246 and other definitive chemoradiation trials (RTOG 85-01, INT 123, SCOPE1) is the higher proportion of adenocarcinoma (73% vs. 15-25%).1,3,4,7,8 Cooper et al. noted that shorter survival was associated with adenocarcinoma patients compared to squamous cell carcinoma after definitive chemoradiation with 5-year survival rates of 13% vs. 21% noted in squamous cell carcinoma.1 The long-term results of NRG Oncology RTOG 0246 suggest that there is no difference between adenocarcinoma and squamous cell cancer patients and both can both be approached in a selective fashion. As Table 1 demonstrates, histology does not appear associated with treatment response and 5 and 7-year survival rates are similar between the groups (Table 2). This is an important observation since an increasing proportion of patients in Western countries are presenting with adenocarcinoma of the esophagus or gastroesophageal junction.13
NRG Oncology RTOG 0246 utilized an esophageal preserving strategy of selective esophageal resection. Following definitive chemoradiation a large portion of patients are Clin Non-CR (Figure 1, 57%). Of those pts who are Clin Non-CR over 80% can be selectively resected with minimal morbidity at high volume centers. All 17 patients taken immediately to surgery for suspected residual disease demonstrated viable tumor in the resected specimen. Long-term survival of this group (Clin Non-CR Surgery) was encouraging with 5 and 7-year survival rates of 41% and 35%, respectively. In the Clin-CR group (n=15), long-term survival was even better with 5 and 7-year rates of 53% and 47% with only 3 patients requiring salvage surgery at a later time for recurrent disease (Figure 1). The benefits of an esophageal preserving strategy include reduced short-term morbidity and mortality from surgery and possible reduced long-term consequences of esophageal resection including reflux, dumping, and dysphagia. 12 Some authors have argued that salvage esophageal resection is associated with increased morbidity and therefore surgical resection should always be performed in a planned fashion. In this study, only 3 patients underwent salvage resection (esophageal resection for recurrent disease identified on follow-up) and there was no operative mortality or esophageal anastomotic leak noted. It is possible that salvage resection in adenocarcinoma populations is associated with lower risk and that techniques have improved over time reducing the risk of salvage resections. MD Anderson Cancer Center recently reported 65 adenocarcinoma patients who underwent salvage resection with an operative mortality similar to planned esophagectomy (5% vs. 3%) suggesting that selective surveillance and salvage can be accomplished with acceptable outcomes in the modern era when performed at a high volume institution on adenocarcinoma populations.14
NRG Oncology RTOG 0246 demonstrates the feasibility and encouraging long-term survival achievable with an esophageal preserving strategy of selective surgical resection after definitive chemoradiation. The question remains whether this strategy is appropriate for all patients with esophageal cancer including squamous cell cancer and adenocarcinoma populations. Table 3 suggests that the long-term survival of NRG Oncology RTOG 0246 is comparable to most trimodality studies including CALGB 9781 and the Urba et al. study.15,16 Only the recently reported CROSS study with 3-year survival of 60% with chemoradiation and surgery demonstrate significantly greater survival than this study at early time points.17
Table 3. Esophageal Multimodality Trials.
In summary, NRG Oncology RTOG 0246 demonstrates the feasibility of and promising survival with an organ preserving selective surgical resection approach. At the present time, a selective approach appears warranted in high risk patients but the question of the optimal approach in good risk populations is still debatable. In the future, selective surgical resection may be further enhanced by molecular markers, clinical factors and non-invasive imaging modalities such as CT-PET to help delineate the patients benefitting from esophageal resection.18,19
Supplementary Material
Figure A: Overall survival for clinical CR and clinical non-CR patients
Figure B: Distant Failure
Acknowledgments
The authors thank Wanda Reese for assistance in preparing the manuscript.
Administrative support: Jaffer A. Ajani, Jennifer Moughan, Christopher G. Willett.
Collection and assembly of data: Kathryn Winter, Ritsuko Komaki (Radiotherapy: QA)
Data analysis and interpretation: Stephen G. Swisher, Jennifer Moughan, Kathryn Winter.
Manuscript writing: Stephen G. Swisher, Jennifer Moughan, Ritsuko R. Komaki, Jaffer A. Ajani, Tsung T. Wu, Wayne Hofstetter, A. Konski, Christopher G. Willett.
Conception and design: Stephen G. Swisher, Jaffer A. Ajani, Ritsuko Komaki, Tsung T. Wu, Christopher G. Willett.
This project was supported by grants U10CA21661, U10CA37422, U10CA180868, and U10CA180822 from the National Cancer Institute (NCI).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of Locally Advanced Esophageal Cancer: Long-term Follow-up of a Prospective Randomized Trial (RTOG 85-01) JAMA. 1999;281:1623–1626. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 2.Minsky BD, Neuberg D, Kelsen DP, et al. Neoadjuvant Chemotherapy Plus Concurrent Chemotherapy and High-Dose Radiation for Squamous Cell Carcinoma of the Esophagus: A Preliminary Analysis of the Phase II Intergroup Trial 0122. J Clin Oncol. 1996;14:149–155. doi: 10.1200/JCO.1996.14.1.149. [DOI] [PubMed] [Google Scholar]
- 3.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05)Phase III Trial of Combined-Modality Therapy for Esophageal Cancer: High-Dose Versus Standard-Dose Radiation Therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 4.Swisher SG, Winter KA, Komaki RU, et al. A PhaseII Study of a Paclitaxel-Based Chemoradiation Regimen with Selective Surgical Salvage for Resectable Locoregionally Advanced Esophageal Cancer: Initial Reporting of RTOG 0246. Int J Radiat Oncol Biol Phys. 2012;82:1967–1972. doi: 10.1016/j.ijrobp.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York, NY: John Wiley & Sons; 1980. pp. 167–169. [Google Scholar]
- 6.Kaplan EL, Meier P. Non-parametric estimation from in complete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 7.Conroy T, Galais MP, Raoul JL, et al. Definitive Chemoradiotherapy with FOLFOX versus Fluorouracil and Cisplatin in Patients with Oesophageal Cancer (PRODIGE5/ACCORD17): Final Results of a Randomised Phase 2/3 Trial. Lancet Oncol. 2014;15:305–314. doi: 10.1016/S1470-2045(14)70028-2. [DOI] [PubMed] [Google Scholar]
- 8.Crosby T, Hurt CN, Falk S, et al. Chemoradiotherapy with or without Cetuximab in Patietns with Oesophageal Cancer (SCOPE1): A Multicentre, Phase 2/3 Randomised Trial. Lancet Oncol. 2013;14:627–637. doi: 10.1016/S1470-2045(13)70136-0. [DOI] [PubMed] [Google Scholar]
- 9.Ajani JA, Xiao L, Roth JA, et al. A phase II randomized trial of induction chemotherapy versus no induction chemotherapy followed by preoperative chemoradiation in patients with esophageal cancer. Annals of Oncol. 2013;11:2844–2849. doi: 10.1093/annonc/mdt339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasini F, de Manzoni G, Zanoni A, et al. Neoadjuvant therapy with weekly docetaxel and cisplatin, 5-fluorouracil continuous infusion, and concurrent radiotherapy in patients with locally advanced esophageal cancer produced a high percentage of long-lasting pathologic complete response. Cancer. 2013;119:939–945. doi: 10.1002/cncr.27822. [DOI] [PubMed] [Google Scholar]
- 11.Mariette C, Dahan L, Mornex F, et al. Surgery Alone Versus Chemoradiotherpy Followed by Surgery for Stage I and II Esophageal Cancer: Final Analysis of Randomized controlled Phase III Trial FFCD 9901. J Clin Oncol. 2014;32:1–10. doi: 10.1200/JCO.2013.53.6532. [DOI] [PubMed] [Google Scholar]
- 12.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 13.Blot WJ, Devesa SS, Kneller RWm, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 14.Marks JL, Hofstetter W, Correa AM, et al. Salvage Esohagectomy after Failed Definitive Chemoradiation for Esophageal Adenocarcinoma. Ann Thorac Surg. 2012;94:1126–1133. doi: 10.1016/j.athoracsur.2012.05.106. [DOI] [PubMed] [Google Scholar]
- 15.Tepper J, Krasn MJ, Niedzwiecki D, et al. Phase III Trial of Trimodality Therapy with Cisplatin, Fluorouracil, Radiotherapy, and Surgery Compared With Surgery Alone for Esophageal Cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urba SG, Orringer MB, Turrisi A, et al. Randomized Trial of Preoperative Chemoradiation Versus Surgery Alone in Patients With Locoregional Esophageal Carcinoma. J Clin Oncol. 2001;19:305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 17.van Hagen P, Hulshoff MCCM, van Lanschot JJB, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 18.Swisher SG, Maish M, Erasmus JJ, et al. Utility of PETCT EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg. 2004;78:1152–1160. doi: 10.1016/j.athoracsur.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 19.Amini A, Ajani J, Komaki R, et al. Factors Associated with Local-Regional Failure After Definitive Chemoradiation for Locally Advanced Esophageal Cancer. Ann Surg Oncol. 2014;21:306–314. doi: 10.1245/s10434-013-3303-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A: Overall survival for clinical CR and clinical non-CR patients
Figure B: Distant Failure


