Abstract
Listeria monocytogenes, the causative agent of listeriosis, can build up to dangerous levels in refrigerated foods potentially leading to expensive product recalls. An important aspect of the bacterium’s growth at low temperatures is its ability to increase the branched-chain fatty acid anteiso C15:0 content of its membrane at lower growth temperatures, which imparts greater membrane fluidity. Mutants in the branched-chain α-keto dehydrogenase (bkd) complex are deficient in branched-chain fatty acids (BCFAs,) but these can be restored by feeding C4 and C5 branched-chain carboxylic acids (BCCAs). This suggests the presence of an alternate pathway for production of acyl CoA precursors for fatty acid biosynthesis. We hypothesize that the alternate pathway is composed of butyrate kinase (buk) and phosphotransbutyrylase (ptb) encoded in the bkd complex which produce acyl CoA products by their sequential action through the metabolism of carboxylic acids. We determined the steady state kinetics of recombinant His-tagged Buk using 11 different straight-chain and BCCA substrates in the acyl phosphate forming direction. Buk demonstrated highest catalytic efficiency with pentanoate as the substrate. Low product formation observed with acetate (C2) and hexanoate (C6) as the substrates indicates that Buk is not involved in either acetate metabolism or long chain carboxylic acid activation. We were also able to show that Buk catalysis occurs through a ternary complex intermediate. Additionally, Buk demonstrates a strong preference for BCCAs at low temperatures. These results indicate that Buk may be involved in the activation and assimilation of exogenous carboxylic acids for membrane fatty acid biosynthesis.
1. Introduction
Listeria monocytogenes, is the dangerous foodborne pathogen that causes listeriosis, a disease characterized by gastroenteritis, meningitis, spontaneous miscarriages and high mortality rate among infected individuals [1]. L. monocytogenes grows actively at temperatures as low as–0.1 °C, building up to dangerous levels, which is enabled by its highly fluid membrane [2]. The cellular membrane of L. monocytogenes is enriched with branched-chain fatty acids (BCFAs) which have greater cross sectional area, disrupt the close packing of membrane fatty acids and thus increase the fluidity of the membrane [3,4]. L. monocytogenes further increases the membrane content of anteiso C15:0, a survival strategy which supports its growth at low temperatures [2,5,6]. Expensive food product recalls due to outbreaks of Listeria underscore the growing need for better control strategies for this organism (http://www.cdc.gov/listeria/outbreaks).
BCFAs, which make up >90% of membrane fatty acid content of L. monocytogenes, are biosynthesized by the activity of branched-chain amino transferase (Bcat) and branched-chain α-keto acid dehydrogenase (Bkd) on branched chain amino acids (BCAAs), followed by elongation of the catabolic products by the bacterial fatty acid biosynthesis pathway (FAS II) [7]. Disruption of this pathway, as in the case of mutants of L. monocytogenes lacking functional Bkd (cld-1, cld-2/MOR401), results in reduced fitness and impairment of the homeoviscous adaptation strategies employed by the organism when exposed to low growth temperatures [4,5,8,9]. Such mutants are deficient in membrane BCFA content, unable to grow at low temperatures, and are significantly affected by unfavorable environmental conditions [4,5,8,10]. Addition of 2-methylbutyrate, the precursor of anteiso C15:0, restores the membrane BCFA content and thus rescues the growth of the mutant and leads to greater survival in macrophages and at low temperatures, as well as greater tolerance for acidity and alkalinity [4,9–12]. Restoration of membrane BCFA content by the addition of 2-methylbutyrate has also been observed in bkd mutants of Staphylococcus aureus and Bacillus subtilis [13,14].
Comprehensive investigation of media supplementation conducted by Kaneda [15] in B. subtilis and Sen et al. [16] in L. monocytogenes show that a wide range of carboxylic acids, which include straight chain carboxylic acids (SCCAs) such as propionate and butyrate, and branched-chain carboxylic acids (BCCAs, including 2-methylbutyrate and isobutyrate) are capable of alteration of membrane fatty acid composition. Additionally, supplementation of unnatural BCCAs such as 2-ethylbutyrate and 2-methylpentanoate result in the incorporation of novel fatty acids in the membrane arising from these compounds [11,15,16]. Since the only known source of BCFAs is via biosynthesis, it is presumed that these compounds are converted into their thioester derivatives by a pathway hitherto uncharacterized, and are utilized as primers by β-keto acyl ACP synthase III (FabH), the enzyme catalyzing the first condensation step in FAS II pathway [17]. The alternate pathway, first suggested by Willecke and Pardee in B. subtilis [14], must therefore exhibit broad substrate specificity to fulfill the requirements of activation of such a large range of substrates.
Homology of the L. monocytogenes bkd operon to the bkd operon in Enterococcus faecalis led to the identification of two genes butyrate kinase (buk) and phosphotransbutyrylase (ptb), present upstream of the lpd gene in the bkd operon [18–20]. Ptb is known to catalyze the reversible conversion of acyl CoA compounds into their corresponding acyl phosphate derivatives [21]. Ptb from L. monocytogenes demonstrates broad substrate specificity and shows a strong preference for branched-chain substrates [22]. Buk catalyzes the phosphorylation of a variety of carboxylic acids and is a BCCA kinase in Spirochaeta isovalerica MA-2 [23,24]. Although the Ptb-Buk pathway has been shown to be the source of butyrate secretion in the important industrial organism Clostridium acetobutylicum, we hypothesize that presence of significant concentrations of exogenous carboxylic acids induces the formation of the acyl CoA products by the sequential activity of Buk and Ptb which are subsequently elongated by the FAS II pathway.
Buk is well characterized in the industrial fermentor, C. acetobutylicum, in which it is an important source of ATP during the acidogenesis stage of fermentation [24]. Other roles such as catabolism of branched-chain amino acids and ATP generation for survival have been attributed to Buk in E. faecalis and S. isovalerica [23,25]. We determined the substrate specificities of Buk by in vitro assay of the recombinant enzyme to investigate the potential role of Buk from L. monocytogenes in the phosphorylation of exogenous carboxylic acids for provision of precursors of fatty acid biosynthesis. Our results indicate that Buk is capable of conversion of a large number of carboxylic acids into their acyl phosphate derivatives.
2. Materials and methods
2.1 Materials
All the materials including antibiotics used as selection agents and acetate kinase were purchased from Sigma-Aldrich (St. Louis, MO).
2.2 Cloning and expression of Buk
Genomic DNA of L. monocytogenes 10403S, grown in Brain Heart Infusion (BHI) media (Becton Dickinson, Sparks, MD), was isolated using a Masterpure genomic DNA purification kit according to the manufacturer’s instructions (Epicenter, Madison, WI). The buk gene was amplified using a buk forward primer with a SacI restriction site (bukF 5′ ATGCGAGCTCATGTCTTTTGATGTTTT) and reverse primer with a PstI restriction site (bukR 5′ ATGCCTGCAGTTAGTACTCTTTTTCTT), which were designed based on the sequence of L. monocytogenes strain EGDe. The restriction sites were used for ligation of the buk gene into the expression vector pRSETa (Thermofisher, Waltham, MA) using T4 DNA ligase (Fermentas, Waltham, MA). Transformation of the plasmid pRSETa-buk into competent E. coli BL21 (DE3) cells was followed by confirmation of overexpression of the protein by Western blotting. DNA purification, ligation and transformation were performed according to the manufacturer’s instructions (Qiagen, Valencia, CA). Ampicillin (50μg/ml) was used as the selection agent for growth of pRSETa and pRSETa-buk carrying E. coli cells in Luria broth (Becton Dickinson, Sparks, MD). Overnight culture of cells carrying the pRSETa-buk vector was diluted into 500 ml of Luria broth until the OD600 reached 0.6. Addition of isopropyl β-D-thiogalactopyranoside (IPTG) to a final concentration of 3 mM was used to induce the overexpression of Buk and the culture was incubated at 37 °C with shaking at 200 rpm for 3 hours. Cell pellets were obtained by centrifugation at 4°C at 3,000 g and were stored at −80 °C until use.
2.3 Purification of Buk
The cell pellet was resuspended in 25 ml binding buffer (200 mM NaCl, 25 mM N-(2-Hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid) [HEPES], 1 mM MgCl2, 5 mM imidazole, 5% glycerol and 5 mM β-mercaptoethanol pH 7.5). The cells were broken using a French press at 16,000 psi and two passes through the machine. Cell debris was removed by centrifugation at 20,000 g for 30 min and the cell-free extract was allowed to bind by gravity flow with nickel-chelated nitrilotriacetic acid (Ni2+-NTA) resin (Thermo Scientific, Waltham, MA), which was pretreated with binding buffer. The resin was then washed with 5 column volumes of wash buffer (200 mM NaCl, 25 mM HEPES, 1 mM MgCl2, 10 mM imidazole, 5% glycerol and 5 mM β-mercaptoethanol, pH 7.5). The bound His6-tagged Buk was then eluted with 4 ml of elution buffer (200 mM NaCl, 25 mM HEPES, 1 mM MgCl2, 250 mM imidazole, 25% glycerol and 5 mM β-mercaptoethanol, pH 7.5). The concentration of protein was determined by the Bradford assay using bovine serum albumin as the standard (Biorad, Hercules, CA). Addition of β-mercaptoethanol (4 mM) and glycerol (25%) was essential for the catalytic activity of Buk. Analysis of the preparation of Buk by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) confirmed the calculated molecular mass of Buk (~ 42 kDa) and the purity of the enzyme preparation (Fig. 1).
Fig 1. Analysis of purified LmBuk by SDS-PAGE.
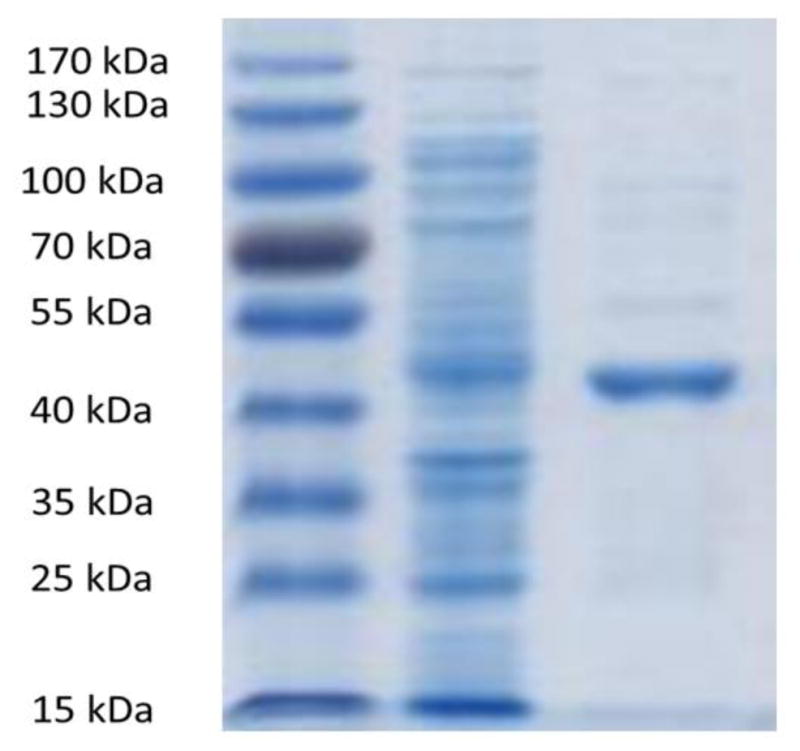
Samples were resolved on 10% (w/v) acrylamide gel and stained with Coomassie Blue. Lane 1 protein molecular weight markers, Lane 2 Cell-free lysate showing expression of LmBuk, Lane 3 Recombinant LmBuk after purification by Ni 2+-affinity chromatography.
2.4 Standardization of Buk assay
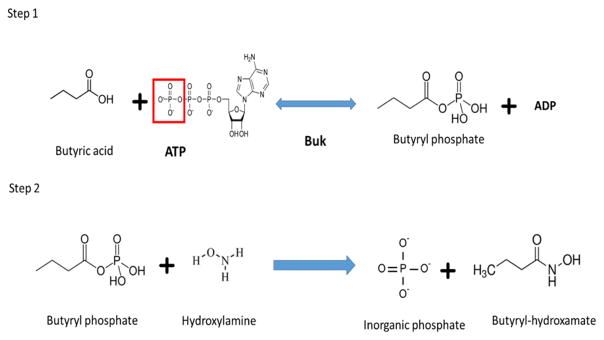
Buk activity was measured in the acyl phosphate forming direction based on the assay developed by Lipmann and Tuttle [26] as described by Hartmanis [24] with minor modifications. Buk catalyzes the reversible transfer of the γ-phosphate of ATP to the carbonyl group of the carboxylic acid resulting in the formation of the high energy compound acyl phosphate as the product (Fig. 2. step 1). The acyl phosphate product was quantitated by the inclusion of neutralized hydroxylamine in the reaction mixture thereby resulting in the formation of its corresponding stable acyl hydroxamate (Fig. 2. Step 2). Acyl hydroxamate in the presence of acidic ferric chloride forms a purple ferric hydroxamic acid complex which was quantified spectrophotometrically at 540 nm. Lipmann and Tuttle [26] analyzed a variety of compounds and showed that color formation was specific for acyl anhydride compounds and color intensity did not vary with differences in the chain length of the compounds.
Fig 2. Reaction catalyzed by Buk.
Step 1 shows the activity of Buk catalyzing the reversible transfer of the terminal phosphate moiety of ATP (highlighted in red) to the carbonyl group of butyric acid resulting in the formation of butyryl phosphate. Step 2 shows the irreversible conversion of the unstable butyryl phosphate to stable butyryl hydroxamate with the release of inorganic phosphate. Butyryl hydroxamate in the presence of acidic ferric chloride forms a purple complex which is quantified at 540 nm.
Commercial acetate kinase was utilized to standardize assay conditions. All assays of Buk activity were performed at 37 °C in a final volume of 200 μl. Buk-dependent formation of the corresponding acyl phosphate product was then studied in the presence of varying concentrations of butyrate and ATP. The assay medium contained 50 mM Tris-hydrochloride buffer (pH 7.5), 4 mM MnSO4, 10 mM ATP, 400 mM neutralized hydroxylamine, 5 mM β-mercaptoethanol and varying amounts of carboxylic acid (100 μM to 300 mM). The reaction was initiated with the addition of purified Buk (1–5 μM final concentration) and arrested by the addition of 40 μl of 50% trichloroacetic acid (TCA). The precipitated protein was removed by centrifugation at 13,000 g for 2 minutes and the supernatant was transferred to a fresh tube. Color was developed by the addition of 400 μl of 1.25% ferric chloride solution in 1M hydrochloric acid and the intensity was measured using a Beckman DU-65 spectrophotometer at 540nm. Initial reaction rates were calculated as the amount of product formed per minute. Acetyl phosphate at varying concentrations (250 μM to 5 mM) was used to determine a standard curve. Assay medium without Buk was utilized as the blank.
2.5 Kinetic analysis of Buk
Initial velocities of Buk in the presence of varying concentrations of the carboxylic acid substrates were determined and plotted as a function of the substrate concentration. Non-linear curve fitting of the data to the Michaelis-Menten equation, v = kcat * [S] / (K0.5 +[S]) where kcat is the turnover number and K0.5 is the substrate concentration that produces half maximal velocity and [S] is the substrate concentration. However, inhibition of product formation was observed at higher concentrations of substrate in the case of hexanoate, 2-ethylbutyrate, 2-methylpentanoate and 3-methylpentanoate. Steady state kinetic parameters were computed for these substrates using KaleidaGraph software by fitting the data to the Michaelis-Menten equation incorporating substrate inhibition, v = kcat * [S] / (K0.5 +[S]*(1+[S]/[Si])) where all variables are the same with the additional factor Si which is the substrate concentration that produces inhibition to half maximal velocity (Kaleidagraph, Synergy software, Reading, PA). Fits to this equation were used to determine the kinetic constants (K0.5 and kcat). At least three replicates of each assay were conducted and the results were presented as means ± SEM. The apparent K0.5 and kcat values were determined for a variety of straight-chain and branched-chain carboxylic acid compounds listed in Table 1.
Table 1.
Steady state kinetic analysis of LmBuk at 37°C.
| Substrate | kcat (min−1) | KM (mM) | kcat / KM (mM−1min−1) |
|---|---|---|---|
| Straight-chain | |||
| Propionate | 155.6 ± 0 | 174.1 ± 68.9 | 0.89 |
| Butyrate | 50.2 ± 0.4 | 50.5 ± 5.8 | 0.99 |
| Pentanoate | 71.7 ± 2.2 | 13.1 ± 3.3 | 5.5 |
| Hexanoate | 1310.9 ± 474.9 | 1856 ± 676 | 0.7 |
| Branched-chain | |||
| Iso butyrate | 23.7 ± 0.4 | 7.6 ± 1.3 | 3.1 |
| Isovalerate | 25 ± 0.1 | 57.1 ± 9.6 | 0.44 |
| 2-methylbutyrate | 15.9 ± 1.5 | 36.2 ± 5.2 | 0.44 |
| 2-ethyl butyrate | 6.7 ± 1.7 | 16.5 ± 0.9 | 0.4 |
| 2-methyl pentanoate | 18.8 ± 2.4 | 44.2 ± 8.5 | 0.4 |
| 3-methyl pentanoate | 21.2 ± 0.4 | 27.3 ± 0.8 | 0.8 |
Initial velocities were measured at 37 °C in the presence of 10 mM ATP at pH 7.5. The kcat and KM were calculated from the least squares fit of the experimental data from the different substrates to the Michaelis-Menten equation. The values indicated are the mean of experiments performed at least in triplicate ± SEM.
3 Results and Discussion
Butyrate kinase (EC 2.7.2.7) belongs to the ASKHA (acetate and sugar kinases / heat shock cognate / actin) superfamily of phosphotransferases and catalyzes the reversible phosphorylation of short-chain carboxylic acids in C. acetobutylicum [24]. The focus of this study was to investigate the substrate preferences of Buk from L. monocytogenes to determine if it was capable of phosphorylating the range of SCCAs and BCCAs which have induced alteration of the membrane fatty acid composition. To this end, Buk heterologously expressed in E. coli was purified by its N-terminal His-tag by affinity purification and assayed for activity by the hydroxamate method. Kinetic constants of Buk were determined in the presence of a saturating concentration of ATP (10 mM) and varying concentrations (0.1–300 mM) of various SCCAs and BCCAs.
3.1 Buk demonstrates broad substrate specificity
3.1.1 SCCAs
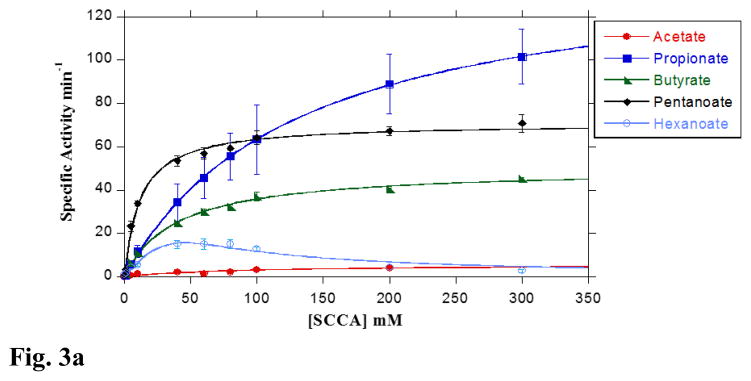
In order to understand the structural limits of substrates preferred by LmBuk, its activity was analyzed with several SCCAs with differing chain lengths (C2-C6). Among the SCCAs that were tested, Buk showed the highest catalytic efficiency (5.5 mM−1 min−1) and affinity for pentanoate (C5) with an apparent K0.5 of 13.1 mM. Butyrate (C4) also served as a good substrate with a corresponding K0.5 value of 50.5 mM (Fig. 3A & Table 1). Buk activity with propionate (C3) showed that substantial product formation was also associated with lower affinity (K0.5 =174.1 mM) compared to C4 and C5 SCCAs. The stronger preference for pentanoate demonstrated by LmBuk was similar to the branched-chain fatty acid kinase from S. isovalerica [23]. Additionally, the K0.5 values exhibited by LmBuk, were relatively higher than reported values for SiBuk utilizing butyrate and propionate (Fig. 3A & Table 1) [23,24].
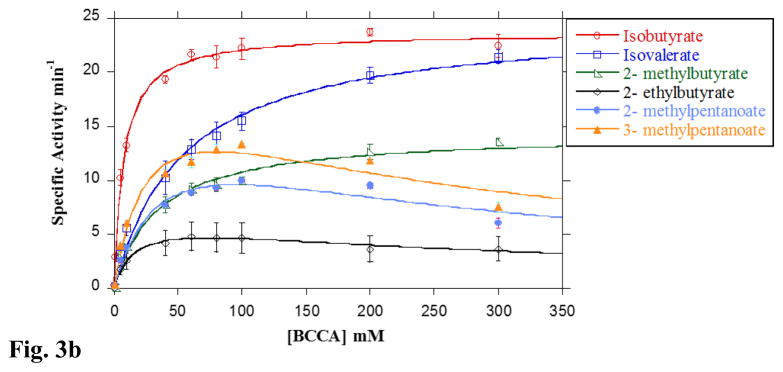
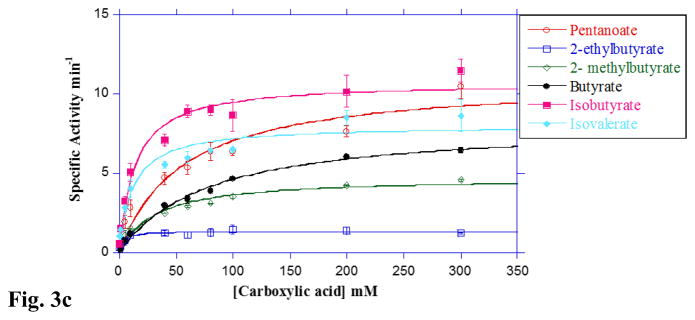
Fig 3. Substrate preference of LmBuk.
A. Concentration-dependent activity of LmBuk in the presence of SCCA substrates at 37 °C. The observed initial rates of reaction for the substrates acetate (closed circles), propionate (closed squares), butyrate (closed triangles), pentanoate (closed diamonds) and hexanoate (open circles) catalyzed by LmBuk were determined at 37 °C in the presence of 10 mM ATP under standard assay conditions (see Materials and Methods). Data were plotted as a function of the substrate concentration and KM and kcat were determined from the fit of the data to the Michaelis-Menten equation using Kaleidagraph software. Data derived for hexanoate was fitted to the Michaelis-Menten equation to include substrate inhibition. Data are means ± SEM of at least three experiments. B. Concentration-dependent activity of LmBuk in the presence of BCCA substrates at 37 °C. The observed initial rates of reaction for the isobutyrate (open circles), isovalerate (open squares), 2-methyl butyrate (open triangles) and 2-ethyl butyrate (open diamonds), 2-methyl pentanoate (closed circles) and 3-methyl pentanoate (closed triangles) catalyzed by LmBuk were determined at 37 °C in the presence of 10 mM ATP under standard assay conditions (see Materials and Methods). Data were plotted as a function of the substrate concentration and KM and kcat were determined from the fit of the data to the Michaelis-Menten equation using Kaleidagraph software. Data derived for 2-ethylbutyrate, 2-methylpentanoate and 3-methylpentanoate was fitted to the Michaelis-Menten equation to include substrate inhibition. Data are means ± SEM of at least three experiments. C. Concentration-dependent activity of LmBuk at 10 °C in the presence of SCCA and BCCA substrates. The observed initial rates of reaction for the isobutyrate (closed squares), isovalerate (closed diamonds), 2-methyl butyrate (open diamonds) and 2-ethyl butyrate (open squares), pentanoate (open circles) and butyrate (closed circles) catalyzed by LmBuk were determined at 10 °C in the presence of 10 mM ATP as described under “Materials and Methods”. Data were plotted as a function of the substrate concentration and KM and kcat were determined from the fit of the data to the Michaelis-Menten equation using Kaleidagraph software. Data plotted are means ± SEM of at least three experiments.
Acetate (C2) and hexanoate (C6) were poor LmBuk substrates (Fig. 3A & Table 1). However, elevated concentrations of acetate (>80 mM) did result in measurable product formation (~10 % of the product with propionate as the substrate). Extremely low activity with acetate indicates that Buk is not involved in the secretion of acetate observed during aerobic and anaerobic growth in L. monocytogenes [27]. Furthermore, Buk is unlikely to be involved in phosphorylation of long chain fatty acids judging by the similarly low activity with hexanoate (C6) as the substrate. Thus, the modest integration of medium and long chain fatty acids reported earlier probably occurs via an alternate pathway [28].
These data indicate the broad substrate specificity and chain length preference (C3-C5) of LmBuk. The substantial activity in the phosphorylation direction (Fig. 3A and 3B; Table 1) supports our hypothesis that presence of sufficient concentrations of carboxylic acid precursors could drive the reaction in the acyl phosphate forming direction. Furthermore, L. monocytogenes Ptb has been shown to form butyryl-CoA from butyryl phosphate thus emphasizing the viability of the reversibility of this pathway [22]. The reversibility of the Ptb-Buk pathway has been utilized in vitro for the production of polyhydroxy alkanoic acids in C. acetobutylicum and Bacillus megaterium and the authors demonstrate the importance of broad substrate specificity of the Ptb–Buk pathway in this context [29,30].
It is also likely that the phosphorylated products of carboxylic acids serve a purpose other than formation of fatty acids. Butyrate is well known as an effector of gene expression. For example, butyrate causes an upregulation of virulence factor expression in enterohemorrhagic E. coli (EHEC) [31]. On the other hand, downregulation of virulence factor expression caused by exposure to butyrate has been observed in Salmonella enterica Enteritidis and L. monocytogenes [10,32]. Intracellular peak concentrations of butyryl phosphate, which likely behaves as a small molecule phosphate donor, is accompanied by altered expression of a large set of genes in C. acetobutylicum [33,34]. Thus, it is probable that Buk plays a significant role in signal transduction in L. monocytogenes, since the organism encounters high concentrations of short chain fatty acids in the mammalian gut during infection [35].
3.1.2 BCCAs
Buk from the marine spirochete MA-2 has been reported to be a true branched-chain fatty acid kinase [23]. We sought to determine if LmBuk exhibited similar behavior and kinetic constants were determined with various BCCAs differing in the size and branch position to this end. All BCCAs tested proved to be good substrates for LmBuk. Curiously, the presence of a methyl branch increased affinity of substrates which had a shorter chain length. For example, the K0.5 values for propionate (174.1 mM) was ~20-fold higher than that of isobutyrate (C3 with a methyl branch at the 2nd position) (7.6 mM) revealing a substantial increase in affinity associated with the branched chain. A modest improvement in affinity was observed with the C4 substrates as demonstrated by a comparison of the K0.5 for butyrate with that for isovalerate, 2-methylbutyrate and 2-ethylbutyrate (Fig. 3B & Table 1). However, the data derived from branched C5 substrates (2-methylpentanoate and 3-methylpentanoate) support lower binding affinity when compared with their straight chain counterparts (Fig. 3B & Table 1). Although the presence of the branch appears to increase affinity it also seems to cause a concomitant reduction in the turnover rate (Fig 3B, Table 1 & 2). Such a change might indicate that branched chain substrates have a reduced off-rate which would produce both of these observed enzymatic characteristics.
Table 2.
Steady state kinetic analysis of LmBuk at 10 °C.
| Substrate | kcat (min−1) | KM (mM) | kcat / KM (mM−1min−1) |
|---|---|---|---|
| Straight-chain | |||
| Butyrate | 8.2 ± 0.1 | 78.1 ± 3.8 | 0.1 |
| Pentanoate | 9.1 ± 0.4 | 42.5 ± 5.2 | 0.2 |
| Branched-chain | |||
| Isobutyrate | 10.2 ± 0.3 | 9 ± 1.5 | 1.1 |
| Isovalerate | 7.6 ± 0.3 | 8.2 ± 1.4 | 0.9 |
| 2-methylbutyrate | 4.3 ± 0.2 | 22 ± 4.3 | 0.2 |
| 2-ethyl butyrate | 1.2 ± 0.1 | 4 ± 2.5 | 0.3 |
Initial velocities were measured at 10 °C in the presence of 10 mM ATP at pH 7.5. The kcat and KM were calculated from the least squares fit of the experimental data from the different substrates to the Michaelis-Menten equation. The values indicated are the mean of experiments performed at least in triplicate ± SEM.
Although Buk activity is likely important in the incorporation of these compounds into the L. monocytogenes fatty acid pool, factors such as the substrate specificity of Ptb, FabH, and the other enzymes that participate in phospholipid biosynthesis probably play a larger role. Consistent with this is that although Buk showed the best catalytic efficiency with pentanoate, Ptb from L. monocytogenes prefers BCCA substrates, and does not utilize pentanoyl-CoA efficiently [22,36]. Additionally, synthesis of BCFAs has been reported to be dependent on the substrate specificity of FabH, which catalyzes the first committed step in fatty acid biosynthesis, thus these endogenous substrates likely outcompete the products of the Buk-Ptb pathway [37].
3.1.3 ATP
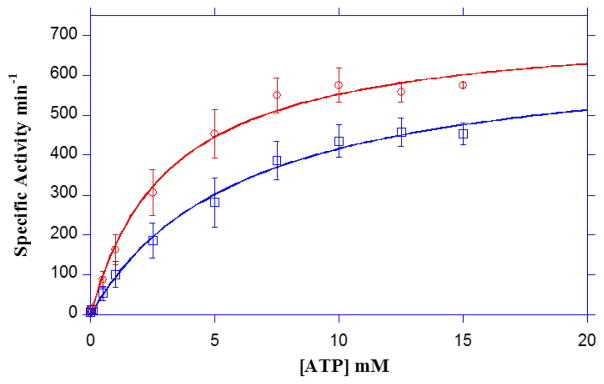
The ATP concentration dependence (10μM – 15mM) for LmBuk was determined in the presence of 200 mM butyrate by fitting the data to the Michaelis-Menten equation as described in the “Materials and Methods”. The K0.5ATP for LmBuk was 3.18 mM (Fig. 4), values which are in agreement with Buk from C. acetobutylicum and the marine spirochete S. isovalerica [23,24]. Additionally, presence of 2 mM ADP demonstrated competitive inhibition of the binding of ATP with an increase in the K0.5ATP to 6.09 mM (Fig. 4).
Fig 4. Concentration dependence of ATP utilization by LmBuk and competitive inhibition by ADP.
LmBuk activity was determined under standard assay conditions in the presence of 200 mM butyrate and varying amounts of ATP (10 μM–15 mM) and data (open circles) were plotted as a function of ATP concentration. Fit of the data to the Michaelis-Menten equation provided values for KM and kcat. ADP inhibition studies were performed using 2 mM ADP (open squares) in each reaction under the same experimental conditions as that of the ATP assay. ADP demonstrated competitive inhibition with respect to ATP. Data plotted are means ± SEM of at least three experiments.
3.2 Buk utilized unnatural BCCAs as its substrates
LmBuk also exhibited significant product formation with unnatural BCCAs such as 2-ethylbutyrate, 2-methylpentanoate, and 3-methylpentanoate (Fig. 3B & Table 1). Enzyme activity in the presence of these substrates was similar to hexanoate where activity begins to slow at higher concentrations. Thus, in these instances data were fitted to an equation that incorporated substrate inhibition (See Methods). Utilization of 2-ethylbutyrate showed the least product formation (kcat 6.7 min−1), which may be attributed to the longer side chain (ethyl) compared to 2-methylbutyrate (kcat 11.3 min−1). Additionally, while the presence of a methyl branch caused an increase in affinity, it also resulted in decreased product formation as evidenced by lower kcat values (Fig. 3B & Table 1). To our knowledge, this is the first demonstration that Buk can utilize unnatural fatty acid precursors capable of altering the membrane fatty acid profile. LmPtb similarly can utilize unnatural BCCA derivatives as substrates indicating that these two enzymes could likely constitute a pathway to altering membrane composition [22]. Sen et al. [16] showed that novel fatty acids arising from these unnatural BCCAs imparted the biophysical characteristics necessary for survival of the organism at low temperatures. Our work here indicates that L. monocytogenes exploits the reversibility of the Ptb-Buk pathway in the presence of sufficient concentrations of unnatural substrates in its environment to supply these fatty acid precursors for elongation by the FAS II pathway. The requirement of higher concentrations in the case of the wild type organism indicates that the domination of Bkd activity in the production of endogenous substrates outcompetes the products from the bypass pathway [11].
3.3 Buk prefers BCCAs at low temperatures
L. monocytogenes cld-2/MOR401 incorporates the products of numerous BCCAs in its membrane at low growth temperatures [8,16]. We determined whether Buk was capable of product formation with these substrates at low temperature as would be required if it contributes to increased membrane fluidity at these temperatures. Product formation at 10 °C was observed with all BCCAs and SCCAs tested (Fig 3C & Table 2). Interestingly, substrate inhibition was not observed at low temperatures. We observed lower kcat values for all of the substrates tested. However, the decrease was more pronounced for SCCAs compared to BCCAs (Fig 3C & Table 2), which may be due in part to an increase in the K0.5 values for the SCCA substrates. This drastic reduction in Buk catalytic efficiency in the presence of SCCAs compared to BCCAs suggests that Buk demonstrates a strong preference for BCCAs at 10 °C (Fig 3C & Table 2). To our knowledge, this is the first report demonstrating a temperature-dependent switch in substrate preference by Buk from any organism. LmFabH also demonstrates a switch in its substrate preference at low temperatures to select for 2-methylbutyryl-CoA, the precursor of the low melting point fatty acid anteiso C15:0 enabling it to survive and grow at low temperatures [36]. The substrate specificities of LmBuk could thus be similar to other organisms which increase the membrane BCFAs in response to lower temperatures and can incorporate exogenous carboxylic acids. Perhaps these unique substrate specificities are indicative of evolutionary adaptations within L. monocytogenes providing a psychrotolerant selective advantage.
3.4 Buk catalysis occurs through a ternary complex intermediate
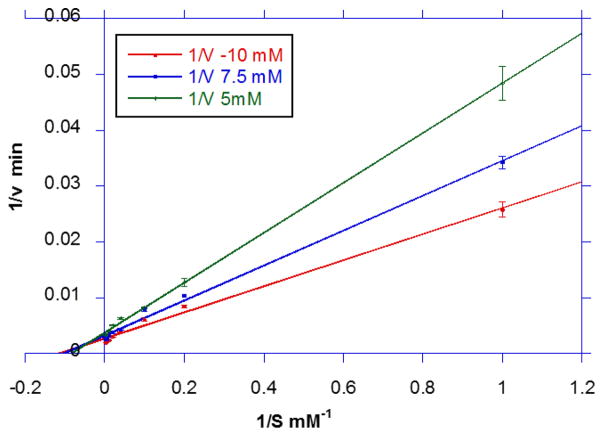
We sought to determine whether Buk catalysis occurs through a sequential or ping-pong mechanism. Enzyme activity was plotted as a function of butyrate concentration at different fixed concentrations of ATP (i.e. 5 mM, 7.5 mM and 10 mM). Double reciprocal plots (Fig. 5) showed that the lines converged to a point in the second quadrant. This is consistent with a reaction mechanism involving a sequential mechanism with the formation of a ternary complex. That is, LmBuk binds both butyrate and ATP forming a ternary complex, prior to catalysis and release of the products butyryl phosphate and ADP. Additionally, competitive binding of ADP (Fig. 4) in the active site is supportive of the sequential mechanism.
Fig 5. LmBuk catalysis occurs through a ternary complex intermediate.
Concentration dependent activity of Buk in the presence varying concentrations of butyrate (0.1– 300 mM) at different fixed concentrations of ATP (5 mM (open diamonds), 7.5 mM (closed squares) and 10 mM (closed circles)) were determined under standard assay conditions at 37 °C. Means of reciprocals of specific activity from at least three experiments ± SEM were plotted.
A similar mode of action was also suggested for the action of other phosphokinases such as acetate kinase (Ack) and glycerol kinase [38]. Although the apparent K0.5 values and relative activity of Buk with various substrates were explored in C. acetobutylicum and the spirochete MA-2, the mechanism of action was not investigated in these organisms [23,24]. Cheek et al., [39] classified and characterized the protein folding of kinases and showed the similarity of the structure of Ack and Buk. Buk is highly conserved among Listeria isolates and also demonstrates a significant identity with Buk from C. acetobutylicum and Thermotoga maritima. Additionally, substrate and nucleotide bound structures of Salmonella typhimurium propionate kinase showed that the carboxyl group of propionate is positioned at a distance of 0.5 nm from the γ-phosphate of ATP in the active site supporting a direct in-line transfer mechanism, i.e. a sequential mechanism [40].
3.5 Possible biological relevance of LmBuk
ptb and buk are immediately upstream of the lpd gene of the branched-chain α-keto acid dehydrogenase operon in L. monocytogenes [8]. The normal physiological function of Ptb and Buk may be in the catabolism of branched-chain α-keto acids to the corresponding free acids with formation of ATP as has been shown in E. faecalis [18], and in the synthesis of butyrate [41]. These two enzymes may function in the reverse direction in the formation of phosphate and CoA derivatives of SCCAs and BCCAs. There clearly is a pathway for the formation of CoA derivatives for fatty acid biosynthesis from BCCAs not involving Bkd and branched chain amino acids. This is most readily seen in L. monocytogenes bkd mutants (cld-1, cld-2 and MOR401) where BCCAs and SCCAs act as precursors for fatty acid biosynthesis [4,8,11,16]. However, even in wild type L. monocytogenes with intact bkd high concentrations of BCCAs such as 2-methyl butyrate, isobutyrate, and isovalerate act as precursors for anteiso odd, iso even and iso odd fatty acids respectively, and butyrate results in the biosynthesis of even straight-chain fatty acids [11]. Furthermore, the C6 BCCAs 2-ethylbutyrate and 2-methylpentanoate lead to the biosynthesis of 12-ethyltetradecanoate and 12-methylpentadecanoate respectively in wild type also. The substrate specificities of Ptb [22] and Buk are compatible with their function in production of CoA derivatives for fatty acid biosynthesis from carboxylic acids. Further verification of the roles of ptb and buk in fatty acid biosynthesis will require construction of mutants in these genes.
It is possible that sufficient concentrations of SCCAs and BCCAs in foods could impact the fatty acid composition of L. monocytogenes. Julotok et al. [11] showed that food preservatives such as acetate and lactate that are metabolized via acetyl CoA did not impact fatty acid composition, whereas propionate and butyrate at 25 mM and higher concentrations did. Both 2-ethylbutyrate and 2-methylpentanoate are used as food flavoring additives but at low levels, e.g.,40 ppm [42], and are therefore unlikely to impact fatty acid composition. Each food would be expected to have a unique concentration of BCCAs and SCCAs, but levels of these constituents are not normally reported as part of the United States Department of Agriculture Survey.
4 Conclusions
LmBuk exhibited substantial phosphorylation with a large number of SCCAs and natural and unnatural BCCA substrates indicating its broad substrate specificity. This suggests that Buk might play a role in provision of precursors for fatty acid biosynthesis under some conditions. Buk is distinct from acetate kinase and prefers substrates with a chain length of C3-C5 and is thus not involved in the activation of acetate or long chain fatty acids. Buk catalysis involves the formation of a ternary complex similar to other members of the phosphokinase protein family. Substantial product formation in the presence of butyrate (present in high concentrations in the mammalian gut) is consistent with a role for Buk in signal transduction during infection by L. monocytogenes.
Highlights.
Buk from L. monocytogenes exhibited broad substrate specificity
Buk preferred substrates with a chain length of 3 to 5 carbons
An alkyl side chain improved binding among shorter chain substrates
Buk could utilize unnatural substrates such as 2-ethylbutyrate, 2-methylpentanoate and 3-methylpentanoate
Buk catalysis involved ternary complex formation
Acknowledgments
This work was supported by grant 1 R15 AI099977-01 from the National Institutes of Health to Brian J Wilkinson and Craig Gatto and 1 R15 GM61583 to Craig Gatto. This work was also supported by the R. D. Weigel grant from the Beta Lambda Chapter of the Phi Sigma Biological Honor Society at Illinois State University. The funding sources had no role in study design, collection, analysis and interpretation of data, writing of this manuscript, or the decision to submit it for publication. We are grateful to Dr. Bart Weimer for his comments on levels of short-chain carboxylic acids in foods.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cossart P. Illuminating the landscape of host-pathogen interactions with the bacterium Listeria monocytogenes. Proc Natl Acad Sci. 2011;108:19484–19491. doi: 10.1073/pnas.1112371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker SJ, Archer P, Banks JG. Growth of Listeria monocytogenes at refrigeration temperatures. J Appl Bacteriol. 1990;68:157–162. doi: 10.1111/j.1365-2672.1990.tb02561.x. [DOI] [PubMed] [Google Scholar]
- 3.Poger D, Caron B, Mark AE. Effect of methyl-branched fatty acids on the structure of lipid bilayers. J Phys Chem. 2014;118:13838–13848. doi: 10.1021/jp503910r. [DOI] [PubMed] [Google Scholar]
- 4.Annous BA, Becker LA, Bayles DO, Labeda DP, Wilkinson BJ. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl Environ Microbiol. 1997;63:3887–3894. doi: 10.1128/aem.63.10.3887-3894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edgcomb MR, Sirimanne S, Wilkinson BJ, Drouin P, Morse RD. Electron paramagnetic resonance studies of the membrane fluidity of the foodborne pathogenic psychrotroph Listeria monocytogenes. Biochim Biophys Acta. 2000;1463:31–42. doi: 10.1016/s0005-2736(99)00179-0. [DOI] [PubMed] [Google Scholar]
- 6.Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974;71:522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oku H, Kaneda T. Biosynthesis of branched-chain fatty acids in Bacillus subtilis A decarboxylase is essential for branched-chain fatty acid synthetase. J Biol Chem. 1988;263:18386–96. [PubMed] [Google Scholar]
- 8.Zhu K, Bayles DO, Xiong A, Jayaswal RK, Wilkinson BJ. Precursor and temperature modulation of fatty acid composition and growth of Listeria monocytogenes cold-sensitive mutants with transposon-interrupted branched-chain α-keto acid dehydrogenase. Microbiology. 2005;151:615–23. doi: 10.1099/mic.0.27634-0. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, O’Riordan MXD. Branched-chain fatty acids promote Listeria monocytogenes intracellular infection and virulence. Infect Immun. 2010;78:4667–4673. doi: 10.1128/IAI.00546-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y, Wilkinson BJ, Standiford TJ, Akinbi HT, O’Riordan MXD. Fatty acids regulate stress resistance and virulence factor production for Listeria monocytogenes. J Bacteriol. 2012;194:5274–5284. doi: 10.1128/JB.00045-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Julotok M, Singh AK, Gatto C, Wilkinson BJ. Influence of fatty acid precursors, including food preservatives, on the growth and fatty acid composition of Listeria monocytogenes at 37 and 10degreesC. Appl Environ Microbiol. 2010;76:1423–32. doi: 10.1128/AEM.01592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giotis ES, McDowell DA, Blair IS, Wilkinson BJ. Role of branched-chain fatty acids in pH stress tolerance in Listeria monocytogenes. Appl Environ Microbiol. 2007;73:997–1001. doi: 10.1128/AEM.00865-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh VK, Hattangady DS, Giotis ES, Singh AK, Chamberlain NR, Stuart MK, Wilkinson BJ. Insertional inactivation of branched-chain α-keto acid dehydrogenase in Staphylococcus aureus leads to decreased branched-chain membrane fatty acid content and increased susceptibility to certain stresses. Appl Environ Microbiol. 2008;74:5882–90. doi: 10.1128/AEM.00882-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willecke K, Pardee AB. Fatty acid-requiring mutant of Bacillus subtilis defective in branched chain alpha-keto acid dehydrogenase. J Biol Chem. 1971;246:5264–72. [PubMed] [Google Scholar]
- 15.Kaneda T. Incorporation of branched-chain C6-fatty acid isomers into the related long-chain fatty acids by growing cells of Bacillus subtilis. Biochemistry. 1970;10:340–347. doi: 10.1021/bi00778a022. [DOI] [PubMed] [Google Scholar]
- 16.Sen S, Sirobhushanam S, Hantak MP, Lawrence P, Thomas Brenna J, Gatto C, Wilkinson BJ. Short branched-chain C6 carboxylic acids result in increased growth, novel “unnatural” fatty acids and increased membrane fluidity in a Listeria monocytogenes branched-chain fatty acid-deficient mutant. Biochim Biophys Acta - Mol Cell Biol Lipids. 2015;1851:1406–1415. doi: 10.1016/j.bbalip.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward DE, van Der Weijden CC, van Der Merwe MJ, Westerhoff HV, Claiborne A, Snoep JL. Branched-chain α-keto acid catabolism via the gene products of the bkd operon in Enterococcus faecalis: a new, secreted metabolite serving as a temporary redox sink. J Bacteriol. 2000;182:3239–3246. doi: 10.1128/jb.182.11.3239-3246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu K, Ding X, Julotok M, Wilkinson BJ. Exogenous isoleucine and fatty acid shortening ensure the high content of anteiso-C15:0 fatty acid required for low-temperature growth of Listeria monocytogenes. Appl Environ Microbiol. 2005;71:8002–8007. doi: 10.1128/AEM.71.12.8002-8007.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson KE, Fouts DE, Mongodin EF, Ravel J, DeBoy RT, Kolonay JF, Rasko DA, Angiuoli SV, Gill SR, Paulsen IT, Peterson J, White O, Nelson WC, Nierman W, Beanan MJ, Brinkac LM, Daugherty SC, Dodson RJ, Durkin AS, Madupu R, et al. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res. 2004;32:2386–2395. doi: 10.1093/nar/gkh562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiesenborn DP, Rudolph FB, Papoutsakis ET. Phosphotransbutyrylase from Clostridium acetobutylicum ATCC 824 and its role in acidogenesis. Appl Environ Microbiol. 1989;55:317–322. doi: 10.1128/aem.55.2.317-322.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirobhushanam S, Galva C, Sen S, Wilkinson BJ, Gatto C. Broad substrate specificity of phosphotransbutyrylase from Listeria monocytogenes: A potential participant in an alternative pathway for provision of acyl CoA precursors for fatty acid biosynthesis. BBA - Mol Cell Biol Lipids. 2016;1861:1102–1110. doi: 10.1016/j.bbalip.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harwood CS, Canale-Parola E. Properties of acetate kinase isozymes and a branched-chain fatty acid kinase from a Spirochete. J Bacteriol. 1982;152:246–254. doi: 10.1128/jb.152.1.246-254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartmanis MGN. Butyrate Kinase from Clostridium acetobutylicum. J Biol Chem. 1987;262:617–621. [PubMed] [Google Scholar]
- 25.Ward DE, Ross RP, van der Weijden CC, Snoep JL, Claiborne A. Catabolism of branched-chain α-keto acids in Enterococcus faecalis: the bkd gene cluster, enzymes, and metabolic route. J Bacteriol. 1999;181:5433–5442. doi: 10.1128/jb.181.17.5433-5442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipmann F, Tuttle LC. A specific micromethod for the determination of acyl phosphates. J Biol Chem. 1945;159:21–28. [Google Scholar]
- 27.Romick TL, Fleming HP, McFeeters RF. Aerobic and anaerobic metabolism of Listeria monocytogenes in defined glucose medium. Appl Environ Microbiol. 1996;62:304–7. doi: 10.1128/aem.62.1.304-307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tokarskyy O, Marshall DL. Mechanism of synergistic inhibition of Listeria monocytogenes growth by lactic acid, monolaurin, and nisin. Appl Environ Microbiol. 2008;74:7126–9. doi: 10.1128/AEM.01292-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu SJ, Steinbüchel A. Exploitation of butyrate kinase and phosphotransbutyrylase from Clostridium acetobutylicum for the in vitro biosynthesis of poly(hydroxyalkanoic acid) Appl Microbiol Biotechnol. 2000;53:545–52. doi: 10.1007/s002530051655. [DOI] [PubMed] [Google Scholar]
- 30.Vazquez GJ, Pettinari MJ, Méndez BS. Evidence of an association between poly(3-hydroxybutyrate) accumulation and phosphotransbutyrylase expression in Bacillus megaterium. Int Microbiol. 2003;6:127–129. doi: 10.1007/s10123-003-0120-5. [DOI] [PubMed] [Google Scholar]
- 31.Tobe T, Nakanishi N, Sugimoto N. Activation of motility by sensing short-chain fatty acids via two steps in a flagellar gene regulatory cascade in enterohemorrhagic Escherichia coli. Infect Immun. 2011;79:1016–1024. doi: 10.1128/IAI.00927-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Thompson A, Hinton JC, Van Immerseel F, Hautefort I. Butyrate specifically down-regulates Salmonella pathogenicity island 1 gene expression. Appl Environ Microbiol. 2006;72:946–949. doi: 10.1128/AEM.72.1.946-949.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Tomas CA, Rudolph FB, Papoutsakis ET, Bennett GN. Intracellular butyryl phosphate and acetyl phosphate concentrations in Clostridium acetobutylicum and their implications for solvent formation. Appl Environ Microbiol. 2005;71:530–537. doi: 10.1128/AEM.71.1.530-537.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCleary WR, Stock JB. Acetyl phosphate and the activation of two-component response regulators. J Biol Chem. 1994;269:31567–31572. [PubMed] [Google Scholar]
- 35.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh AK, Zhang Y, Zhu K, Subramanian C, Li Z, Jayaswal K, Gatto C, Rock CO, Wilkinson BJ. FabH selectivity for anteiso branched-chain fatty acid precursors in low temperature adaptation in Listeria monocytogenes. FEMS Microbiol Lett. 2009;301:1–8. doi: 10.1111/j.1574-6968.2009.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi KHHK, Heath RJ, Rock CO. β-ketoacyl-acyl carrier protein synthase III ( FabH ) is a determining factor in branched-chain fatty acid biosynthesis. J Bacteriol. 2000;182:365–370. doi: 10.1128/jb.182.2.365-370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blattler WA, Knowles JR. Stereochemical course of phosphokinases The use of adenosine [g-(S)-16O,17O,18O] triphosphate and the mechanistic consequences for the reactions catalyzed by glycerol kinase, hexokinase, pyruvate kinase, and acetate kinase. Biochemistry. 1979;18:3927–3933. doi: 10.1021/bi00585a013. [DOI] [PubMed] [Google Scholar]
- 39.Cheek S, Zhang H, Grishin NV. Sequence and structure classification of kinases. J Mol Biol. 2002;320:855–881. doi: 10.1016/s0022-2836(02)00538-7. [DOI] [PubMed] [Google Scholar]
- 40.Murthy AMV, Mathivanan S, Chittori S, Savithri HS, Murthy MRN. Structures of substrate- and nucleotide-bound propionate kinase from Salmonella typhimurium: substrate specificity and phosphate-transfer mechanism. Acta Crystallogr Sect D Biol Crystallogr. 2015;71:1640–1648. doi: 10.1107/S1399004715009992. [DOI] [PubMed] [Google Scholar]
- 41.Vital M, Howe C, Tiedje M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. 2014;5:1–11. doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burdock GA, editor. Fenaroli’s Handbook of flavor ingredients. 6. CRC Press; Boca Raton Florida: 2016. [Google Scholar]