Abstract
Study Objectives:
To compare the effectiveness of a custom-made (MRDc) versus ready-made (MRDr) mandibular repositioning devices (MRD) in the management of obstructive sleep apnea (OSA).
Methods:
A randomized crossover trial design was adopted in which patients with a confirmed diagnosis of OSA were randomly allocated to receive either a 3-month period of ready-made or custom-made MRD, with an intervening washout period of 2 weeks, prior to crossover. Treatment outcomes included both objective sleep monitoring and patient-centered measures (daytime sleepiness, partner snoring and quality of life).
Results:
Twenty-five patients, with a mild degree of OSA (apnea-hypopnea index of 13.3 [10.9–25] events/h) and daytime sleepiness (Epworth Sleepiness Scale of 11 [6–16]), completed both arms of the trial. The MRDc achieved a complete treatment response in 64% of participants, compared with 24% with the MRDr (p < 0.001). A significant difference was observed in treatment failures, when comparing the MRDr (36%) with the MRDc (4%). Excessive daytime sleepiness (Epworth Sleepiness Scale ≥ 10) persisted in 33% (MRDc) and 66% (MRDr) of OSA subjects, following treatment. A statistically significant improvement was observed in quality of life scales following MRDc therapy only. Significant differences were observed in relation to both the number of nights per week (p = 0.004) and hours per night (p = 0.006) between the two different designs of device.
Conclusions:
The study demonstrates the significant clinical effectiveness of a custom-made mandibular repositioning device, particularly in terms of patient compliance and tolerance, in the treatment of OSA.
Citation:
Johal A, Haria P, Manek S, Joury E, Riha R. Ready-made versus custom-made mandibular repositioning devices in sleep apnea: a randomized clinical trial. J Clin Sleep Med. 2017;13(2):175–182.
Keywords: clinical studies, clinical trial, appliance, therapy, sleep disorders
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by repeated collapse of the upper airway during sleep resulting in sleep fragmentation, loud snoring and diurnal sleepiness. The latter, reduces the ability to concentrate, affects mood, reduces quality of life and can affect driving, with an increased risk of accidents. Untreated moderate-to-severe OSA is an independent risk factor for hypertension and can increase the risk of cardiometabolic morbidity and mortality.1–3
OSA can be treated with continuous positive airway pressure (CPAP), which is the current treatment recommended by the National Institute for Health and Clinical Excellence.4 Unfortunately, not all patients tolerate CPAP treatment. Mandibular repositioning devices (MRDs) have been shown to be an effective alternative treatment option for adult patients with mild-moderate OSA as either first line therapy or for those with severe OSA who refuse CPAP therapy and are not considered suitable candidates for surgery.5 MRDs are worn in the mouth at night and act to hold the lower jaw in a forward position, thus reducing the tendency of the upper airway to collapse. They can be either custom-made or bought ready-made over the counter. Ready-made MRDs offer the potential advantages of being easily available, at relatively low cost. Some over the counter designs permit a degree of adjustment to make them easier for a patient to wear. In contrast, the advantages of custom-made MRDs are that they fit the mouth better and permit the patient to gradually learn to advance the jaw forward, improving their effectiveness.6 They are likely to be more comfortable and used more frequently by the patient to control OSA.7 However, these devices need to be constructed and fitted by a trained dentist and laboratory technician and therefore incur greater initial expense.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The role of mandibular repositioning devices (MRD) in the management of obstructive sleep apnea (OSA) is now recognized. However, data comparing the effectiveness of a custom-made (MRDc) versus ready-made (MRDr) repositioning devices is contradictory and may reflect the inherent limitations in study design.
Study Impact: The present study demonstrates the significant therapeutic benefit of a custom-made MRD, in terms of both clinician-and patient- centered outcomes and highlights the key shortcomings of ready-made MRDs.
There is limited evidence as to which type of MRD is the most effective in the treatment of mild-moderate OSA. To date, crossover trials comparing these devices have been limited by their selection to a mono-block design,8,9 which permits no subsequent titration of mandibular advancement, following placement, despite the demonstrated dose-dependent relationship with outcome6; the time interval of evaluation of the relative devices has been limited to 4 weeks of active treatment,9 which may be insufficient to judge their clinical effectiveness10 and a combination of sleep disorders, including non-apneic snoring8 making it difficult to determine their true effectiveness in obstructive sleep apnea alone.
This trial aimed to compare the effectiveness of ready-made and custom-made MRDs in the treatment of mild-to-moderate OSA, in terms of both objective (sleep physiology) and a range of subjective measures, including patient-centered outcomes.
METHODS
Design and Setting
A single-center, hospital-based, randomized crossover trial design was adopted to compare the effectiveness of a ready-made MRD (MRDr) and custom-made MRD (MRDc) over a 3-month period, with an intervening 2-week washout interval. The study was performed at a center specializing in the provision of MRD treatment.
Participants
The selection criteria for the trial were: adults (> 18 years), with a confirmed diagnosis of mild-moderate OSA (apneahypopnea index [AHI] of 5–30 events/h); sufficient healthy teeth to retain an MRD; the absence of periodontal disease or temporomandibular joint dysfunction and no previous history of MRD use. Full Research Ethics (09/H0805/44) and local Research and Development approval was obtained. Participants meeting the selection criteria were invited to participate in the trial and written informed consent obtained. Treatment allocation was performed centrally, by a statistician within the University, whereby random identification numbers were compiled from Altman's randomization table11 to one of the two treatment groups (A or B), using a block randomization method to enable even distribution between the groups. Patients were randomized to either sequence group A (MRDc for 3 months, followed by a 2-week washout period, before receiving MRDr or group B (the reverse treatment sequence). Opaque envelopes to conceal the allocation were labelled with identification numbers only.
A sample size estimation was based on the primary outcome measure, the AHI. On the basis of the data from a previous study designed to compare custom-made and thermoplastic oral appliances, the standard deviation was reported as 12 events per hour, with a clinically relevant difference of 8 events per hour.8 For a study of 80% power, and an alpha of 0.05%, an estimated sample size of 18 patients was required for a within person study.12 In view of the experienced high dropout rate in similar crossover trials of 30% to 40%, the sample size was inflated to 35 patients.8,10
Mandibular Repositioning Device Design
The custom-made MRD (MRDc; Figure 1) selected was the “Medical Dental Sleep Appliance” (R.J. and V.K. Bird, Middle Park, Victoria, Australia) which had been previously evaluated.10 The appliance design was regarded to meet the gold standard in light of the fact it exhibits minimal opening, is self-adjustable, and allows incremental advancement of the mandible, up to a maximum of 9 mm. The appliance was constructed in a single laboratory, based on working models of the teeth and an inter-occlusal registration in the intercuspal position. It was fitted by an experienced orthodontist and the incremental method of advancing the mandible demonstrated. Subjects were advised to turn the screw on a weekly basis until sleep improved and symptoms resolved.
Figure 1. The custom-made mandibular repositioning device (MRDc), that permitted incremental mandibular advancement to be achieved.
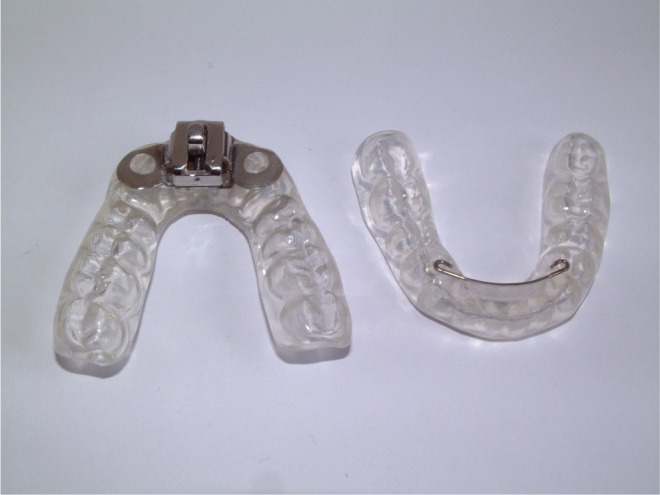
The ready-made MRD (MRDr; Figure 2) selected was a preformed thermoplastic appliance, the “Snoreshield” (S4S, Sheffield, UK). Patients were instructed to fit the appliance as per the manufacturer's instructions, by soaking the device in warm water and fitting to the upper arch. The mandible was then protruded into the device. The appliance could be re-heated at home for further manipulation as required, with a maximum permissible protrusion of 6 mm.
Figure 2. The ready-made mandibular repositioning device (MRDr).

Outcome Measures
The primary treatment outcome was the AHI, measured by an overnight sleep study, performed in the participant's home, using the “Visi-Lab Greyflash” (Stowood Scientific Instruments Ltd, Beckley, Oxford, UK). This recorded airflow, respiratory effort, blood oxygenation and AHI. Data were transferred from the sleep monitor using “Visi-Download” software for Microsoft Windows (XP / VISTA 7, Stowood Scientific Instruments Ltd.). Apnea was defined as a cessation of airflow > 10 s. Hypopnea was defined as ≥ 30% reduction in nasal pressure signal, lasting > 10 s, accompanied by a fall ≥ 4% in oxygen saturation. The AHI was determined by dividing the total numbers of apneas and hypopneas by the total sleep time. A complete response was defined as a reduction of AHI < 5 events/h, while a partial response was defined as a 50% reduction in the AHI. Treatment failure was defined as < 50% reduction in AHI. The overnight sleep study was performed at baseline and immediately following 3 months of MRD use, prior to the device being withdrawn and the washout period instigated, in respect of each of the two designs (MRDc and MRDr).
Secondary outcomes included changes in subjective daytime sleepiness (Epworth Sleepiness Scale13); the Functional Outcomes of Sleep Questionnaire (FOSQ14); the Medical Outcomes Study 36-item Short Form Health Survey (SF-3615); and an oral appliance outcome questionnaire (OAOQ) after 3 months of appliance wear, designed to assess self-reported treatment compliance and problems experienced.16 In addition, patient preference was documented at trial exit. Compliance failure was defined as the patient no longer wishing to continue with MRD treatment.
Statistical Analysis
All data were checked for errors, outliers and missing data prior to analysis and reported in line with the CONSORT guidelines. Data analysis, was undertaken blind to the interventions by a statistician with coded data, using SPSS (New York, USA). Means (standard deviation [SD]), medians (Quartile 1–Quartile 3 [Q1–Q3]) and frequencies (%) were used to describe the continuous normal and non-normally distributed variables as well as categorical variables, respectively. The Kolmogorov-Smirnov test was used for assessing normality of distribution. An analysis of variance for repeated measures, with a Bonferroni correction, was applied to test for treatment effects and a Wilcoxon matched pairs test was subsequently performed as a post hoc test. An Intention-to-treat analysis was carried out and the presence of any significant difference in baseline characteristics between dropouts and the participants who completed the trial was checked using independent samples t-test, Mann-Whitney U test and χ2 test, as required. McNemar test was performed to compare treatment effects on categorical outcomes. If period and/or period by treatment interaction effects were detected in any variable, treatment effects were tested by performing a generalized linear mixed model, adjusted for the orders, in view of the crossover design adopted in the present study. Level of significance was set at the 0.05 level.
RESULTS
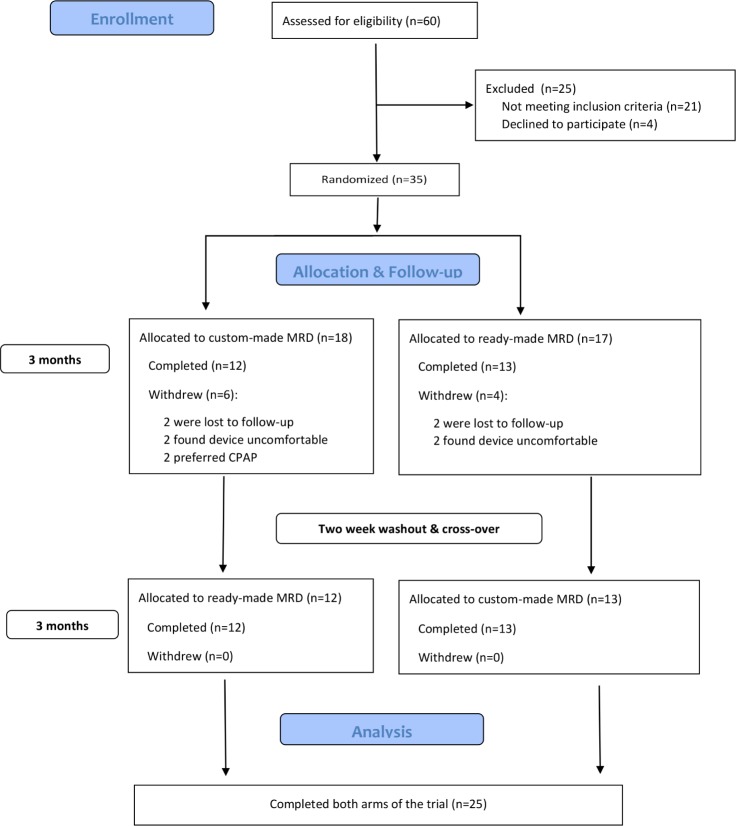
Thirty-five patients were randomized to receive either the MRDc (n = 18) or MRDr (n = 17) as shown in Figure 3, in accordance with CONSORT guidelines. A total of 10 (29%) patients withdrew from the trial, all after the first 3-month treatment interval, of which 6 were from the custom-made (2 were lost to follow-up, 2 found device uncomfortable, and 2 preferred CPAP) and 4 were from the ready-made (2 lost to follow-up and 2 found device uncomfortable) MRD group, respectively. There were no significant observed differences in baseline characteristics between those who completed and those who withdrew from the trial, except for ESS score, which was higher in dropouts. Intention-to-treat analysis demonstrated that dropouts did not affect the validity of the current study results.
Figure 3. Consort flow diagram.
Baseline Characteristics
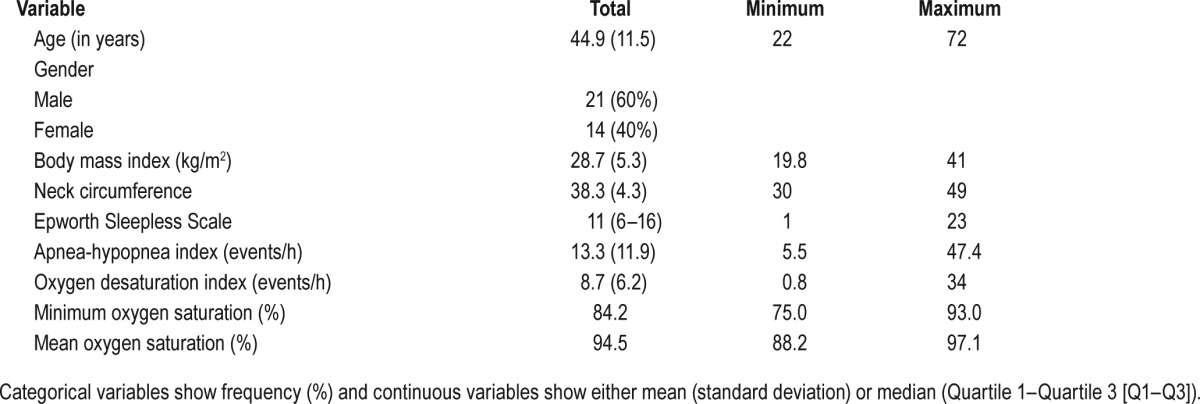
Table 1 demonstrates the baseline characteristics of participants. The mean age of the patients was 44.9 (SD 11.5) years, of wom 21 (60%) were male. Patients were overweight with a BMI of 28.7 (SD 5.3) kg/m2, with a neck circumference of 38.3 (SD 4.3) cm. The patients demonstrated a mild degree of OSA, with an AHI of 13.3 (10.9–25) and oxygen desaturation index (ODI) of 8.7 (6.2) events/h and an ESS of 11 (6–16).
Table 1.
Baseline characteristics of all recruited patients (n = 35).
Primary Outcome
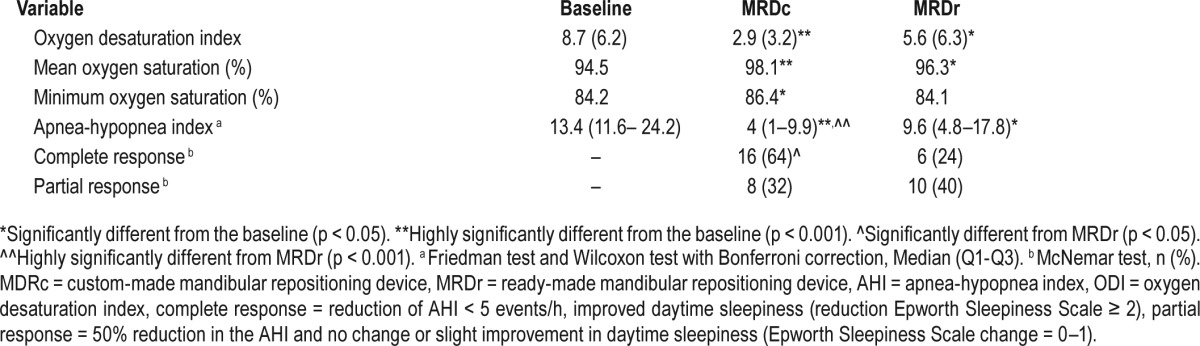
The MRDc resulted in a statistically significant difference (p < 0.001) in terms of total treatment success (96%; n = 24), of which a complete response was observed in 64% (n = 16) and a partial response in 32% (n = 8; Table 2). In contrast, The MRDr resulted in a total treatment success of 64% (n = 16), of which a complete response was observed in 24% (n = 6) and a partial response in 40% (n = 10). A significant difference was observed in treatment failures, when comparing the MRDr (36%) with the MRDc (4%). Furthermore, 89% of treatment failures with the MRDr observed either a complete (67%) or partial (22%) response with MRDc therapy. No period or carryover effects were observed in relation to the primary outcome.
Table 2.
Treatment effects of the MRDc and MRDr design on AHI, ODI, mean oxygen saturation, minimum oxygen saturation, and complete and partial response (n = 25).
Secondary Outcomes
The generalized linear mixed model showed that, compared with baseline, ESS scores were significantly improved with MRDc (p = 0.032) and just reached statistical significance (p = 0.048) with MRDr treatment (Table 3). Furthermore, excessive daytime sleepiness (ESS ≥ 10) was reported in 12 subjects at baseline and persisted in 33% and 66% of OSA subjects, following MRDc and MRDr therapy, respectively.
Table 3.
Treatment effects of the MRDc and MRDr design on ESS, FOSQ and SF-36.
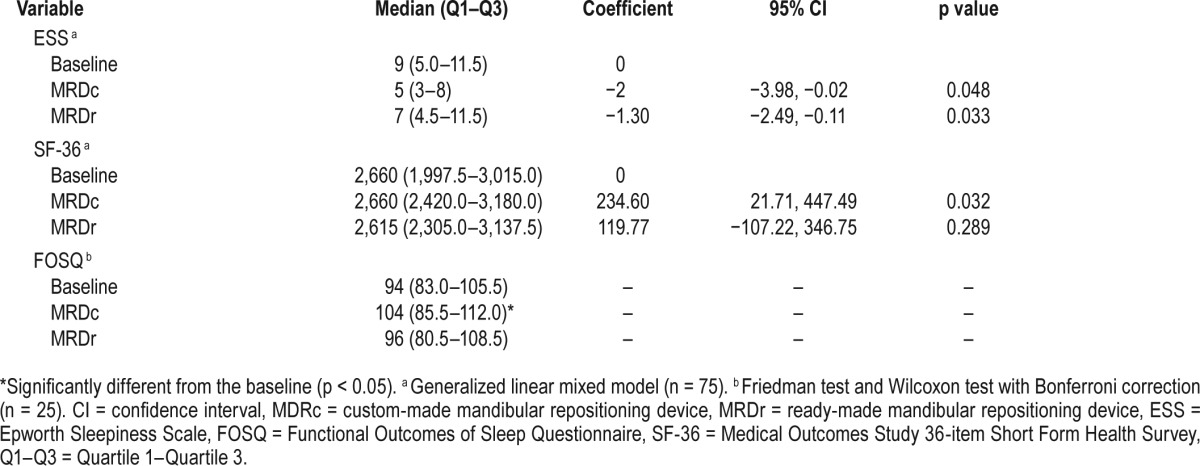
In terms of quality of life scales, a statistically significant improvement was observed in relation to both the FOSQ and SF-36, following MRDc therapy only (Table 3).
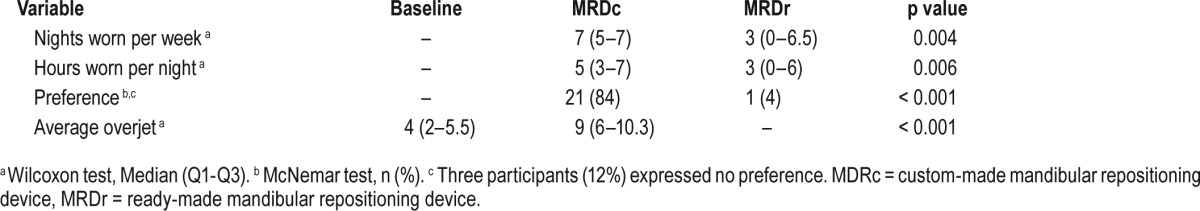
A statistically significant difference was observed in relation to both the number of nights per week (p = 0.004) and hours per night (p = 0.006) of self-reported use between the 2 different designs of device (Table 4). Subjects used their MRDc a median of 7 (range 5–7) nights per week and 5 (range 3–7) h/night compared with the MRDr with a median score of 3 (range 0–6.5) nights per week and 3 (range 0–6) h/night. Furthermore, a statistically significant difference (p < 0.001) was observed in response to device preference, with 21 (84%) patients expressing preference for the MRDc as compared to 1 (4%) in favor of the MRDr. Three patients expressed no preference. The difference in maximum protrusion, determined from the overjet achievable between baseline and MRDc was statistically significant (p < 0.01; Table 4).
Table 4.
Participant adherence, treatment preference and overjet expressed in relation to the MRDc and MRDr designs (n = 25).
A period by treatment interaction effect was seen in relation to the secondary outcome variables of ESS and SF-36. However, no statistically significant change was detected in either the BMI or neck circumference during the trial period.
DISCUSSION
The current trial was designed to address an important but relatively unanswered question regarding the clinical effectiveness of ready-made versus custom-made MRDs in the treatment of mild-to-moderate OSA, using a range of clinician- and patient-based outcome measures.
The importance of MRD therapy in the management of obstructive sleep apnea has been established, as both a principle treatment choice and as an acceptable alternate where CPAP therapy is not accepted or tolerated. The goal of treatment being to normalize nocturnal respiration and abolish the reported symptoms and thus minimize any potential cardiovascular and health-related consequences.
This trial demonstrated that whilst both designs of MRD could lower the baseline AHI and ODI values, there was a significant difference in their relative effectiveness. Importantly, both the observed difference in complete response and failure rate, seen with the MRDc compared well against the MRDr. These results compare more favorably than those reported by Quinnell et al.9 in their crossover RCT comparing 2 ready-made and 1 custom-made MRD, in which a complete or partial response to treatment was observed 38%, 49% and 45%, respectively and are more in line with reported findings of Vanderveken et al.,8 in which they reported total treatment success, combining complete and partial response as being 60% and 31%, with their custom- and ready-made MRDs, respectively. This difference can be explained on the basis of the study design and selection of MRD. Quinnell et al.9 assessed treatment effectiveness after only 4 weeks of active treatment, following a 2-week acclimatization period and with an intervening 1-week washout period. This could be simply regarded as too short a period for any patient to adapt and achieve maximum therapeutic benefit from their MRD.10 Vanderveken et al.8 applied a 4-month treatment period and the results may be more representative. A further significant difference between the current study is that to date, crossover trials comparing MRDs have acknowledged the limitation of selecting a mono-block design,8,9,12 which not only permit no subsequent titration of the amount of mandibular advancement, following placement, but furthermore necessitate the patient (ready-made) or the clinician protruding the mandible forward during their construction to an estimated degree. This in turn can not only significantly limit their clinical effectiveness due to the dose-dependent relationship between treatment outcome and degree of mandibular advancement,6,12,17 but also induce a greater degree of short-term muscle and dental discomfort.5,18,19 The latter was evident in the current study by the demonstrable effect of using a custom-made MRD which allowed the patient to incrementally adjust the degree of mandibular advancement to help achieve maximum therapeutic benefit, with a significant 5-mm difference (p < 0.001) observed in the degree of mandibular protrusion achieved compared to baseline.10
Increasingly the role of patient-centered measures is being recognized in treatment. While clinical trials remain the gold standard in evaluating the effectiveness and safety of interventions, it is crucial that appropriate outcomes are selected, measured and clearly defined in the process. Increasing evidence is accumulating to support the notion that consumers of care should be more involved in the process of identifying important outcomes, which may have otherwise been overlooked.20 A recent review of systematic reviews, which aimed to determine which outcomes to measure in clinical trials involving adults with OSA treated with MRDs, did not identify any group that directly involved patients in the process of selecting outcomes.21 In relation to patient-centered measures of outcome: daytime sleepiness, quality of life, adherence and device preference were assessed in the current study.
Both designs of MRD achieved a reduction in reported daytime sleepiness, which was similar in magnitude to previous reports.8,9 Excessive daytime sleepiness persisted in 33% and 66% of OSA patients, following MRDc and MRDr therapy, respectively. These results compare with those of Vanderveken et al.,8 in which excessive daytime sleepiness persisted in 45% and 55% of patients with the custom-made and ready-made MRDs, respectively.
In terms of reported quality of life scales, the custom-made MRD achieved greatest improvement in terms of both generic (SF-36) and specific (FOSQ) measures. Quinnell et al.9 also reported improved FOSQ scores in response to MRD treatment and similarly an improvement in the SF36 scores, in response to their custom-made MRD only. The authors acknowledged that the cost-effectiveness and clinical advantages of an adjustable MRD compared to a non-adjustable ready-made MRD required further exploration.9
A highly significant finding in the present study was the difference patients reported in their MRD adherence and are comparable with the findings of Quinnell et al.9 in relation to the ready-made MRD and better than those reported for the custom-made MRD, in terms of both the number of nights per week used and hours per night, particularly given the very short duration of their trial. Vanderveken et al.8 also reported poor use of the ready-made device. The potential benefits of a custom-made MRD, with incremental advancement, permitting better adaptation to the device may explain the more favorable results of the present study and had to date been minimally explored despite the guidance.5 MRD therapy is an entirely patient-dependent treatment and consequently an overriding principle in its success has to be patient comfort and consequent use. This was strikingly evident when patient preference was explored, with 21 (84%) patients expressing preference for the MRDc as compared to 1 (4%) in favor of the MRDr. Vanderveken et al.8 similarly observed that the end of their study, 19 of the 23 (82%) patients that completed both arms of their trial preferred the MRDc. In contrast, patients in the Quinnell et al.9 study reported a 10% difference in preference between the MRD designs but unlike the present study or that of Vanderveken et al.,8 reported 4 serious adverse events, and more importantly, 96% of patients reported minor adverse events, which related predominantly to discomfort. This, however, may reflect the short duration of the study, permitting insufficient time for adaptationn.9 A constant theme of both the current study and previous crossover trials comparing MRDs, is the reported difficulties of retaining the device in the mouth during sleep. This is an essential requirement in order to have any true benefit and highlights the difficulty in selecting a one-size fits all approach, with ready-made MRDs. The aim of this study was to replicate as far as possible the “natural” state of these devices and therefore to provide an evidence-base to the current practice for their use. Patients and sleep physicians frequently find that due to either monetary constraints or service access, they are recommending or purchasing, respectively, the ready-made MRD devices, not least because they are easily available from multiple sources and relatively cheap. Thus, in undertaking this crossover trial we very much wanted to reflect their everyday clinical use and thus patients were instructed to “follow the manufacturer's instructions” with regards to the ready-made MRD and provided a custom-made MRD by a dental professional, in order to highlight both the clinical and patient-centered aspects of their use. The latter significantly correlated with the former, in that patients overwhelmingly found the ready-made MRD difficult to tolerate and this was reflected in both the clinical outcomes and expressed preference. Ready-made MRDs unfortunately are limited in their design, by the very fact that the manufacturer is attempting to cater to the needs of a very diverse population, with inherent differences in the size of their jaws and ability to protrude their mandible. In turn, the inherent malleability of these devices potentially compromises their fit.
Potential limitations to the current study include the small number of patients recruited. However, the power to detect a difference was retained, by accounting for the potential dropouts in the original sample size determination. Additionally, no period or carryover effects were observed in relation to this primary outcome (AHI). However, a period by treatment interaction effect was seen in relation to the secondary outcome variables of ESS and SF-36. An explanation for this could be a gain in weight, with a resultant reduction in airway compliance. However, to minimize the risk of bias, patients had their BMI and neck circumference assessed at all visits, with no significant change detected. Self-reported outcomes do have attendant biases, but this has been a feature of all but a single-center study in MRDs reported in the literature.19 The value of compliance meters for MRDs are increasingly likely to be recognized in coming years and add further to our understanding of the role of these devices in managing OSA. It was not possible for blinding of either the clinician or participants after assignment to the interventions. However, all outcomes were blindly assigned, by a statistician, by coding the identity of participants.
CONCLUSIONS
The present study demonstrates the significant clinical effectiveness of a custom-made mandibular device, particularly in terms of patient compliance and tolerance, in the treatment of OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hyponea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcomes of Sleep Questionnaire
- MRD
mandibular repositioning device
- MRDc
custom-made mandibular repositioning device
- MRDr
ready-made mandibular repositioning device
- OAOQ
oral appliance outcome questionnaire
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- RCT
randomized controlled trial
- SD
standard deviation
- SF-36
Medical Outcomes Study 36-item Short Form Health Survey
REFERENCES
- 1.Horne J, Reyner L. Sleep related vehicle accidents. BMJ. 1995;310(6979):565–567. doi: 10.1136/bmj.310.6979.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: a population study. BMJ. 2008;320(7233):479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pataka A, Riha RL. Continuous positive airway pressure and cardiovascular events in patients with obstructive sleep apnea. Curr Cardiol Rep. 2013;15(8):385. doi: 10.1007/s11886-013-0385-z. [DOI] [PubMed] [Google Scholar]
- 4.Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. National Institute for Health and Care Excellence Web site. [Accessed January 5, 2017]. https://www.nice.org.uk/guidance/ta139. Published March 26, 2008.
- 5.Ramar K, Dort LC, Katz SG, et al. Clinical practice guidelines for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015;11(7):773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuiki S, Lowe AA, Almeida FR, Fleetham JA. Effects of an anteriorly titrated mandibular position on awake airway and obstructive sleep apnea severity. Am J Orthod Dentofacial Orthop. 2004;125(5):548–555. doi: 10.1016/j.ajodo.2003.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013;68(1):91–96. doi: 10.1136/thoraxjnl-2012-201900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med. 2008;178(2):197–202. doi: 10.1164/rccm.200701-114OC. [DOI] [PubMed] [Google Scholar]
- 9.Quinnell TG, Bennett M, Jordan J, et al. A crossover randomised controlled trial of oral mandibular advancement devices for obstructive sleep apnoeahypopnoea (TOMADO) Thorax. 2014;69(10):938–945. doi: 10.1136/thoraxjnl-2014-205464. [DOI] [PubMed] [Google Scholar]
- 10.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170(6):656–664. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 11.Altman DG. Practical Statistics for Medical Research. London, UK: Chapman and Hall; 1991. [Google Scholar]
- 12.Johnston CD, Gleadhill IC, Cinnamond MJ, Gabbey J, Burden DJ. Mandibular advancement appliances and obstructive sleep apnoea: a randomized clinical trial. Eur J Orthod. 2002;24(3):251–262. doi: 10.1093/ejo/24.3.251. [DOI] [PubMed] [Google Scholar]
- 13.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 14.Weaver TE, Kribbs NB, Pack AI, et al. Night to night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 16.Johal A, Battagel JM. An investigation into the changes in airway dimension and the efficacy of mandibular advancement appliances in subjects with obstructive sleep apnoea. Brit J Orthod. 1999;26(3):205–210. doi: 10.1093/ortho/26.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Otsuka R, Ono T, et al. Effect of titrated mandibular advancement and jaw opening on the upper airway in nonapneic men: a magnetic resonance imaging and cephalometric study. Am J Orthod Dentofac Orthop. 2004;125(2):191–199. doi: 10.1016/s0889-5406(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 18.Marklund M, Franklin KA, Persson M. Orthodontic side-effects of mandibular advancement devices during the treatment of snoring and obstructive sleep apnoea. Eur J Orthod. 2001;23(2):135–144. doi: 10.1093/ejo/23.2.135. [DOI] [PubMed] [Google Scholar]
- 19.Dieltjens M, Bream MJ, Vroegop AV, et al. Objectively measured vs. self-reported compliance during oral appliance therapy for sleep-disordered breathing. Chest. 2013;144(5):1495–1502. doi: 10.1378/chest.13-0613. [DOI] [PubMed] [Google Scholar]
- 20.Sinha I, Gallagher R, Williamson PR, Smyth R. Determining the clinical relevance of outcomes for evaluating regular therapies for children with asthma—a survey of clinicians, parents and young people. Arch Dis Child. 2010;95(Suppl 1):A59. [Google Scholar]
- 21.Johal A, Fleming P, Manek S, Marinho VC. Mandibular advancement splint (MAS) therapy for obstructive sleep apnoea--an overview and quality assessment of systematic reviews. Sleep Breath. 2015;19(3):1101–1108. doi: 10.1007/s11325-015-1148-4. [DOI] [PubMed] [Google Scholar]