Abstract
Study Objectives:
The aim of this study was to evaluate the effects of nasal dilator strip (NDS) as a placebo intervention compared with continuous positive airway pressure (CPAP) treatment in patients with severe obstructive sleep apnea (OSA).
Methods:
Patients were treated with both NDS and nasal CPAP. The sequence was randomized and interposed by 15 days of washout. Polysomnography was performed at baseline and on the first night of intervention with NDS and CPAP (titration). The Epworth Sleepiness Scale (ESS), Functional Outcomes of Sleep Questionnaire (FOSQ), and Beck Depression Inventory (BDI) were completed at baseline and at the end of both interventions. A questionnaire on the comfort and satisfaction (0 = no to 10 = total) was completed at the end of each intervention.
Results:
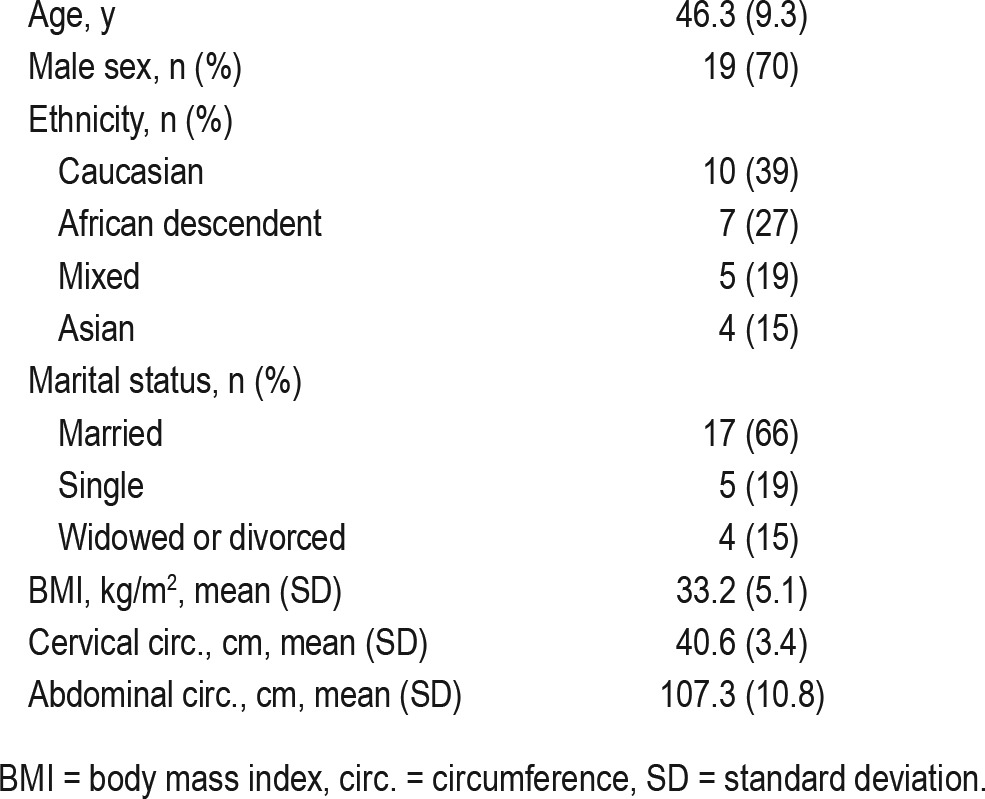
Twenty-six patients with OSA were evaluated (19 male; age 46.3 ± 9.3 y; body mass index 33.2 ± 5.1 kg/m2; ESS 15.8 ± 4.1; apnea-hypopnea index 60.7 ± 25.2). Adherence was high in both NDS (98%) and CPAP interventions (94%; 5.8 ± 1.7 h/night). In contrast to the baseline values, NDS intervention had no significant effect on all polysomnographic parameters, but NDS improved somnolence (ESS 13.0 ± 5.4, p = 0.001) and depressive symptoms (BDI 7.7 ± 6.9, p = 0.005). Reported satisfaction was significantly higher for CPAP than for NDS (sleep quality 9.2 ± 0.8 vs. 6.1 ± 2.1; wake up at morning: 8.6 ± 1.2 vs. 6.0 ± 2.2; daily activities: 8.9 ± 1.4 vs. 5.8 ± 1.5; quality of life: 8.3 ± 2.1 vs. 3.8 ± 3.5, p < 0.001), but similar low levels of difficulty for both interventions were observed (1.3 ± 2.2 vs. 0.3 ± 1.3, p = 0.098).
Conclusions:
Our data indicate that NDS is an attractive placebo intervention for randomized controlled trials evaluating the effects of CPAP in sleepy patients with OSA.
Citation:
Yagihara F, Lorenzi-Filho G, Santos-Silva R. Nasal dilator strip is an effective placebo intervention for severe obstructive sleep apnea. J Clin Sleep Med. 2017;13(2):215–221.
Keywords: CPAP, nasal dilator strip, obstructive sleep apnea, placebo
INTRODUCTION
Obstructive sleep apnea (OSA) is a highly prevalent sleep disorder with significant public health outcomes.1–7 Continuous positive airway pressure (CPAP) is considered the primary medical treatment for patients with moderate to severe OSA,8,9 and several randomized controlled trials (RCT) have shown the effectiveness of this treatment.10
An RCT is the most rigorous methodology to evaluate a treatment's effectiveness, but it requires an appropriate control group, blinding of the treatment group assignment for the investigator and participants, and attention to the use of an elaborate placebo control device.11 However, RCTs evaluating the effectiveness of CPAP differ from pharmacologic agents because there are practical difficulties to finding an ideal placebo. The sham-CPAP has been considered the placebo intervention of choice in the RCTs of several authors.12–15 One could argue that the placebo effects of a sham-CPAP may be vulnerable to the discomfort and frustration caused by the necessity of wearing a mask that delivers a suboptimal treatment pressure. Moreover, some studies have shown a lower adherence of sham-CPAP compared with that of active CPAP,14,16,17 as well as significantly worse sleep quality and increased hypopnea and oxyhemoglobin desaturation with sham-CPAP compared with that of baseline values.15 These data suggest that sham-CPAP may adversely affect sleep-related outcomes and may also influence the integrity of a particular study.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Randomized controlled trials have shown the effectiveness of continuous positive airway pressure (CPAP) treatment in patients with obstructive sleep apnea (OSA), but there are practical difficulties to finding the ideal placebo. The current study sought to evaluate the effects of nasal dilator strip as a placebo intervention compared with CPAP treatment in patients with severe OSA in a randomized controlled crossover protocol.
Study Impact: This study showed high adherence and low levels of difficulty for nasal dilator strip as a placebo intervention in a randomized controlled crossover protocol. Our data indicate that the nasal dilator strip could be used as an alternative placebo intervention in randomized controlled trials of patients with severe OSA, as supported by the absence of effects in clinical sleep outcomes (sleep architecture and respiratory events) compared with CPAP.
Some studies have evaluated the effects of a nasal dilator strip (NDS) on patients with OSA, showing little effect on snoring and no effect on the apnea-hypopnea index (AHI).18–22 Therefore, NDS is an attractive placebo to be compared with CPAP.23
Thus, the aim of this study was to evaluate the effects of the NDS as a placebo intervention compared with that of the CPAP treatment in patients with severe OSA in a randomized controlled crossover study.
METHODS
Consecutive patients were recruited from the outpatient sleep clinic from the Heart Institute of Faculdade de Medicina, Universidade de São Paulo. Patients aged 30 to 60 y were included if they had a previous diagnosis of severe OSA (AHI greater than 30) confirmed by polysomnography (PSG) and diurnal somnolence (Epworth Sleepiness Scale score greater than 9). The exclusion criteria were previous treatment for sleep-disordered breathing; the presence of any other sleep disorders; the presence of previous chronic diseases that were decompensated or untreated; a history of chronic use of alcohol, drugs, or sedatives; and increased risk of motor vehicle or professional accidents.
The protocol was approved by the ethics committee of the Faculdade de Medicina, Universidade de São Paulo (CAAE:11829213.7.0000.0068) and registered at ClinicalTrials. gov (NCT02117271). All patients were informed that the study goal was ‘to test two different types of OSA treatments’ before they provided written consent.
Study Design
The patients were randomized and crossed over into two study arms: 1 mo using NDS and 1 mo using nasal CPAP. A washout period of 2 w was completed between both NDS and CPAP conditions. The patients were submitted to additional PSG on the first night of each study period, i.e., PSG for CPAP titration and PSG on the first night using NDS. One trained professional followed each patient to ensure the proper use of both the CPAP and NDS throughout the intervention period. After the first 3 days of the intervention, the patients received a telephone call to ensure their adherence and to solve any potential doubts. After each week of intervention, the patients returned to the sleep laboratory, where the adherence data were gathered from the memory card or NDS count, and the patients were reinformed about the study protocol.
Subjective Evaluation
The patients were evaluated by the following questionnaires: the Epworth Sleepiness Scale (ESS),24 the Functional Outcomes of Sleep Questionnaire,25 and the Beck Depression Inventory (BDI).26 All of the questionnaires were completed at baseline and on the last day of each intervention. A survey of comfort and satisfaction was also completed the last day of NDS and CPAP intervention periods.
Sleep Study
Each patient was submitted to 3 full nights of PSG: the first PSG confirmed the OSA diagnosis, the second PSG was for manual CPAP titration (REMstar Pro M Series, Philips Electronics N.V., Somerset, NJ, USA) and the third PSG used the NDS (Breathe Right, GlaxoSmithKline Brazil, RJ, Brazil). All of the PSG were performed by digital equipment (Alice 5 Diagnostic Sleep System, Philips Electronics N.V., Somerset, NJ, USA). The recordings included electroencephalogram (EEG), electrooculogram, electromyogram (chin and anterior tibial muscle), electrocardiogram (modified D2 derivation), airflow (thermocouple and pressure transducer), chest and abdomen movements (inductive plethysmography), snoring, body position, and oxyhemoglobin saturation (SpO2). The patient's sleep and associated events were recorded and scored according to the recommended criteria for sleep studies.27 The CPAP titration was performed according to the protocol proposed by the American Academy of Sleep Medicine.9
Placebo Intervention
The patients were instructed to use the NDS (Breathe Right) every night of the placebo intervention period. On the night that the PSG using NDS was performed, patients were trained on the proper way to place the NDS: “(1) Wash and thoroughly dry your nose before applying the NDS; (2) Remove the protective liner to expose the adhesive; (3) Apply the NDS on your nose -the strip should be centered along the width of your nose with the tabs on the flaring part of the nostril. The tabs should not cover the flaring part of the nostril entirely, but rather should sit just above; and (4) Gently rub the strip to secure it to your nose.” After the first 3 days of the intervention, the patients received a telephone call to ensure the use of NDS and to answer any questions about the intervention. Weekly, the patients returned to the sleep laboratory to refill the number of NDS that they used during the week, and they were reinformed about the study protocol and the appropriate use of the intervention. Patients were also instructed to call a professional to solve any problems concerning the intervention. Adherence was evaluated by the NDS count and by a sleep diary that included questions about the use of the NDS. Adherence was measured in percentage (total number of days using NDS divided by the total number of days in the study arm period × 100). A survey on the comfort and satisfaction of using NDS was completed after the period of intervention.
CPAP Treatment
One CPAP device (System One REMstar Pro M Series, Philips Electronics N.V., Somerset, NJ, USA) that was set to the optimal pressure according to the PSG was provided to each patient. They also received a mask (WISP - Clear Frame, with headgear, Philips Electronics N.V., Somerset, NJ, USA) with three nasal cushions (small, medium, and large), and they used whichever one best fit their face. The patients were followed by a trained professional and received all necessary information on the device use before starting treatment. After the first 3 days of treatment, the patients received a telephone call to ensure the use of the CPAP device and to answer any questions about the treatment. Weekly, the patients returned to the sleep laboratory to be checked for the effectiveness and adherence to the treatment. Objective therapy data were gathered from the CPAP device memory card. Subjective therapy data were evaluated by sleep diary that included the use of CPAP questions. Adherence was measured in percentage (total number of days using CPAP divided by total number of days of the treatment arm period × 100). The patients were also instructed to call a professional to solve any problems concerning the treatment. A survey on the comfort and satisfaction of using the nasal CPAP was completed after the period of treatment.
Statistical Analyses
Statistical analyses were performed using SPSS software (IBM SPSS Statistics for Windows, Version 21.0, Armonk, NY, USA). A general linear model for repeated-measures data was used for the analysis of the PSG parameters, questionnaires, and satisfaction survey. Bonferroni post hoc testing was used. No covariates were added to the model since the sampling process was established, considering the expected proportion of OSA between sex (4:1 for men) and a particular age group (30–60 y). Power analysis was done using the G-Power 3 based on AHI variable, which was our main outcome. Based on our results of objective sleep data evaluated by the PSG at baseline and after intervention for both the placebo and CPAP interventions, achieving an effect size of 84% and a significance level of 5%, with a sample size of 26 patients previously recruited, an observed power of 100% was found. Effect sizes were estimated assessing the magnitude of the difference between groups (ETA square).28 The significance level was set at p ≤ 0.05.
RESULTS
Of the 70 patients who were enrolled, 44 were excluded due to the reasons described in Figure 1. A total of 26 patients was randomized and their baseline characteristics are presented in Table 1. The analysis of the objective and subjective data from the 13 patients who start the study using CPAP compared with data from the 13 patients who start using NDS showed no effect of the order of intervention (p = 0.74).
Figure 1. Flow chart of the included patients.
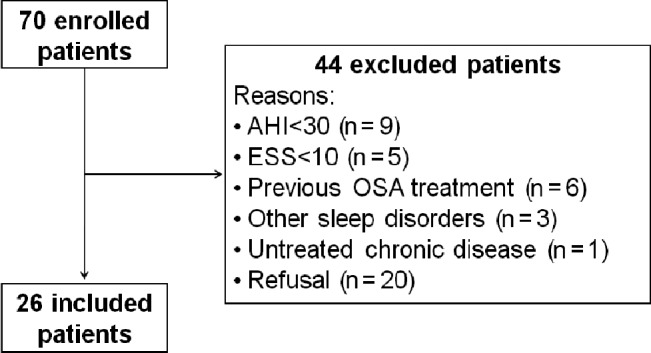
AHI = apnea-hypopnea index, ESS = Epworth Sleepiness Scale, OSA = obstructive sleep apnea,
Table 1.
Demographic data of the patients included in the research protocol (n = 26).
The total period (mean ± standard deviation) of the placebo intervention was 31.9 ± 5.9 days, and NDS was effectively used for 31.3 ± 6.0 days (overall adherence = 98%). The total period of the CPAP treatment was 33.7 ± 4.4 days. The CPAP device was effectively used for 31.5 ± 5.7 days, indicating an adherence of 94% during the total period of CPAP treatment. The number of hours per night with CPAP treatment was 5.8 ± 1.7 h. The CPAP pressure was 12.7 ± 2.5 cm H2O.
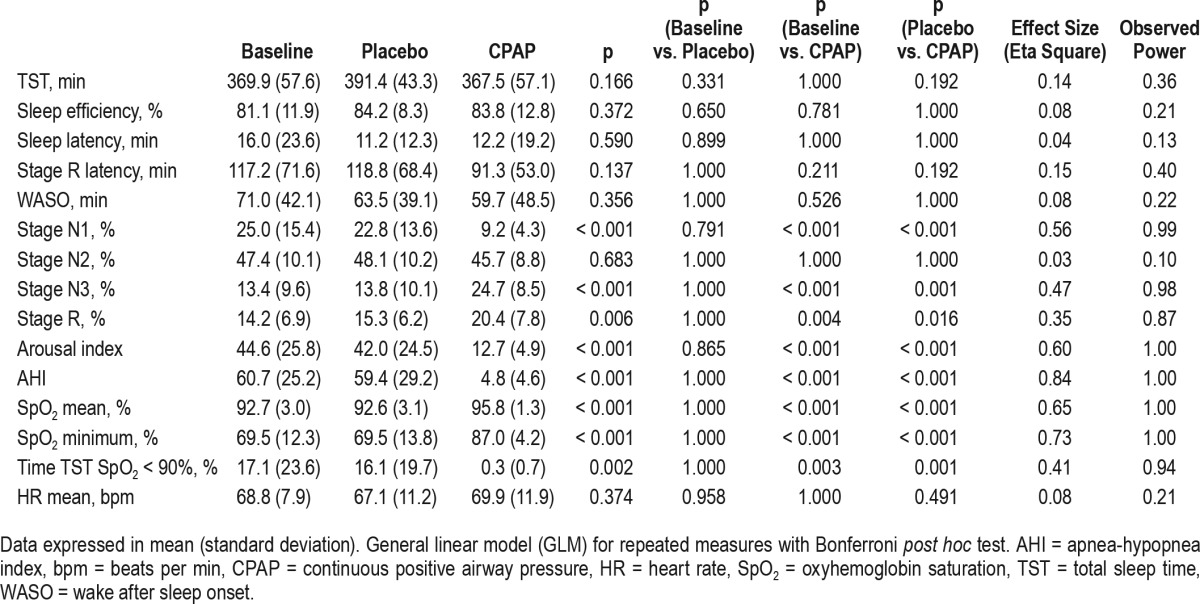
The objective sleep data evaluated by the PSG at baseline and after intervention for both the placebo and CPAP interventions are shown in Table 2. There was significant improvement in sleep parameters (stage N1 [p < 0.001], stage N3 [p < 0.001], stage R [p = 0.006] and arousal index [p < 0.001]), the AHI (p < 0.001) and SpO2 (mean and minimum values) (p < 0.001) after the CPAP treatment compared with those at baseline and compared with those after the placebo intervention.
Table 2.
Polysomnographic data at baseline, after 1 mo of placebo treatment and after 1 mo of continuous positive airway pressure treatment (n = 26).
The questionnaire scores at the three experimental conditions (baseline, after 1 mo of placebo intervention and after 1 mo of CPAP treatment) are shown in Table 3 and Figure 2. Normal values of ESS and Functional Outcomes of Sleep Questionnaire scores were observed after CPAP treatment (7.6 ± 4.1 and 17.2 ± 3.5, respectively). However, the ESS and BDI scores were decreased after both the placebo intervention and the CPAP treatment compared with those at baseline.
Table 3.
Questionnaires scores at baseline, after 1 mo of placebo treatment and after 1 mo of continuous positive airway pressure treatment (n = 26).
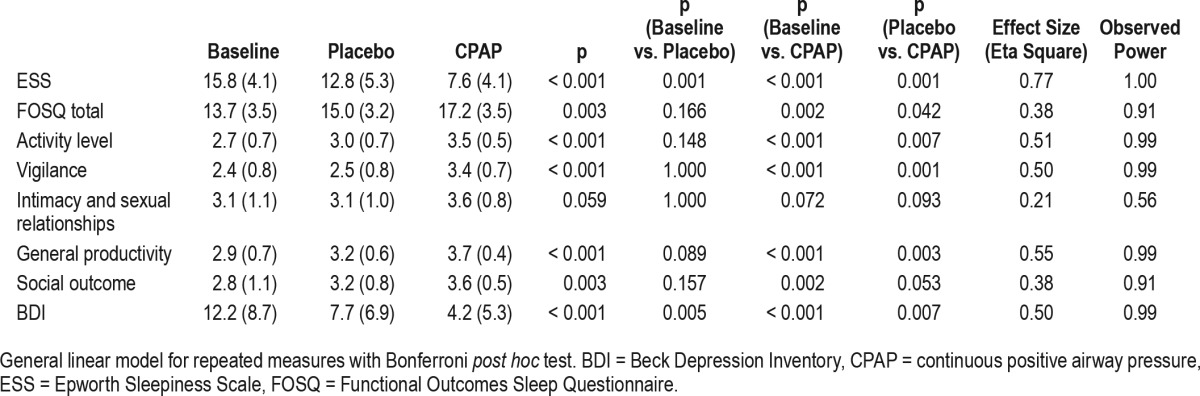
Figure 2. Questionnaires scores as a function of three experimental conditions (baseline, after 1 mo of placebo intervention, and after 1 mo of continuous positive airway pressure treatment).
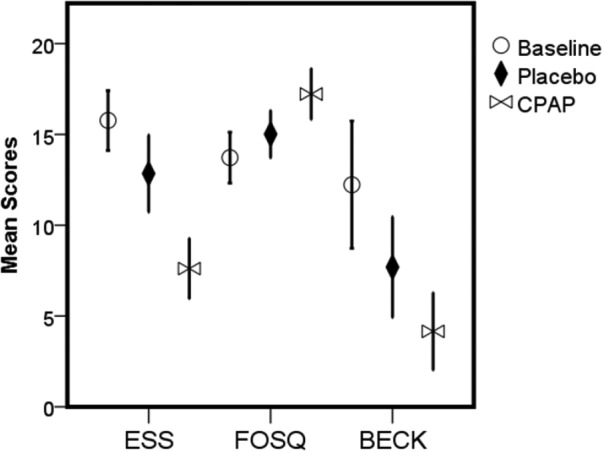
General linear model for repeated measures with Bonferroni post hoc test. All differences were significant, except for baseline and placebo conditions of FOSQ scores. Error bars = 95% CI. ESS = Epworth Sleepiness Scale, FOSQ = Functional Outcomes Sleep Questionnaire, BECK = Beck Depression Inventory.
The results of the comfort and satisfaction survey completed after both the placebo intervention and CPAP treatment are shown in Figure 3. After use of the CPAP device, compared with those who used the NDS, the patients reported having better sleep quality (p < 0.001), feeling better when they woke up in the morning (p < 0.001), having an improved effect on daily activities (p < 0.001) and having a better quality of life (p < 0.001). In addition, the patients reported little difficulty in using both conditions (p = 0.098).
Figure 3. The results of the comfort and satisfaction survey completed after the period of both the placebo and CPAP treatments.
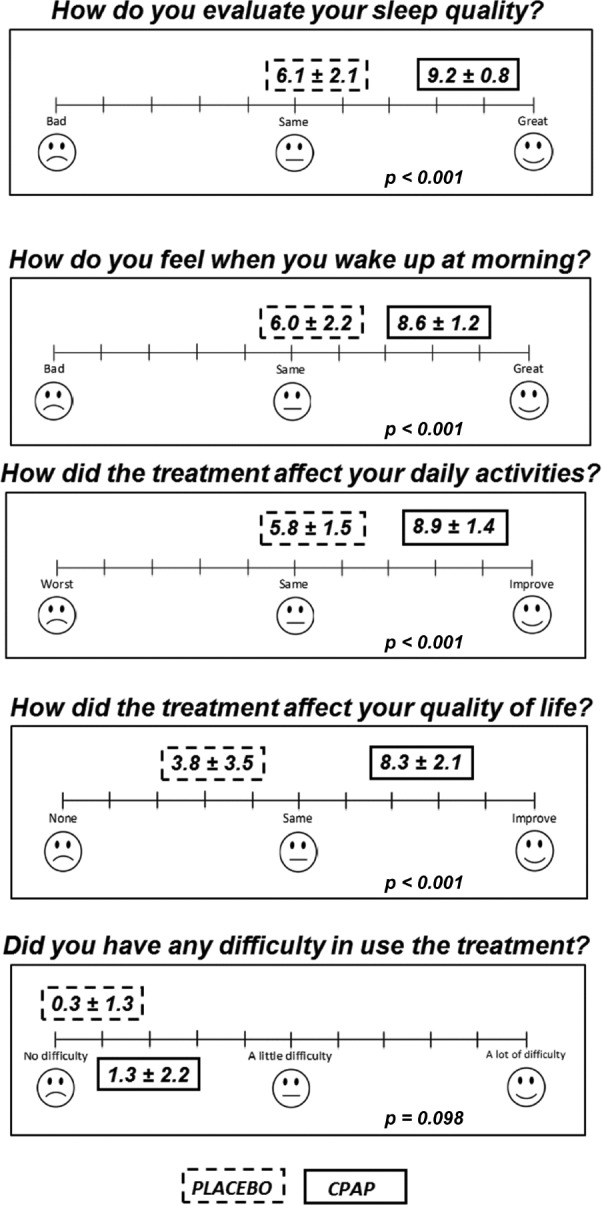
The instructions for the patients included: “You must answer the questions according to your perception after 1 mo of using the treatment during sleep”. The data are expressed as the mean ± standard deviation. The analysis was performed with general linear model (GLM) for repeated measures.
DISCUSSION
In this randomized crossover study, we sought to evaluate NDS as a placebo intervention compared with CPAP treatment in patients with severe OSA. As expected, in contrast with the CPAP device, the NDS showed no effect on objective sleep parameters but some subjective improvements in sleepiness and depressive symptoms were observed. However, the standard scores for the applied scales were achieved only after CPAP treatment.
The current study also tested objective and subjective data according to the order of interventions, and no differences were found. Thus, the observed results are likely free from the interference of the type of intervention that was first experienced by the patients. Previous studies comparing 2 or more nights of diagnostic PSG have reported a ‘first night effect’ on objective sleep parameters.29,30 Interestingly, we did not find differences in any studied parameter according to the randomization order in this study using the NDS as a placebo intervention.
Our results did not show any differences in objective sleep parameters using NDS compared with baseline. We reasoned that an ideal placebo should be used every night but not adversely influence the sleep quality, similar to CPAP. However, several authors had suggested that sham-CPAP should be considered as the best choice for the placebo intervention in a RCT that evaluates sleep breathing disorders.12–15 Even so, some evidence showed decreased objective sleep quality and slightly increased the AHI and oxyhemoglobin desaturation index on the first night using sham-CPAP compared to the baseline.15 Changes in the AHI were due to the shift in the type of respiratory event while using the sham-CPAP. Those authors observed a decreased number of obstructive apneas and an increasing in the number of hypopneas on the sham-CPAP compared to the baseline. Several potential hypotheses for this shift in respiratory events were described, including, among others, the increasing arousal index and wakefulness after sleep onset episodes while using the sham-CPAP. Although the clinical significance of these very subtle differences are unknown, and the observed effect sizes in the study were quite minor, those data can reinforce the adverse effect of sham-CPAP in objective sleep quality and, as mentioned earlier, such effects could be a bias in RCTs evaluating effectiveness of CPAP and using sham-CPAP as placebo intervention.
Our data showed that overall adherence to both the CPAP and NDS interventions was very high and satisfactory. It is also important to note that the patients reported little trouble in using both types of interventions, probably due to the same carefully applied follow-up protocol during both intervention periods. One could argue that obtaining a reliable measure of NDS adherence is difficult. Indeed, this should be one limitation of using the NDS as a placebo in RCT. Moreover, the time spent/intensity issues of counseling to adhere to CPAP versus shorter time needed to reinforce use in NDS may affect measures of daytime functional outcomes. However, it is important to consider that the follow-up protocol is crucial for the effectiveness of interventions and the quality of outcome data in any RCT.31 We suggest that NDS should be considered as an effective placebo for RCTs that evaluate patients with severe OSA, but it may be less applicable to asymptomatic OSA patients and patients with milder forms of OSA. Further, among the crossover studies using sham-CPAP as a placebo compared with active CPAP, all but one study demonstrated lower rates in the sham-CPAP arm.32–36 The only study showing similar rates of adherence between the arms was a study that included patients with mild OSA.37
One previous study also suggested that NDS was an effective placebo intervention in a randomized crossover protocol evaluating 3 mo of treatment with CPAP in 12 patients with acromegaly and moderate to severe OSA.23 Those authors found no effects of NDS on AHI compared with CPAP but an improvement of subjective parameters, which was compatible with a placebo effect. The current study showed that patients reported decreased diurnal sleepiness, and it also demonstrated reduced scores for the depression inventory after 1 mo of the NDS intervention compared with baseline. These observations, called a placebo effect, were previously discussed by other authors. The placebo effect is a clinical improvement following the administration of an inert treatment or procedure.38 Some evidence indicates that the placebo effect is a genuine psychobiological phenomenon and is dependent on complex cognitive processing information that includes a threat analysis in a given context, expectations of treatment outcome, and a desire for relief.39–41 Additionally, some neurobiological studies have shown that placebos activate the same brain circuits of effective drugs.39,42 Moreover, in studies involving depression, the placebo intervention partially imitates selective serotonin reuptake inhibitor-mediated brain activation.40
Another important discussion about RCTs that evaluate sleep breathing disorders involves practical, scientific, and ethical issues for any placebo intervention. Any degree of unblinding is undesirable; however, practical difficulties in preserving double blinding are problematic in those studies.17 Many research groups conducted RCTs without fully disclosing information to patients who received a placebo intervention, sham-CPAP in these cases, and they showed decreased adherence rates in the placebo arm compared with that in the CPAP treatment.32–36 Unblinding was a critical limitation in our study. Because it was not possible to “mask” the NDS treatment, the current findings should be pondered under of this potential bias and considered as a control may be more adequate. However, during the consenting process, we informed patients that our study aimed to test ‘two different types of OSA treatments’ rather than telling them that one intervention would be ineffective. Djavadkhani and colleagues described that the best way to preserve patient blinding in a sham-CPAP crossover trial was to avoid disclosing to patients the existence of a placebo, but it could involve an ethical dilemma that requires extra attention.17 As discussed previously, our data showed that adherence of both CPAP and NDS interventions was high, and patients reported little difficulty in using both types of interventions but better sleep quality and improved daytime activities were only observed after CPAP treatment.
In conclusion, the results of this randomized crossover study suggested that NDS could be used as a placebo intervention in RCT of patients with severe OSA who are sleepy as supported by the absence of effects on clinical sleep outcomes (sleep architecture and respiratory events) compared with CPAP.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP (#13/12301-5 to Fabiana Yagihara; #13/14025-5 to Dr. Santos-Silva) and the Núcleo Interdisciplinar da Ciência do Sono - NICS. The authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BDI
beck depression inventory
- BMI
body mass index
- CPAP
continuous positive airway pressure
- ESS
epworth sleepiness scale
- FOSQ
functional outcomes of sleep questionnaire
- NDS
nasal dilator strip
- OSA
obstructive sleep apnea
- PSG
polysomnography
- RCT
randomized controlled trials
REFERENCES
- 1.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes: a historical cohort study. Am J Respir Crit Care Med. 2014;190(2):218–225. doi: 10.1164/rccm.201312-2209OC. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182(2):269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166(16):1709–1715. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 8.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 9.Kushida CA, Littner MR, Hirshkowitz M, et al. American Academy of Sleep Medicine. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29(3):375–380. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 10.Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29(3):381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 11.Friedman LM, Furberg CD, DeMets D, Reboussin DM, Granger CB. Fundamentals of Clinical Trials. 5th ed. Switzerland: Springer International Publishing Switzerland; 2015. [Google Scholar]
- 12.Farré R, Hernández L, Montserrat JM, Rotger M, Ballester E, Navajas D. Sham continuous positive airway pressure for placebo-controlled studies in sleep apnoea. Lancet. 1999;353(9159):1154. doi: 10.1016/S0140-6736(99)01056-9. [DOI] [PubMed] [Google Scholar]
- 13.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164(4):608–613. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 14.Chasens ER, Drumheller OJ, Strollo PJ., Jr Success in blinding to group assignment with sham-CPAP. Biol Res Nurs. 2013;15(4):465–469. doi: 10.1177/1099800412461711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodway GW, Weaver TE, Mancini C, et al. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. Sleep. 2010;33(2):260–266. doi: 10.1093/sleep/33.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kushida CA, Nichols DA, Quan SF, et al. The Apnea Positive Pressure Long-term Efficacy Study (APPLES): rationale, design, methods, and procedures. J Clin Sleep Med. 2006;2(3):288–300. [PubMed] [Google Scholar]
- 17.Djavadkhani Y, Marshall NS, D'Rozario AL, et al. Ethics, consent and blinding: lessons from a placebo/sham controlled CPAP crossover trial. Thorax. 2015;70(3):265–269. doi: 10.1136/thoraxjnl-2014-206354. [DOI] [PubMed] [Google Scholar]
- 18.Höijer U, Ejnell H, Hedner J, Petruson B, Eng LB. The effects of nasal dilation on snoring and obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1992;118(3):281–284. doi: 10.1001/archotol.1992.01880030069015. [DOI] [PubMed] [Google Scholar]
- 19.Ulfberg J, Fenton G. Effect of Breathe Right nasal strip on snoring. Rhinology. 1997;35(2):50–52. [PubMed] [Google Scholar]
- 20.Todorova A, Schellenberg R, Hofmann HC, Dimpfel W. Effect of the external nasal dilator Breathe Right on snoring. Eur J Med Res. 1998;3(8):367–379. [PubMed] [Google Scholar]
- 21.Liistro G, Rombaux P, Dury M, Pieters T, Aubert G, Rodenstein DO. Effects of Breathe Right on snoring: a polysomnographic study. Respir Med. 1998;92(8):1076–1078. doi: 10.1016/s0954-6111(98)90358-4. [DOI] [PubMed] [Google Scholar]
- 22.Schönhofer B, Franklin KA, Brünig H, Wehde H, Köhler D. Effect of nasal-valve dilation on obstructive sleep apnea. Chest. 2000;118(3):587–590. doi: 10.1378/chest.118.3.587. [DOI] [PubMed] [Google Scholar]
- 23.Amaro AC, Duarte FH, Jallad RS, Bronstein MD, Redline S, Lorenzi-Filho G. The use of nasal dilator strips as a placebo for trials evaluating continuous positive airway pressure. Clinics. 2012;67(5):469–474. doi: 10.6061/clinics/2012(05)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 25.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–843. [PubMed] [Google Scholar]
- 26.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 27.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus CL, Vaughn BV. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2012. Version 2.0. [Google Scholar]
- 28.Sullivan GM, Feinn R. Using effect size-or why the P value is not enough. J Grad Med Educ. 2012;4(3):279–282. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agnew HWJ, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2(3):263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 30.Verhulst SL, Schrauwen N, De Backer WA, Desager KN. First night effect for polysomnographic data in children and adolescents with suspected sleep disordered breathing. Arch Dis Child. 2006;91(3):233–237. doi: 10.1136/adc.2005.085365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SM, Wallace E, O'Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2016;3:CD006560. doi: 10.1002/14651858.CD006560.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27:1229–1235. doi: 10.1183/09031936.06.00062805. [DOI] [PubMed] [Google Scholar]
- 33.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29(4):720–727. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 34.Cross MD, Mills NL, Al-Abri M, et al. Continuous positive airway pressure improves vascular function in obstructive sleep apnoea/hypopnoea syndrome: a randomized controlled trial. Thorax. 2008;63(7):578–583. doi: 10.1136/thx.2007.081877. [DOI] [PubMed] [Google Scholar]
- 35.Phillips CL, Yee BJ, Marshall NS, Liu PY, Sullivan DR, Grunstein RR. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: a randomized, placebo-controlled crossover trial. Am J Respir Crit Care Med. 2011;184(3):355–361. doi: 10.1164/rccm.201102-0316OC. [DOI] [PubMed] [Google Scholar]
- 36.Jones A, Vennelle M, Connell M, et al. The effect of continuous positive airway pressure therapy on arterial stiffness and endothelial function in obstructive sleep apnea: a randomized controlled trial in patients without cardiovascular disease. Sleep Med. 2013;14(12):1260–1265. doi: 10.1016/j.sleep.2013.08.786. [DOI] [PubMed] [Google Scholar]
- 37.Marshall NS, Neill AM, Campbell AJ, Sheppard DS. Randomised controlled crossover trial of humidified continuous positive airway pressure in mild obstructive sleep apnoea. Thorax. 2005;60(5):427–432. doi: 10.1136/thx.2004.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benedetti F. Placebo and the new physiology of the doctor-patient relationship. Physiol Rev. 2013;93(3):1207–1246. doi: 10.1152/physrev.00043.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia imaging a shared neuronal network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 40.Diederich NJ, Goetz CG. The placebo treatments in neurosciences: New insights from clinical and neuroimaging studies. Neurology. 2008;71(9):677–684. doi: 10.1212/01.wnl.0000324635.49971.3d. [DOI] [PubMed] [Google Scholar]
- 41.Colloca L, Miller FG. Role of expectations in health. Curr Opin Psychiatry. 2011;24(2):149–155. doi: 10.1097/YCO.0b013e328343803b. [DOI] [PubMed] [Google Scholar]
- 42.Piedimonte A, Benedetti F, Carlino E. Placebo-induced decrease in fatigue: evidence for a central action on the preparatory phase of movement. Eur J Neurosci. 2015;41(4):492–497. doi: 10.1111/ejn.12806. [DOI] [PubMed] [Google Scholar]


