Abstract
Study Objectives:
Autotitrating continuous positive airway pressure (CPAP) devices adjust pressure in response to changes in airflow and are an alternative to attended in-laboratory titration polysomnography (PSG) to determine optimal pressure levels. The aim of this study was to compare the performance of the System One RemStar Auto A-Flex (Philips Respironics, Murrysville, PA, USA) automatically adjusted positive airway pressure (APAP) mode to manually titrated, fixed pressure CPAP and to validate the device's breathing event detection capabilities against attended in-laboratory PSG.
Methods:
Sixty-one patients investigated in five centers for moderate to severe obstructive sleep apnea between May 2012 and June 2013 were invited to participate. Participants underwent two full-night attended polysomnograms in random order with manually titrated, fixed pressure CPAP versus APAP.
Results:
Fifty-three participants with a mean apnea-hypopnea index (AHI) of 45.9 ± 23 completed two sleep studies and were included in the analysis. There were significant but not clinically relevant differences between APAP and CPAP respectively: Apnea index [1.0 (2.8 ± 0.8), median (mean ± standard deviation)] versus [1.8 (5.3 ± 11.5)], p = 0.004; percentage of N1 sleep [12.3 (15.9 ± 0.5)] versus [14.3 (18.9 ± 12.7)], p = 0.028. AHI values differed between PSG [2.8 (5.5. ± 9.3)] and device [3.7 (6.0 ± 8.6)], p = 0.003). Regarding residual events detection, intraclass correlation coefficients for AHI were strong (0.956, p < 0.001) and the area under the curve was 0.988 (AHI cut-off value of 10).
Conclusions:
The new APAP modality was effective and residual apnea-hypopnea indices calculated by the device strongly correlated to those assessed by PSG.
Commentary:
A commentary on this article appears in this issue on page 167.
Citation:
Gagnadoux F, Pevernagie D, Jennum P, Lon N, Loiodice C, Tamisier R, van Mierlo P, Trzepizur W, Neddermann M, Machleit A, Jasko J, Pépin JL. Validation of the System One RemStar Auto A-Flex for obstructive sleep apnea treatment and detection of residual apnea-hypopnea index: a European randomized trial. J Clin Sleep Med. 2017;13(2):283–290.
Keywords: APAP, CPAP, residual event detection, sleep apnea
INTRODUCTION
Obstructive sleep apnea-hypopnea syndrome (OSA) is a highly prevalent disease characterized by recurrent episodes of partial or complete obstruction of the upper airway during sleep. Most recent estimates of OSA prevalence suggest that 13% of men and 6% of women have clinically significant OSA.1–3 Continuous positive airway pressure (CPAP), as the first-line therapy for moderate to severe OSA, improves daytime alertness and health-related quality of life and reduces arterial pressure.4,5 Observational prospective cohort studies indicate that regular CPAP therapy is also associated with a lower risk of driving-related accidents and cardiovascular events.6,7
BRIEF SUMMARY
Current Knowledge/Study Rationale: Automatically adjusted positive airway pressure (APAP) offers potential clinical advantages when compared to fixed pressure continuous positive airway pressure (CPAP) by adjusting pressure to changes in airflow and providing data about residual respiratory events under treatment. However, algorithms for events detection and automatic pressure adjustments are device specific and have to be evaluated by adequately powered studies.
Study Impact: This European randomized trial demonstrates that APAP with the System One RemStar Auto A-Flex device is as effective as manually titrated fixed-pressure CPAP in abolishing obstructive breathing events with no deleterious effect of pressure adjustments through the night on sleep structure and fragmentation. The System One RemStar Auto A-Flex device has a high accuracy for breathing events detection when compared to full-night polysomnography.
After a patient receives a diagnosis of OSA, the optimal pressure for maintaining upper airway patency in all sleep stages whatever the body position has to be determined.8 For years, the standard operating procedure was to perform a full-night attended in-laboratory titration polysomnography (titration PSG) during which CPAP was adjusted by the sleep technician throughout the recording period. However, full-night attended PSG is labor intensive and may be hampered by long waiting lists in the sleep laboratories. A single-night estimation of optimal pressure does not reflect real-life conditions, including alcohol intake. Automatically adjusted positive airway pressure (APAP) devices provide potential solutions to the aforementioned problems and are now widely used. These devices can be used either for titration to determine the optimal fixed CPAP level or as a long term treatment modality for chronic use at home.9 Data from randomized controlled trials, including patients with moderate to severe OSA and without signifi-cant comorbidities, showed that treatment effects are similar between APAP and CPAP.10 However, it has been suggested that OSA patients with cardiovascular comorbidities are at higher risk of residual respiratory events under APAP.11 Furthermore, findings on efficacy of APAP devices cannot be generalized, as the algorithms for automatic pressure adjustments are device specific.12 One of the advantages of APAP devices is to provide data about residual respiratory events under treatment. Although potentially useful in clinical practice, the accuracy of this downloaded information is device dependent and has to be evaluated in an adequately powered study.
The System One RemStar Auto A-Flex device (Philips Respironics, Murrysville, PA, USA) measures changes in airflow by an internal pneumotachograph to identify respiratory events. It has not only the ability to detect airway obstruction from respiratory effort related arousals (RERAs) to hypopneas and apneas, but also to identify Cheyne-Stokes respiration. The aim of this randomized controlled trial was to compare the efficacy of the System One RemStar Auto A-Flex to fixed-pressure CPAP established by titration PSG for the treatment of OSA, and to validate the device's breathing event detection capabilities compared to full-night, attended PSG.
METHODS
The study was conducted in accordance with the amended Declaration of Helsinki with ethics committee approval from the CPP Ouest-II and AFSSAPS (UEC/AnnR/DA/2011.231) in France; De Videnskabsetiske Komileer: Region Hovedstaden (CVR/SL-nr:29 19 06 23) in Denmark; Medisch-Ethische Toetsingcommissie (M11-842) in the Netherlands and Ethik Kommission der Arztekammer Westfalen-Lippe und der Medizinischen Fakultat der Westfalischen Wilhelms-Universitat (2011-627-f-M) in Germany. The study was registered under ISRCTN Register Nr ISRCTN 19824122.
Study Population
Adult patients who had an apnea-hypopnea index (AHI) > 15 and > than 50% obstructive events as confirmed by a recent full night attended PSG (within 14 days before participating in the study started) were eligible for the study.
Exclusion criteria included acute upper respiratory infection or surgery of the upper airway within 90 days prior to study enrollment, associated sleep disorders including severe insomnia, restless legs syndrome, use of sedatives or other drugs which may impair sleep, previous exposure to CPAP therapy, acute dermatitis or other skin lesions or trauma interfering with the application of a mask, shift workers, and other major medical conditions that, at the discretion of the site investigator, precluded participation. Patients were also excluded in case of CPAP titration failure (inability to determine a CPAP pressure reducing AHI < 10 during titration PSG8). Participants were informed that the purpose of the study was to evaluate a positive airway pressure device with two different modes. No additional information about the modes was provided. All participants provided written informed consent. Participants were recruited between May 2012 and June 2013.
Study Design
This was a double-blind, randomized, crossover study performed at five European sites. Prior to the titration PSG, patients underwent a daytime CPAP acclimatization session. After interface selection and mask fitting, CPAP was introduced gradually. Patients were first asked to hold the mask and breathe through it, without the CPAP hose connected. Then, the machine was connected, a CPAP pressure of approximately 4 cmH2O was selected and the patients practiced breathing with the interface for approximately 5 min. When the patient was comfortable with the CPAP pressure, instructions were provided on how to fit the mask and headgear and approximately 15 to 20 min were spent acclimatizing to CPAP at 4 cmH2O. For the titration PSG, CPAP was initiated at 4 cmH2O and was increased in 1-cmH2O increments to the point where disordered breathing events, including hypopneas, RERAs, snoring, and flow limitation were eliminated. In line with current clinical guidelines,8 a successful titration should reduce AHI < 10 events/h and include a periods of supine position and rapid eye movement sleep at the selected pressure.
Following the titration PSG, participants were randomly assigned to 1 night of APAP and 1 night of fixed CPAP at the pressure determined previously. APAP and fixed pressure CPAP were delivered by the System One RemStar Auto A-Flex on 2 consecutive nights and within 2 w of the titration PSG. On the APAP night, the pressure was set to a range of 4 to 20 cmH2O. The same interface was used on each PSG.
Patients were subsequently treated at home with either fixed CPAP or APAP based on their preference under physician guidance.
Sleep Recordings
On each study night, attended full-night PSG on therapy was performed using an Alice 5 (Philips Respironics, Murrysville, PA, USA) or similar system. The PSG was initiated near the participant's usual bedtime and ended at approximately 06:00. A standard montage13 was used including electroencephalogram, electro-oculogram, chin electromyogram, leg electromyogram, electrocardiogram, effort parameters, airflow parameters, oxygen saturation, and body position. Pressure and flow measurements acquired with a pneumotachograph were included. Four channels of data acquired from the therapy device (pressure, flow, leak, and breathing events) were included in the PSG montage. The only airflow measurement was that of the pneumotachograph in the therapy device, and this flow channel was scored by the Somnolyzer (Koninklijke Philips N.V.) to obtain AHIPSG and by the therapy device to obtain AHIFLOW.
PSG Scoring
Scoring was performed at a single center using Somnolyzer 24 × 7 (Koninklijke Philips N.V.) system, a validated, computer assisted-automated PSG scoring software program.14,15 Each recording was subjected to automated analysis by the Somnolyzer system followed by expert review. The specialized scoring was used so that the pressure pulses delivered by the CPAP device were not misclassified as hypopneas. According to American Academy of Sleep Medicine (AASM) guidelines, hypopneas were defined by a ≥ 30% decline in airflow for at least 10 sec accompanied by an oxyhemoglobin desaturation of ≥ 4% (Recommended Rule 4A16). Electronic copies of the PSG recordings were deidentified by a PSG technician prior to transferring the file to the Somnolyzer 24 ×7 system. These data were used to determine the efficacy of the autotitrating algorithm in treating OSA. The expert reviewer was blinded to the participant's health information.
Study Device
The same device was used for both fixed pressure CPAP and APAP (System One RemStar Auto A-Flex). The System One devices measure changes in airflow by an internal pneumotachograph to identify respiratory events. A moving window of 3 or 4 min is established and, if flow decreases by 40% to 80% for at least 10 sec, the event is labeled a hypopnea; a decrease in flow by more than 80% for at least 10 sec is labeled an apnea. A RERA is defined as a sequence of breaths that exhibit both a subtle reduction in airflow and progressive flow limitation that is terminated by a sudden increase in airflow without flow limitation, and the event does not meet the conditions for an apnea or hypopnea.17
Statistical Analysis
Baseline data were summarized among all participants who contributed to the analysis population. The primary analysis compared PSG variables between the APAP and CPAP modes. The paired differences in the apnea indices (APAP versus CPAP) exhibited an asymmetric distribution; therefore, the PSG measures were examined with the nonparametric Wilcoxon signed-rank test. For the event detection analysis comparing PSG to device scores, the indices for Somnolyzer-scored events were computed as the number of events per hour of sleep. The indices for device-detected events were the number of events per hour of therapy time (i.e., device recording time). Agreement of respective indices between the two detection methods was assessed using the Wilcoxon signed-rank test. Intraclass correlation coefficient (ICC) was performed using a two-way random model measuring absolute agreement for single measures. In addition, Bland-Altman plots of the AHI were generated for visualization of the bias and limits of agreement. The sensitivity, specificity, and positive and negative predictive values for given device-detected AHI cutoff values were calculated, and receiver operating characteristic (ROC) curves were constructed. The data were analyzed using computer programs: SPSS (IBM, version 20; Chicago, IL, USA) and Analyse-it (Analyse-it Software, Ltd. version 2.26; UK). Descriptive statistics include mean, standard deviation, and median values. Statistical trends were considered significant at p < 0.05.
RESULTS
Patient Characteristics
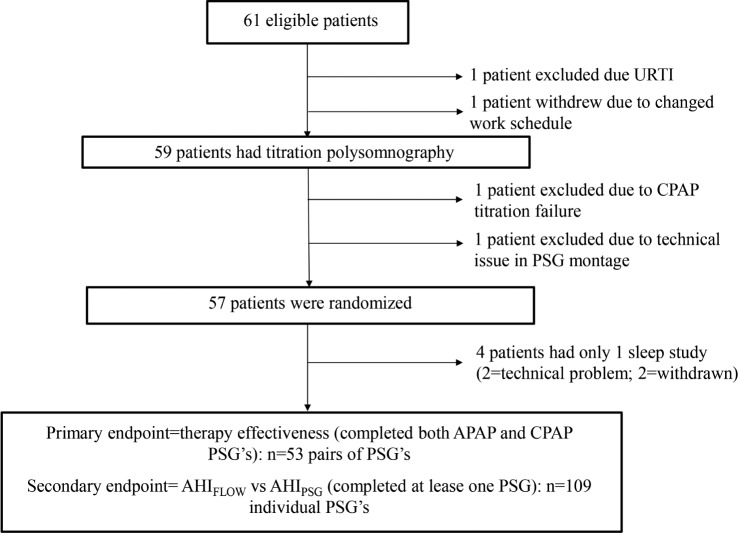
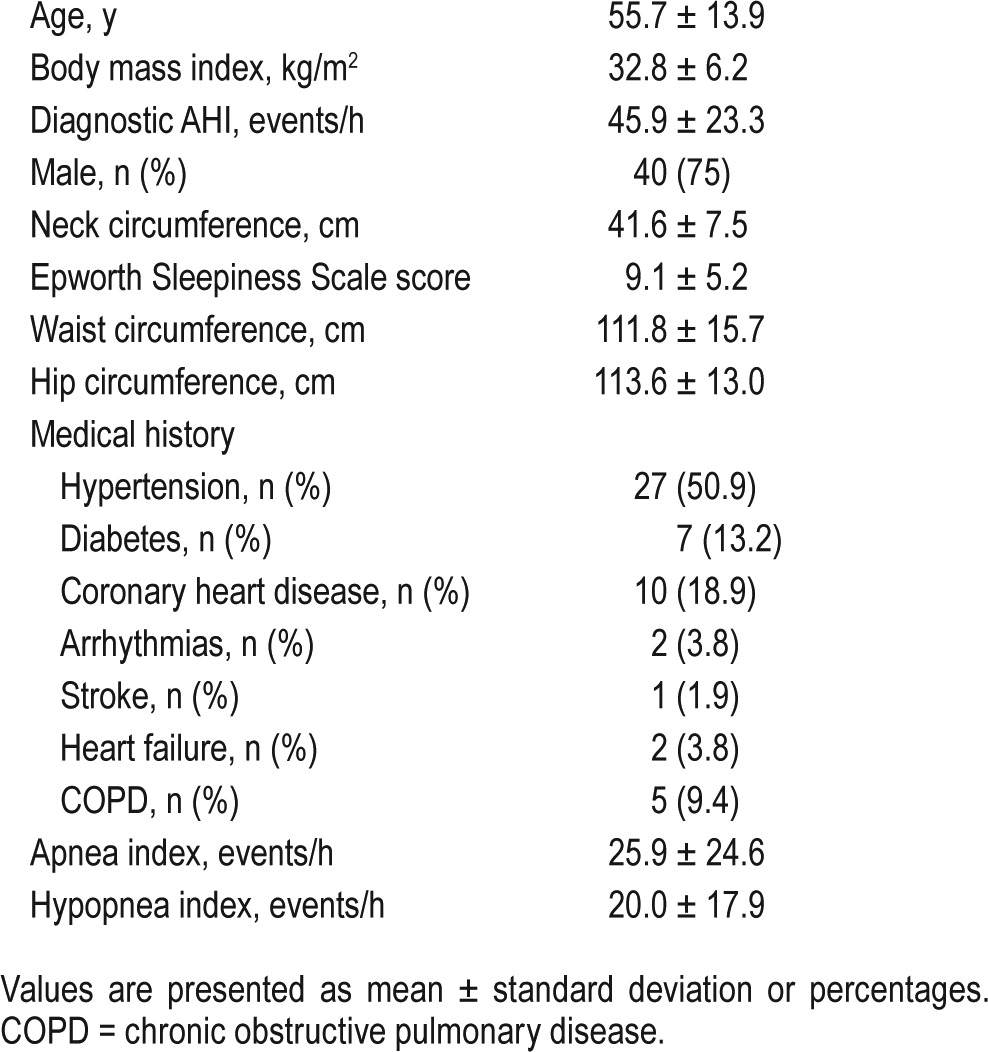
Sixty-one participants among 2,338 patients with moderate to severe OSA referred during the same period in the five participating centers met the inclusion criteria and were invited to participate to the study. Four patients withdrew prior to randomization. One participant was excluded due to an upper respiratory tract infection and one withdrew due to changed work schedule. One patient was excluded due to CPAP titration failure, and in one participant titration PSG recording was hampered by technical issues (Figure 1). Four of 57 randomized patients completed only 1 sleep study; therefore, the final sample included in the primary endpoint analysis was 53. Participants' characteristics are presented in Table 1. The mean diagnostic AHI was 45.9 ± 23.3. The study population was a classic moderate to severe OSA population—predominantly male and obese with frequent cardiovascular comorbidities. Positive airway pressure was delivered through an oronasal mask in 17 participants and a nasal mask in 30 participants. Information about mask type was not available in six participants.
Figure 1. Flowchart of study.
APAP = automatically adjusted positive airway pressure, CPAP = continuous positive airway pressure, PSG = polysomnography, URTI = upper respiratory tract infection.
Table 1.
Participants' characteristics (n = 53).
Comparative Efficacy of APAP Versus Fixed-Pressure CPAP
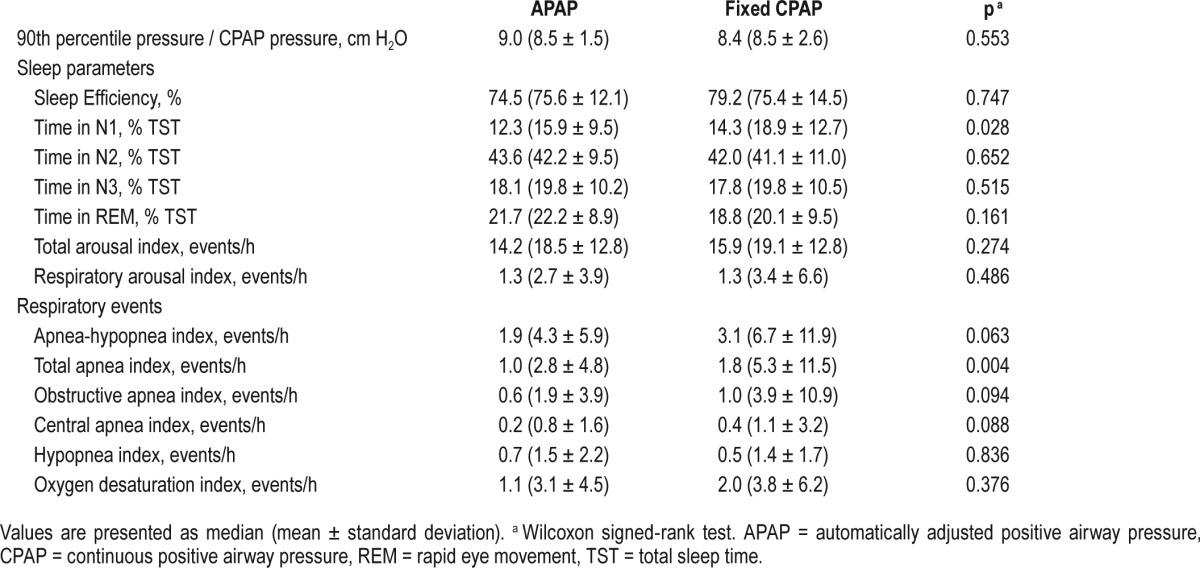
There were no significant differences between median (mean ± standard deviation) values of 90th percentile pressure on APAP mode and CPAP pressure on fixed CPAP mode (9.0 [8.5 ± 1.5] cmH2O versus 8.4 [8.5 ± 2.6] cmH20 respectively; p = 0.553 (Table 2). The comparison of polysomno-graphic data between APAP and fixed pressure CPAP showed no significant differences in sleep efficiency and the percent of total sleep time spent in N2, N3, and rapid eye movement sleep stages. The amount of N1 sleep was slightly lower on APAP compared to CPAP (12.3 [15.9 ± 9.5]) versus 14.3 [18.9 ± 12.7] respectively, p = 0.028). Because APAP adjusts pressure throughout the night, arousal indexes were evaluated in detail. No significant differences were observed between APAP and CPAP for the total arousal index and the respiratory arousal index. Participants were treated effectively with both APAP and fixed pressure CPAP with AHI values of 1.9 (4.3 ± 5.9) versus 3.1 (6.7 ± 11.9) respectively, p = 0.063. The apnea index was significantly lower for APAP compared to fixed pressure CPAP (1.0 [2.8 ± 4.8], versus 1.8 [5.3 ± 11.5], p = 0.004). There were no significant differences between APAP and fixed pressure CPAP for the hypopnea index, the oxygen desaturation index, and the mean nocturnal oxygen saturation.
Table 2.
Comparison of polysomnography data on automatically adjusted positive airway pressure versus fixed-pressure continuous positive airway pressure.
Assessment of Residual Respiratory Events
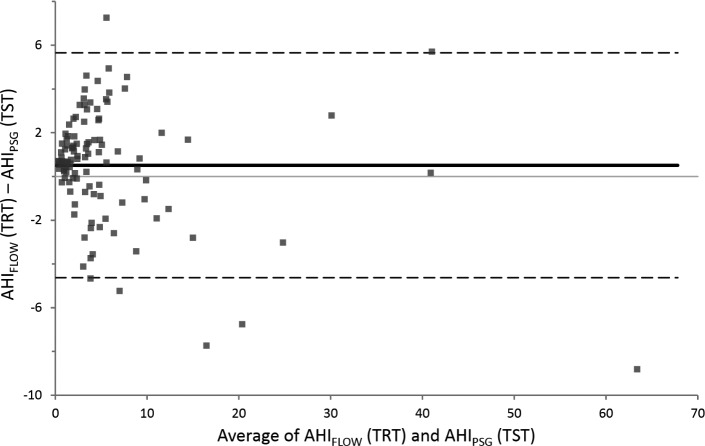
The residual AHIs computed by the device (AHIFLOW) were compared to the AHI automated scored on PSG using Somnolyzer system (AHIPSG). AHIFLOW and AIFLOW were strongly correlated to AHIPSG and AIPSG with ICC of 0.956 and 0.973, respectively (Table 3 and Figure 2). The ICC was lower for hypopnea index (0.249) but still significant (p = 0.003). The ICC did not reach significance for RERA. The agreement between AHIFLOW and AHIPSG according to the Bland and Altman method is presented in Figure 3. The device slightly overestimates the AHI.
Table 3.
Comparison of respiratory event indices detected by the device and scored by polysomnography.
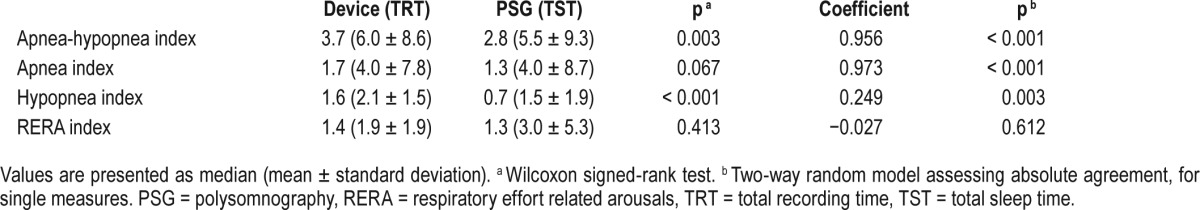
Figure 2. Scatterplots.
Scatterplots showing the relationship between apnea-hypopnea index detected by the device (AHIFLOW) and manually scored on polysomnography (AHIPSG) (A) and between apnea index detected by the device (AIFLOW) and manually scored on polysomnography (AIPSG) (B). TRT = total recording time, TST = total sleep time.
Figure 3. Bland and Altman plots.
Difference versus mean apnea-hypopnea index identified by the device (AHIFLOW) and manually scored on polysomnography (AHIPSG). TRT = total recording time, TST = total sleep time.
Fourteen of the 109 PSGs analyzed showed an AHIPSG ≥ 10; of these 14 PSGs, 12 (85.7%) also had an AHIFLOW ≥ 10. The remaining 95 PSGs had an AHIPSG < 10, and of these, 94 (98.9%) also had an AHIFLOW < 10. The mean difference in AHI was 0.51 (95% confidence interval, 0.02 – 1.01) and the limits of agreement were −4.6 to 5.6.
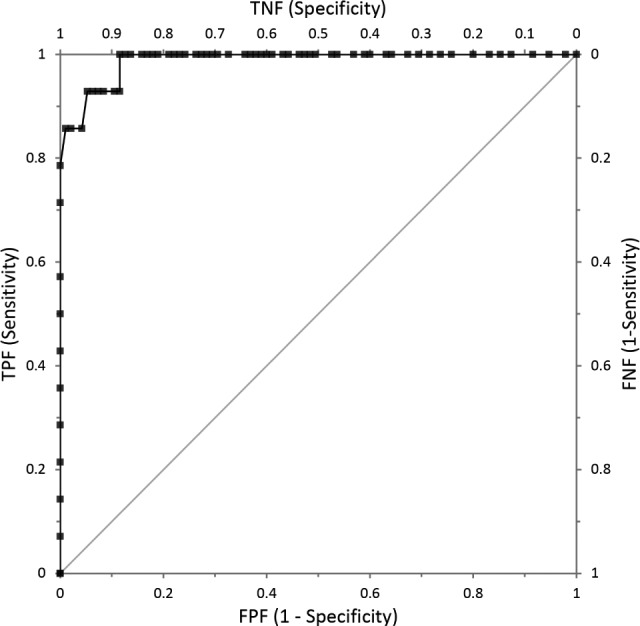
The area under the curve for the AHI cutoff of ≥ 10 was 0.988 (Figure 4). For an AHIFLOW cut-off of 10, the sensitivity was 0.86, the specificity was 0.99, the positive predictive value was 0.92, and the negative predictive value was 0.98. An AHIFLOW cutoff of 7.8 had a sensitivity of 0.929 and a specificity of 0.926, with positive predictive value of 0.65 and a negative predictive value of 0.99.
Figure 4. Receiver operating characteristic plot using an apnea-hypopnea index (AHI) polysomnography (PSG) cutoff of 10 events/h.
DISCUSSION
There are three main findings in the current study. First, APAP with the System One RemStar Auto A-Flex device was as effective as CPAP at a fixed pressure established by titration PSG in abolishing obstructive breathing events during sleep. Second, pressure adjustments through the night under APAP had no deleterious effect on sleep structure and fragmentation. Third, the residual AHI calculated by the device strongly correlated to those assessed by PSG.
The use of APAP is growing, because of its efficacy in reducing sleep-disordered breathing and daytime sleepiness, associated with a reduction of the costs of titration and improvement in the time from diagnosis to therapy when compared to conventional titration PSG.10,18 Our finding that APAP and fixed-pressure CPAP are equivalent in terms of normalization of respiratory events, and effect on sleep quality is in accordance with data from previous reviews and meta-analyses.9,10 In a recent meta-analysis including 16 trials providing sufficient data for analysis of residual AHI during treatment, none of the studies reported a statistically significant difference in AHI between APAP and CPAP.10 However, bench studies clearly demonstrated that specific APAP devices respond quite differently to the same condition and display considerable differences in pressure profiles, as also observed in clinical trials.9,12 This implies that the effectiveness and tolerance of APAP in treating sleep-disordered breathing should be evaluated individually for each APAP device.
In the current study, the System One RemStar Auto A-Flex device was highly effective in reducing the AHI and improving oxygen saturation during sleep. The residual AHI tended to be lower under APAP, and statistical significance was reached for the apnea index. This finding may be explained by several factors including night-to-night variability of sleep-disordered breathing,19 how the device detects respiratory events and adjusts pressure, and suboptimal therapeutic pressure titration. As pointed out in a previous review,20 an inherent risk of performing only 1 titration night is the inability to find an appropriate pressure level.
One potential limitation of the current study is the relatively low rate of inclusion that was explained by the study needs requiring four polysomnograms. However, the study population was a typical OSA population that was predominantly male, obese, and with comorbidities and moderate to severe OSA, suggesting no selection bias. Another potential limitation is that the comparison between APAP and fixed-pressure CPAP was limited to polysomnographic data with no clinical outcomes. A recent meta-analysis concluded that APAP improved compliance by approximately 11 min per night and reduced sleepiness as measured by the Epworth Sleepiness Scale by approximately 0.5 points compared with fixed pressure CPAP.10 As noted by the authors, the clinical relevance of such statistically significant differences is unclear. A recent study suggested that APAP might be less effective than CPAP in reducing cardiovascular risk factors including blood pressure and insulin resistance.21 However, it should be noted that APAP was also less effective in reducing AHI and the oxygen desaturation index in this report, which was not the case in the current study. In a subsequent report from the same group,22 APAP treatment was characterized by a greater sympathetic activation during sleep compared with CPAP that might account for the different blood pressure-lowering effects induced by the two treatments. It has also been reported that APAP is associated with an increased sleep fragmentation that may contribute to increase nighttime blood pressure.23,24 Interestingly, we found no increase in sleep fragmentation indices under APAP therapy with the System One RemStar Auto A-Flex device when compared to fixed-pressure CPAP.
CPAP adherence tracking systems provide a strong platform to generate outcome data in OSA including adherence, hours of use, mask leak, and residual AHI.25 CPAP adherence can be reliably determined from CPAP tracking systems. Conversely, data on residual breathing events derived from CPAP tracking systems are more difficult to interpret as event detection algorithms differ among the manufacturers. There have been only a few studies examining the accuracy of these event detection algorithms.11,25–31 A review of the more recent studies concluded that the assessment of the residual apnea index was more robust than the hypopnea index, and that the event detection algorithms tended to underestimate the AHI at higher manually scored AHI values (AHI > 10 events/h) and overestimate the AHI at lower manually scored AHI values (AHI < 10 events/h).25–28,30,31 From a clinical perspective, the authors concluded that these event detection algorithms can be used if the data are at either end of the spectrum. Treatment of OSA is likely to be effective if AHI < 10 events/h, and is likely to be inadequate if AHI > 20 events/h. The latter is especially the case if detected events are apneas. As previously reported27,28,30,31 we found that the correlation between device-detected and manually scored breathing events was higher for apneas than for hypopneas. This is not surprising because the polysomnographic definition of hypopnea includes oxygen desaturations and arousals that are not detected by the APAP device.25 In our study population with relatively low PSG-scored residual breathing events, we demonstrated that the System One RemStar Auto A-Flex device has a high accuracy for breathing events detection. In line with a recent American Thoracic Society statement,25 our results suggest that a follow-up sleep recording under positive airway pressure therapy is not required in patients with a clinical improvement device (AHI < 10 events/h).
An update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events was published in October 2012.13 Since our study began in May 2012, we used AASM 2007 recommended criteria.16 The 2012 AASM criteria were found to increase the incidence of hypopneas were compared to 2007 recommended criteria.32 It seems unlikely that using AASM 2012 criteria would have modified the findings of the within-patient comparison between APAP and CPAP. Conversely, changing hypopnea definition may have modified the agreement between AHIFLOW and AHIPSG. As the device tended to overestimate the AHI in our study, increasing HIPSG by using AASM 2012 criteria may have contributed to improve the agreement between AHIFLOW and AHIPSG. It should also be noted that our patients were treated with relatively low-pressure levels. The current findings may not be generalized to patient populations requiring substantially higher pressure.
CONCLUSIONS
APAP was effective and did not differ from treatment with CPAP at a fixed pressure established by titration PSG. The residual AHI identified by the device strongly correlated to the AHI identified on the PSG's. The algorithm of the System One RemStar Auto A-Flex device is proprietary and therefore the current findings are not generalizable to other devices.
DISCLOSURE STATEMENT
The study was supported by unrestricted grants from Philips Respironics. Data analysis and interpretation were under responsibility of academic authors. Frédéric Gagnadoux has received research grants from Philips Respironics, Resmed Inc, and Fisher and Paykel Healthcare. He has received speaker fees from Philips Respironics, Resmed Inc, Fisher and Paykel Healthcare, and Vitalaire. Dirk Pevernagie, Poul Jennum, Martina Neddermann, and Annika Machleit, have received research grants from Philips Respironics. Renaud Tamisier has received research grants from Resmed Foundation. Jeffrey Jasko is employed by Philips Respironics. Jean-Louis Pépin has received unrestricted research funds from Philips Respironics, Resmed Inc., GSK, Fondation de la recherche medicale. He has also received lecture fees from Resmed Inc., Perimetre, Philips Respironics, Fisher and Paykel Healthcare, Astra-Zeneka. He has also received conference-traveling grant from Agiradom and Teva. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- AHIFLOW
AHI as scored by the therapy device
- AHIPSG
AHI as scored by the Somnolyzer
- APAP
automatically adjusted positive airway pressure
- COPD
chronic obstructive pulmonary disease.
- CPAP
continuous positive airway pressure
- ICC
Intraclass correlation coefficient
- OSA
obstructive sleep apnea
- PSG
polysomnography
- REM
rapid eye movement
- RERAs
respiratory effort related arousals
- ROC
receiver operating characteristic
- TRT
total recording time
- TST
total sleep time
- URTI
upper respiratory tract infection
REFERENCES
- 1.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips B, Gozal D, Malhotra A. What is the future of sleep medicine in the United States? Am J Respir Crit Care Med. 2015;192(8):915–917. doi: 10.1164/rccm.201508-1544ED. [DOI] [PubMed] [Google Scholar]
- 4.Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314(21):2280–2293. doi: 10.1001/jama.2015.16303. [DOI] [PubMed] [Google Scholar]
- 5.McDaid C, Griffin S, Weatherly H, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess. 2009;13(4):iii–iv. xi–xiv, 1–119, 143–274. doi: 10.3310/hta13040. [DOI] [PubMed] [Google Scholar]
- 6.Antonopoulos CN, Sergentanis TN, Daskalopoulou SS, Petridou ET. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev. 2011;15(5):301–310. doi: 10.1016/j.smrv.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-de-la-Torre M, Campos-Rodriguez F, Barbe F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1(1):61–72. doi: 10.1016/S2213-2600(12)70051-6. [DOI] [PubMed] [Google Scholar]
- 8.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- 9.Hertegonne K, Bauters F. The value of auto-adjustable CPAP devices in pressure titration and treatment of patients with obstructive sleep apnea syndrome. Sleep Med Rev. 2010;14(2):115–119. doi: 10.1016/j.smrv.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Ip S, D'Ambrosio C, Patel K, et al. Auto-titrating versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: a systematic review with meta-analyses. Syst Rev. 2012;1:20. doi: 10.1186/2046-4053-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang HC, Hillman DR, McArdle N. Control of OSA during automatic positive airway pressure titration in a clinical case series: predictors and accuracy of device download data. Sleep. 2012;35(9):1277–1283. doi: 10.5665/sleep.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farre R, Montserrat JM, Rigau J, Trepat X, Pinto P, Navajas D. Response of automatic continuous positive airway pressure devices to different sleep breathing patterns: a bench study. Am J Respir Crit Care Med. 2002;166(4):469–473. doi: 10.1164/rccm.2111050. [DOI] [PubMed] [Google Scholar]
- 13.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderer P, Moreau A, Woertz M, et al. Computer-assisted sleep classification according to the standard of the American Academy of Sleep Medicine: validation study of the AASM version of the Somnolyzer 24 × 7. Neuropsychobiology. 2010;62(4):250–264. doi: 10.1159/000320864. [DOI] [PubMed] [Google Scholar]
- 15.Punjabi NM, Shifa N, Dorffner G, Patil S, Pien G, Aurora RN. Computer-assisted automated scoring of polysomnograms using the Somnolyzer System. Sleep. 2015;38(10):1555–1566. doi: 10.5665/sleep.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 17.Li QY, Berry RB, Goetting MG, et al. Detection of upper airway status and respiratory events by a current generation positive airway pressure device. Sleep. 2015;38(4):597–605. doi: 10.5665/sleep.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Planes C, D'Ortho MP, Foucher A, et al. Efficacy and cost of home-initiated auto-nCPAP versus conventional nCPAP. Sleep. 2003;26(2):156–160. doi: 10.1093/sleep/26.2.156. [DOI] [PubMed] [Google Scholar]
- 19.Gagnadoux F, Pelletier-Fleury N, Philippe C, Rakotonanahary D, Fleury B. Home unattended vs hospital telemonitored polysomnography in suspected obstructive sleep apnea syndrome: a randomized crossover trial. Chest. 2002;121(3):753–758. doi: 10.1378/chest.121.3.753. [DOI] [PubMed] [Google Scholar]
- 20.Morgenthaler TI, Aurora RN, Brown T, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep. 2008;31(1):141–147. doi: 10.1093/sleep/31.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patruno V, Aiolfi S, Costantino G, et al. Fixed and autoadjusting continuous positive airway pressure treatments are not similar in reducing cardiovascular risk factors in patients with obstructive sleep apnea. Chest. 2007;131(5):1393–1399. doi: 10.1378/chest.06-2192. [DOI] [PubMed] [Google Scholar]
- 22.Patruno V, Tobaldini E, Bianchi AM, et al. Acute effects of autoadjusting and fixed continuous positive airway pressure treatments on cardiorespiratory coupling in obese patients with obstructive sleep apnea. Eur J Intern Med. 2014;25(2):164–168. doi: 10.1016/j.ejim.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99(1):106–109. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marrone O, Insalaco G, Bonsignore MR, Romano S, Salvaggio A, Bonsignore G. Sleep structure correlates of continuous positive airway pressure variations during application of an autotitrating continuous positive airway pressure machine in patients with obstructive sleep apnea syndrome. Chest. 2002;121(3):759–767. doi: 10.1378/chest.121.3.759. [DOI] [PubMed] [Google Scholar]
- 25.Schwab RJ, Badr SM, Epstein LJ, et al. An official American Thoracic Society statement: continuous positive airway pressure adherence tracking systems. The optimal monitoring strategies and outcome measures in adults. Am J Respir Crit Care Med. 2013;188(5):613–620. doi: 10.1164/rccm.201307-1282ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakker J, Campbell A, Neill A. Randomised controlled trial of auto-adjusting positive airway pressure in morbidly obese patients requiring high therapeutic pressure delivery. J Sleep Res. 2011;20(1 Pt 2):233–240. doi: 10.1111/j.1365-2869.2010.00846.x. [DOI] [PubMed] [Google Scholar]
- 27.Berry RB, Kushida CA, Kryger MH, Soto-Calderon H, Staley B, Kuna ST. Respiratory event detection by a positive airway pressure device. Sleep. 2012;35(3):361–367. doi: 10.5665/sleep.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai H, Patel A, Patel P, Grant BJ, Mador MJ. Accuracy of autotitrating CPAP to estimate the residual apnea-hypopnea index in patients with obstructive sleep apnea on treatment with autotitrating CPAP. Sleep Breath. 2009;13(4):383–390. doi: 10.1007/s11325-009-0258-2. [DOI] [PubMed] [Google Scholar]
- 29.Mulgrew AT, Lawati NA, Ayas NT, et al. Residual sleep apnea on polysomnography after 3 months of CPAP therapy: clinical implications, predictors and patterns. Sleep Med. 2010;11(2):119–125. doi: 10.1016/j.sleep.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Prasad B, Carley DW, Herdegen JJ. Continuous positive airway pressure device-based automated detection of obstructive sleep apnea compared to standard laboratory polysomnography. Sleep Breath. 2010;14(2):101–107. doi: 10.1007/s11325-009-0285-z. [DOI] [PubMed] [Google Scholar]
- 31.Ueno K, Kasai T, Brewer G, et al. Evaluation of the apnea-hypopnea index determined by the S8 auto-CPAP, a continuous positive airway pressure device, in patients with obstructive sleep apnea-hypopnea syndrome. J Clin Sleep Med. 2010;6(2):146–151. [PMC free article] [PubMed] [Google Scholar]
- 32.Duce B, Milosavljevic J, Hukins C. The 2012 AASM respiratory event criteria increase the incidence of hypopneas in an adult sleep center population. J Clin Sleep Med. 2015;11(12):1425–1431. doi: 10.5664/jcsm.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]