Abstract
The use of wearable sleep tracking devices is rapidly expanding and provides an opportunity to engage individuals in monitoring of their sleep patterns. However, there are a growing number of patients who are seeking treatment for self-diagnosed sleep disturbances such as insufficient sleep duration and insomnia due to periods of light or restless sleep observed on their sleep tracker data. The patients' inferred correlation between sleep tracker data and daytime fatigue may become a perfectionistic quest for the ideal sleep in order to optimize daytime function. To the patients, sleep tracker data often feels more consistent with their experience of sleep than validated techniques, such as polysomnography or actigraphy. The challenge for clinicians is balancing educating patients on the validity of these devices with patients' enthusiasm for objective data. Incorporating the use of sleep trackers into cognitive behavioral therapy for insomnia will be important as use of these devices is rapidly expanding among our patient population.
Citation:
Baron KG, Abbott S, Jao N, Manalo N, Mullen R. Orthosomnia: are some patients taking the quantified self too far? J Clin Sleep Med. 2017;13(2):351–354.
Keywords: insomnia, technology, cognitive behavioral therapy
INTRODUCTION
It is estimated that 10% of US adults use a wearable fitness/ sleep tracking device on a regular basis, and 50% would consider purchasing one. This includes brands such as Fitbit, Apple Watch, Nike Fuel Band, and Jawbone Up.1 Despite the growing interest among consumers, sleep professionals have been wary of incorporating these devices into treatment2 because of low concordance with polysomnography and actigraphy.3,4 There are an increasing number of patients who are seeking treatment as a result of their sleep tracker data because of concerns over both sleep duration and quality. Three cases are presented in this case series, along with suggestions for accommodating patients' sleep trackers into treatment with cognitive behavioral therapy for insomnia (CBT-I). We termed this condition “orthosomnia,” with “ortho” meaning straight or correct, and “somnia” meaning sleep, because patients are preoccupied or concerned with improving or perfecting their wearable sleep data. We chose this term because the perfectionist quest to achieve perfect sleep is similar to the unhealthy preoccupation with healthy eating, termed orthorexia.5
REPORT OF CASES
Case 1: Mr. R
Mr. R, a 40-y-old male, was referred by his sleep medicine physician for CBT-I because of a complaint of light and fragmented sleep that developed 5 y prior during the transition to a new job. He began monitoring his sleep using a sleep tracking device 1 y prior, after he was given a sleep tracker as a gift by his girlfriend. He was seeking treatment due to irritability, cognitive difficulties (poor attention, memory, and concentration), and fatigue during the day. However, the patient stated that these symptoms would only occur on days he obtained less than 8 h of sleep based on his sleep tracker record. Throughout the session he presented his sleep schedule and symptoms as “according to my data.” Furthermore, the patient did not bring in a sleep diary to the session and instead displayed his sleep tracker data on his phone as evidence of his sleep schedule and sleep duration. He reported a sleep schedule from 17:30–06:00 on weekdays and 22:00–08:00 on weekends, with the last 3 h comprising light and fragmented sleep according to both the tracker data and the patient's subjective report. He stated his average sleep duration was “7 h and 45 minutes” based on the tracker data and that he felt pressure every night to ensure his tracker would display at least 8 h of sleep. His treatment goal was “to achieve at least 8 h of deep sleep each night.” He denied depression and anxiety but endorsed often feeling stressed about his work. He described having a high number of work hours (40 h per week at his job, plus working many hours in the evening and weekends on side projects). He often worked right until bedtime and slept with his phone next to the bed. He occasionally checked his phone because he received texts and emails during the night. He attributed his physical or cognitive symptoms to poor sleep quality. He did not have medical comorbidities or report symptoms of sleep-disordered breathing or restless legs. We recommended that he return for sessions of CBT-I and started treatment by beginning sleep compression6 (23:00–06:30) and recommending a consistent sleep schedule and increased wind-down time before bed. We also suggested psychotherapy because of the patient's elevated stress/anxiety symptoms. However, he did not return for follow-up visits. Although he did not provide a reason for lack of follow-up, he appeared reticent about the time commitment for repeated sessions and expressed doubt that treatment would be helpful for his sleep problem.
Case 2: Ms. B
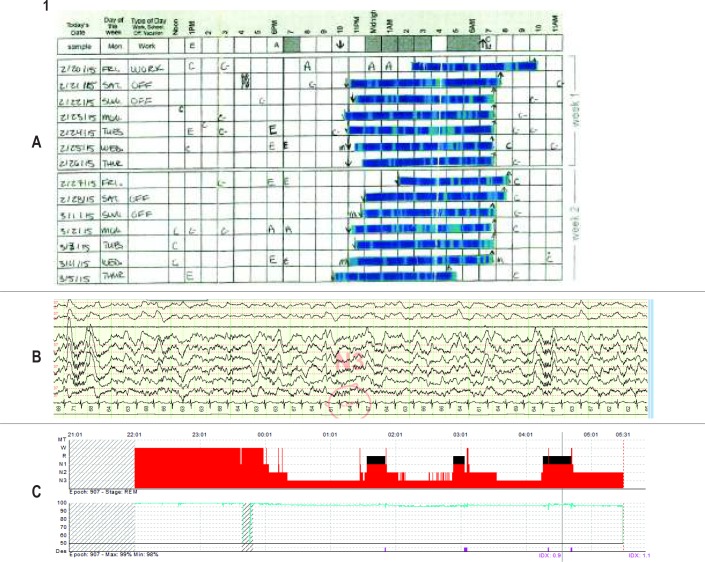
Ms. B, a 27-y-old woman, was initially referred for a sleep medicine evaluation from internal medicine due to difficulty initiating and maintaining sleep in the setting of symptoms of restless legs syndrome (RLS). She presented her sleep tracker data in the initial evaluation to demonstrate the severity of her sleep problems. Her sleep tracker reported frequent restless sleep and an average sleep efficiency of only 60%. She thought these data were consistent with her subjective experience of her sleep. She even completed a sleep diary in which she scaled and pasted in the printout from her sleep tracker (Figure 1A). She had a normal Epworth Sleepiness Scale score (ESS = 4), and her general and neurologic examination were notable only for a body mass index of 30 kg/m2 and a Mallampati class III airway. A diagnosis of RLS was made. The patient's ferritin level was checked and found to be in the low-normal range at 26.5 ng/mL (reference range 11–307), which improved after iron supplementation (144 ng/mL). She was also prescribed gabapentin for her RLS symptoms. Although this combination of medications completely controlled the patient's symptoms of RLS, at a follow-up visit 2 mo later she continued to report restless and unrefreshing sleep and correspondingly no improvement in her tracker data. She had a typical bedtime of 23:00, and after a short sleep latency, she thought that she slept lightly throughout the night but without any prolonged awakenings. She awoke to an alarm at 07:50 each morning feeling unrefreshed (though not sleepy). The sleep physician introduced the concept of sleep restriction and set a 6-h sleep window from 01:00 to 07:00. After only 10 days of sleep restriction, she contacted her physician saying she did not benefit from sleep restriction, and at that point the physician ordered a home sleep apnea test, which was negative for sleep-disordered breathing. At a follow-up visit 5 mo later, despite a 20-lb intentional weight loss, the patient continued to report light and unrefreshing sleep. She was scheduled for an in-laboratory overnight polysomnogram, which again showed no sleep-disordered breathing (apnea-hypopnea index/respiratory disturbance index 0.7); however, alpha frequency intrusion into stage N2 and N3 sleep was noted (Figure 1B). Although there was a prolonged sleep latency of 97 minutes, the arousal index was normal (5.9/h) and the amount of deep (stage N3) sleep was increased as a proportion of the total sleep time (Figure 1C). Despite hearing that she slept deeply on the in-laboratory polysomnogram, the patient asked, “Then why does my Fitbit say I am sleeping poorly?” It was recommended that she follow up for a course of CBT-I, but she did not follow up because of a lack of insurance coverage for that type of service.
Figure 1.
(A) Ms. B's 2-week sleep diary, created by the patient cutting and pasting (by hand) sleep tracker data. (B) Segment of Ms. B's polysomnogram demonstrating alpha frequency in N2 and N3 sleep. (C) Ms. B's hypnogram and oxygen saturation, demonstrating increased proportion of N3 sleep, average number of awakenings, and few oxygen desaturations.
Case 3: Mr. S
Mr. S, a 69-y-old male, was referred for CBT-I due to reports of light and fragmented sleep that did not improve after diagnosis with obstructive sleep apnea (OSA) and continuous positive airway pressure (CPAP) treatment. He had been monitoring his sleep using a sleep tracking device for approximately 1.5 y after his wife purchased the device for him as a gift. After he started monitoring his sleep, he became concerned about periods of restless sleep during the night. He reported his observation to his neurologist, who referred him for overnight polysomnography. He was found to have severe OSA (AHI = 54 events/h) and was started on CPAP with excellent adherence (8.5 h of usage per night). The patient credited his sleep tracker with alerting him to the presence of sleep apnea. When his sleep quality did not improve with CPAP, his sleep medicine physician prescribed doxepin (6 mg), which Mr. S found to be helpful at improving his sleep duration and quality (both on the tracker and by subjective report). He was referred for a behavioral sleep medicine evaluation to discuss nonpharmacologic interventions for insomnia. At the intake session, he reported 8.5 to 9 h in bed each night and a sleep duration of 7 h, based on tracker data, with multiple brief awakenings from 02:00–05:00. He was not aware of these periods of early morning awakenings when they occurred but noted them in his tracker data. He did not endorse excessive daytime sleepiness but reported fatigue, irritability, and cognitive difficulties that he attributed to poor sleep quality. He denied symptoms of depression and anxiety. We recommended that he return for sessions of CBTI and started treatment by suggesting improving sleep habits, such as limiting time in bed to 8 h and keeping a consistent schedule. We also suggested that he switch his sleep tracking device from sensitive to normal mode. We coordinated a plan with his sleep medicine physician to taper his hypnotic medication after one or two CBT-I sessions. He returned for several follow-up sessions, and despite endorsing a desire to sleep without medication, he made no changes in his medications or behaviors. Finally, between sessions three and four, he successfully tapered off hypnotic medications. He had some worsening of his insomnia in his first attempt to taper off medications, but in the second attempt he was successful. He reported that observing the changes in his sleep tracker data was helpful for tapering off his hypnotic medications because it showed him how his sleep quality began to improve after a few days of stopping medications. He was pleased with his progress and thought that his mood and functioning at work had improved because of treatment. He even remarked that his coworkers noticed the change in his mood due to improvements in sleep.
DISCUSSION
These three cases demonstrate that sleep trackers may pose unique challenges in CBT-I and reinforce sleep-related anxiety or perfectionism for some patients. Each patient was seeking treatment due to perceived insufficient sleep or periods of restlessness or light sleep. Despite multiple validation studies that have demonstrated consumer-wearable sleep tracking devices are unable to accurately discriminate stages of sleep and have poor accuracy in detecting wake after sleep onset, we found patients' perceptions difficult to alter.3,4 In addition, lack of transparency in the device algorithms makes it impossible to know how accurate they are even under the best circumstances. These cases suggest there may be unintended effects of sleep tracking for a subset of patients. For example, all three patients were spending excessive time in bed in attempts to increase the sleep duration reported by the sleep tracker, which may have exacerbated their insomnia. Given that these devices tend to overestimate sleep, they may have served to reinforce poor sleep habits by encouraging extending time in bed.
One limitation of our report is that we do not know about the patients' sleep before the sleep tracker to know whether the tracker caused the sleep problem. As such, we do not know whether they started tracking their sleep because they were concerned about insomnia or they “discovered” their insomnia as a result of reviewing their data. Interestingly, two of the three patients in our case report did not purchase their device and instead received them as gifts from their spouse or partner. It is unknown whether the gifts were purchased for the enjoyment of self-discovery or whether they had hoped to nudge their partners to higher physical activity or better sleep.
Despite education and even two objective recordings in one case, patients' beliefs about the sleep tracker data were unchanged. Ms. B's belief that she was not getting enough “deep sleep” was unchanged even after seeing data from her in-laboratory polysomnogram. It is well known that laboratory studies are often different than sleep at home. However, most studies demonstrate worse sleep in the laboratory (i.e., the “first night effect.”).7 There have also been reports of “reverse first night effects”8 and data suggesting that objective sleep parameters are worse at home among individuals with bed partners, etc.9 With Ms. B, the clinician hoped that she would be reassured that she was indeed receiving deep sleep, in fact higher than average for her age. However, her belief persisted. The technique of discussing objective sleep data has been tried with patients who have paradoxical insomnia and also did not influence perceptions of sleep quality among this population.10
The cases also demonstrate that these patients were difficult to engage in CBT-I interventions. Mr. R chose not to follow up with CBT-I for unknown reasons. Ms. B was recommended to start sleep restriction but abandoned this technique shortly because of lack of perceived benefit. Mr. S was able to follow a structured taper schedule of his hypnotic medication but never made any changes to his sleep schedule, despite suggestions for the use of sleep restriction for his worsening insomnia symptoms during the taper. He was never able to verbalize specific barriers to his adherence (e.g., anxiety about sleep loss, belief that it would not work) and instead would steer the conversation back to his observations of sleep tracker data, which may have indicated his discomfort with making any change that would decrease his sleep duration, even if only temporarily. We suspect that he felt more confident with his own data and was not persuaded by the suggestions of the psychologist to try sleep restriction. In the end he did not need to use the technique because he was able to taper off his hypnotic medication with eventual resolution of his insomnia.
For other patients who are less persistent in their beliefs, it may be possible to integrate CBT-I techniques with sleep tracker data. For patients who bring up their sleep tracker data in the initial behavioral sleep medicine evaluation, we review the patient's sleep tracker data with them and discuss limitations to validity. We typically discuss that trackers measure movement and not electroencephalograms and, therefore, are unable to discriminate between light versus deep sleep (example: reading their phone in bed will often be recorded as light sleep although they are clearly awake). We also discuss the best use of these devices is probably to monitor their sleep pattern, including how much time they are spending in bed, rather than specifically the minutes spent awake and asleep. We also request that patients complete a written sleep log in addition to wearing a tracker, which some but not all patients agree to do (and others fill out based on tracker data while in the waiting room). Despite our best attempts to take the sleep tracker data into account, the patients we discuss in this case series were aware that we did not feel as strongly about their data as they did (Mr. S would preface his statements with “I know you don't like this, but my Fitbit tells me…”). In fact, our doubts about the tracker data may have affected the therapeutic relationship by undermining the patients' confidence in our approach.
In patients with less strongly held beliefs about their sleep trackers, therapists may also be able to integrate sleep tracker data to challenge catastrophic thinking. Therapists may suggest collecting data about the correlation between sleep duration/quality and daytime performance (often not as tightly correlated as they think) or work with the patient on black-and- white or catastrophic thinking about the tracker data (“I absolutely can't function, and the day is a total failure if I do not sleep for 7 h according to my recording”). These are techniques similar to the cognitive interventions with sleep diaries. If clinicians suspect that sleep trackers themselves are making patients more preoccupied with sleep, they may consider engaging patients in a behavioral experiment to track their subjective sleep quality and daytime function with and without the sleep tracker to determine if the preoccupation is making the patient more stressed about their sleep and thus negatively impacting their perceived sleep quality.
Finally, patients who are overly reliant on their sleep tracking devices may also have other problematic technology use patterns, such as smartphone notifications or calls interrupting sleep and working or watching TV from a laptop or phone at night. Although none of these patients were specifically logging into their sleep applications to check on their sleep patterns during the night, it is conceivable that patients who are highly anxious about their sleep may be tempted to do so. Clinicians should be sure to address poor sleep habits such as phone use during the night that may be exacerbating factors in insomnia and daytime fatigue.
In conclusion, the use of wearable sleep trackers is increasing, and most consumers are unaware that the claims of these devices often outweigh the science to support them as devices to measure and improve sleep. More research is needed on whether sleep tracker technology can be integrated into CBT-I because regardless of poor validation data, consumers are enthusiastic about this technology and often think the data are highly consistent with their experience of their sleep. Working with patients to integrate devices into treatment provides the opportunity to increase communication between patients and providers and reduce participant burden. We should also be aware of the potential for unintended effects on sleep beliefs and behaviors.
DISCLOSURE STATEMENT
Ms. Mullen has received research support from AbbVie, Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Michelle Garrison, PhD and Hrayr Attarian, MD for comments on our manuscript draft and Meghan Lavin for grammatical editing.
REFERENCES
- 1.Fleming G. The State of Consumers and Technology Benchmark 2015, US. Cambridge, MA: Forrester; 2015. [Google Scholar]
- 2.Russo K, Goparaju B, Bianchi MT. Consumer sleep monitors: is there a baby in the bathwater? Nat Sci Sleep. 2015;7:147–157. doi: 10.2147/NSS.S94182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meltzer LJ, Hiruma LS, Avis K, Montgomery-Downs H, Valentin J. Comparison of a commercial accelerometer with polysomnography and actigraphy in children and adolescents. Sleep. 2015;38(8):1323–1330. doi: 10.5665/sleep.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery-Downs HE, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep Breath. 2012;16(3):913–917. doi: 10.1007/s11325-011-0585-y. [DOI] [PubMed] [Google Scholar]
- 5.Bratman S, Knight D. Health Food Junkies: Orthorexia Nervosa: Overcoming the Obsession with Healthful Eating. New York, NY: Broadway; 2001. [Google Scholar]
- 6.Riedel BW, Lichstein KL, Dwyer WO. Sleep compression and sleep education for older insomniacs: self-help versus therapist guidance. Psychol Aging. 1995;10(1):54–63. doi: 10.1037//0882-7974.10.1.54. [DOI] [PubMed] [Google Scholar]
- 7.Agnew HW, Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2(3):263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 8.Hauri PJ, Olmstead EM. Reverse first night effect in insomnia. Sleep. 1989;12(2):97–105. doi: 10.1093/sleep/12.2.97. [DOI] [PubMed] [Google Scholar]
- 9.Edinger JD, Glenn DM, Bastian LA, et al. Sleep in the laboratory and sleep at home II: comparisons of middle-aged insomnia sufferers and normal sleepers. Sleep. 2001;24(7):761–770. doi: 10.1093/sleep/24.7.761. [DOI] [PubMed] [Google Scholar]
- 10.Geyer JD, Lichstein KL, Ruiter ME, Ward LC, Carney PR, Dillard SC. Sleep education for paradoxical insomnia. Behav Sleep Med. 2011;9(4):266–272. doi: 10.1080/15402002.2011.607022. [DOI] [PMC free article] [PubMed] [Google Scholar]