Abstract
While apolipoprotein (Apo)E4 is linked to increased incidence of Alzheimer’s disease (AD), there is growing evidence that it plays a role in functional brain irregularities that are independent of AD pathology. However, ApoE4-driven functional differences within olfactory processing regions have yet to be examined. Utilizing knock-in mice humanized to ApoE4 versus the more common ApoE3, we examined a simple olfactory perceptual memory that relies on the transfer of information from the olfactory bulb (OB) to the piriform cortex (PCX), the primary cortical region involved in higher order olfaction. In addition, we have recorded in vivo resting and odor-evoked local field potentials (LPF) from both brain regions and measured corresponding odor response magnitudes in anesthetized young (6-month-old) and middle-aged (12-month-old) ApoE mice. Young ApoE4 compared to ApoE3 mice exhibited a behavioral olfactory deficit coinciding with hyperactive odor-evoked response magnitudes within the OB that were not observed in older ApoE4 mice. Meanwhile, middle-aged ApoE4 compared to ApoE3 mice exhibited heightened response magnitudes in the PCX without a corresponding olfactory deficit, suggesting a shift with aging in ApoE4-driven effects from OB to PCX. Interestingly, the increased ApoE4-specific response in the PCX at middle-age was primarily due to a dampening of baseline spontaneous activity rather than an increase in evoked response power. Our findings indicate that early ApoE4-driven olfactory memory impairments and OB network abnormalities may be a precursor to later network dysfunction in the PCX, a region that not only is targeted early in AD, but may be selectively vulnerable to ApoE4 genotype.
Keywords: Apolipoprotein E, olfaction, local field potential, olfactory bulb, piriform cortex, memory deficits
The human APOE gene exists as three alleles – ε2, ε3, and ε4 – which have a worldwide prevalence of 10%, 70%, and 20% respectively (Mahley et al., 2006). Compared to the more common apolipoprotein ε3 allele (ApoE3), the apolipoprotein ε4 allele (ApoE4) is strongly associated with several adverse clinical outcomes, including cardiovascular disease and Alzheimer’s disease (AD) (Farrer, 1997, Mahley et al., 2006, Mahley, 2016). Additionally, ApoE4 has been shown to influence cognitive deficits that arise prior to detectable AD pathology and in healthy older adults (Small et al., 2004, Caselli et al., 2009, Wisdom et al., 2011), suggesting that ApoE4 expression adversely impacts brain pathways independently of AD pathology. In fact, a growing body of neuroimaging research has reported functional differences in human ApoE4 carriers versus noncarriers prior to the onset of cognitive decline within diverse brain regions (such as in hippocampi and specific regions of cortex) (Small et al., 2000, Reiman et al., 2004, Bookheimer and Burggren, 2009, Filippini et al., 2009, Dennis et al., 2010, Sheline et al., 2010, Brown et al., 2011). However, ApoE4-driven functional differences within regions that might specifically explain olfactory dysfunction have largely been ignored.
Prior to broad cognitive decline, human ApoE4 carriers exhibit odor identification impairments (Graves et al., 1999, Murphy, 1999, Wilson et al., 2007, Schubert et al., 2008, Olofsson et al., 2016) that may precede later cognitive deficits (Graves et al., 1999, Wilson et al., 2007, Schubert et al., 2008). Normal olfactory activity relies on proper functioning within the olfactory network, the neural mechanisms of which are increasingly well established (Wilson, 2009): central odor processing initiates within the olfactory bulb (OB), which then transmits information directly to the piriform cortex (PCX), a region that is crucial for procedures involved in odor habituation and olfactory learning (Barnes et al., 2008, Wilson and Linster, 2008, Wilson, 2009, Gottfried, 2010). Olfactory information ultimately enters the entorhinal cortex and finally the hippocampus – a site of memory storage and retrieval (Staubli et al., 1984, Sosulski et al., 2011, Kay, 2014). Olfactory event related potential (OERP) recordings during olfactory memory tasks in humans have demonstrated differences in peak latencies and amplitudes that distinguish ApoE4 carriers from non-carriers (Corby et al., 2012, Green et al., 2013). However, interpreting the relationship between brain activity and OERP signals measured on the scalp remains challenging (He et al., 2011). Electrode recordings of local field potentials (LFPs) in animal models allows more direct assessment of local activity in circuits directly involved in specific behaviors (Buzsaki et al., 2012). Additionally, odor-evoked LFP oscillations of the rodent olfactory system in the frequencies of 15–40Hz (beta band; reflects long-range communication) and 40–80Hz (gamma band; may reflect more local processing) have been shown to be especially indicative of behaviorally relevant odor processing (Neville and Haberly, 2003, Wesson et al., 2011, Kay, 2014, Martin and Ravel, 2014, Sadrian et al., 2014) and are sensitive to neuropathology related to aging (Wesson et al., 2011, Xu et al., 2015). Using LFP recordings from mice that express human ApoE, we have demonstrated within distinct olfactory regions that both ApoE4 genotype and aging play a factor in influencing the signal-to-noise ratio of an odor-evoked response, which may contribute to olfactory memory impairment.
Experimental procedures
Study Approval
All experimental procedures involving animals in this study were approved by and complied with the guidelines of the Institutional Animal Care and Use Committee of the Nathan Kline Institute.
Mice
The mice in this study were purchased from Taconic farms (Germantown, NY) and were homozygous for ApoE4 and ApoE3 on a C57BL/6 background. These targeted-replacement mice were developed to express human ApoE under the control of the endogenous murine promoter (Sullivan et al., 1997), which allows for the expression of human ApoE at physiologically regulated levels in the same temporal and spatial pattern as endogenous murine ApoE. Mice were investigated at 6 or 12 months of age and both sexes were examined. Separate cohorts of mice were tested for odor habituation and physiology.
Odor habituation
For odor habituation testing (done as described previously in detail (Wesson et al., 2010)), monomolecular odors (2-heptanone, isoamyl acetate, (+) enantiomer of limonene, ethyl valerate; Sigma Aldrich, St. Louis, MO) were diluted in mineral oil to a concentration of 100 ppm based on vapor pressure and applied to a cotton-applicator stick which was then enclosed in a piece of odorless plastic tubing to prevent contact of the liquid odor with the testing chamber or animal, yet allowing for volatile odor delivery. In the listed order, each odor was delivered over 4 trials of 20 sec each separated by a 30 sec interval, by inserting the odor stick into a port on the side of the animal's home cage. The duration of time spent investigating, defined as snout-oriented sniffing within 1 cm of the odor presentation port, was recorded by a single observer blinded to animal genotype. Home cages were cleaned with fresh corn-cob bedding added 24 hrs prior to behavioral testing, and the food bin and water bottle were removed from cages immediately prior to testing.
In vivo electrophysiology
Mice were anesthetized with urethane (1.25 g/kg, i.p.), and positioned in a stereotaxic apparatus for in vivo LFP recordings. The stereotaxic frame was outfitted with a water-filled heating pad to maintain core body temperature (37°C). Skin was removed to expose the dorsal skull, and 1.5 mm diameter ipsilateral holes were drilled over the anterior PCX and the OB (as previously described (Wesson et al., 2011)). Monopolar tungsten recording electrodes were lowered into the PCX and into the OB for data acquisition. Proper electrode placement in the PCX was confirmed by appropriate evoked responses following direct stimulation in the OB.
To assess odor-evoked LFPs, odors were presented to anesthetized mice using a flow-dilution olfactometer positioned 2 cm from the animal’s nose. Odor vapor was introduced with a computer-controlled pinch valve at a rate of 0.1 liter per minute to a constant 1 liter per minute flow of nitrogen gas, as we have done previously (Wesson et al., 2011, Xu et al., 2015). Stimuli included three monomolecular odorants (mesital oxide, ethyl valerate, and isoamyl acetate; Sigma Aldrich, St. Louis, MO). Stimuli were introduced for 2 sec per trial with at least a 30 sec inter-stimulus interval. Each odor was presented for 4 trials. These odors were chosen based on previous experience to evoke strong oscillatory responses in the mouse olfactory system and to overlap with those used in the behavioral tests.
Data and statistical analyses
For the analysis of behavior data, all raw investigatory values (in sec) were organized within animals according to odor presentation number (trial number; as described previously (Wesson et al., 2011)). As a measure of odor habituation, raw investigatory values (trials 1–4) were pooled within each group and analyzed following normalization to the initial investigation duration/animal for each odor (trial 1). The initial investigation duration was assigned a value of “1” and the following investigation times a proportion of 1 for display of habituation curves and ANOVA. A second, simplified magnitude of habituation score was also calculated as 1 minus the sum of the normalized investigatory times in trials 2–4. For this measure, a score of 1 signifies complete habituation while a score of 0 signifies no habituation. Data by trial were analyzed using repeated measures 4×4 ANOVA (trial × group). As post-hoc comparisons, two-tailed t-tests assuming equal variance were used to make pair-wise genotype comparisons of total odor habituation as well as within each trial.
LFP’s along with stimulus presentation events were acquired using Spike2 software (Cambridge Electronic Design Ltd., Cambridge, England). Analysis of electrophysiological data was performed using Spike2 scripts. Spontaneous and odor-evoked LFP’s were analyzed with an off-line Fast-Fourier transform (FFT) operation to extract different frequency components: beta (15–40 Hz), and gamma (40–80 Hz) frequency bands. Spontaneous power was defined as the activity 2 sec before stimulus onset, while odor-evoked power was defined as the activity 2 sec after stimulus onset. Isoamyl acetate produced the greatest responses in our ApoE mice. Therefore, for consistency across animals, odor-evoked and spontaneous powers were averaged across 3 trials of this odorant for each frequency band for each animal. To calculate the odor-evoked response magnitude, we normalized the odor-evoked power spectrum to the spontaneous power spectrum. Two-tailed t-tests assuming unequal variance were used to make pair-wise genotype and age comparisons within each frequency band.
Results
Behavioral olfactory deficits in young ApoE4 mice
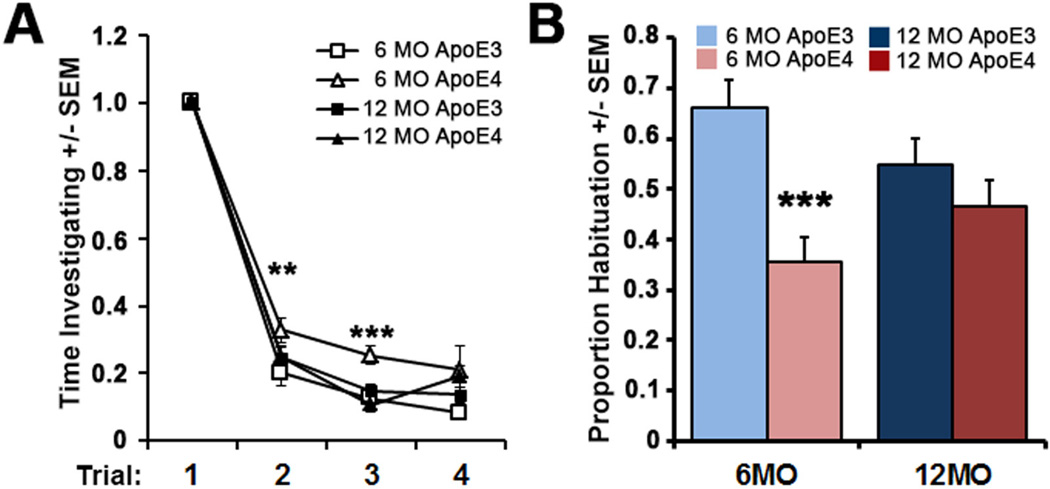
ApoE mice were initially evaluated using a simple olfactory habituation task. As we have previously described (Wilson, 2009, Wesson et al., 2011), the length of time that a mouse spends investigating novel odors decreases with repeated presentation when olfaction is intact. Analysis of the results by trial (Fig. 1A) with a repeated measures ANOVA (trial × group) on all 4 groups (6 mo ApoE3, 6 mo ApoE4, 12 mo ApoE3, and 12 mo ApoE4) found a main effect of both trial (F (3,276) =637.6, p<.0001) and group (F(3, 276)=6.36, p<0.001). There were no statistical differences in the period of time that the groups sniffed during trial 1, with the mice sniffing a combined average of 3.5±0.1 seconds. Post-hoc t-tests revealed that ApoE4 mice at 6 months of age spent a significantly longer period of time sniffing presented odors at trials 2 (t(149)=−2.45, p<0.01) and 3 (t(149)=−3.66, p<0.001) compared to young ApoE3 mice. While odor habituation was found to be impaired in young ApoE4 mice, differences between the two genotypes were not detected at 12 months-of-age (Fig. 1B). At this age, the time spent exploring odors over multiple exposures was similar comparing ApoE4 to ApoE3 mice in all trials (p=0.27) (Fig. 1A and B). Thus, the extent of odor habituation was both genotype- and age-dependent, with impairment specific to young ApoE4 mice.
Figure 1. Olfactory deficits in young ApoE4 mice.
(A) Mean time spent investigating odors, normalized to trial 1 (see Methods) across 4 successive odor presentation trials in 6 mo and 12 mo old ApoE3 and ApoE4 mice. (6 mo ApoE3 n=18, ApoE4 n=24; 12 mo ApoE3 n=18, ApoE4 n=14) ** p<0.01, *** p<0.001, 6 mo ApoE4 vs. ApoE3, between groups 2-tailed t-tests. (B) Magnitude of odor habituation in 6 and 12 mo old ApoE3 and ApoE4 mice totaled from trials 2 through 4. A score of 1 signifies complete habituation while a score of 0 signifies no habituation.
Heightened LFP response to odors within the beta band of young ApoE4 OB
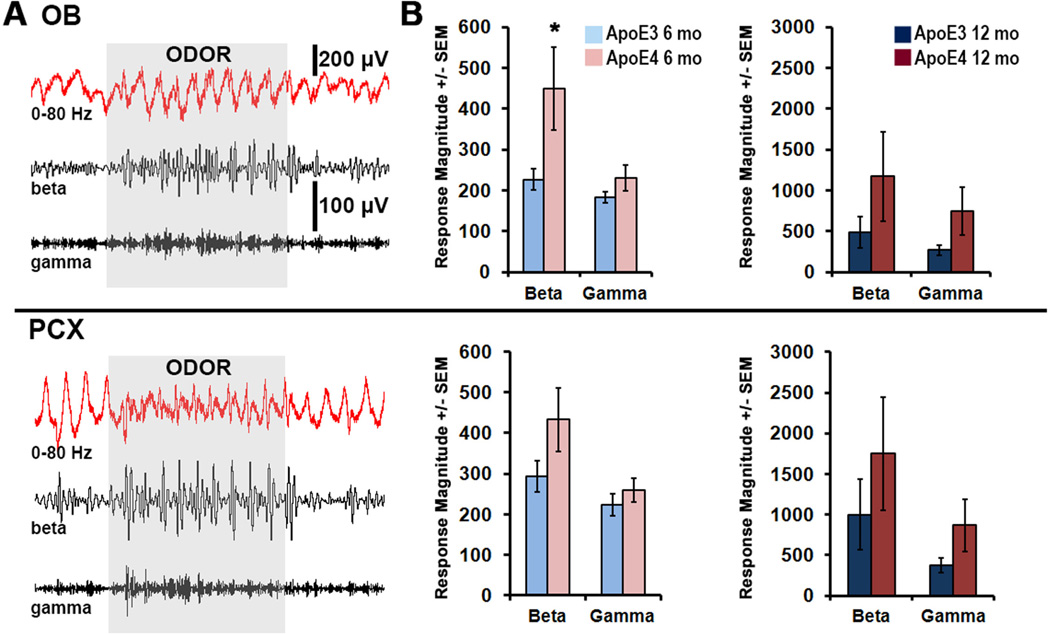
The neural networks required for short-term odor habituation reside within the OB and PCX (Wilson and Linster, 2008, Wilson, 2009). As such, we recorded odor-evoked LFP activity simultaneously from these two brain regions of ApoE mice under anesthesia to control for changes in behavioral state (Fig. 2A). We applied FFT analysis to segregate recorded LFP waveforms into distinct frequency components of interest: beta band activity (15–40 Hz) is often enhanced during olfactory learning, while gamma band activity (40–80 Hz) may be critical for fine odor discrimination (Neville and Haberly, 2003, Kay, 2014, Martin and Ravel, 2014). Consistent with the odor habituation data, significant differences between groups appeared in the OB odor-evoked response (as percent of pre-odor baseline) of young ApoE mice: heightened response magnitudes in the beta band (2-fold increase; t(13.6)=−2.11, p<0.05) but not gamma band (p=0.19) were observed in young ApoE4 compared to ApoE3 mice. Group differences in PCX response magnitudes (as percent of pre-odor baseline) were not significant (p=0.12 beta; p=0.38 gamma) (Fig. 2B). Thus, when young ApoE4 mice display impaired odor habituation, there is enhanced OB odor-evoked activity compared to ApoE3 mice, but no differences detected in PCX activity.
Figure 2. Heightened response to odors within the beta band of young ApoE4 OB, plotted as a function of genotype.
(A) OB (top) and PCX (bottom) LFPs from a representative 6 mo ApoE4 mouse is shown in response to a 2 sec odor presentation. Data are filtered (2nd-order band-pass) to display full band (0–80Hz), beta (15–40Hz), and gamma (40–80Hz). Odor-evoked response magnitudes in the OB (top) and PCX (bottom) were analyzed for 6 mo (B) and 12 mo (C) ApoE mice as activity 2 sec during odor / activity 2 sec pre-odor (n=7) * p<0.05, 2-tailed t-test.
Odor-evoked response magnitudes in ApoE4 mice increase in the PCX with age
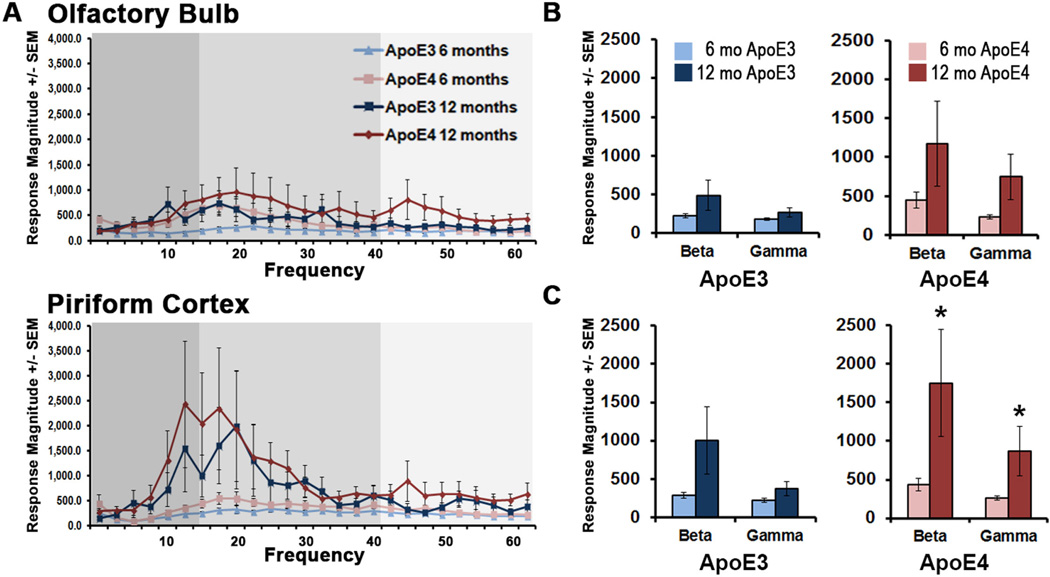
To complement the aging component of our short-term habituation data, odor-evoked LFPs were additionally recorded from the OB and PCX of middle-aged anesthetized ApoE mice. We detected no between-group differences in middle-aged odor evoked responses (as percent of pre-odor baseline) in either region (Fig 2C). However, two-way ANOVA analysis on odor-evoked response magnitudes uncovered an age effect in both beta (p<0.01) and gamma (p<0.05) bands within the PCX. This effect was predominantly ApoE4-driven, with a prominent 4-fold increase in beta (t(9.2)=−1.88, p<0.05) and 3-fold increase in gamma band (t(9.1)=−1.89, p<0.05) odor-evoked response magnitudes from 6 mo to 12 mo (Fig. 3A and C). ApoE3 beta band response magnitudes in the PCX revealed a trending increase with age with no significant differences observed (3-fold increase; p=0.053) (Fig. 3A and C). While PCX responses increased with aging in ApoE4 mice, odor-evoked response magnitudes within the aging OB of ApoE3 and ApoE4 mice remained relatively unchanged compared to their young counterparts (Fig. 3A and B), consistent with evidence that OB neuronal populations and axonal growth remain stable with aging (Mirich et al., 2002).
Figure 3.
Odor-evoked response magnitudes in ApoE4 mice increase in the PCX with age Same data as shown in Figure 2, but re-plotted as a function of age. (A) Average response magnitudes are shown in the OB (top) and PCX (bottom). Beta (medium gray) and gamma (light gray) frequency ranges are indicated. The progression of odor-evoked magnitudes from 6 mo to 12 mo of age is shown in OB (B) and PCX (C). * p<0.05, 2-tailed t-tests.
Spontaneous LFP activity is reduced in aged ApoE4 mice
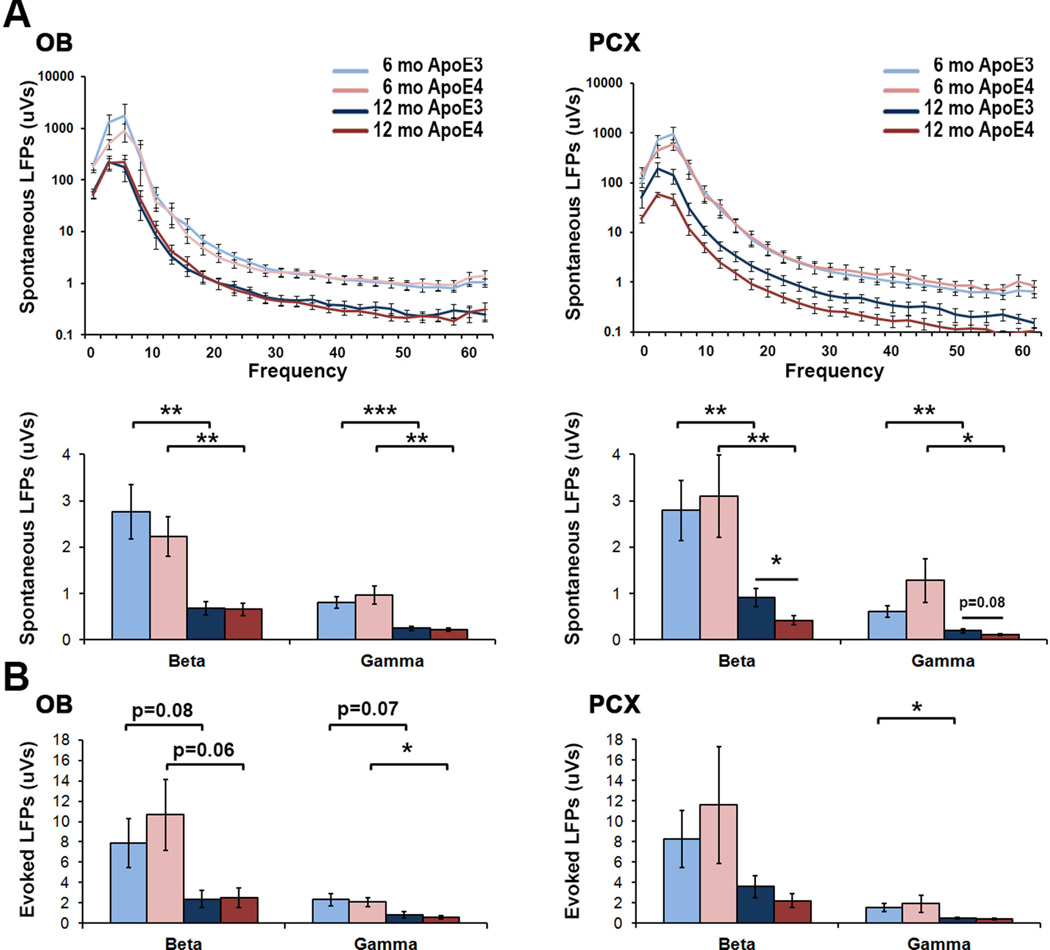
Extensive experimental evidence supports the idea that spontaneous brain activity contributes to the processing of sensory information, biasing perception and behavioral response to stimuli (Tsodyks et al., 1999, Raichle, 2006, Boly et al., 2007, Hesselmann et al., 2008, Luczak et al., 2009, Sadaghiani et al., 2010, Berkes et al., 2011). Therefore, in addition to the brief periods of pre-stimulus activity used in assessing odor-evoked responses, we recorded spontaneous LFPs for a consecutive 10 min from anesthetized ApoE mice for a more complete assessment of genotype and aging effects on spontaneous activity. Young mice exhibited no ApoE-genotype driven differences in spontaneous activity in either the OB (p=0.47 beta; p=0.52 gamma) or the PCX (p=0.78 beta; p=0.18 gamma) (Fig. 4A). However, spontaneous LFPs in both groups significantly decreased with age within both the OB (t(13.4)=3.46, p<0.01 ApoE3 beta; t(14.3)=3.5, p<0.01 ApoE4 beta; t(15.6)=4.2, p<0.001 ApoE3 gamma; t(12.8)=3.7, p<0.01 ApoE4 gamma) and the PCX (t(14.1)=2.8, p<0.01 ApoE3 beta; t(12.3)=3.0, p<0.01 ApoE4 beta; t(14.4)=3.2, p<0.01 ApoE3 gamma; t(12.0)=2.5, p<0.05 ApoE4 gamma) (Fig. 4A). Furthermore, between-group comparison revealed a further enhanced decrease in middle-aged ApoE4 spontaneous beta activity (t(16)=2.5, p<0.05) that was localized to the PCX (Fig. 4A). This enhanced decrease in spontaneous ApoE4 PCX activity appeared to significantly contribute to the observed increase in odor-evoked response magnitude, as non-normalized odor-evoked LFP power remained largely unchanged between groups at 12 mo (Fig. 4B). Thus, the odor-evoked response increase in middle-aged ApoE4 mice was primarily driven by a dampening of baseline spontaneous activity rather than an increase in evoked response power.
Figure 4. Reduced spontaneous LFP activity in aged ApoE4 mice accounts for heightened odorevoked response magnitudes.
(A) Spontaneous LFPs in both OB (left) and PCX (right) from 6 to 12 mo within beta and gamma bands of both ApoE3 and ApoE4 mice. There is a decrease in spontaneous activity in of ApoE4 vs. ApoE3 mice that is localized to the PCX of 12 mo mice. *p<0.05, **p<0.01, 2-tailed t-tests. (B) Evoked LFP activity is shown for the OB (left) and PCX (right) of 6 and 12 mo ApoE mice.
Discussion
While previous work has demonstrated impaired olfactory guided behavior in ApoE knockout mice (Nathan et al., 2004), the present results are the first to investigate specific ApoE genotype effects on olfactory behavior and physiology in vivo. Young (6 mo) ApoE4 compared to age matched ApoE3 mice display both impaired behavioral odor habituation and OB hyperactivity during odor stimulation, suggesting a relationship between olfactory dysfunction onset and early stage sensory network hyperactivity that is specific to the ApoE4 mouse model. While there is no genotype difference in PCX odor-evoked activity at 6 mo, comparison of a middle-aged (12 mo) cohort with the young cohort show an ApoE4-driven shift of odor-evoked physiological dysfunction from the OB to the PCX with age while memory impairment declined: while genotype-dependent differences in odor habituation and OB physiology diminish, genotype-dependent changes in PCX physiology emerged. Furthermore, there is a significant effect of aging on PCX spontaneous activity, particularly in ApoE4 mice. These results suggest early emerging, age- and genotype-dependent changes in both odor behavior (habituation) and olfactory system physiology in ApoE4 mice.
It is presently recognized that not only does spontaneous activity carry on during task performance (Arieli et al., 1996, Fox et al., 2006, Fransson, 2006), but that the spontaneous condition of a system at the time of stimulus presentation may modulate the neuronal response as well as account for the variability often seen in response to the stimulus (Arieli et al., 1996, Fox et al., 2006). Our findings suggest that responses in ApoE mice may likewise represent a superposition of task-evoked and ongoing spontaneous activity. We show in middle-aged versus young ApoE4 mice that PCX odor-evoked response magnitudes appear enhanced relative to a decrease in power of spontaneous beta and gamma oscillations. However, the raw power of the middle-aged evoked response was likewise notably dampened, much like the spontaneous activity of the PCX, when compared to young ApoE4 mice. Since this superposition of task-evoked and ongoing spontaneous activity appears to be linear over this age-range, it is likely the further dampening of spontaneous activity in middle-aged ApoE4 versus ApoE3 mice that contributes to the perceived enhancement of PCX odor-evoked response magnitudes.
The mechanisms of ApoE genotype-dependent effects on olfactory system function and aging are less clear. ApoE plays an important role in neural repair and neurite extension (Nathan et al., 1994, Poirier, 1994, Laskowitz et al., 1998, Horsburgh et al., 2000). Given the lifelong neurogenesis that occurs in both the olfactory epithelium and OB (Brann and Firestein, 2014, Lledo and Valley, 2016), and the necessarily associated synaptic and circuit remodeling, the OB may be particularly sensitive to ApoE genotype. In fact, the extreme condition of ApoE knock-out induces substantial changes in olfactory anatomy (Nathan et al., 2010) and behavior (Nathan et al., 2004). Early changes in OB output activity could then translate to changes in PCX function (Best and Wilson, 2003, Poo and Isaacson, 2007), perhaps contributing to the delayed changes observed in PCX LFP’s. Induced changes in excitatory or inhibitory synaptic function, or cell excitability, could contribute to the observed changes in LFP oscillations. In fact, a recent study reports a loss of synaptic AMPA receptors in aging PCX, suggesting there may be subtle changes in glutamatergic synaptic function (Gocel and Larson, 2013).
In humans, the deleterious impacts of ApoE4 on olfactory function increase with age such that the gene has effects on a limited set of olfactory behavioral or physiological functions in young and middle-aged individuals (Corby et al., 2012), whereas older ε4 carriers are more severely impaired (Murphy et al., 1998, Olofsson et al., 2009, Olofsson et al., 2010). Similarly, the effects of ApoE4 in mice vary with age. Young ApoE4 mice demonstrate olfactory habituation deficits at an early-, but not middle-, age when compared to ApoE3 mice. More detailed assessments of olfactory function in middle-age or aged ApoE4 are warranted, but given the shift in pathophysiology from OB to PCX, differences in olfactory perceptual outcomes are expected. We speculate that age-dependent changes in human ApoE4 carriers may represent two separate phenomenon: that the subtle early deficits observed in young ApoE4 olfaction are a foreshadowing of upcoming abnormalities in regional circuitry, while future pathological factors such as the deposit of AD-related proteins emerge to affect olfactory function at a substantially older age.
Highlights.
Olfactory deficits are shown in young, but not middle-aged, ApoE4 vs. ApoE3 mice.
Young ApoE4 vs. ApoE3 mice exhibit hyperactive LFP response to odors in the OB.
Odor-evoked response magnitudes in ApoE4 mice increase in the PCX with age.
Spontaneous LFPs in ApoE mice decrease with age in both the OB and the PCX.
Spontaneous LFP activity is further reduced in middle-aged ApoE4 vs. ApoE3 mice.
Acknowledgments
We thank Dr. Monika Pawlik for her expert assistance with our mouse colonies. KYP did the experimentation and initial data analysis. All authors contributed to the experimental design and to the manuscript.
Funding: This work was supported by the NIA (P01-AG017617 to PMM and EL; and R01-AG037693 to DAW and EL). Katherine Peng was additionally supported by a training program (T32-GM066704, Dr. Erika Bach) and the Sackler Institute of Graduate Biomedical Sciences, New York University School of Medicine. The funding sources were not involved in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Abbreviations
- ApoE
Apolipoprotein E
- ApoE3
Apolipoprotein E3
- ApoE4
Apolipoprotein E4
- LPF
local field potentials
- OB
olfactory bulb
- PCX
piriform cortex
- AD
Alzheimer’s disease
- FFT
Fast-Fourier transform
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Barnes DC, Hofacer RD, Zaman AR, Rennaker RL, Wilson DA. Olfactory perceptual stability and discrimination. Nature neuroscience. 2008;11:1378–1380. doi: 10.1038/nn.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes P, Orban G, Lengyel M, Fiser J. Spontaneous cortical activity reveals hallmarks of an optimal internal model of the environment. Science. 2011;331:83–87. doi: 10.1126/science.1195870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best AR, Wilson DA. A postnatal sensitive period for plasticity of cortical afferents but not cortical association fibers in rat piriform cortex. Brain research. 2003;961:81–87. doi: 10.1016/s0006-8993(02)03847-7. [DOI] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci U S A. 2007;104:12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S, Burggren A. APOE-4 genotype and neurophysiological vulnerability to Alzheimer's and cognitive aging. Annual review of clinical psychology. 2009;5:343–362. doi: 10.1146/annurev.clinpsy.032408.153625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann JH, Firestein SJ. A lifetime of neurogenesis in the olfactory system. Front Neurosci. 2014;8:182. doi: 10.3389/fnins.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Terashima KH, Burggren AC, Ercoli LM, Miller KJ, Small GW, Bookheimer SY. Brain network local interconnectivity loss in aging APOE-4 allele carriers. Proc Natl Acad Sci U S A. 2011;108:20760–20765. doi: 10.1073/pnas.1109038108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nature reviews Neuroscience. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, Locke DE, Snyder CH, Alexander GE, Rademakers R, Reiman EM. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. The New England journal of medicine. 2009;361:255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corby K, Morgan CD, Murphy C. Abnormal event-related potentials in young and middle-aged adults with the ApoE epsilon4 allele. Int J Psychophysiol. 2012;83:276–281. doi: 10.1016/j.ijpsycho.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh-Bohmer KA, Cabeza R. Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2010;6:303–311. doi: 10.1016/j.jalz.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA. Effects of Age, Sex, and Ethnicity on the Association Between Apolipoprotein E Genotype and Alzheimer Disease<subtitle>A Meta-analysis</subtitle>. JAMA: The Journal of the American Medical Association. 1997;278:1349. [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nature neuroscience. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Gocel J, Larson J. Evidence for loss of synaptic AMPA receptors in anterior piriform cortex of aged mice. Front Aging Neurosci. 2013;5:39. doi: 10.3389/fnagi.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA. Central mechanisms of odour object perception. Nature reviews Neuroscience. 2010;11:628–641. doi: 10.1038/nrn2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, Larson EB. Impaired olfaction as a marker for cognitive decline: Interaction with apolipoprotein E 4 status. Neurology. 1999;53:1480–1480. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- Green AJ, Cervantez M, Graves LV, Morgan CD, Murphy C. Age and apolipoprotein E epsilon4 effects on neural correlates of odor memory. Behavioral neuroscience. 2013;127:339–349. doi: 10.1037/a0031891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Yang L, Wilke C, Yuan H. Electrophysiological imaging of brain activity and connectivity-challenges and opportunities. IEEE Trans Biomed Eng. 2011;58:1918–1931. doi: 10.1109/TBME.2011.2139210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann G, Kell CA, Eger E, Kleinschmidt A. Spontaneous local variations in ongoing neural activity bias perceptual decisions. Proc Natl Acad Sci U S A. 2008;105:10984–10989. doi: 10.1073/pnas.0712043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh K, McCarron MO, White F, Nicoll JA. The role of apolipoprotein E in Alzheimer's disease, acute brain injury and cerebrovascular disease: evidence of common mechanisms and utility of animal models. Neurobiology of aging. 2000;21:245–255. doi: 10.1016/s0197-4580(00)00097-x. [DOI] [PubMed] [Google Scholar]
- Kay LM. Circuit oscillations in odor perception and memory. Progress in brain research. 2014;208:223–251. doi: 10.1016/B978-0-444-63350-7.00009-7. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Horsburgh K, Roses AD. Apolipoprotein E and the CNS response to injury. J Cereb Blood Flow Metab. 1998;18:465–471. doi: 10.1097/00004647-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Valley M. Adult Olfactory Bulb Neurogenesis. Cold Spring Harb Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A, Bartho P, Harris KD. Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron. 2009;62:413–425. doi: 10.1016/j.neuron.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders. J Mol Med (Berl) 2016;94:739–746. doi: 10.1007/s00109-016-1427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Ravel N. Beta and gamma oscillatory activities associated with olfactory memory tasks: different rhythms for different functional networks? Frontiers in behavioral neuroscience. 2014;8:218. doi: 10.3389/fnbeh.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirich JM, Williams NC, Berlau DJ, Brunjes PC. Comparative study of aging in the mouse olfactory bulb. The Journal of comparative neurology. 2002;454:361–372. doi: 10.1002/cne.10426. [DOI] [PubMed] [Google Scholar]
- Murphy C. Loss of olfactory function in dementing disease. Physiology & behavior. 1999;66:177–182. doi: 10.1016/s0031-9384(98)00262-5. [DOI] [PubMed] [Google Scholar]
- Murphy C, Bacon AW, Bondi MW, Salmon DP. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Ann Ny Acad Sci. 1998;855:744–750. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Gairhe S, Nwosu I, Clark S, Struble RG. Reconstitution of the olfactory epithelium following injury in apoE-deficient mice. Experimental neurology. 2010;226:40–46. doi: 10.1016/j.expneurol.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan BP, Yost J, Litherland MT, Struble RG, Switzer PV. Olfactory function in apoE knockout mice. Behavioural brain research. 2004;150:1–7. doi: 10.1016/S0166-4328(03)00219-5. [DOI] [PubMed] [Google Scholar]
- Neville KR, Haberly LB. Beta and gamma oscillations in the olfactory system of the urethane-anesthetized rat. Journal of neurophysiology. 2003;90:3921–3930. doi: 10.1152/jn.00475.2003. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Josefsson M, Ekstrom I, Wilson D, Nyberg L, Nordin S, Nordin Adolfsson A, Adolfsson R, Nilsson LG, Larsson M. Long-term episodic memory decline is associated with olfactory deficits only in carriers of ApoE-ε4. Neuropsychologia. 2016;85:1–9. doi: 10.1016/j.neuropsychologia.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Wiens S, Hedner M, Nilsson LG, Larsson M. Odor identification impairment in carriers of ApoE-varepsilon4 is independent of clinical dementia. Neurobiology of aging. 2010;31:567–577. doi: 10.1016/j.neurobiolaging.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Ronnlund M, Nordin S, Nyberg L, Nilsson LG, Larsson M. Odor identification deficit as a predictor of five-year global cognitive change: interactive effects with age and ApoE-epsilon4. Behavior genetics. 2009;39:496–503. doi: 10.1007/s10519-009-9289-5. [DOI] [PubMed] [Google Scholar]
- Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends in neurosciences. 1994;17:525–530. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. An early critical period for long-term plasticity and structural modification of sensory synapses in olfactory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:7553–7558. doi: 10.1523/JNEUROSCI.1786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. Neuroscience. The brain's dark energy. Science. 2006;314:1249–1250. [PubMed] [Google Scholar]
- Reiman EM, Chen KW, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. P Natl Acad Sci USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Friston KJ, Kleinschmidt A. The relation of ongoing brain activity, evoked neural responses, and cognition. Front Syst Neurosci. 2010;4:20. doi: 10.3389/fnsys.2010.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadrian B, Lopez-Guzman M, Wilson DA, Saito M. Distinct neurobehavioral dysfunction based on the timing of developmental binge-like alcohol exposure. Neuroscience. 2014;280:204–219. doi: 10.1016/j.neuroscience.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CR, Carmichael LL, Murphy C, Klein BE, Klein R, Cruickshanks KJ. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc. 2008;56:1517–1521. doi: 10.1111/j.1532-5415.2008.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D'Angelo G, Liu C, Dixit S, Benzinger T, Fagan A, Goate A, Mintun MA. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Backman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychology and aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DHS, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, Mazziotta JC, Saxena S, Wu HM, Mega MS, Cummings JL, Saunders AM, Pericak-Vance MA, Roses AD, Barrio JR, Phelps ME. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. P Natl Acad Sci USA. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Ivy G, Lynch G. Hippocampal denervation causes rapid forgetting of olfactory information in rats. Proc Natl Acad Sci U S A. 1984;81:5885–5887. doi: 10.1073/pnas.81.18.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. The Journal of biological chemistry. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science. 1999;286:1943–1946. doi: 10.1126/science.286.5446.1943. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Borkowski AH, Landreth GE, Nixon RA, Levy E, Wilson DA. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer's beta-amyloidosis mouse model. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:15962–15971. doi: 10.1523/JNEUROSCI.2085-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Levy E, Nixon RA, Wilson DA. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer's disease mouse model. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:505–514. doi: 10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. Olfaction as a model system for the neurobiology of mammalian short-term habituation. Neurobiol Learn Mem. 2009;92:199–205. doi: 10.1016/j.nlm.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Linster C. Neurobiology of a simple memory. Journal of neurophysiology. 2008;100:2–7. doi: 10.1152/jn.90479.2008. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Archives of general psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- Wisdom NM, Callahan JL, Hawkins KA. The effects of apolipoprotein E on non-impaired cognitive functioning: a meta-analysis. Neurobiology of aging. 2011;32:63–74. doi: 10.1016/j.neurobiolaging.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Xu W, Fitzgerald S, Nixon RA, Levy E, Wilson DA. Early hyperactivity in lateral entorhinal cortex is associated with elevated levels of AbetaPP metabolites in the Tg2576 mouse model of Alzheimer's disease. Experimental neurology. 2015;264:82–91. doi: 10.1016/j.expneurol.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]