Abstract
Antigen-mediated cross-linking of IgE-FcεRI complexes activates mast cells and basophils, initiating the allergic response. Of 34 donors recruited having self-reported shrimp allergy, only 35% had significant levels of shrimp-specific IgE in serum and measurable basophil secretory responses to recombinant Pen a 1 (shrimp tropomyosin). We report that degranulation is linked to the number of FcεRI occupied with allergen-specific IgE, as well as the dose and valency of Pen a 1. Using CRISPR-based gene editing, rat basophilic leukemia (hRBLrαKO) cells were created that exclusively express the hFcεRIα subunit. Pen a 1 specific-IgE (IgEPen a 1) was affinity purified from shrimp positive plasma. Cells primed with a range of IgEPen a 1 and challenged with Pen a 1 show a bell-shaped dose response for secretion, with optimal Pen a 1 doses of 0.1–10 ng/ml. Mathematical modeling provided estimates of receptor aggregation kinetics based upon FcεRI occupancy with IgE and allergen dose. Maximal degranulation was elicited when ~2700 IgE-FcεRI complexes were occupied with specific IgE and challenged with Pen a 1 (IgE epitope valency of 8+), although measurable responses were achieved with only a few hundred FcεRI were occupied. Prolonged periods of pepsin-mediated Pen a 1 proteolysis, which simulates gastric digestion, were required to diminish secretory responses. Recombinant fragments (60–79 amino acids), that together span the entire length of tropomyosin, were weak secretagogues. These fragments have reduced dimerization capacity, compete with intact Pen a 1 for binding to IgE-FcεRI complexes, and represent a starting point for the design of promising hypoallergens for immunotherapy.
Introduction
Food allergies are IgE-mediated immunological reactions that affect up to 10% of the human population. (1). Increased consumption of seafood, particularly shrimp, over the past few decades has led to increased incidences of allergic reactions. Over 6 million Americans suffer from seafood allergies (2). Ingestion of shellfish can cause a potentially life-threatening anaphylactic reaction and poses a growing worldwide health problem (3). The major allergen in shrimp has been identified as the muscle protein tropomyosin, a coiled-coiled dimer composed of two identical alpha helices of 33 kDa each (4). Pen a 1, the tropomyosin of brown shrimp (Penaeus aztecus), is one of the most common shrimp allergens and is reactive with circulating IgE from >80% of shrimp allergic patients (5). Tropomyosin is not only a major allergen in crustacean species (i.e., crabs and lobsters)(6, 7), but also in mollusks (squid, snail)(8), house dust mites (9) and cockroaches (10). The concept of tropomyosin as a pan-reactive invertebrate allergen is strongly supported by studies describing shrimp cross-reactive IgE in the serum of individuals strictly following a kosher diet that restricts shrimp (11).
Previous studies have identified five major IgE binding regions within Pen a 1, which bear ~8 epitopes based upon analysis of synthetic overlapping peptides using the SPOTS technique (12). These studies provided critical insight into shrimp allergy, through characterization of IgE binding to Pen a 1-derived peptides as well as early evaluation on their ability to modulate the activation of mast cells (13, 14). As with other allergic reactions, activation of mast cells and basophils occurs when multi-valent allergen crosslinks specific IgE that is tightly bound to FcεRI on the cell surface (15, 16).
Allergen-specific immunotherapies (AIT, SIT) have been used for over a century (17) to alter adaptive immune responses, with the best successes in aeroallergens and insect hypersensitivity (18). Current guidelines reflect the standards of practice in allergy clinics, including the common use of stock extracts that contain whole allergens (19, 20). In addition to commercial extracts, there is an increased move towards development of recombinant proteins that will offer better standardization (21). However the potential risk of adverse events, for both subcutaneous administration (SCIT) and newer sublingual (SLIT) delivery, has driven research towards alternative approaches to traditional immunotherapy (17, 22). Promising new results has suggested that early exposure of infants to peanuts and peanut products can lead to long term tolerance (23, 24). Oral food challenges are commonly practiced in allergy clinics (25), which are time-intensive and clinically-supervised procedures. There is a critical need for additional knowledge about the properties of food allergens (26, 27) leading to the design of safe and effective diagnostic tools and immunotherapies.
The current strategies for the development of hypoallergens for immunotherapy include genetically engineered allergens or chemically-fixed allergoids that reduce or abolish IgE reactivity, while preserving the T cell reactivity for the modulation of an immune response (22). One limitation of these approaches is that hypoallergens that lack IgE-binding epitopes altogether may not elicit desirable IgG production capable of blocking IgE binding to allergens. We propose that rationally designed hypoallergens can be created by carefully integrating the properties of allergen valency and structure, as they relate specifically to the crosslinking of IgE-FcεRI complexes on the surface of mast cells and basophils. Our goal is to create a formulation that retains the full spectrum of IgE-binding and T cell epitopes, while avoiding FcεRI crosslinking conditions that trigger the release of inflammatory mediators. The design of such hypoallergens requires detailed insight regarding the allergen-mediated aggregation of the FcεRI, including the number and orientation of the Immuno Tyrosine Activation Motifs (ITAMS)(28) in the FcεRI γ subunits within allergen-IgE-FcεRI complexes and the formation of activation-competent signaling clusters in the plasma membrane (29). To retain the potential for the immunotherapy formulation to shift the Th1/Th2 paradigm to the recruitment of regulatory T cells, researchers should also consider the available information about T cell epitopes for specific allergens (30).
As proof of principle for the improved design of hypoallergens based upon structural limitations for activating the FcεRI, we began with characterization of Pen a 1-mediated responses in human basophils from allergic subjects. We recruited shrimp allergic subjects to evaluate the range of responses based upon IgE reactivity against shrimp extract, as well as basophil histamine release. Using a CRISPR-engineered basophilic cell line (hRBLrαKO) and a rule-based theoretical model, we characterized the relationship between Pen a 1-specific IgE dose, the number of FcεRI occupied and Pen a 1 dose-dependent mast cell degranulation. Five overlapping recombinant fragments spanning the sequence of Pen a 1 were generated to characterize their allergenic/degranulation activity. We show that, although recombinant Pen a 1 fragments are IgE-reactive and are likely a mix of monomers and dimers, the resulting FcεRI aggregates have reduced potency for cell activation. We discuss the potential for these hypoallergenic fragments to form the basis of safe and effective immunotherapy.
MATERIALS AND METHODS
Cell isolation and histamine release assays
Histamine release was measured as described previously (31, 32). Briefly, Percoll gradient centrifugation was used to prepare basophil-enriched cell populations (1–55% basophils) from 54 ml anticoagulated blood. Enriched cell fractions were activated by incubating with anti-IgE antibody, Pen a 1 full length peptides, or digested fragments at 37°C. Spontaneous degranulation was measured by adding Hanks only to the cells. Calcium ionophore A23187 was used as an internal positive control. Cells were activated for 30 min and reactions were terminated by addition of excess ice-cold buffer. Post centrifugation, histamine in cell supernatants was measured by ELISA (Genway Biotech) according to the manufacturer’s instructions. Total histamine was measured in supernatants generated by lysis of identical cell aliquots using three freeze-thaw cycles. The net FcεRI-mediated histamine released in response to ligand was expressed as a percentage of total histamine release after subtraction of spontaneous release.
Study Subjects
Individuals with shrimp allergy were recruited based on self-reporting. Shrimp allergy was confirmed by Immunocap assay performed at Tricore Reference Laboratories (Albuquerque, NM), which determines IgE reactivity with crude shrimp extract. Values >0.35 kU/L were considered positive. Subjects that were positive for shrimp allergy, as well as control subjects, were clinically assessed. Total IgE levels were measured by Immunetech Reference Laboratory (Foster City, CA). As a source of standardized Pen a 1-specific IgE, serum of shrimp allergic individuals was purchased from Plasmalabs (Everett, WA). Serum samples were stored at −20°C.
Purification of IgEPena1
Pen a 1-specific IgE was purified on an affinity column, prepared using full length rPen a1 according to manufacturer’s instructions (Abcam, Cambridge, MA). IgG was removed by incubating elution fractions with protein A/G beads (Invitrogen, Grand Island, NY) for 2 hrs. Absence of IgG in fractions was confirmed by immunoblotting with anti-IgG HRP (Invitrogen, Grand Island, NY).
CRISPR-edited hRBLrαKO
Cas9-mediated DNA cleavage was used to knock out both alleles encoding endogenous rat α-subunit in RBL-2H3 cells. A highly specific gRNA, directed against the first exon of the rat FcεRIα genomic sequence, was designed using the http://crispr.mit.edu/portal and then sub-cloned into the PX458 vector (Addgene plasmid #48138) for simultaneous expression of the gRNA, WT Cas9 and a GFP reporter. For the sub-cloning, two partially complementary oligonucleotides were ordered from Integrated DNA Technologies, Inc. (Coralville, IA, USA) and assembled by PCR.
-
Fwd rFcεRIα KO gRNA:
5′TTTCTTGGCTTTATATATCTTGTGGAAAGGACGAAACACCGAGTGTCCTTGGACCCACCG-3′;
-
Rvse rFcεRIα KO gRNA:
5′GACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAACCGGTGGGTCCAAGGACACTC-3′.
Gel purified PCR products were cloned into BbsI-digested PX458 using Gibson Assembly (NEB, MA, USA) following the manufacturer’s specifications. The engineered plasmid was used to transfect RBL-2H3 cells using the Amaxa system (Lonza, Basel, Switzerland). Positive, GFP-expressing cells were selected by flow cytometry using an iCyt cell sorter. After transient GFP expression was cleared, transfected cells were primed overnight with mouse IgE labelled with Pacific Blue 410, mIgEPB410 at 1 μg/ml. GFP-negative and mIgEPB410-negative cells were selected by flow cytometry and named hRBLrαKO. These cells were subsequently transfected with a vector containing the cDNA coding for human FcεRIα and geneticin resistance. Stably transfected cells flow sorted after overnight labeling with Alexa 488-conjugated human IgE (hIgEAlexa-488) at 1 μg/ml and positive cells, named hRBLrαKO.
Evaluation of IgE binding
hRBLrαKO cells were primed with hIgEAlexa-488 as indicated in figure legends. Cells were then harvested, washed twice to remove unbound IgE and cell-associated fluorescence was measured in a Becton Dickinson LSR Fortessa Flow Cytometer. To estimate the association rate constants, suspension cultures of hRBLrαKO or RBL-2H3 cells (5×105 cells in 400 μL medium) were incubated with Alexa-conjugated hIgE or mIgE at doses ranging from 60 to 20,000 μg/ml for 30 min and 60 min, followed by quantification of binding by flow cytometry. To measure the rate of hIgE dissociation from chimeric FcεRI, suspension cells (5×105 cells in 400 μL medium per well) were primed with 4 μg/ml hIgEAlexa488 for 2 days. After washing, resuspended cells were incubated with 10 μg/ml unlabeled IgE for defined intervals and loss of labeled IgE was monitored by flow cytometry. For quantification of bound hIgE under priming conditions used for degranulation assays, hRBLrαKO cells were primed for 2 hrs with human IgEAlexa-488 as described in legends. The number of FcεRI bound to IgE on the cell surface was determined based upon calibration using Quantum MESF Standard Beads (Bangs Laboratories, Fishers, IN) and measured F:P ratios for each batch of IgEAlexa-488.
Model for IgE-FcεRI binding
Interaction of IgE with FcεRI is consistent with a simple reaction scheme (33, 34): L+R=B where L represents free IgE, R represents free FcεRI, and B represents the IgE-FcεRI complex. Binding is not diffusion controlled (35), and internalization/turnover of the IgE-FcεRI complex is minimal (36). Under assumptions of mass-action kinetics and conservation of IgE and FcεRI (over time scales of interest), the time-dependence of IgE-FcεRI abundance is governed by the following equation:
where α = 2(kfL0R0 − krB0), β = kf(L0 + R0) + kr, and . Here, B0, L0, and R0 represent the abundances of IgE-FcεRI complex, free IgE, and free FcεRI at time t = 0; B(t) is the time-dependent abundance of IgE-FcεRI; kf is the forward rate constant for IgE binding to FcεRI; and kr is the reverse rate constant. The equilibrium abundance of IgE-FcεRI complex, Beq, is limt→∞ B(t).
In experiments where the amount of unlabeled IgE in solution is in large excess of labeled IgE on cells, such that rebinding of dissociated labeled IgE is negligible, decay of labeled receptor-bound IgE is governed by the following equation, which is a special case of the equation given above: B(t) = B0 exp(−krt). Parameters were estimated using nonlinear least squares fitting (37). Confidence limits on estimates were determined using bootstrapping (38).
From flow cytometric measurements of decay of labeled cell-surface hIgE in the presence of excess unlabeled hIgE in solution, we estimated that B0 =1.27×105 copies per cell and kr = 1.53×10−5 s−1. The 68% confidence limits on these estimates are (1.26, 1.28)×105 copies per cell and (1.43, 1.68)×10−5 s−1.
To characterize the kinetics of hIgE association with human FcεRI, we set kr at our estimated value of 1.53×10−5 s−1. For mIgE interaction with human FcεRI, we set kr at ρMH (1.53×10−5 s−1) = 2.20×10−5 s−1, where ρMH = 9.89/6.87 is a ratio of dissociation rate constants measured by Ishizaka et al. (1985) for murine and hIgE binding to human FcεRI. For mIgE interaction with rat FcεRI, we set kr at ρRH (1.53×10−5 s−1) = 2.20×10−5 s−1, where ρRH = 2.73/6.87 is the ratio of dissociation rate constants measured by Sterk and Ishizaka (1982)(39) and Ishizaka et al. (1985)(40) for mIgE binding to rat and human FcεRI. Our parameter estimates are insensitive to the particular fixed values chosen for dissociation rate constants, provided that these values are sufficiently small (i.e., such that the bond lifetime 1/kr is much longer than the duration of the experiment).
Theoretical modeling of allergen-mediated FcεRI aggregation
In our rule-based model, IgE-bound receptors have a valency of two and dimerized Pen a 1 molecules have 10 binding sites. We simplify the model by assuming that all binding sites have the same affinity. Receptor-antigen complexes are limited to 20 receptors per aggregate, as slow diffusion of large complexes and constraints on accessibility are expected to constrain aggregate size. All dissociation events have the same off-rate (koff = 0.01 s−1). Unbound antigen is assumed to diffuse over the entire 3D volume of the well; the on-rate for receptor binding (kon) is given by the off-rate koff divided by the dissociation constant KD. However, if the antigen is already bound to one or more receptors on the cell surface, its on-rate is effectively higher as there is a reduction of the number of degrees of freedom of the search space for binding events. In our model, the crosslinking on-rate is given by kon times the crosslinking factor α, a dimensionless constant. Rules also reflect overall concentration of antigen (ie, limiting or in excess as compared to the number of receptors). We simulate the entire cell in BioNetGen, with number of receptors and Pen a 1 molecules derived experimentally; the volume of extracellular fluid is set at 2.3 × 10−6 ml. The molecular weight of Pen a 1 is 72,000 g/mol. For each IgE priming condition, we have 2692 molecules of IgE (120 ng/ml), 1211 molecules of IgE (60 ng/ml), 716 molecules of IgE (30 ng/ml), and 324 molecules of IgE (15 ng/ml). Pen a 1 concentration is varied logarithmically, from 10−4 ng/ml to 104 ng/ml, for a total of 9 data points. We run our model with BioNetFit (41), an optimization algorithm that couples BioNetGen rule-based model computations and a genetic algorithm that finds the values of free parameters that give a best fit for the output of the simulation as compared to experimental results. The free parameters that are optimized during fitting are the dissociation constant (kD=off-rate/on-rate) and a crosslinking factor α. These parameters are varied randomly within constraints: The kD is varied between 10−10 to 10−8 M while α is varied between 1 and 5000. For fitting, we make the simple assumption (42) that secretion percentage is directly proportional to the number of receptors in aggregates, thus directly comparing simulation output (number of receptors in aggregates) to experimental degranulation data. This is a first approximation of the relationship between aggregation and secretion since the two events are separated by a series of subsequent intracellular events, implicating a more complex relationship. Thus, to test the hypothesis that the two distinct observables can indeed be compared in this manner, we perform training and testing on the data: First, we determine the free parameters of the model for the IgE concentration of 120 ng/ml using BioNetFit. Then, we use these same parameters for simulations of the same model under different IgE concentrations (60, 30, and 15 ng/ml), and compare the number of receptors in aggregates to the percentage secretion in each case. The secretion percentage data for [IgE] = 120 ng/ml is normalized to the highest secretion value, resulting in a set of values between 0 and 1. This dataset is the input file used in BioNetFit. The simulation output that is compared to the normalized secretion is the number of receptors in aggregates divided by total number of receptors. BioNetGen runs are stochastic simulations performed with NFSim (42). During the fit, data was collected in 18-second intervals and reported as the overall fraction of receptors in aggregates at 3 minutes. After the fit, constants were used in BioNetGen with NFSim to compare the total number of receptors in aggregates to the percentage secretion for each IgE concentration. Number of receptors in aggregates was estimated from kinetics by calculating the area under the curve from zero to 3 minutes.
Electron Microscopy
Methods for preparation of mast cell membrane sheets and immunogold labeling FcεRI have been described (Wilson et al., 2000). Digital images were acquired using a Hitachi H600 transmission electron microscope, followed by image processing to capture FceRI gold particle distribution and use of published methods for statistical analysis of clustering. Statistical differences were also computed using one-way ANOVA test in MATLAB.
Cloning
Pen a 1 cDNA (GenBank: DQ151457.1 Farfantepenaeus aztecus) was synthesized by Genewiz (South Plainfield, NJ). Five fragments of Pen a 1 were amplified using PCR and cloned into pET101 vector (Invitrogen, Grand Island, NY). Primers were synthesized by IDT (Coralville, IA). All constructs were expanded in One Shot® TOP10 chemically competenent E. coli (Invitrogen, Grand Island, NY) and plasmid DNA was isolated using Nucleospin Plasmid kits (Macherey Nagel, Duren, Germany. Sequencing was performed using Genewiz (South Plainfield, NJ). Primers used for amplification are as follows: F1 - 5′ CACCATGGAC GCCATCAAGAAGAAGATGC 3′; R1- 5′ AGAGAGGGCCTTGTCCTTGTC 3′; F2- 5′ CACCATGAACATCCAGCTTGTGGAGAA 3′; R2 - 5′ GTTCTCGAGCACCTTGCGCAT 3′ F3 - 5′ CACCATGAAGGTGCTCGAGAACC 3; R3 - 5′ CTCCTCAGCACGCTCAAGGT 3′ F4 - 5′ CACCATGGACCTTGAGCGTG 3′; R4 - 5′ CTCAGCCGCCTTCAGCTTGTT 3′; F5 5′ CACCATGCAGATTAAGACACTTACCAACAAG 3′; R5 - 5′ GTAGCCAGACAGTTCGCTGAAAGTCT 3′.
Expression and Purification of Recombinant Allergens
All recombinant proteins were expressed in BL21 starTM(DE3) cells (Invitrogen, Grand Island, NY) induced with 1mM isopropylthio-β-galactoside for 4 h. Cells were harvested by centrifugation and stored at −80°C overnight. Next day, pellets were dissolved in native buffer (50 mM Na phosphate, 0.5 M NaCl, 1 mg/ml lysozyme, protease inhibitors, pH 8.0). Cells were sonicated using the Misonix system and cell debris was removed by centrifugation at 5000 rpm, 15 min. Proteins were extracted and purified using Ni-NTA purification system (Invitrogen, Grand Island, NY) according to the manufacturer’s instructions using native or denaturing buffer conditions. Proteins were dialyzed against phosphate-buffered saline (PBS, pH 7.4) followed by final purification using size exclusion chromatography using the SEC s-3000 column (Phenomenex, Torrance, CA) and the Agilent 1100 system. Concentrations were determined by densitometric analysis of SDS-PAGE gels stained with Coomasie brilliant blue, using known amounts of natural shrimp tropomyosin (Indoor Biotechnologies, Charlottesville, VA) as standard.
Circular Dichroism Spectroscopy
Proteins dialyzed against PBS were adjusted to a concentration of 200 μg/ml. Circular Dichroism spectroscopy was performed with a Model 420 AVIV spectropolarimeter (Biomedical Inc., USA) with a constant nitrogen flushing at 20°C. Proteins were scanned with a spectral range of 185–255 nm, with a step width of 0.2 nm and band width of 1nm.
Immunoblotting Analysis
Proteins were fractionated by SDS-PAGE under reducing conditions using 4–12% gradient precast gels (Invitrogen, Grand Island, NY) and bands were detected by Coomasie staining. For immunoblotting, Pen a 1 and fragments separated by SDS-PAGE were transferred onto nitrocellulose membranes using the iBlot (Thermo Fischer), blocked with 3% BSA in TBST (0.1% tween in TBS) and incubated overnight with the pool of patient sera diluted in blocking buffer. Bound IgE was detected using 1: 1000 dilution of anti-IgE HRP (Invitrogen, Grand Island, NY). For detection and quantification of purified IgETM, elution fractions mixed with sample buffer without DTT were run on SDS-PAGE, transferred onto nitrocellulose membrane, and blocked and detected with anti-IgE HRP as described above. In between incubations, membranes were washed three times with TBST for 5 min each with gentle shaking at RT. For dot blots, 0.5 μg of each protein was spotted on to nitrocellulose membrane, followed by blocking with 5% milk in TBS-T for 1 hr at RT. Membranes were incubated overnight with either normal or IgG depleted patient serum diluted in blocking buffer, then washed with TBST. Bound antibody was detected using 1: 1000 dilution of anti-IgE HRP or 1:10,000 dilution of anti-IgG HRP. For depletion of IgG, 800 μl serum was incubated with 2 ml protein G beads ((Thermofisher, MA, USA) for 2 hours at RT. After incubation, beads were spun down and serum was recovered.
ELISA assay for reactivity of IgE in human plasma to Pen a 1
In brief, recombinant Pen a 1 or fragments were coated on the bottom of a high-binding, flat-bottom 96-well plates (Corning Inc., Corning, N.Y., USA) at 1.25 ug/ml in carbonate-bicarbonate buffer pH 9.6. Plates were blocked with 5% nonfat dry milk in PBST overnight, followed by incubation with atopic sera at 1:20 dilution for 1 h at room temperature. For IgE detection, wells were thoroughly washed and incubated with polyclonal goat anti-human IgE-HRP (Invitrogen, Grand Island, NY). Optical density was read at 450 nm following detection with 1-step ultra TMB-ELISA substrate (Thermofisher, MA, USA) and 2M sulfuric acid as stop solution.
Digestion of Pen a 1
Digestion of recombinant tropomyosin was performed using simulated gastric fluid (SGF, 35 mM NaCl, 84 mM HCl, pH 1.2) conditions as described in Mattison et al (2014) (43). Recombinant tropomyosin (1 μg) was added to SGF containing half-log (3.16-fold) dilutions of porcine pepsin (Sigma Aldrich, St. Louis, MS, USA) beginning with 40 units of enzyme. Pepsin was diluted in SGF prior to use, and was omitted from the zero time point sample. Reactions were incubated at 37 °C for 10 minutes, and then halted by the addition of Tris buffer (pH 8.5) to 100mM on ice. Samples were frozen and stored at −80 °C prior to analysis.
Basophil purification and acid stripping of IgE
Venous blood was collected from normal donors at United Blood Services (Albuquerque, NM). Basophils were isolated and enriched from blood by using HetasepTM for RBC depletion and EasySepTM human basophil enrichment kit (Stem cell technologies, Vancouver, Canada) for negative selection according to manufacturer’s instructions. Enriched basophils were stripped of bound IgE by incubating with 0.01 M lactate-buffered NaCl, KCl solution, pH 3.9 for 20s followed by neutralization with Tris buffer. Stripped basophils were primed with media containing shrimp-reactive serum (20%), followed by stimulation and collection of supernatant for histamine release as described above.
RESULTS
Variability in Pen a 1 specific IgE levels and basophil responsiveness in shrimp-allergic subjects
Shrimp allergic patients were recruited based on self-reporting and clinical history, followed by testing for positive reaction to shrimp extract (Supplemental Figure 1A). As shown in Figure 1A, only 12 of the 34 subjects (35%) had clinically detectable levels of shrimp-reactive IgE. Data in Figure 1B report shrimp–specific IgE levels for the 10 positive subjects, based upon by the MID Clinical Laboratories management options for ImmunoCAP specific IgE blood tests. Also reported in Fig. 1B are histamine release data from donor basophils after stimulation with 0.1 μg/ml recombinant Pen a 1 (rPen a 1), a principal allergen in shrimp extraction. As controls, we also report histamine release in response to 5 μg/ml anti-IgE. Statistical analysis indicates there is poor correlation between specific to total IgE ratio and Pen a 1-mediated histamine release (Fig 1B and Supplemental Figure 1B). There is also no correlation between anti-IgE mediated secretion and specific/total IgE ratio.
Figure 1. Pen a 1-specific IgE levels and basophil releasability in shrimp allergic blood donors.
A) Scoring system for classification of allergic patients based on circulating levels of specific IgE. Pie chart shows the distribution of self-reported shrimp allergic donors according to the classification. B) Inset table indicates the circulating levels of specific IgE, total IgE, ratio of specific to total IgE and corresponding basophil secretory response upon stimulation with either 0.1μg/ml rPen a 1 or 5 ug/ml anti-IgE in allergic donors. Plots show the dose-dependent basophil releasability profile upon challenge with rPen a 1 for three allergic donors.
Dose response studies over a range of rPen a 1 (0.001–5 μg/ml) were also performed using basophils from these donors. Data are shown for Donors B,D and F, which represent a wide range of shrimp-reactive IgE concentrations (expressed in kU/L). Since the circulating levels of specific IgE do not reliably predict degranulation responses, we speculate that other factors contribute to overall responses. These factors could be circulating cytokines, IgE epitope specificity, aggregation potential and the percent of the individual’s total IgE repertoire that is antigen-specific. Although there was considerable donor-to-donor variation in the total histamine release, the optimal dose of Pen a 1 typically fell between 0.1–1 μg/ml.
Quantification of dose-dependent Pen a 1-mediated degranulation responses and corresponding FcεRI occupancy
For further studies, we employed rat basophilic leukemia cells modified for exclusive expression of the human α-subunit of FcεRI (hRBLrαKO). To accomplish this, the rat FcεRI α-subunit was disrupted in RBL-2H3 cells using CRISPR-Cas 9 gene editing methods, followed by transfection with an expression vector coding for the human FcεRI α-subunit and flow sorting for human IgE (hIgE) binding. The stably transfected cells are thus unique, in that there are no rodent FcεRI α tetrameric receptors to compete with the human α-bearing FcεRI tetramers for coupling with signaling partners. The specificity of the chimeric FcεRI (human α, rat β/γ) in these cells for hIgE was verified by flow cytometry analysis (Supplemental Figure 2). Additional characteristics of the hRBLrαKO model system include: 1) low surface accumulation of IgE-FcεRI complexes after prolonged incubation of hRBLrαKO cells with hIgE, which peaks within 3–4 days (Supplemental Figure 2B) and 2) hIgE binds to the chimeric FcεRI with a kf of 6.23 × 103 M−1s−1 and dissociates at a kr of 1.53 × 10−5s−1, with a KD =kr/kf= 2.4 nM (Supplemental Figure 3, Supplemental Table 1). The marked rise in human FcεRI levels over days is consistent with prior reports of IgE-mediated stabilization of human FcεRI (44). For most experiments in hRBLrαKO cells, IgE priming was consistently performed for 2 hrs (maximal 62,000 IgE-bound FcεRI, see Table 1). This is important to compare results with stripped human primary basophils (Fig. 6), since basophils have a limited lifetime after isolation from peripheral blood. In addition, results in Supplemental Figure 2C show that 2 hr priming conditions avoid the need to account for receptor internalization and recycling.
Table 1.
Quantification of IgE-bound FcεRI
| IgE (ng/ml) | # IgE | % Receptor Occupied |
|---|---|---|
| 20000 | 62632 | 100 |
| 15000 | 58432 | 93 |
| 10000 | 52500 | 83 |
| 8000 | 49450 | 78 |
| 4000 | 37909 | 60 |
| 1000 | 17641 | 28 |
| 120 | 2692 | 4.00 |
| 60 | 1211 | 1.89 |
| 30 | 716 | 1.10 |
| 15 | 324 | 0.44 |
| 7.5 | 162 | 0.26 |
| 3.75 | 129 | 0.16 |
hRBLrαko cells were primed for 2 hours with human IgEAlexa-488 over specified range of [IgE] and fluorescence was quantified using flow cytometry. Number of FcεRI bound to IgE on the cell surface were measured using Quantum MESF Standard Beads (Bangs Laboratories) and F:P ratio of IgEAlexa-488. Results are expressed as mean of two independent experiments.
Figure 6. Pen a 1 fragments cause reduced cell activation and inhibit responses to intact Pen a 1.
A) hRBLrko cells were primed with atopic sera and stimulated with recombinant Pen a 1 and fragments (concentrations shown in legend). Cells were incubated with serum from shrimp allergic individual for 2 h before addition of recombinant Pen a 1, isolated fragments or fragments pooled together (concentrations shown in legend). B) Addition of fragment mix in specified molar concentrations to cells pre-incubated with 1 μg/ml Pen a 1 for 5 mins. Degranulation response is based on the percent of total β-hexosaminidase released from cells after stimulation for 30 min. C) Histamine release in basophils expressed in ng/ml, after subtraction of spontaneous release. Basophils isolated from donors were stripped of bound IgE, primed with atopic serum and stimulated with indicated allergen. Error Bars represent standard deviation. FR1–FR5 = Pen a 1 fragments; Mix = five Pen a 1 fragments pooled together in equivalent amounts at indicated concentrations.
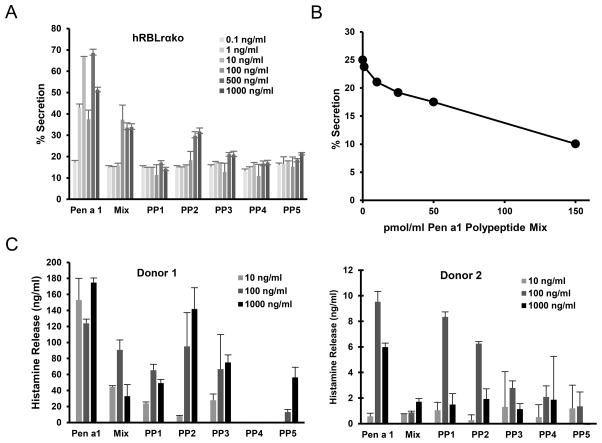
Figure 2 reports secretion data from hRBLrαKO cells after 2 hr priming with a range of concentrations of Pen a 1-specific IgE (IgEpena1) that was affinity purified from shrimp-reactive plasma. Cells were activated with increasing doses of rPen a 1 (rPen a 1) or anti-IgE (as a positive control). The data are plotted two ways: as a function of IgE priming conditions (Figures 2A,B) or as a function of crosslinker concentration (Figures 2C,D). Saturating concentrations of IgE occurred at approximately 500 ng/ml (Figures 2A,B). The typical bell-shaped secretory response, seen with structurally defined ligands (45) as well as natural allergens (46), is reproduced in the hRBLrαKO cells challenged with Pen a 1 (Fig. 2C). The optimal dose of rPen a 1 was 10 ng/ml under all priming conditions, underscoring that the number of receptors occupied with IgEpena1 is a key factor in setting the threshold for response.
Figure 2. Threshold and dose-dependency of Pen a 1 mediated response.
A,B,C,D) hRBLrαko cells were primed with IgEpena1 as indicated, incubated with a range of anti-IgE or Pen a 1 concentrations to crosslink IgE-FcεRI complexes, and degranulation responses measured. Error bars represent Standard Deviation. Results are representative of three independent experiments. E) Simulated number of IgE-FceRI complexes in aggregates as a function of time for each Pen a 1 concentration. F) Comparison of percent secretion (dashed lines) with average number of receptors in aggregates of any size, computed via integration of kinetic data as shown in (E). Curves in E–F are averages of >20 stochastic runs of the rule-based model with BioNetGen. Error bars are standard deviations. Values for the dissociation constant and crosslinking factor were KD = 4.21 × 10−1 nM and α = 3.98 × 103.
We used a flow-based assay to quantitate occupancy of FcεRI with allergen-specific IgE (Table 1). The minimal IgEPena1 priming conditions for stimulating secretion from hRBLrαKO cells occurred after 2 hrs exposure to IgEPena1 at 15 ng/ml (6.25 kU/L (47)). This translates to a minimum of 300 FcεRI required for measurable responses to Pen a 1 (Figure 2C, yellow line). Maximal secretory responses to rPen a 1 were achieved when less than 3000 FcεRI were primed with IgEPena1 (Figure 2C, red line; Table 1).
Rule-based model predicts FcεRI aggregation kinetics over a range of Pen a 1 doses
We next applied a rule-based mathematical model to estimate the number of IgE-FcεRI in aggregates as a function of IgE occupancy and allergen dose. Results of the optimized rule-based model (see Methods) are shown in Figure 2E, comparing the computed aggregation kinetics for 2692 IgE-FcεRI complexes as a function of Pen a 1 dose. As expected, high doses of antigen bind and initiate receptor aggregation within seconds. Dotted lines in Figure 2F report the total number of number of receptors bound in aggregates of any size over the simulation time course, based upon the total area under the curve for each condition of IgE priming over the entire range of antigen doses used. For comparison, dashed lines in Figure 2F provide the experimentally measured values for secretion used for fitting in the mathematical model.
The general shape of the dose-response curve, which increases as the allergen concentration increases until it peaks and then monotonically decreases for higher allergen concentrations, meets theoretical expectations (48). We expect that at low allergen concentrations, there will be small aggregates due to the low number of allergens per cell. As the allergen concentration rises, the average aggregate size will correspondingly increase until it reaches a size that causes maximum response. Further increases in allergen dose begin to favor monovalent allergen-receptor binding, which limits crosslinking. These results are consistent with prior explanations for “high-dose inhibition” of human basophils (49).
Pen a 1 induces FcεRI clustering
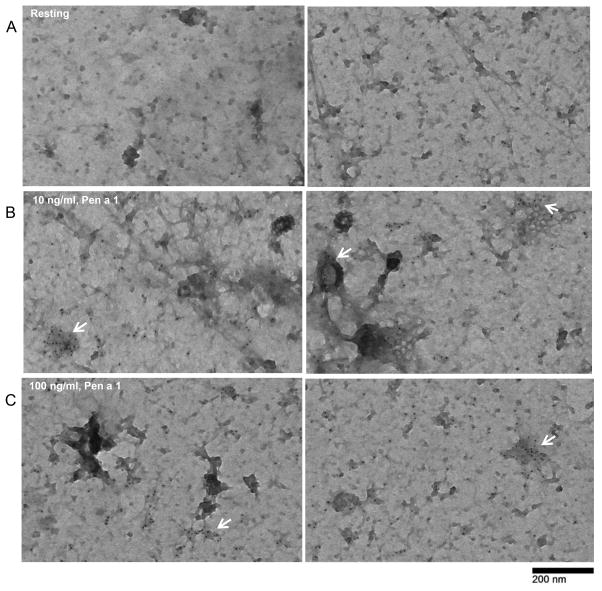
We next evaluated the spatial redistribution of FcεRI on the surface of Pen a 1-treated cells, using established electron microscopy methods. hRBLrαKO cells were primed with a saturating conditions of IgEPena1, then incubated +/− Pen a 1 prior to preparing membrane sheets (“rip-flips”) on EM grids. Samples were labeled with anti-FcεRIβ immunogold and imaged by transmission electron microscopy. Images in Figure 3A show that resting receptors are distributed across the cell membrane, alone and in small clusters that likely represent transient co-confinement in membrane domains (50). Figures 3B and 3C show that activation with Pen a 1 (10 or 100 ng/ml) leads an increase in the size of FcεRI clusters on the plasma membrane (arrows). These clusters appear to represent linear and branching chains (white arrowheads), as well as globular patches (black arrows) that likely represent heterogenous mixtures of Pena1-IgE-FcεRI aggregates of various sizes.
Figure 3. Pen a 1 cross-linking induces FcεRI clustering on the cell surface.
A, B, C) Transmission Electron Microscopy images of membrane sheets prepared from cells primed with 500 ng/ml IgEPen a 1 overnight, followed by stimulation with 0, 10 or 100 ng/ml Pen a 1 and immunogold labeling (6 nm gold) for FcεRI β. Arrows point to signaling patches, typical after addition of antigen. Bar = 0.2 μm.
Simulated gastric digestion produces rPen a 1 IgE-binding peptides with modestly attenuated mediator release
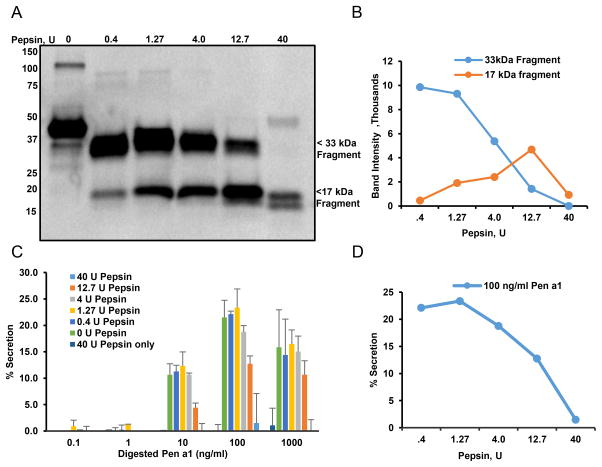
As a food allergen, Pen a 1 undergoes proteolytic cleavage during digestion. To evaluate the changing profile of Pen a 1 as a secretagogue during this process, rPen a 1 was incubated with pepsin under conditions designed to mimic gastric digestion. Immunoblot analysis of digested rPen a 1 (Figure 4A,B) revealed that IgE from the serum of an atopic donor strongly recognized major fragments migrating at 17 and 33 kDa, that persist even after 10 min digestion with 0.4–12.7U pepsin. These fragments retain their ability to crosslink IgEPena1-FcεRI complexes and robustly stimulate secretion, as shown in Figure 4C. Extensive digestion (40U pepsin) was required to reduce the secretory response of hRBLrαKO cells primed with IgEPena1 (Fig. 4D), consistent with the life-threatening clinical sensitivity of allergic subjects during exposure to dietary shrimp tropomyosin.
Figure 4. Effect of pepsin digestion on IgE binding to Pen a 1 and basophil degranulation.
A) Immunoblot analysis of IgE from serum of atopic individual binding to rPen a 1 digested with increasing concentrations of pepsin for 10 min at 37oC. Bound IgE was detected using HRP conjugated anti-IgE. B) Quantification of two major fragments obtained with rPen a 1 digestion in SDS-PAGE (B). C) hRBL-2H3 cells were primed with serum of atopic individual and challenged with rPen a 1 digested with increasing concentration of pepsin (concentrations shown in legend). D) Quantification of secretory responses induced by 100 ng/ml rPen a 1 digested with increasing concentrations of pepsin from plot B.
Design and characterization of Pen a 1-derived polypeptides
While pepsin-cleaved fragments of natural Pen a 1 are potent initiators of degranulation, it has been reported that short, recombinant Pen a 1-derived polypeptides encompassing a single predominant IgE binding epitope have diminished capacity (51). As shown in Figure 5A, we designed and expressed five sequential Pen a 1-recombinant polypeptides (FR11–79, FR268–127, FR3121–181, FR4172–236, FR5224–284), each 60–79 amino acids in length. These products covered the entire sequence of Pen a 1, retained known IgE and T-cell epitopes (13, 52), and had overlaps of 7–12 amino acids. Each polypeptide covered one major IgE binding region based on prior studies (53). Recombinant His-tagged proteins were expressed in E. coli, purified using Ni-NTA columns and separated using size exclusion chromatography to yield a single major band on SDS-PAGE (Figure 5B) and identified with an anti-His antibody on a western blot (Figure 5C). The minor, slower-migrating bands in Fig. 5C suggest that minute fractions of PP1, PP2 and PP5 migrate as dimers.
Figure 5. Design and recombinant expression of Pen a 1 and fragments.
A) Amino acid sequence of Pen a 1 and the five fragments. Sequence does not include N-terminal His tag. B) SDS-PAGE. Size-exclusion chromatography purified proteins were separated and detected by coomassie staining. C) Anti-His western blotting. Size-exclusion chromatography purified proteins were separated and detected by probing with anti-His antibodies. D) CD spectroscopy in PBS. Intact Pen a 1 but not the fragments showed a typical spectrum for α helix with minima at 208 and 222 nm. The percentage of a-helical content was calculated for each molecule by using mean residue ellipticity at 222 nm as described previously E) Immunoblot analysis of IgE binding to rPen a 1 and its fragments with serum of atopic (shrimp allergic) individual. Bound IgE was detected using HRP conjugated anti-IgE. F) ELISA to measure reactivity of IgE from atopic sera to rPen a 1 or fragments. C = control serum. Error bars represent standard deviation. G) Dot blot analysis of IgE or IgG binding to rPen a 1 and its fragments with normal or IgG depleted serum from atopic (shrimp allergic) individual. Bound antibody was detected using HRP conjugated anti-IgE or anti-IgG.
Secondary structures of recombinant intact Pen a 1 and the truncated polypeptides were evaluated using circular dichroism (CD) (Fig. 5D). rPen a 1 exhibited a typical α-helical structure, with characteristic minima at 208 and 222 nm and maxima at 193 nm (54). The Pen a 1-derived polypeptides exhibited varying amounts of α-helical content that was significantly lower than that of the parent molecule. Since tropomyosin coiled-coil dimerization is dependent on α-helical structure (55), this is consistent with the blotting results that estimate only a small fraction of the recombinant polypeptides are in the dimer state in these solutions.
The binding of IgE from serum from a representative shrimp allergic subject was confirmed by western blotting (Fig. 5E, left), as well as ELISA-based measurements (Fig. 5F). As expected, intact recombinant Pen a 1 showed strong binding to IgE in shrimp-positive serum. Of the truncated products produced as recombinant polypeptides, PP1, PP2 and PP3 were highly reactive in immunoblots probed with IgE from the same serum, with modest reactivity with PP5 and weak reactivity with PP4 (Fig 5E, left panel). Results were confirmed by ELISA (Figure 5F). Note that IgE from control plasma (ie, negative by the Immunocap assay) failed to bind either whole Pen a 1 or the truncated polypeptides (data not shown).
The binding of peptides to IgG present in the same shrimp-positive serum was evaluated using dot blot analysis. Proteins were spotted on nitrocellulose membrane and incubated with serum, followed by detection with anti-IgG HRP or anti-IgE HRP (Fig 5G). Similar to IgE, IgG against all peptides and intact Pen a 1 was detected. Specificity was confirmed by the absence of binding detection when IgG-depleted serum was used.
Recombinant Pen a 1 truncated polypeptides are poor secretagogues and act as competitive inhibitors for intact Pen a 1
Based upon data in Figure 5, our preparations of truncated Pen a 1 polypeptides are predominately monomers, with a small fraction of dimers. These peptides have been reported in the literature to each have a single dominant linear epitope or an overlapping string of epitopes.(12) We expected that preparations with a large fraction of monomers (valency = 1), and small fraction of dimers (valency = 2), would be a poor aggregating stimulus for IgE-FcεRI complexes. To test this, hRBLrαKO cells were sensitized with IgE by incubation with serum from a shrimp-allergic subject, followed by a challenge with whole rPen a 1 or each of the five polypeptides alone (Fig. 6A). We observed reduced secretory response to each of five fragments when administered alone, even when challenging cells with the highest dose (1 μg/ml). Even when all five truncated polypeptides were pooled, where cross-reactivity between epitopes might enhance crosslinking of IgE-FcεRI complexes, the secretory response was approximately half that of intact antigen.
Results in Figure 6B show that Pen a 1-truncated polypeptides also compete with intact Pen a 1, to competitively inhibit mediator release from hRBLrαKO cells. IgE-sensitized cells were incubated first for 5 min with 1μg/ml Pen a 1, followed by addition of pooled fragments at defined concentrations for 25 minutes. Competitive inhibition by the truncated polypeptides was dose dependent.
Finally, we confirmed these results using primary cells (Fig. 6C). Human basophils were freshly isolated from two donors by negative selection procedures from the blood of a normal donor, then stripped of native IgE and primed for 1 hour with 20% serum from the same shrimp allergic subject as described above. The basophils were then challenged with either intact rPen a 1 or the truncated Pen a 1 polypeptides. Results for donor 1 show that basophil histamine release was markedly lower following challenge with PP1, PP3 and PP5 and not measurable to PP4. High concentrations of PP2 did stimulate histamine release that was nearly as robust as intact allergen, suggesting that the donor’s IgE repertoire is enriched in IgE recognizing epitope(s) in the PP2 peptide and/or that primary cells may have an even lower threshold for activation than the hRBLrαKO cell line. For donor 2, Pen a 1 elicited a higher response at 100 and 1000 ng/ml concentration when compared to all polypeptides added individually or pooled together. However, the difference was less pronounced with 100 ng/ml PP1 and PP2 that stimulated relatively high levels of histamine. The low concentration of 10 ng/ml caused a barely detectable histamine release regardless of stimulant. These results demonstrate the donor to donor variability resulting from differences in activation thresholds and underscore the need to validate results obtained with cultured cells with primary basophils or mast cells.
DISCUSSION
The sequence, structure and stability of the major shellfish allergen, Pen a 1, has been well characterized (56). B and T cell epitopes of Pen a 1 have also been identified using extensive binding studies (57, 58). However, little is known about the relationship between threshold or activating doses of Pen a 1 and their relationship to the IgE-receptor (FcεRI) aggregate properties and effector cell responses that constitute allergy. Since the density of cell surface FcεRI expression in basophils and mast cells are generally expected to correlate with circulating/free levels of IgE (59, 60), the detection of IgE antibodies to shrimp or specific epitopes of tropomyosin in serum are assumed to be clinically relevant (57, 61). The magnitude of effector cell responses have been shown to depend on the ratio of allergen-specific IgE to total IgE, which varies with age, total serum IgE and heterogeneity in IgE (62). In our study, we do not observe correlation between specific IgE levels in individual blood samples and magnitude of responses of basophils isolated from the same donor source upon challenge with allergen. Therefore, number of factors contribute to variability amongst individuals, including the basal levels of inflammatory cytokines such as IL3 that are well known to potentiate FcεRI-stimulated responses (63). In addition, there is a persistent and wide gap between the number of individuals who believe they have food allergies and the true prevalence (1).These results support the use of clinically-approved in vitro basophil activation tests (64), to evaluate allergen reactivity for patients in the allergy clinic.
We report here the first use of hRBLrαKO cells that exclusively express the human FcεRI α subunit. Our characterization of this cell line (Supplemental Figures 2,3) reveals a slower on rate for IgE binding compared to previous estimates for human FcεRI on primary cells (40), but with a somewhat higher affinity than transgenic mice expressing chimeric FcεRI comprised of human α subunit with mouse β and γ2 (2.4 nM vs 6.4 nM)(65).
In this study, we initially characterized the relationship between Pen a 1 and IgEPena1 priming using both experimental and rule-based modeling approaches, enabling us to determine the relationship between the number of FcεRI occupied and cellular responses. Since the occupancy of FcεRI with IgE is saturable, we expected that a key variable is the fraction of the total IgE repertoire that is allergen specific. Our data using “humanized” RBL cells indicate that measurable degranulation responses to Pen a 1 can be achieved with only a few hundred receptors engaged on the cell surface, while maximal secretion can occur when less than 2700 FcεRI are primed with allergen-specific IgE. Human basophils express from 70,000–230,000 FcεRI on the cell surface (66, 67). Thus, if a similar threshold applies to human primary cells expressing 230,000 FcεRI, occupancy of between 0.0017–0.01% of FcεRI with Pen a 1-specific IgE might be sufficient to trigger histamine release. We note that, although hRBLrαko do serve as a useful surrogate for primary basophils, ~ten-fold higher Pen a 1 doses were required for optimal degranulation from basophils from individual, shrimp-allergic donors (see Donors B,F in Figure 1).
Other key variables for FcεRI activation are the valency and dose of allergen, which together exert strong influence over the aggregation potential of a given allergen. We developed a rule-based model to generate predictions of receptor aggregation achieved over a range of IgE and Pen a 1 doses. The mathematical model matches aggregating conditions that are optimal for Pen a 1-mediated degranulation, which crosslink around 2000 receptors for 120 ng/ml of IgE, and predicts a higher number of receptors in aggregates at maximum secretion for 15, 30, and 60 ng/ml of IgE. Using immuno-electron microscopy of membrane sheets (Figure 3), we observed that receptor clusters grow in size under these crosslinking conditions. Some of the larger clusters seen by EM are likely to be composites of smaller aggregates in the same signaling patch (29).
Specific immunotherapy using natural allergen extracts is the most common treatment option employed today for allergen-specific disease modification (68). The risk of adverse reactions during immunotherapy for food allergens is considerable (69). Avoidance remains the primary recommendation for patients diagnosed with shrimp allergy, with one recent study evaluating sublingual immunotherapy with shrimp extract (70). It is particularly remarkable that defined formulations of recombinant tropomyosin have not been evaluated for shrimp-specific immunotherapy, despite the successful use of recombinant allergens in clinical trials with grass and birch pollen allergens (71, 72). Caution is understandably merited for the full length Pen a 1 protein, due to its unique filamentous, dimer structure and well-spaced epitopes. With a valency of ten or more, tropomyosin is an exceptionally efficient cross-linking stimulus for the IgE receptor. It makes sense to consider these structural features for the design of hypoallergenic allergy vaccines (73). Improved safety profiles might be expected after reengineering Pen a 1 to have reduced or abolished IgE binding (13) or by lowering the crosslinking capacity (14, 51). Mutagenesis and epitope deletion strategies have been shown to reduce allergenicity in the shrimp allergen, Met e 1 (74). Because tropomyosin is a pan allergen, causing adverse reactions across shellfish and other invertebrate species (ie, dust mite) (75), it is particularly important to reevaluate these strategies with the goal of developing Pen a 1 hypoallergen formulations that retain T cell epitopes and lower the potential for adverse events during therapy.
One of the methodologies for reducing IgE binding includes destroying the structural conformation of the allergen with heat-induced denaturation or in vivo digestion. However, the structure of Pen a 1 is extremely stable even after boiling (76), retaining full allergenicity after heat treatment (77). We characterized the IgE binding and effector cell activation of Pen a 1 under conditions that mimic gastric digestion. The profile of digested fragments obtained in our study differed from previously reported studies (78, 79), possibly because of differences in IgE epitopes recognized by patients in the different studies as well as experimental variables (pepsin concentrations, incubation times and protein production). Importantly, we show that extensive digestion is needed to lower the allergenic potential of Pen a 1. Apart from the already established route of gut absorption, allergic symptoms have been shown to emerge with mere inhalation of cooking vapors or handling of seafood (80), thus serving as an alternative route of triggering an allergic response.
Protein engineering represents an important strategy to reduce allergen valency and allergenicity (73). We designed overlapping recombinant fragments that span the entire length of Pen a 1 and evaluated IgE binding and effector cell responses. Similar to digested Pen a 1, these polypeptides retained the ability to bind IgE in both denatured and native forms. These polypeptide formulations each bear a single major linear IgE epitope or have a group of adjacent epitopes that would likely be effectively monovalent due to steric constraints once IgE was bound to one epitope. The Pen a 1-derived polypeptides were relatively weak stimulators of hRBLrαKO cells primed with allergen-specific IgE, even at high concentrations and when pooled together. Their low alpha helical content suggests that the bulk of the polypeptides remain monomeric in solution and are thus monovalent. For the small fraction that do form dimers based upon SDS-PAGE analysis, it is intriguing to note that the coiled-coil orientation of two identical epitopes in any pair of dimers renders their distance very close (<2 nm). Our preparations showed reduced allergenicity by comparison to an earlier study (51), presumably due to differences in design, affinity purification and/or concentration steps that will be critical to carry forward for potential clinical development.
The Pen a 1-derived polypeptides also had a markedly reduced capacity to stimulate normal human basophils from two individual donors. Both of these basophil preparations were subjected to rapid acid-stripping of bound IgE, followed by priming with the same source of allergic serum containing Pen a 1-specific IgE. The differences in histamine releasibility for these basophils after challenge with intact or fragmented Pen a 1 likely reflect differences that are unique to the donors, including basal levels of surface FcεRI and exposure of the basophils in vivo to circulating cytokines.
We expect that differences in primary cells versus hRBLrαKO cells reflect variable expression levels of signaling molecules like Syk, Lyn and SHIP, which serve as positive and negative regulators of mediator release (31, 81). Studies with birch pollen allergen Bet v 1 additionally suggest that the basophil activation assay may not always correlate with the potency of an allergen in triggering an in vivo response (82).
In conclusion, we have demonstrated the concept of dividing a linear complex allergen like tropomyosin into overlapping recombinant polypeptides. In total, they retain all known IgE and T-cell epitopes while simultaneously reducing allergenicity. These formulations represent a starting point for the design and development of a therapeutic vaccine for shrimp allergy that is efficacious and safe with minimal side effects in patients. It is notable that IgG against all polypeptides was also detected in allergic serum, thus confirming that these polypeptides bear some IgG binding epitopes. Further studies are now warranted to examine the potential of these polypeptides to induce a protective blocking-antibody response in vivo.
Supplementary Material
Acknowledgments
This study was supported by NIH P50GM085273 (BSW) and NSF CAREER Award III-1553266 (LT). This research was supported in part by funds from the U.S. Department of Agriculture, Agricultural Research Service (to CM).
The authors acknowledge the UNM Clinical and Translational Science Center (CTSC) for recruitment of human subjects, as well as the UNM Electron Microscopy Facility, Center for Advanced Research Computing, and Cancer Center Core Facilities for Flow Cytometry and Fluorescence Microscopy. We thank Dr. Eva Chi and Adeline Fanni for their help with HPLC experiments. Mention of trade names, commercial products, or companies in this paper is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
References
- 1.Chafen JJ, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, Sundaram V, Paige NM, Towfigh A, Hulley BJ, Shekelle PG. Diagnosing and managing common food allergies: a systematic review. JAMA. 2010;303:1848–1856. doi: 10.1001/jama.2010.582. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114:159–165. doi: 10.1016/j.jaci.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Lopata AL, O’Hehir RE, Lehrer SB. Shellfish allergy. Clin Exp Allergy. 2010;40:850–858. doi: 10.1111/j.1365-2222.2010.03513.x. [DOI] [PubMed] [Google Scholar]
- 4.Smillie L. Structure and functions of tropomyosins from muscle and non-muscle sources. Trends Biochem Sci. 1979;4:151–155. [Google Scholar]
- 5.Daul CB, Slattery M, Reese G, Lehrer SB. Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Int Arch Allergy Immunol. 1994;105:49–55. doi: 10.1159/000236802. [DOI] [PubMed] [Google Scholar]
- 6.Mykles DL, Cotton JL, Taniguchi H, Sano K-i, Maeda Y. Cloning of tropomyosins from lobster (Homarus americanus) striated muscles: fast and slow isoforms may be generated from the same transcript. J Muscle Res Cell Motil. 1998;19:105–115. doi: 10.1023/a:1005352410725. [DOI] [PubMed] [Google Scholar]
- 7.Leung PS, Chen Y-c, Gershwin ME, Wong HS, Kwan HS, Chu KH. Identification and molecular characterization of Charybdis feriatus tropomyosin, the major crab allergen. J Allergy Clin Immunol. 1998;102:847–852. doi: 10.1016/s0091-6749(98)70027-2. [DOI] [PubMed] [Google Scholar]
- 8.Miyazawa H, Fukamachi H, Inagaki Y, Reese G, Daul CB, Lehrer SB, Inouye S, Sakaguchi M. Identification of the first major allergen of a squid (Todarodes pacificus) J Allergy Clin Immunol. 1996;98:948–953. doi: 10.1016/s0091-6749(96)80011-x. [DOI] [PubMed] [Google Scholar]
- 9.Aki T, Kodama T, Fujikawa A, Miura K, Shigeta S, Wada T, Jyo T, Murooka Y, Oka S, Ono K. Immunochemical characterization of recombinant and native tropomyosins as a new allergen from the house dust mite, Dermatophagoides farinae. J Allergy Clin Immunol. 1995;96:74–83. doi: 10.1016/s0091-6749(95)70035-8. [DOI] [PubMed] [Google Scholar]
- 10.Asturias JA, Gómez-Bayón N, Arilla MC, Martínez A, Palacios R, Sánchez-Gascón F, Martínez J. Molecular characterization of American cockroach tropomyosin (Periplaneta americana allergen 7), a cross-reactive allergen. J Immunol (Baltimore, Md : 1950) 1999;162:4342–4348. [PubMed] [Google Scholar]
- 11.Fernandes J, Reshef A, Patton L, Ayuso R, Reese G, Lehrer SB. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy. 2003;33:956–961. doi: 10.1046/j.1365-2222.2003.01722.x. [DOI] [PubMed] [Google Scholar]
- 12.Ayuso R, Lehrer S, Reese G. Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin) Int Arch Allergy Immunol. 2002;127:27–37. doi: 10.1159/000048166. [DOI] [PubMed] [Google Scholar]
- 13.Reese G, Viebranz J, Leong-Kee SM, Plante M, Lauer I, Randow S, Moncin MS, Ayuso R, Lehrer SB, Vieths S. Reduced allergenic potency of VR9-1, a mutant of the major shrimp allergen Pen a 1 (tropomyosin) J Immunol. 2005;175:8354–8364. doi: 10.4049/jimmunol.175.12.8354. [DOI] [PubMed] [Google Scholar]
- 14.Albrecht M, Kuhne Y, Ballmer-Weber BK, Becker WM, Holzhauser T, Lauer I, Reuter A, Randow S, Falk S, Wangorsch A, Lidholm J, Reese G, Vieths S. Relevance of IgE binding to short peptides for the allergenic activity of food allergens. J Allergy Clin Immunol. 2009;124:328–336. 336 e321–326. doi: 10.1016/j.jaci.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 15.Ishizaka K, Tomioka H, Ishizaka T. Mechanisms of passive sensitization. I. Presence of IgE and IgG molecules on human leukocytes. J Immunol. 1970;105:1459–1467. [PubMed] [Google Scholar]
- 16.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 17.Durham SR. Allergen immunotherapy: 100 years on. Clin Exp Allergy. 2011;41:1171. doi: 10.1111/j.1365-2222.2009.03843.x. [DOI] [PubMed] [Google Scholar]
- 18.Marth K, Focke-Tejkl M, Lupinek C, Valenta R, Niederberger V. Allergen Peptides, Recombinant Allergens and Hypoallergens for Allergen-Specific Immunotherapy. Current treatment options in allergy. 2014;1:91–106. doi: 10.1007/s40521-013-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson MR, Petersen MM, Wolverton WO, Mikita CP. Allergen immunotherapy extract treatment set preparation: making a safer and higher quality product for patients. Current allergy and asthma reports. 2013;13:399–405. doi: 10.1007/s11882-013-0362-z. [DOI] [PubMed] [Google Scholar]
- 20.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, Nelson M, Weber R, Bernstein DI, Blessing-Moore J, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph C, Schuller DE, Spector SL, Tilles S, Wallace D. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127:S1–55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 21.Pauli G, Malling HJ. The current state of recombinant allergens for immunotherapy. Curr Opin Allergy Clin Immunol. 2010;10:575–581. doi: 10.1097/ACI.0b013e32833fd6c5. [DOI] [PubMed] [Google Scholar]
- 22.Casale TB, Stokes JR. Future forms of immunotherapy. J Allergy Clin Immunol. 2011;127:8–15. doi: 10.1016/j.jaci.2010.10.034. quiz 16–17. [DOI] [PubMed] [Google Scholar]
- 23.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, Turcanu V, Sever ML, Gomez Lorenzo M, Plaut M, Lack G, Team LS. Randomized trial of peanut consumption in infants at risk for peanut allergy. The New England journal of medicine. 2015;372:803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, Brough HA, Santos AF, Harris KM, Radulovic S, Basting M, Turcanu V, Plaut M, Lack G L.-O. S. T. Immune Tolerance Network. Effect of Avoidance on Peanut Allergy after Early Peanut Consumption. The New England journal of medicine. 2016;374:1435–1443. doi: 10.1056/NEJMoa1514209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pongracic JA, Bock SA, Sicherer SH. Oral food challenge practices among allergists in the United States. J Allergy Clin Immunol. 2012;129:564–566. doi: 10.1016/j.jaci.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Lopata AL, Lehrer SB. New insights into seafood allergy. Current opinion in allergy and clinical immunology. 2009;9:270–277. doi: 10.1097/ACI.0b013e32832b3e6f. [DOI] [PubMed] [Google Scholar]
- 27.Lehrer SB, Ayuso R, Reese G. Current understanding of food allergens. Ann N Y Acad Sci. 2002;964:69–85. doi: 10.1111/j.1749-6632.2002.tb04133.x. [DOI] [PubMed] [Google Scholar]
- 28.Cambier JC. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 29.Wilson BS, Pfeiffer JR, Oliver JM. Observing FcepsilonRI signaling from the inside of the mast cell membrane. J Cell Biol. 2000;149:1131–1142. doi: 10.1083/jcb.149.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smarr CB, Bryce PJ, Miller SD. Antigen-specific tolerance in immunotherapy of Th2-associated allergic diseases. Critical Reviews™ in Immunology. 2013:33. doi: 10.1615/critrevimmunol.2013007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kepley CL, Youssef L, Andrews RP, Wilson BS, Oliver JM. Multiple defects in Fc epsilon RI signaling in Syk-deficient nonreleaser basophils and IL-3-induced recovery of Syk expression and secretion. J Immunol. 2000;165:5913–5920. doi: 10.4049/jimmunol.165.10.5913. [DOI] [PubMed] [Google Scholar]
- 32.Youssef LA, Wilson BS, Oliver JM. Proteasome-dependent regulation of Syk tyrosine kinase levels in human basophils. J Allergy Clin Immunol. 2002;110:366–373. doi: 10.1067/mai.2002.127562. [DOI] [PubMed] [Google Scholar]
- 33.Kulczycki A, Jr, Metzger H. The interaction of IgE with rat basophilic leukemia cells. II. Quantitative aspects of the binding reaction. J Exp Med. 1974;140:1676–1695. doi: 10.1084/jem.140.6.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendoza G, Metzger H. Distribution and valency of receptor for IgE on rodent mast cells and related tumour cells. Nature. 1976;264:548–550. doi: 10.1038/264548a0. [DOI] [PubMed] [Google Scholar]
- 35.Wank SA, DeLisi C, Metzger H. Analysis of the rate-limiting step in a ligand-cell receptor interaction: the immunoglobulin E system. Biochemistry. 1983;22:954–959. doi: 10.1021/bi00273a038. [DOI] [PubMed] [Google Scholar]
- 36.Isersky C, Rivera J, Mims S, Triche TJ. The fate of IgE bound to rat basophilic leukemia cells. J Immunol. 1979;122:1926–1936. [PubMed] [Google Scholar]
- 37.More JJ, Garbow BS, Hillstrom KE. User Guide for MINPACK-1. Argonne National Laboratory; 1980. [Google Scholar]
- 38.Press WH, Teukolsky SA, Vetterling WT, Flannery BP. Numerical Recipes 3rd Editon: The Art of Scientific Computing. New York: Cambridge University Press; 2007. [Google Scholar]
- 39.Sterk AR, Ishizaka T. Binding properties of IgE receptors on normal mouse mast cells. J Immunol. 1982;128:838–843. [PubMed] [Google Scholar]
- 40.Ishizaka T, Dvorak AM, Conrad DH, Niebyl JR, Marquette JP, Ishizaka K. Morphologic and immunologic characterization of human basophils developed in cultures of cord blood mononuclear cells. J Immunol. 1985;134:532–540. [PubMed] [Google Scholar]
- 41.Thomas BR, Chylek LA, Colvin J, Sirimulla S, Clayton AH, Hlavacek WS, Posner RG. BioNetFit: a fitting tool compatible with BioNetGen, NFsim, and distributed computing environments. Bioinformatics. 2015:btv655. doi: 10.1093/bioinformatics/btv655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sneddon MW, Faeder JR, Emonet T. Efficient modeling, simulation and coarse-graining of biological complexity with NFsim. Nat Methods. 2011;8:177–183. doi: 10.1038/nmeth.1546. [DOI] [PubMed] [Google Scholar]
- 43.Mattison CP, Grimm CC, Wasserman RL. In vitro digestion of soluble cashew proteins and characterization of surviving IgE-reactive peptides. Mol Nutr Food Res. 2014;58:884–893. doi: 10.1002/mnfr.201300299. [DOI] [PubMed] [Google Scholar]
- 44.MacGlashan DW, Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, Sterbinsky SA, Hamilton RG, Lichtenstein LM. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–1445. [PubMed] [Google Scholar]
- 45.Mahajan A, Barua D, Cutler P, Lidke DS, Espinoza FA, Pehlke C, Grattan R, Kawakami Y, Tung CS, Bradbury AR, Hlavacek WS, Wilson BS. Optimal aggregation of FcepsilonRI with a structurally defined trivalent ligand overrides negative regulation driven by phosphatases. ACS Chem Biol. 2014;9:1508–1519. doi: 10.1021/cb500134t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilsson OB, Adedoyin J, Rhyner C, Neimert-Andersson T, Grundstrom J, Berndt KD, Crameri R, Gronlund H. In vitro evolution of allergy vaccine candidates, with maintained structure, but reduced B cell and T cell activation capacity. PloS one. 2011;6:e24558. doi: 10.1371/journal.pone.0024558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dolen W. IgE antibody in the serum–detection and diagnostic significance. Allergy. 2003;58:717–723. doi: 10.1034/j.1398-9995.2003.00281.x. [DOI] [PubMed] [Google Scholar]
- 48.Sulzer B, Perelson AS. Equilibrium binding of multivalent ligands to cells: effects of cell and receptor density. Math Biosci. 1996;135:147–185. doi: 10.1016/0025-5564(96)00022-3. [DOI] [PubMed] [Google Scholar]
- 49.Dembo M, Goldstein B. A model of cell activation and desensitization by surface immunoglobin: the case of histamine release from human basophils. Cell. 1980;22:59–67. doi: 10.1016/0092-8674(80)90154-3. [DOI] [PubMed] [Google Scholar]
- 50.Wilson BS, Oliver JM, Lidke DS. Spatio-temporal signaling in mast cells. Adv Exp Med Biol. 2011;716:91–106. doi: 10.1007/978-1-4419-9533-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myrset HR, Faeste CK, Kristiansen PE, Dooper MM. Mapping of the immunodominant regions of shrimp tropomyosin Pan b 1 by human IgE-binding and IgE receptor crosslinking studies. Int Arch Allergy Immunol. 2013;162:25–38. doi: 10.1159/000350791. [DOI] [PubMed] [Google Scholar]
- 52.Ravkov EV, I, Pavlov Y, Martins TB, Gleich GJ, Wagner LA, Hill HR, Delgado JC. Identification and validation of shrimp-tropomyosin specific CD4 T cell epitopes. Human Immunol. 2013;74:1542–1549. doi: 10.1016/j.humimm.2013.08.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ayuso R, Lehrer SB, Reese G. Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin) Int Arch Allergy Immunol. 2002;127:27–37. doi: 10.1159/000048166. [DOI] [PubMed] [Google Scholar]
- 54.Holzwarth G, Doty P. THE ULTRAVIOLET CIRCULAR DICHROISM OF POLYPEPTIDES. J Am Chem Soc. 1965;87:218–228. doi: 10.1021/ja01080a015. [DOI] [PubMed] [Google Scholar]
- 55.Gimona M, Watakabe A, Helfman DM. Specificity of dimer formation in tropomyosins: influence of alternatively spliced exons on homodimer and heterodimer assembly. Proc Natl Acad Sci U S A. 1995;92:9776–9780. doi: 10.1073/pnas.92.21.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reese G, Ayuso R, Lehrer SB. Tropomyosin: an invertebrate pan–allergen. Int Arch Allergy Immunol. 1999;119:247–258. doi: 10.1159/000024201. [DOI] [PubMed] [Google Scholar]
- 57.Ayuso R, Sanchez-Garcia S, Pascal M, Lin J, Grishina G, Fu Z, Ibanez MD, Sastre J, Sampson HA. Is epitope recognition of shrimp allergens useful to predict clinical reactivity? Clin Exp Allergy. 2012;42:293–304. doi: 10.1111/j.1365-2222.2011.03920.x. [DOI] [PubMed] [Google Scholar]
- 58.Ravkov EV, I, Pavlov Y, Martins TB, Gleich GJ, Wagner LA, Hill HR, Delgado JC. Identification and validation of shrimp-tropomyosin specific CD4 T cell epitopes. Hum Immunol. 2013;74:1542–1549. doi: 10.1016/j.humimm.2013.08.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gomez G, Jogie-Brahim S, Shima M, Schwartz LB. Omalizumab reverses the phenotypic and functional effects of IgE-enhanced FcεRI on human skin mast cells. J Immunol. 2007;179:1353–1361. doi: 10.4049/jimmunol.179.2.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MacGlashan D. IgE receptor and signal transduction in mast cells and basophils. Curr Opin Immunol. 2008;20:717–723. doi: 10.1016/j.coi.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Gámez C, Sánchez-García S, Ibáñez M, López R, Aguado E, López E, Sastre B, Sastre J, Del Pozo V. Tropomyosin IgE-positive results are a good predictor of shrimp allergy. Allergy. 2011;66:1375–1383. doi: 10.1111/j.1398-9995.2011.02663.x. [DOI] [PubMed] [Google Scholar]
- 62.Hamilton RG, MacGlashan DW, Jr, Saini SS. IgE antibody-specific activity in human allergic disease. Immunologic research. 2010;47:273–284. doi: 10.1007/s12026-009-8160-3. [DOI] [PubMed] [Google Scholar]
- 63.MacGlashan D., Jr Subthreshold desensitization of human basophils re-capitulates the loss of Syk and FcepsilonRI expression characterized by other methods of desensitization. Clin Exp Allergy. 2012;42:1060–1070. doi: 10.1111/j.1365-2222.2012.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos AF, Douiri A, Becares N, Wu SY, Stephens A, Radulovic S, Chan SM, Fox AT, Du Toit G, Turcanu V, Lack G. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J Allergy Clin Immunol. 2014;134:645–652. doi: 10.1016/j.jaci.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fung-Leung WP, De Sousa-Hitzler J, Ishaque A, Zhou L, Pang J, Ngo K, Panakos JA, Chourmouzis E, Liu FT, Lau CY. Transgenic mice expressing the human high-affinity immunoglobulin (Ig) E receptor alpha chain respond to human IgE in mast cell degranulation and in allergic reactions. J Exp Med. 1996;183:49–56. doi: 10.1084/jem.183.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saini SS, MacGlashan DW, Jr, Sterbinsky SA, Togias A, Adelman DC, Lichtenstein LM, Bochner BS. Down-regulation of human basophil IgE and FC epsilon RI alpha surface densities and mediator release by anti-IgE-infusions is reversible in vitro and in vivo. J Immunol. 1999;162:5624–5630. [PubMed] [Google Scholar]
- 67.Oliver JM, Tarleton CA, Gilmartin L, Archibeque T, Qualls CR, Diehl L, Wilson BS, Schuyler M. Reduced FcepsilonRI-mediated release of asthma-promoting cytokines and chemokines from human basophils during omalizumab therapy. Int Arch Allergy Immunol. 2010;151:275–284. doi: 10.1159/000250436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, Cox L, Demoly P, Frew AJ, O’Hehir R, Kleine-Tebbe J, Muraro A, Lack G, Larenas D, Levin M, Nelson H, Pawankar R, Pfaar O, van Ree R, Sampson H, Santos AF, Du Toit G, Werfel T, Gerth van Wijk R, Zhang L, Akdis CA. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–568. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 69.Wood RA. Food allergen immunotherapy: Current status and prospects for the future. J Allergy Clin Immunol. 2016;137:973–982. doi: 10.1016/j.jaci.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Refaat MM, Attia MY, Saber HM. Desensitization Efficacy by Sublingual Immunotherapy of Shrimps Extract in Asthmatic, Rhinitis and Urticaria Allergic Patients. Food and Nutrition Sciences. 2014;5:1704. [Google Scholar]
- 71.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J Allergy Clin Immunol. 2005;116:608–613. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 72.Pauli G, Larsen TH, Rak S, Horak F, Pastorello E, Valenta R, Purohit A, Arvidsson M, Kavina A, Schroeder JW. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122:951–960. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 73.Valenta R, Linhart B, Swoboda I, Niederberger V. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy. 2011;66:775–783. doi: 10.1111/j.1398-9995.2011.02565.x. [DOI] [PubMed] [Google Scholar]
- 74.Wai CY, Leung NY, Ho MH, Gershwin LJ, Shu SA, Leung PS, Chu KH. Immunization with Hypoallergens of shrimp allergen tropomyosin inhibits shrimp tropomyosin specific IgE reactivity. PloS one. 2014;9:e111649. doi: 10.1371/journal.pone.0111649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scala E, Alessandri C, Palazzo P, Pomponi D, Liso M, Bernardi ML, Ferrara R, Zennaro D, Santoro M, Rasi C, Mari A. IgE recognition patterns of profilin, PR-10, and tropomyosin panallergens tested in 3,113 allergic patients by allergen microarray-based technology. PloS one. 2011;6:e24912. doi: 10.1371/journal.pone.0024912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lehrer S, Ibanez M, McCants M, Daul C, Morgan J. Characterization of water-soluble shrimp allergens released during boiling. J Allergy Clin Immunol. 1990;85:1005–1013. doi: 10.1016/0091-6749(90)90044-5. [DOI] [PubMed] [Google Scholar]
- 77.Leung PS, Chu KH, Chow WK, Ansari A, Bandea CI, Kwan HS, Nagy SM, Gershwin ME. Cloning, expression, and primary structure of Metapenaeus ensis tropomyosin, the major heat-stable shrimp allergen. J Allergy Clin Immunol. 1994;94:882–890. doi: 10.1016/0091-6749(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 78.Gamez C, Zafra MP, Sanz V, Mazzeo C, Ibanez MD, Sastre J, del Pozo V. Simulated gastrointestinal digestion reduces the allergic reactivity of shrimp extract proteins and tropomyosin. Food Chem. 2015;173:475–481. doi: 10.1016/j.foodchem.2014.10.063. [DOI] [PubMed] [Google Scholar]
- 79.Liu GM, Huang YY, Cai QF, Weng WY, Su WJ, Cao MJ. Comparative study of in vitro digestibility of major allergen, tropomyosin and other proteins between Grass prawn (Penaeus monodon) and Pacific white shrimp (Litopenaeus vannamei) J Sci Food Agric. 2011;91:163–170. doi: 10.1002/jsfa.4167. [DOI] [PubMed] [Google Scholar]
- 80.Jeebhay M, Robins T, Lehrer S, Lopata A. Occupational seafood allergy: a review. Occup Environ Med. 2001;58:553–562. doi: 10.1136/oem.58.9.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.MacGlashan DW. Relationship between spleen tyrosine kinase and phosphatidylinositol 5′ phosphatase expression and secretion from human basophils in the general population. J Allergy Clin Immunol. 2007;119:626–633. doi: 10.1016/j.jaci.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 82.Holm J, Gajhede M, Ferreras M, Henriksen A, Ipsen H, Larsen JN, Lund L, Jacobi H, Millner A, Würtzen PA. Allergy vaccine engineering: epitope modulation of recombinant Bet v 1 reduces IgE binding but retains protein folding pattern for induction of protective blocking-antibody responses. J Immunol. 2004;173:5258–5267. doi: 10.4049/jimmunol.173.8.5258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.