Abstract
Background and Purpose
Immune responses to brain antigens after stroke contribute to poor outcome. We hypothesized that splenectomy would lessen the development of such responses and improve outcome.
Methods
Male Lewis rats (275–350 g) underwent 2 hrs middle cerebral artery occlusion (MCAO) immediately after splenectomy or sham-splenectomy. Animals were survived to 4 wks and immune responses to myelin basic protein (MBP) determined at sacrifice. Infarct volume was determined in a subset of animals sacrificed at 72 hrs. Behavioral outcomes were assessed to 672 hrs.
Results
Splenectomy was associated with worse neurological scores early after stroke, but infarct size at 72 hrs was similar in both groups. Behavioral outcomes and immune responses to MBP were also similar among splenectomized and sham-operated animals 672 hrs after MCAO.
Conclusions
Splenectomy did not alter the immune responses to brain antigens or improve outcome after stroke. Differences between this study and other studies of splenectomy and stroke are examined.
Keywords: stroke, splenectomy, lymphocytes, inflammation, outcome
We previously showed that immune responses to brain antigens, particularly myelin basic protein (MBP), are associated with worse outcome after experimental stroke.1 The spleen, the largest repository of lymphocytes within the body, undergoes profound atrophy following stroke.2 There is a simultaneously increase in the number of mononuclear cells (lymphocytes and monocytes) within brain, with the implication being the spleen is the source of these cells.3 Many studies show that splenectomy prior to or immediately after stroke improves outcome in rats.4–7 And splenectomy prior to hypoxic-ischemic injury was also found to be beneficial.8 In mice, splenectomy 2 weeks prior to MCAO improved outcome in males but not females9, while splenectomy at the time of MCAO was of no benefit.10
We hypothesized that splenectomy immediately prior to MCAO would decrease mononuclear cell infiltration into the brain and attenuate immune responses to brain antigens, thereby improving outcome.
Methods
Animals
Male Lewis rats (275–350 grams) were purchased from Harlan-US. All experiments were approved by the University of Washington Institutional Animal Care and Use Committee. Animals were randomly assigned to splenectomy or sham-splenectomy; outcomes were assessed by individuals masked to treatment status. Sample size calculations were based on extant literature regarding splenectomy and stroke outcomes.4–7
Splenectomy
Anesthesia was induced with 5% and maintained with 1.5% isoflurane. The splenic artery, veins and nerves were ligated and the spleen removed through a small lateral peritoneal incision. For sham-splenectomy, the spleen was identified but not removed.
Middle Cerebral Artery Occlusion (MCAO)
MCAO was performed immediately after splenectomy or sham-splenectomy. A midline neck incision was made and the right common and internal carotid arteries ligated. A monofilament suture (Doccol©; 4.0) was inserted into the common carotid and advanced into the internal carotid artery to block the origin of the MCA. Animals were maintained at normothermia during surgery and reperfused 2 hrs after MCAO. Rectal temperatures and body weight were assessed at set times. Animals were sacrificed at 72 hrs or 672 hrs after surgery.
Behavioral Outcomes
Stroke severity was determined using a modified Bederson score.11 Additional tests included the rotarod, foot fault and the alternating T-maze. Animals were trained on the rotarod prior to MCAO; the ability to stay on the rotarod for 100 second intervals was tested. Performance on the foot fault test was expressed as the percentage of foot faults per total steps taken. And the percentage of appropriately alternating choices over 25 trials in a T maze was calculated before and at 1 month after MCAO.
Infarct Volume
A subset of animals was sacrificed 72 hrs after MCAO, brains were removed, sectioned at 2 mm intervals and stained with 2,3,5-triphenyl-2H-tetrazolium chloride (TTC). Infarct volume was calculated using ImageJ.
ELISPOT Assays
Lymphocytes were isolated from the brain by separating the hemispheres and homogenizing the tissue through a 70 micron screen. The homogenate was spun over a Ficoll®-Paque gradient to separate the lymphocytes from brain tissue. Lymphocytes (1×105 cells/well) were cultured in media alone or media supplemented with antigen or the mitogen concanavalin A (ConA; Sigma) for 48 hours in 96 well plates (Multiscreen®-IP, Millipore). ELISPOT assays were used to detect rat MBP (NeoBioSci™) and ovalbumin (OVA; Sigma) specific secretion of interferon (IFN)-γ, interleukin (IL)-17 and transforming growth factor (TGF)-β1. Antigens were used at a concentration of 50 μg/mL and ConA at 5 ug/mL. Responses were assessed in triplicate.
Plates were developed using standard protocols (R & D Systems). Spots were counted with the aid of a semi-automated system (AID iSPOT®) and expressed as the ratio of the relative increase in antigen-specific IFN-γ secreting cells to that of TGF-β1 secreting cells (TH1 response) or as the ratio of the relative increase in antigen-specific IL-17 secreting cells to that of TGF-β1 secreting cells (TH17 response).
Statistics
Parametric data are displayed as mean ± standard deviation (sd) and compared using the t-test. Non-parametric data are displayed as median (interquartile range [IQR]) and compared using the Mann-Whitney U test. Categorical data are compared using the χ2-test. Significance was set at P<0.05.
Results
Mortality was similar in animals undergoing splenectomy and sham-splenectomy. The increase in body temperature after MCAO was attenuated in animals undergoing splenectomy (Figure 1a); changes in body weight were similar in both groups (Figure 1b). Infarct volume at 72 hrs was similar in animals undergoing splenectomy and sham-splenectomy (230±136 cc vs 225±221 cc). Neurological scores were higher early after MCAO in splenectomized animals (Figure 2a). Performance on the foot fault test, rotarod and alternating T-maze was similar in both groups (Figure 2b, c, d).
Figure 1.
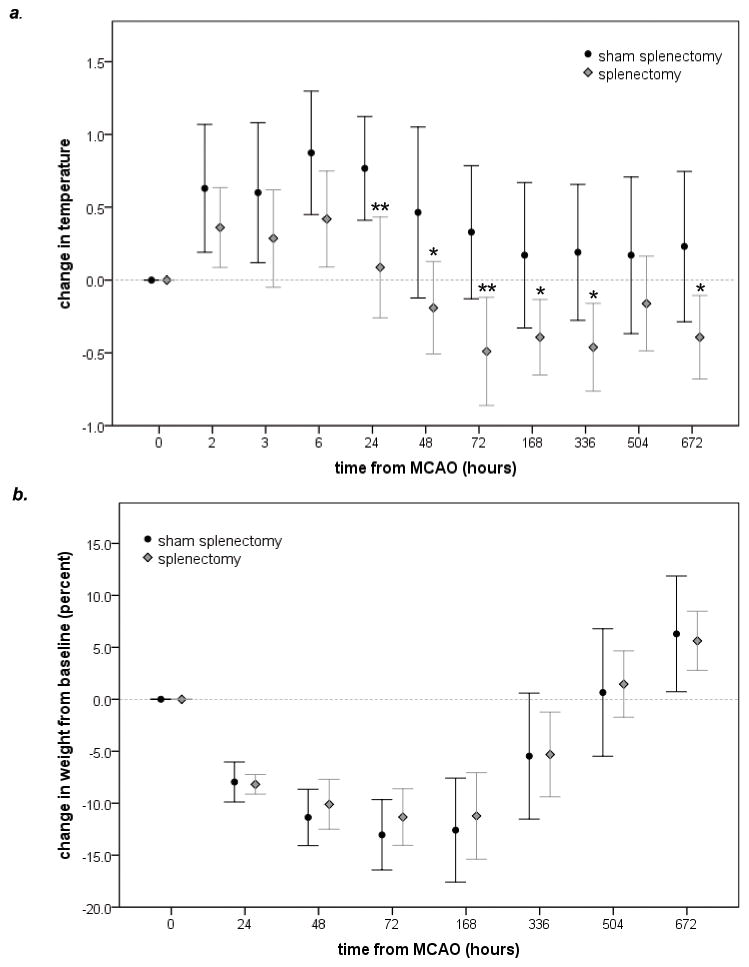
Splenectomy is associated with lower temperatures following MCAO (a). Splenectomized rats and sham-operated rats gain weight at the same rate after MCAO (b). Statistics are by t-test. *P<0.05, **P<0.01.
Figure 2.
Neurological scores were higher (worse) at 3 and 6 hrs after MCAO in animals undergoing splenectomy (a), but performance on the foot fault (b), the rotarod (c) and the alternating T-maze (d) was similar between groups. Statistics are by Mann-Whitney U test. *P<0.05, **P<0.01.
There were no differences in the numbers of mononuclear cells isolated from the brains of animals undergoing splenectomy and sham-splenectomy (15.4±4.3×106 vs. 12.2±3.6×106 in the infarcted hemisphere and 11.5±3.1×106 vs. 8.9±3.9×106 in the non-infarcted hemisphere). Further, there were no differences in cellular immune responses (data not shown).
Discussion
We did not find a beneficial effect of splenectomy on either early or late outcomes after stroke. Our study differs from previous studies in several ways. Ours is the only study done in Lewis rats, which are more prone to immune mediated diseases than Sprague-Dawley rats.12 We performed splenectomy at the time of stroke as opposed to 2 wks before, as in most prior studies.4–6 The duration of follow-up was limited in prior studies, and few of these studies evaluated behavioral outcomes.4–7 Table 1 outlines the characteristics of studies evaluating the effect of splenectomy on stroke outcome.
Table 1.
Splenectomy and stroke outcome in rodents.
| strain | stroke model | timing of splenectomy (relative to stroke) | survival | outcome (method of infarct analysis) | behavior | reference |
|---|---|---|---|---|---|---|
| mice | ||||||
| C57BL/6 | 30 min MCAO | immediately before | 3 d | • no change in infarct size (phase contrast microscopy) | • none | 10 |
| 7 d | ||||||
| C57BL/6 | 60 min MCAO | 2 wks before | 4 d | • ↓′d infarct size in males (TTC) | • none | 9 |
| rats | ||||||
| Lewis | 120 min MCAO | immediately before | 3 d | • no change in infarct size (TTC) • worse neurological scores early after MCAO |
• yes | current study |
| 4 wks | • no change in behavioral outcome | |||||
| Sprague-Dawley | 90 min MCAO | immediately after reperfusion | 7 d | • ↓′d infarct size (MRI) • no difference in neurological scores |
• limited | 7 |
| Sprague-Dawley | permanent focal ischemia | 2 wks before | 24 hrs | • ↓′d infarct size (Nissl) | • none | 5 |
| Sprague-Dawley | permanent focal ischemia | 2 wks before | 4 d | • ↓′d infarct size (fluorojade) | • none | 6 |
| Sprague-Dawley | permanent focal ischemia | 2 wks before | 4 d | • ↓′d infarct size (fluorojade) | • none | 4 |
| Sprague-Dawley | HI injury | 3 days prior | 3 hrs | • no change in infarct size (TTC) | • yes | 8 |
| 3 d | • ↓′d infarct size (TTC) | |||||
| 3 wks | • improved behavioral outcome | |||||
ns=not specified, MCAO=middle cerebral artery occlusion, TTC=2,3,5-triphenyl-2H-tetrazolium chloride, HI=hypoxia/ischemia
There are no data comparing strain related differences in the immune response after stroke, but such differences could impact the effect of splenectomy on outcome. Strains prone to immune mediated diseases should benefit more from splenectomy if inflammation worsens stroke outcome. It is thus surprising that no improvement was seen in Lewis rats, yet strains resistant to immunological diseases (ie. Sprague-Dawley) benefitted from splenectomy. In a study done in C57BL/6 mice, splenectomy immediately prior to MCAO did not improve outcome.10 In most “positive” studies, splenectomy was performed 2 weeks prior to MCAO, a time frame in which multiple systemic changes may occur. Further, most “positive” studies induced permanent ischemia4–6, 9, while those showing no benefit induced transient ischemia.10 If splenocytes contribute to poor stroke outcome, the beneficial effect should be more pronounced in models that establish reperfusion.
Following stroke, the spleen atrophies2 and the number of circulating lymphocytes decreases by 50%.13 We previously found that the number of splenocytes decreases by about 103×106 and the number of mononuclear cells in brain increases by 0.4×106 at 72 hrs after MCAO1, suggesting that most splenocytes “lost” from the spleen do not migrate to brain. Given the lymphopenia early after stroke, these “lost” splenocytes are not entering the circulation. Further, we found that at 4 wks after MCAO, animals undergoing splenectomy had just as many mononuclear cells in the brain as those with intact spleens.
We hypothesized that splenectomy would decrease autoimmune responses to brain after stroke, but found that TH1 and TH17 responses to MBP were similar in animals undergoing splenectomy and sham-splenectomy. In summary, splenectomy at the time of MCAO did not decrease infarct volume, improve neurological outcome, or affect the immune responses to brain antigens. Additional studies are needed to explore the contribution of the spleen to ischemic brain injury.
Supplementary Material
Acknowledgments
Funding
This study was funded by National Institutes of Neurological Disorders and Stroke R01NS056457.
Footnotes
Conflicts of Interest
None.
References
- 1.Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 3.Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012;7:1017–1024. doi: 10.1007/s11481-012-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajmo CT, Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, et al. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang BJ, Men XJ, Lu ZQ, Li HY, Qiu W, Hu XQ. Splenectomy protects experimental rats from cerebral damage after stroke due to anti-inflammatory effects. Chin Med J (Engl) 2013;126:2354–2360. [PubMed] [Google Scholar]
- 6.Seifert HA, Leonardo CC, Hall AA, Rowe DD, Collier LA, Benkovic SA, et al. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis. 2012;27:131–141. doi: 10.1007/s11011-012-9283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belinga VF, Wu GJ, Yan FL, Limbenga EA. Splenectomy following mcao inhibits the tlr4-nf-kappab signaling pathway and protects the brain from neurodegeneration in rats. J Neuroimmunol. 2016;293:105–113. doi: 10.1016/j.jneuroim.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Fathali N, Ostrowski RP, Hasegawa Y, Lekic T, Tang J, Zhang JH. Splenic immune cells in experimental neonatal hypoxia-ischemia. Transl Stroke Res. 2013;4:208–219. doi: 10.1007/s12975-012-0239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dotson AL, Wang J, Saugstad J, Murphy SJ, Offner H. Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J Neuroimmunol. 2015;278:289–298. doi: 10.1016/j.jneuroim.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim E, Yang J, Beltran CD, Cho S. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J Cereb Blood Flow Metab. 2014;34:1411–1419. doi: 10.1038/jcbfm.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 12.Becker KJ. Strain-related differences in the immune response: Relevance to human stroke. Transl Stroke Res. 2016;7:303–312. doi: 10.1007/s12975-016-0455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Relton JK, Sloan KE, Frew EM, Whalley ET, Adams SP, Lobb RR. Inhibition of alpha4 integrin protects against transient focal cerebral ischemia in normotensive and hypertensive rats. Stroke. 2001;32:199–205. doi: 10.1161/01.str.32.1.199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



