Abstract
The role of IL-33 particularly in tumor growth and tumor immunity remains ill defined. We show here that exogenous IL-33 can induce robust antitumor effect through a CD8+ T cell-dependent mechanism. Systemic administration of recombinant IL-33 (rIL-33) alone was sufficient to inhibit growth of established tumors in both transplant and de novo melanoma tumorigenesis models. Notably, in addition to a direct action on CD8+ T cell expansion and IFN-γ production, rIL-33 therapy activated myeloid dendritic cells (mDCs) in tumor-bearing mice, restored antitumor T cell activity and increased antigen cross-presentation within the tumor microenvironment. Furthermore, combination therapy with rIL-33 and agonistic anti-CD40 antibodies demonstrated synergistic antitumor activity. Specifically, MyD88, an essential component of the IL-33 signaling pathway, was required for the IL-33-mediated increase in mDC number and upregulation of costimulatory molecule expression. Importantly, we identified that the IL-33 receptor ST2, MyD88 and STAT1 cooperate to induce costimulatory molecule expression on mDCs in response to rIL-33. Our study has thus revealed a novel IL-33-ST2-MyD88-STAT1 axis that restores mDC activation and maturation in established cancer, and thereby the magnitude of anti-tumor immune responses, suggesting a potential use of rIL-33 as a new immunotherapy option to treat established cancer.
Introduction
Interleukin 33 (IL-33), a new IL-1 family member (1), is mainly and constitutively expressed by non-hematopoietic cells such as fibroblasts, epithelial cells and endothelial cells (2). IL-33 has been identified as the functional ligand for the orphan receptor, ST2 (IL-1R-like-1, IL-1RL1) (3, 4). IL-33 signaling via ST2 and IL-1R accessory protein (IL-1RAcP) dimers (5), results in the recruitment of MyD88, which induces activation of various signaling pathways, including NF-κB and MAP kinase pathways (1, 4, 6-8). Accumulating evidence demonstrates that IL-33 has an important role in promoting allergic responses (4, 9), and other Th2-related diseases such as asthma (10), atopic dermatitis (11), and anaphylaxis (12). On the other hand, IL-33 may also protect against inflammation-associated atherosclerosis (13) or infection-induced tissue damage (14, 15). Therefore, IL-33 has a dual functionality in different diseases, depending on the immune mechanism underlying the pathogenesis of various disease condition.
Although IL-33 has been studied primarily for its role in the context of inflammation, allergy and auto-immunity (1, 6, 16), its role in tumor immunity and tumor growth is only beginning to be appreciated (17). It is now becoming clear that IL-33 plays a role far beyond the realm of Th2 immunity by promoting Th1 immune responses (18), regulating the development of antiviral CD8+ T cells and driving the effector function of CD8+ T cells (19, 20). There are several recent studies showing IL-33 can promote antitumor CD8+ T cell responses in experimental mouse tumor models (21-23). However, the molecular and cellular mechanisms of how IL-33 influences the antitumor CD8+ T cells responses still remain elusive.
Tumor can escape host immune surveillance by fostering a highly suppressive microenvironment (24-26). There is clear evidence for the role of tumor-induced DCs dysfunction as one important mechanism for tumor induced immune escape (27, 28), as DC function as key professional antigen presenting cells (APC) to induce tumor-specific immune responses, particularly via cross-priming through MHC-class I antigen presentation (29, 30). DC defects are caused by abnormal differentiation leading to decreased production of fully competent APCs and increased accumulation of immature tolerogenic DCs. In fact, the inability to mount a potent antitumor immune response has often been attributed to DC defects. Limited data are available on the role of IL-33 in DC activity. IL-33 has been shown to promote bone marrow DC generation in vitro (31). Moreover, IL-33-activated DCs drove an atypical Th2-type immune response (32), and exacerbated allergic lung inflammation (33). No studies, however, have examined the effects of IL-33 on DC phenotype and function in the context of cancer.
In this study, we showed that systemic administration of recombinant IL-33 (rIL-33) alone was sufficient to inhibit the growth of established B16 melanoma, EG7 lymphoma and clinically more relevant BrafV600EPTEN inducible melanomas. Furtheremore, the therapeutic efficacy of rIL-33 was primarily dependent of CD8+ T cells rather than NK+ cells and CD4+ T cells. In addition to CD8+ T cells, dysfunctional mDCs of tumor-bearing mice were activated directly by rIL-33 to express costimulatory molecules potentiating antitumor immune responses. As a result, synergistic antitumor activity was acheived with rIL-33 and agonistic anti-CD40 combination therapy. Mechanistically, we identified a novel IL-33-ST2-MyD88-STAT1 axis that regulated DC activation and maturation, and thereby the magnitude of antitumor immune responses. Our results may thus have direct impact for developing rIL-33 as a novel and promising approach to treat established cancer.
Materials and Methods
Mice, cell lines and reagents
C57BL/6 WT, C57BL/6 MyD88−/−, Rag1−/−, BRAF(V600E)/PTEN (Tyr∷CreER; BrafCA/+; Ptenlox5/lox5) and Pmel-1 mice were purchased from Jackson Laboratory. OT-1 Rag1−/− mice were purchased from Taconic. Dr. Hans Schreiber (University of Chicago) provided EG7, B16F10, B16-OVA, B16-SIY cell lines, SIYRYYGL (SIY) peptides and the 2C transgenic mice. The BPS-1 mouse melanoma was generated from a spontaneously arising tumor in BRAF(V600E)/PTEN (Tyr∷CreER; BrafCA/+; Ptenlox5/lox5) transgenic mice (34, 35). We have demonstrated the presence of the BRAFV600E transversion in BPS-1 cells, and Braf inhibitors PLX4032 can effectively inhibit the growth of BPS-1 melanomas in vivo (data not shown). All the cell lines were routinely tested for mycoplasma infections by culture and DNA stain, and maintained in complete medium composed of RPMI 1640 with 5% FBS. All animal experiments were approved by institutional animal use committees of the University of Texas Health Science Center at San Antonio and Northwestern University. H-2Db/gp100 tetramers were provided by the National Institutes of Health Tetramer Core Facility. 1B2 antibodies (clonotypic anti-2C TCR) were provided by Dr. Justin Klein from University of Chicago. Phospho-STAT mAbs were purchased from Cell Signaling Technology. All other flow mAbs were obtained from eBiosciences and BioLegend. Proliferation dye eFluro450 was from eBiosciences. 4-hydroxytamoxifen (4-HT, Z-isomer) was purchased from Sigma Aldrich. Agonistic anti-CD40 (clone FGK4.5), depleting mAb clone GK1.5 (anti-CD4), clone 53.6.7 (anti-CD8α), clone PK136 (anti-NK1.1) and control IgG were purchased from BioXcell. Recombinant murine IL-33 and GM-CSF were purchased from Biolegend. ST2 blocking antibody, clone DJ8, was purchased from MD Bioproducts. The peptide hgp10025-33 was purchased from GenScript. For MTT assay, Cell Proliferation Kit I (MTT), was purchased from Roche.
Analysis of cells by flow cytometry
All samples were initially incubated with 2.4G2 to block antibody binding to Fc receptors. Single-cell suspensions were stained with 1μg of relevant mAbs and then washed twice with cold PBS. H-2Db/gp100 tetramer staining, Foxp3 staining, and intracellular IFN-γ staining were performed as previously described (36). For STAT staining, cells were treated as indicated, then 106 cells were fixed in 4% formaldehyde for 10 minutes at room temperature. Cells were then washed with ice cold PBS containing 2% BSA followed by another wash step with ice cold PBS. Cells were resuspended in 80% methanol and incubated for 30 minutes at −20°. Pellet was washed twice with ice cold PBS. mAbs were added in a final volume of 100 μl ice cold PBS and incubated at 4° for 45 minutes. Cells were washed with ice cold PBS containing 2% BSA and analyzed by flow cytometry. Samples were conducted on a MACSQuant Analyzer (Miltenyi Biotec) and data were analyzed with FlowJo software.
T cell and DC Purification
Splenic CD8+ T cells from WT, 2C, Pmel-1, and OT-1 mice were selected using IMag™ CD8 magnetic particles (BD Biosciences) with >95% purity routinely checked by flow cytomtety. For DC selection, DLNs or spleen cells were suspended in cold PBS at a concentration of 108 cells/ml and incubated with anti-mouse CD11c-Biotin for 30 minutes at 4°. Cells were then washed and incubated with magnetic streptavidin particles for 30 minutes (Streptavidin Particles Plus-DM, BD Biosciences) and purified according to manufacturer protocol with an expected purity of 80-90%.
ELISPOT assay
For evaluation of ex-vivo DC priming ability ELISPOT assay was performed. 96-well MiltiScreen-IP Filter plates (Millilpore) were used for plating and all other reagents were from Mouse IFN-γ ELISPOT Ready-SET-GO (eBioscience). All steps were done according to manufacturer protocol. Each well contained 105 DCs along with 105 CD8+ T cells in a final volume of 200μl/well. Negative controls were CD8+ T cells alone, without addition of DCs. Samples were incubated for 48 hour before harvest. The numbers and diameters of spots were counted in triplicates and calculated by an automatic ELISPOT counter.
In vitro cell treatment
For in vitro treatment of DCs purified from tumor-bearing mice or tumor-free mice, rIL-33 was added at 10 ng/ml for 10 minutes before STAT staining. To exclude the potential role of T cells in IL-33-induced DC activation, T cells were depleted from splenocytes using CD90.1 selection prior to purification of CD3−CD11c+ cells by FACS for >99% purity of cells. Sorted CD3−CD11c+ WT and eFluro450-labeled MyD88−/− DCs were mixed at a 1:1 ratio and treated with/without rIL-33. The next day cells were analyzed for STAT1 phosphorylation by flow cytometry. WT DCs were gated as e450−, while MyD88−/− DCs were CD3− CD11c+ e450+. Anti-ST2 (clone DJ8) was added at 10 μg/ml when used. For in vitro treatment of CD8+ T cells, purified splenic CD8+ T cells from tumor-free mice were labeled with eFluro450 as described previously (36). rIL-33 was added at 10 ng/ml for 48 hours before proliferation and IFN-γ analysis.
Tumor challenge and treatments
B16-F10, B16-SIY, BPS-1 or EG7 cells (1 × 106) in suspension were injected s.c. into the rear right flank of mice. For DC transfer experiment, purified splenic CD11c+ DCs from WT or MyD88−/− mice were stimulated with rIL-33 (10 ng/ml). Three days later, B16-SIY-bearing mice were injected with these IL-33-activated WT or MyD88−/− DCs. The following day naïve 2C CD8+ T cells were stained with e450 cell proliferation dye and transferred i.v. After four days DLNs and tumor tissues were harvested to measure proliferation and IFN-γ production of transferred 2C T cells. For in vivo rIL-33 treatment, groups of mice received i.p. injection of either PBS or 1μg of rIL-33 dissolved in PBS, daily, starting on day 8-10 after injection, or when tumors had reached approximately 150-300 mm3. Depletion of CD4+ T cells, CD8+ T cells, or natural killer (NK) cells was achieved as previously described (36) by twice a week i.p. injection of depleting mAb clone GK1.5 (anti-CD4, 200 μg), clone 53.6.7 (anti-CD8α, 200 μg) or clone PK136 (anti-NK1.1, 200 μg) starting one day prior to rIL-33 treatment. Induction of melanoma in BRAF(v600E)/PTEN mice was performed as described previously (Braf cooperates with Pten loss to induce metastatic melanoma) (34, 35). 4-HT was dissolved to 2 mg/ml in DMSO, and 6-4 μl were painted on the hind flank for three consecutive days. For the BRAF(v600E)/PTEN model, rIL-33 treatment was initiated when tumors became measureable. For combination therapy, agonistic anti-CD40 (clone FGK4.5, 150 μg) was administered i.p. on the same time or one day after initiation of rIL-33 treatment, and then administered every third day. In all experiments the size of tumor was determined at 2-3 day intervals. Tumor volumes were measured along orthogonal axes (a,b, and c) and calculated as abc/2.
Statistical analysis
Mean values were compared using an unpaired Student’s two-tailed t test. The statistical differences between the survival of groups of mice were calculated according to the log-rank test. Probability values >0.05 were considered non-significant.
Results
Antitumor effect of rIL-33 is dependent on CD8+ T cells
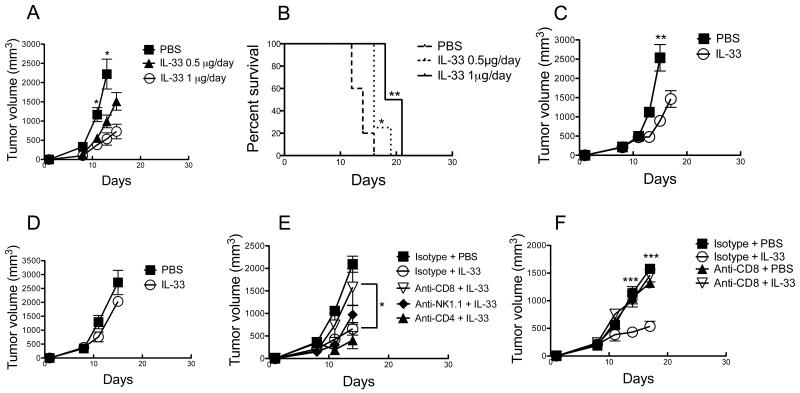
The effects of exogenous IL-33 on tumorigenesis have been reported in several recent studies (21-23). However, the exact roles of IL-33 in regulating antitumor immunity and antitumor growth are barely understood. We examined the effect of daily i.p. injection of rIL-33 on the growth of B16F10 melanoma. Given the importance of tumor size and duration of tumor growth in experimental therapy for clinical implication, mice bearing established tumors (approximately 150-250 mm3) were randomized and treated beginning on day 8-10. A dose response relationship on tumor growth (Fig. 1A) and mice survival (Fig. 1B) was initially demonstrated from 0.5 μg to 1.0 μg i.p. daily. Similarly, systemic administration of rIL-33 (1 μg/day) significantly inhibited the growth of established EG7 lymphoma (Fig. 1C). The in vivo antitumor activity of rIL-33 was dependent on adaptive anti-tumor immune responses, because rIL-33 therapy was ineffective against B16F10 tumors in Rag1−/− mice (lacking T and B lymphocytes) (Fig. 1D). As expected, rIL-33 did not affect B16F10 tumor growth in vitro (not shown), consistent with previous studies (23). Furthermore, depletion of CD8α+ cells rather than CD4+ cells or NK cells, abrogated the tumor-inhibiting activity of rIL-33 treatment in EG7-bearing mice (Fig. 1E) and B16F10-bearing mice (Fig. 1F), which indicates that the tumor-inhibiting activity of rIL-33 administration was primarily dependent on CD8+ cells, and independent of CD4+ cells or NK cells.
Figure 1. Administration of rIL-33 alone is sufficient to inhibit tumor growth in a CD8+ T cell dependent manner.
(A and B) The rIL-33-induced antitumor effect is dose dependent. C57BL/6 mice were injected s.c. with 106 B16-F10 cells and treated i.p. daily with the indicated dose of rIL-33 starting from d9. In (B) mice were sacrificed when tumor volume reached 2 cm3. *; p<0.05. **; p<0.01, PBS vs. 0.5 μg/day or 1 μg/day treatment. (C) Inhibition of EG7 tumor growth by rIL-33. C57BL/6 mice were injected s.c. with 106 EG7 cells and treated i.p. daily with 1 μg rIL-33 starting from d11. **; p<0.01. (D) Tumor inhibition by rIL-33 is dependent on adaptive immunity. C57BL/6 Rag1−/− mice were injected s.c. with 106 B16-F10 cells and treated i.p. daily with 1 μg rIL-33 starting from d10. (E and F) rIL-33 inhibits tumor growth in a CD8+ T cell dependent manner. Depletion of CD4+ T cells, CD8+ T cells or NK cells was achieved by twice weekly injection of anti-CD4, anti-CD8 or anti-NK1.1 depleting Abs, respectively, on the same day in EG7-bearing mice (E) or two days prior to rIL-33 or PBS administration in B16-F10-bearing mice (F). In all experiments rIL-33 per mouse was administered when tumor volume was approximately 150-250 mm3. *; p<0.05. Data (mean ± SEM) are representative of at least 3 independent experiments with 5 mice per group.
rIL-33 treatment promotes antitumor T cell immunity
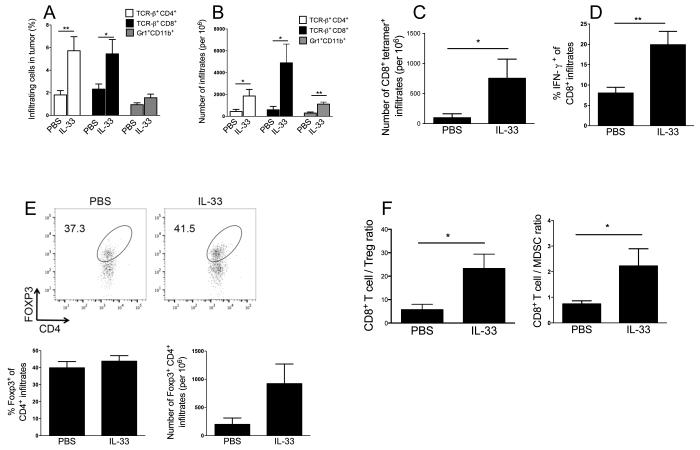
We next characterized the exogenous rIL-33-induced antitumor T cell immunity. Phenotype and cytokine profile of tumor-infiltrating immune cells were examined 8-10 days after rIL-33 or PBS treatment. We found that rIL-33 treatment remarkably enhanced the infiltration of CD4+, CD8+ T cells (Fig. 2A, B) and tumor antigen gp100–specific CD8+ T cells (Fig. 2C). Moreover, there was significantly increased IFN-γ production (Fig. 2D) and KLRG1 expression (not shown) by tumor-infiltrating CD8+ T cells from rIL-33-treated mice compared to the PBS group. The percentage of Foxp3+ among CD4+ T cells remained unchanged. There was a trend towards an increase in the absolute number of CD4+Foxp3+ Tregs by rIL-33 treatment, but the difference did not reach statistical significance (Fig. 2E). rIL-33 treatment resulted in a significant increase in absolute number of tumor-infiltrating Gr1+CD11b+ myeloid-derived suppressor cells (MDSCs) (Fig. 2B). In addition, IL-33 was effective in inhibiting the growth of immunogenic B16-SIY (expressing SIY antigen) (Supple. Fig. 1A), which also corresponded to the infiltration of greater numbers of CD8+ T cells but less CD4+Foxp3+ Tregs (Supple. Fig. 1B,C). IL-33 treatment promoted CD8+ effector T cell proliferation within the B16-SIY tumors, as measured by expression of the cell cycle associated protein Ki67 (Supple. Fig. 1D), as previously reported. In both B16-F10 (Fig. 2F) and B16-SIY (Supple. Fig. 1E) tumor models, intratumoral ratios of CD8+ T effector cells to Tregs and CD8+ T effector cells to MDSCs were markedly increased by rIL-33 therapy. Furthermore, we observed a significant increase in splenic frequencies of Tregs (Supple. Fig. 2A) and MDSCs (Supple. Fig. 2B) in IL-33-treated mice. Collectively, these data suggest that systemic administration of rIL-33 promoted antigen-specific CD8+ T cell expansion and effector function, despite the fact that this expansion was accompanied by an expansion of Tregs and MDSCs in the periphery and tumor.
Figure 2. rIL-33 therapy increases antitumor CD8+ T cell immunity.
Percent (A) and absolute number (B) of B16-F10 tumor-infiltrating TCRVb+CD4+, TCRVb+CD8+, Gr1+ CD11b+ cells, as determined by flow cytometry (n=5). (C) rIL-33 treatment increased gp100-specific tetramer+ TCRVb+CD8+ cells per 106 tumor infiltrates, calculated by flow cytmometry (n=10). (D) rIL-33 treatment increased IFN-γ production from tumor-infiltrating CD8+ cells, as determined by flow cytometry (n=10). (E) Percent and absolute number of B16-F10 tumor-infiltrating CD4+Foxp3+, as determined by flow cytometry (n=5). (F) IL-33 shifts the ratio of CD8+ T cell to suppressive cells in favor of increased proportion of CD8+ T cells, as determined by flow cytometry percentages. Cells were collected for flow cytometry 8-10 d after PBS or rIL-33 treatment. *; p<0.05, **;p<0.01. Data (mean ± SEM) are representative of at least 2 independent experiments.
Defective tumor-associated DC function is reversed by exogenous IL-33
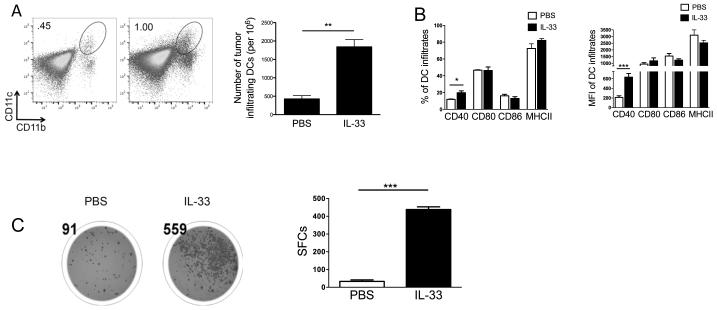
Although we showed that rIL-33 had a direct effect on CD8+ T cell expansion and IFN-γ production (Supple. Fig. 3), it is very likely that IL-33 can potentiate antitumor T cell immunity through other immune cells, such as DCs. Indeed, In B16-SIY-bearing mice, the absolute number of tumor-infiltrating CD11b+CD11c+ myeloid DCs was greatly increased by rIL-33 treatment (Fig. 3A). IL-33 also induced up-regulation of CD40 among these DC infiltrates (Fig. 3B). Similarly, there was significantly increased expression of CD40 and CD80 in the IL-33-treated B16-F10-bearing mice compared to the control group (not shown). To examine further the functional significance of these tumor-associated DCs by rIL-33 therapy, CD11c+ DCs were purified from either PBS-treated or rIL-33-treated B16-SIY-bearing mice. As shown in Fig. 3C, DCs from IL-33-treated mice induced more number of IFN-γ-producing SIY-specific 2C CD8+ T cells, indicating the possibility that more DCs from IL-33- than PBS-treated mice present the SIY antigen, resulting in more 2C T cells recognizing their cognate peptides. Likewise, in B16-OVA-bearing mice, DCs from IL-33-treated mice but not from PBS-treated mice were able to cross-present OVA antigen to trigger IFN-γ production from OT-1 CD8+ T cells (Supple. Fig. 4). The data indicate that expogenous IL-33 may induce DC activation and maturation within the tumor microenvironment, thereby promoting antitumor T cell immunity.
Figure 3. IL-33 activates tumor-associated DCs to restore their T cell cross-priming ability.
(A) rIL-33 increased the absolute number of B16-SIY-infiltrating DCs gated as CD45+Gr1−CD11b+CD11c+. (B) Percentage and median fluorescence intensity (MFI) of CD40, CD80, CD86 and MHC-II expression were determined among tumor-infiltrating DCs (n=10). (C) DCs from IL-33-treated B16-SIY-bearing mice have rescued cross presentation and priming ability compared to those from PBS-treated B16-SIY-bearing mice, quantified with an IFN-γ-based ELISPOT assay. DCs were cultured with naïve 2C CD8+ T cells without addition of exogenous antigen at a 1:1 ratio for 48 hours. Representative ELISPOT panel shown on left (n=3). *; p<0.05, **; p<0.01, ***; p<0.001. Data (mean ± SEM) are representative of 2 independent experiments.
Antitumor activity of exogenous IL-33 against de novo BrafV600EPTEN-driven melanoma
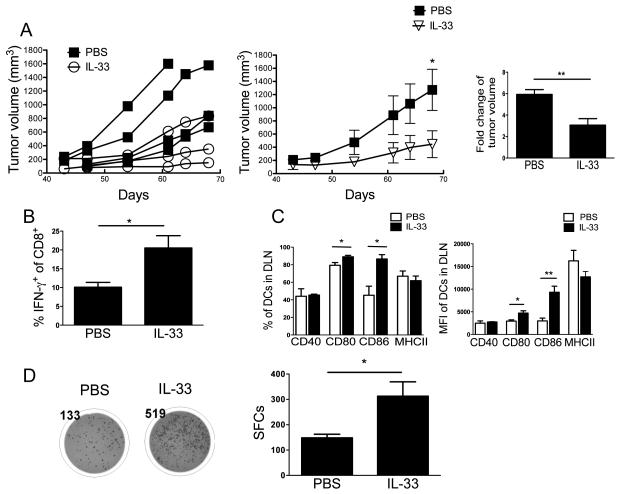
Given the potential limitation of transplantable tumor models above, we further examined the antitumor effect of IL-33 administration in a more clinically relevant de novo melanoma model, i.e. the BrafV600EPTEN mouse model (34, 35). As shown in Fig. 4A, systemic administration of rIL-33 inhibited the growth of established BrafV600EPTEN inducible melanomas. Moreover, we observed significantly increased numbers of CD8+ T cells producing IFN-γ in rIL-33-treated mice compared to the control group (Fig. 4B). rIL-33 treatment also augmented expression levels of CD80 and CD86 but not CD40 or MHC-II on mDCs from DLNs (Fig. 4C). Furthermore, DCs from rIL-33-treated mice had increased ability to cross-prime antigen-specific CD8+ T cells (Fig. 4D). The data obtained from the BrafV600EPTEN melanoma model are thus in line with that from the transplantable tumor models above, suggesting that rIL-33 treatment directly activates the tumor-associated mDCs in vivo to express costimulatory surface molecules, thereby boosting antitumor T cell immunity. The discrepancy of IL-33-activated costimulatory molecules may be explained by the different nature of each tumor model tested and the time of harvest.
Figure 4. rIL-33 inhibits tumor growth and promotes the T cell cross-priming ability of tumor-associated DCs in BrafV600EPTEN model.
(A) rIL-33 inhibited melanoma growth in BRAF/PTEN model. 42 days after 4-HT induction, mice were treated with either 1 μg/day IL-33 or PBS for 25 days. Fold change is between start and endpoint of treatment. (B) rIL-33 treatment increased IFN-γ production from CD8+ cells from PBS or IL-33-treated melanoma-bearing mice, as determined by flow cytometry (n=3). (C) Percentage and MFI of CD40, CD80, CD86 and MHC-II expression were determined among DLN CD11b+CD11c+Gr-1−DCs (n=3). (D) DCs from IL-33-treated melanoma-bearing mice have increased T cell cross-priming ability compared to those from PBS-treated melanoma-bearing mice, quantified with an IFN-γ-based ELISPOT assay. Purified CD11c+ cells from DLNs were cultured with naïve Pmel CD8+ T cells without addition of exogenous antigen at a 1:1 ratio for 48 hours. Representative ELISPOT panel shown on left (n=3). *; p<0.05, **; p<0.01, ***; p<0.001. Data (mean ± SEM) are representative of 2 independent experiments.
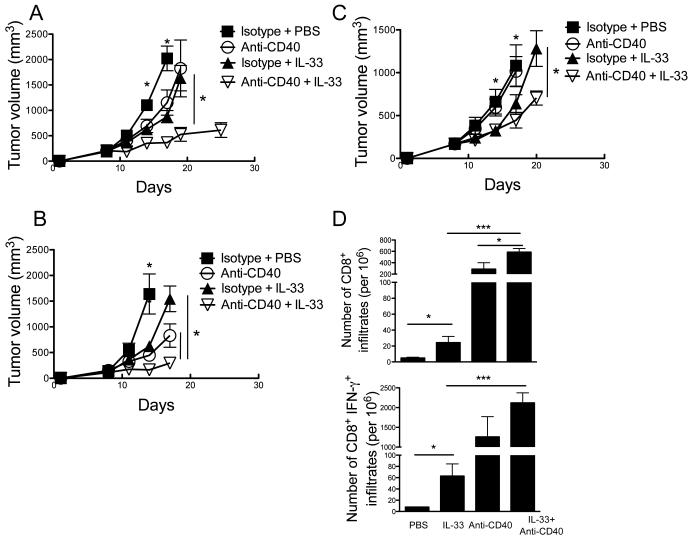
Synergistic antitumor activity of rIL-33 and agonistic anti-CD40 combination therapy
rIL-33 significantly upregulated CD40 expression in tumor-associated DCs in vivo (Fig. 3B), and promoted DC-mediated antitumor T cell activity (Fig. 3C). Therefore, it is reasonable to combine agonistic anti-CD40 antibody therapy and IL-33 administration in cancer treatment. Consistent with prior published data (37), we confirmed the significant antitumor activity of anti-CD40 alone and comparable effect of rIL-33 alone against the established B16-F10 (Fig. 5A) and B16-SIY (Fig. 5B) tumors. More strikingly, the antitumor activity of anti-CD40 was significantly enhanced by rIL-33 treatment, with all tumor-bearing mice efficiently arresting and inducing regression of some of established B16-F10 (Fig. 5A) and B16-SIY (Fig. 5B) tumors. A similar combinatorial synergy was observed for IL-33 and anti-CD40 mAb, but it had a lower efficacy in suppressing growth of the more immune-resistant BPS-1 melanomas (Fig. 5C) that originally generated from a spontaneously arising tumor in BrafV600EPTEN mice. The reduced efficacy of anti-CD40/rIL-33 against BPS-1 melanomas correlated with a lower frequency of CD8+ T cells naturally infiltrating the tumors (1.5%±1.4) when compared with B16 tumors (Fig. 2B). As expected, either rIL-33 or anti-CD40 alone increased tumor infiltration of CD8+ T cells and IFN-γ production from these CD8+ T cells. Combination therapy of rIL-33 and anti-CD40 showed further improved activity over either treatment alone (Fig. 5D).
Figure 5. Combination of anti-CD40 and rIL-33 has a synergistic effect to delay tumor growth.
C57BL/6 mice (n=5) were injected s.c. with 106 B16-F10 (A) or B16-SIY (B) tumor cells, and received daily either PBS or 1 μg/day rIL-33 starting from d8. Agonistic anti-CD40 was i.p. administered every 3 days starting from d9. (C) Inhibition of BPS-1 tumor growth by combination therapy. Mice (n=5) were injected s.c. with 106 BPS-1 tumor cells and received same therapy as A and B. (D) rIL-33 treatment increased number of B16-SIY-infiltrating CD8+ and CD8+ IFN-γ+ T cells. *; p<0.05, **; p<0.01, ***; p<0.001. Data (mean ± SEM) are representative of 2 independent experiments.
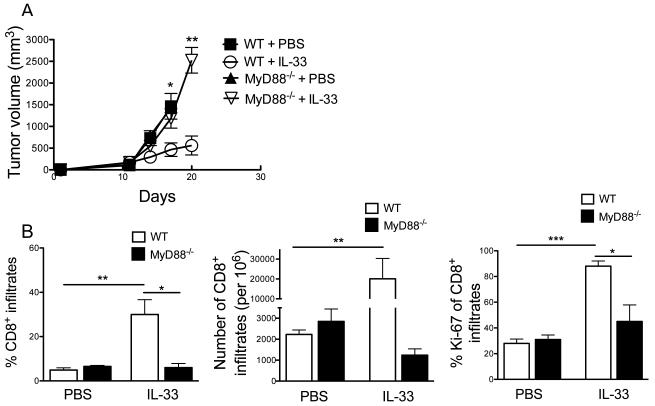
Exogenous IL-33-mediated antitumor effect requires MyD88
IL-33 signals through the adaptor protein MyD88 (38); however, the role of MyD88 in regulating tumor immunity by IL-33 has not been studied in detail. We found that rIL-33 treatment delayed the growth of established B16-SIY tumors in WT mice, but this antitumor effect was completely abrogated in Myd88−/− mice (Fig. 6A), suggesting an essential role of MyD88 for rIL-33-mediated antitumor activity. Furthermore, rIL-33 treatment failed to enhance the frequency, absolute number and proliferation (ki-67 expression) of intratumoral CD8+ T cells from Myd88−/− mice compared with that from WT mice (Fig. 6B), as would be predicted. We also noticed that MyD88 signaling was dispensible for tumor growth and antitumor CD8+ T cell activity in the absence of rIL-33 therapy (Fig. 6A, B).
Figure 6. rIL-33-mediated antitumor effect is dependent on MyD88 signaling pathway.
(A) C57BL/6 WT or MyD88−/− mice were injected s.c. with 106 B16-SIY tumor cells and received either PBS or 1 μg/day rIL-33 daily starting from d9 (n=5). (B) Percent and absolute number of tumor-infiltrating TCRVb+CD8+ T cells, and percent of Ki-67 as determined by flow cytometry (n=5). MyD88−/− mice exhibited decreased CD8+ T cell infiltration and proliferation in response to rIL-33. *; p<0.05, **; p<0.01, ***; p<0.001. Data (mean ± SEM) are representative of 2 independent experiments.
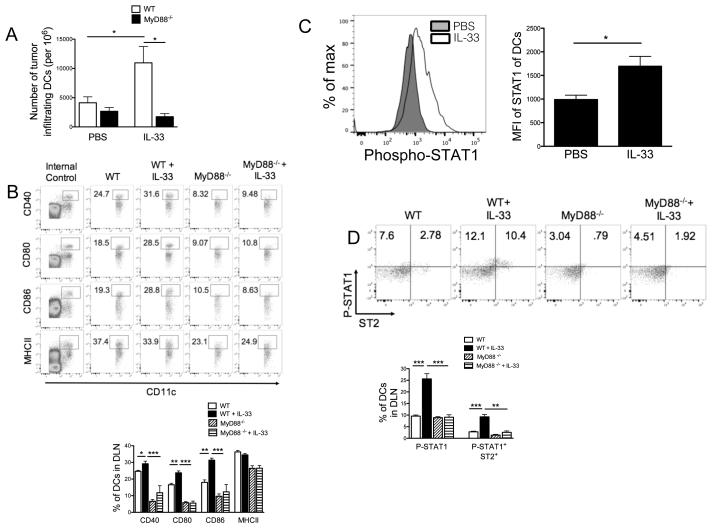
Exogenous IL-33 signaling in DCs requires ST2, MyD88 and STAT1
Given the ability of rIL-33 to rescue the tumor-associated DC activity, we tested whether rIL-33 therapy acts on tumor-associated DCs through MyD88 signaling. Examination of tumor-free Myd88−/− mice showed similar proportions of mDCs in the LNs and spleen, compared with that of WT mice (not depicted), indicating that MyD88 is not required for DC development or differentiation. In the B16-SIY model, however, IL-33 treatment increased the absolute number of intratumoral DCs in WT rather than Myd88−/− mice (Fig. 7A). We also observed an up-regulation of costimulatory molecules (CD86, CD40, CD80 and MHC-II) on DCs from DLNs in WT-tumor-bearing mice in response to rIL-33 therapy, but this effect was completely abrogated in Myd88−/− mice (Fig. 7B). To dissect further the molecular mechanism of MyD88-mediated signaling downstream of exogenous IL-33 in DC activation, we examined the involvement of STAT activation by IL-33. As shown in Fig. 7C, rIL-33 treatment specifically induced phosphorylation of STAT1, but not STAT5 in mDCs from WT tumor-bearing mice (not shown). More interestingly, frequency of ST2+STAT1+ in mDCs was significantly reduced in response to rIL-33 therapy in tumor-bearing MyD88−/− mice compared to WT mice (Fig. 7D), suggesting a potential functional link between STAT1 and ST2-MyD88-mediated signaling downstream of IL-33.
Figure 7. IL-33 signaling in tumor-associated DCs requires MyD88 and STAT1.
(A) rIL-33 increased the number of B16-SIY-infiltrating Gr-1−CD11b+CD11c+ DCs from WT but not MyD88−/− mice (n=5). (B) Representative flow cytometry analysis of frequency of CD40, CD80, CD86, or MHC-II in DCs from DLNs of WT or MyD88−/− tumor-bearing mice (n=5) killed 8-10 d after PBS or rIL-33 treatment. Internal controls were gated from cell populations within the analyzed sample that do not express DC costimulatory molecules. (C) Gr1−CD11c+ DCs from DLNs of tumor-bearing WT mice were rested overnight and then stained intracellularly for phosphorylated STAT1 by flow cytometry. (D) Representative flow cytometry analysis of phosphorylated STAT1 and ST2 by gated CD11b+CD11c+ DCs from DLNs of rIL-33-treated tumor-bearing WT or MyD88−/− mice. *; p<0.05, **; p<0.01, ***; p<0.001. Data (mean ± SEM) are representative of 3 independent experiments.
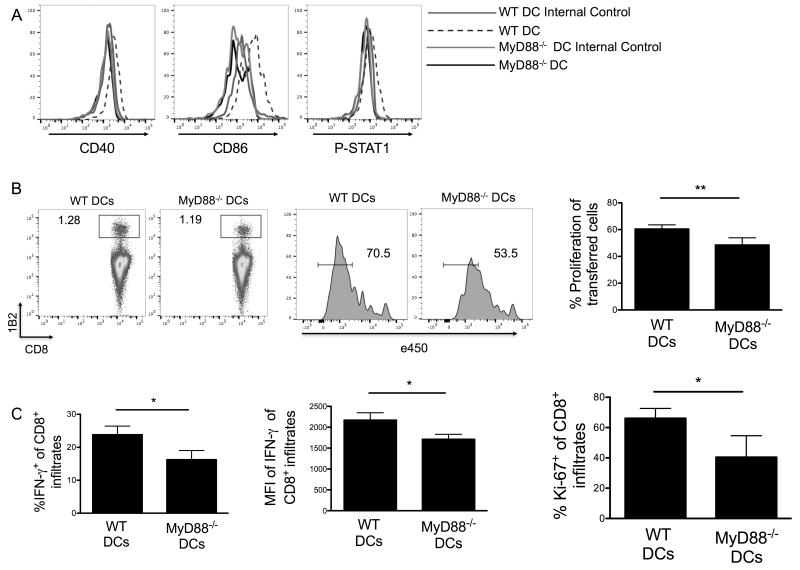
We next examined whether MyD88 signaling in the DC compartment was responsible for IL-33-mediated CD8+ T cell priming. As expected, WT but not MyD88−/− DCs increased costimulatory molecules and STAT1 in response to rIL-33 (Fig. 8A). Further, transfer of rIL-33-activated WT DCs triggered a greater proliferation of antigen-specific CD8+ T cells in DLNs from B16-SIY-bearing mice than transfer of rIL-33-activated MyD88−/− DCs (Fig. 8B). Similarly, a significant increase in frequencies of tumor-infiltrating CD8+IFN-γ+ T cells and proliferating Ki-67+CD8+ T cells was observed in these B16-SIY-bearing mice following the transfer of IL-33-activated WT DCs compared to MyD88−/− DCs (Fig. 8C).
Figure 8. IL-33 induces DC maturation and T cell priming via MyD88.
(A) rIL-33 increased costimulatory molecules and STAT1 on WT DCs but not in MyD88−/− DCs. Purified splenic DCs were stimulated with 10 ng/mL of IL-33 daily for three days. (B) IL-33-activated DCs from (A) were injected into mice harboring established B16-SIY tumors (n=5). The following day naïve 2C CD8+ T cells (1B2+) were stained with e450 cell proliferation dye and transferred i.v. After four days DLN were harvested to measure proliferation of transferred cells. Flowjo software was used to calculate the percentage of individual generations by e450 dye dilution. (C) Flow cytometry was used to analyze the percentages of IFN-γ+ and Ki-67+ among 2C CD8+ T cells in tumor tissues. *; p<0.05, **;p<0.01. Data (mean ± SEM) are representative of at least 2 independent experiments.
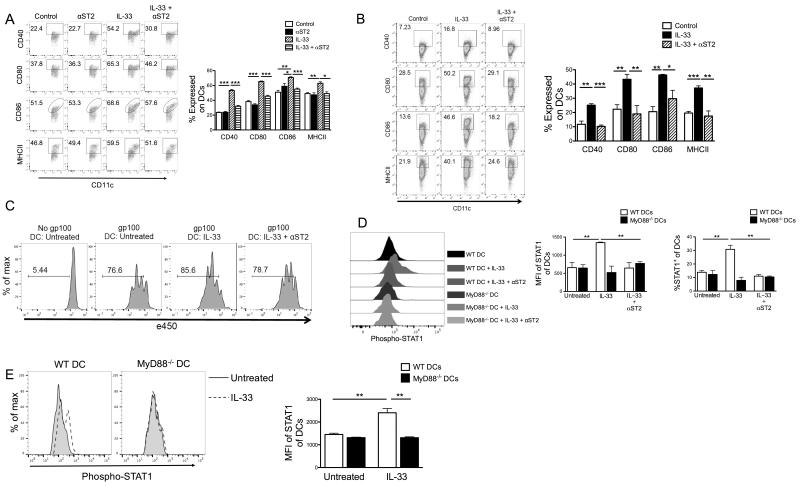
To test whether ST2 signal could promote DC activation and maturation, we activated WT splenic DCs from tumor-bearing mice in the presence of GM-CSF and rIL-33 with or without anti-ST2 blocking mAb. Consistent with a requirement for MyD88 for IL-33-induced DC activation, as shown in Fig. 9A, expression of costimulatory molecules (CD86, CD40, CD80 and MHC-II) was up-reguated by rIL-33. Anti-ST2 inhibited rIL-33-induced up-reguation of these costimulatory molecules (Fig. 9A). Similar results were also observed using splenic DCs from tumor-free mice (Fig. 9B). These data suggest that that ST2 mediates costimulatory molecule expression on DCs in response to rIL-33, in line with a requirement for MyD88 for IL-33-induced DC activation. Purified CD11+ DCs from IL-33-stimulated versus IL-33+anti-ST2-stimulated DC cultures were then compared for their ability to activate Pmel CD8+ T cells when pulsed with gp100 peptides. As expected, IL-33-stimuated DCs exhibited greater T cell proliferation, whereas anti-ST2 inihibited IL-33-induced T cell proliferation (Fig. 9C). To test whether STAT1 activation is through ST2-MyD88-mediated signaling downstream of IL-33, we examined IL-33-mediated STAT1 phosphorylation in DCs from MyD88−/− mice or treated by anti-ST2. Notably, STAT1 phosphorylation was stimulated by rIL-33 in DCs from WT mice compared with the control. However, this stimulatory effect was abolished by anti-ST2 (Fig. 9D). Moreover, STAT1 phosphorylation was also increased by rIL-33 in WT DCs but not MyD88−/− DCs (Fig. 9D). T cells can express CD11c, so they were depleted from splenocytes using CD90.1 selection prior to purification of CD11c+ cells, to exclude the potential role of T cells in IL-33-induced DC activation. We further confirmed that IL-33 increased STAT1 phosphorylation only in sorted CD3− CD11c+ WT DCs when they were mixed with MyD88−/− DCs (Fig. 9E), suggesting that IL-33-induced STAT1 phosphorylation is DC intrinsic. Overall, the data indicate that exogenous IL-33 can activate DCs to express costimulatory molecules for maturation through ST2-MyD88-mediated STAT1 signaling.
Figure 9. IL-33, ST2, MyD88 and STAT1 orchestrate activation and maturation of DCs.
Purified splenic CD11c+ cells from B16-SIY-bearing mice (A) or tumor-free mice (B) were incubated with the indicated reagents for 48 hours, and frequency of CD40, CD80, CD86, or MHC-II in Gr-1−CD11c+ cells was assessed by flow cytometry. (B) IL-33 induced maturation of naïve DCs is also ST2 dependent. (C) rIL-33-treated DCs promote antigen-specific CD8+ T cell proliferation. CD11c+ cells were treated with the indicated reagents for 48 hours as in (B), then pulsed with gp100 peptides for 2 hours. After pulsing they were cocultured with eFluro450-labeled naïve Pmel CD8+ T cells for 3 days and proliferation was measured by eFluro450 dilution. (D) rIL-33-induced STAT1 activation is ST2 and MyD88 dependent. Purified splenic WT or MyD88−/− CD11c+ cells were incubated with indicated reagents and then stained intracellularly for phosphorylated STAT1 by flow cytometry. (E) rIL-33-induced STAT1 phosphorylation is DC intrinsic. Purified CD3−CD11c+ WT and eFluro450-labeled MyD88−/− DCs were mixed at a 1:1 ratio and treated with/without rIL-33. The next day cells were analyzed for STAT1 phosphorylation by flow cytometry. WT DCs were gated as e450−, while MyD88−/− DCs were CD3−CD11c+ e450+. *; p<0.05, **; p<0.01, ***; p<0.001. Data (mean ± SEM) are representative of 3 independent experiments.
Discussion
The roles of IL-33 in tumor growth and tumor immunity have been not well defined. Here we demonstrate that rIL-33 adminstration inhibited the growth of estabished tumors in a CD8+ T-cell-dependent manner, consistent with the previous studies (21-23). However, our therapeutic approach using rIL-33 is different than that from these studies (Gao et al. used IL-33 transgenic mice (21); Villarreal et al. used the IL-33 DNA vaccination (22); the other used the cancer cells expressing ectopic IL-33 (23)). More importantly, we are developing the IL-33-based therapy against established tumors, rather than targeting very small tumors (0.5–120 mm3 in volume or have only grown for 0–4 d in mice), and we utilized experimental models of cancer to represent as closely as possible clinical cancers with the hope to mimic a clinical setting.
It is noted that there are several studies that show an opposite tumor-promoting role of IL-33. One study reported that systemic injection of low levels of rIL-33 increases tumor metastasis (39). Two other studies showed that systemic daily injection of rIL-33 alone (40) or with oncogene (41) for a long time (>8 weeks) promotes tumorigenesis. We speculate that differences in the dosage and duration of IL-33 treatment, and the primary target cells of IL-33 in these experimental settings are accountable for the different in vivo effects. In addition, it has been also reported that cancer patients tend to have higher IL-33 levels in sera and tumor tissues than healthy donors (42, 43). Clinically, the levels of circulating soluble ST2 are correlated with tumor burden (44, 45). These data may suggest a role of endogenous IL-33/ST2 signaling in promoting cancer progression (46-48), which are fairly different from the results from our lab and others using exogenous IL-33 based on the therapeutic milieu perspective.
One major obstacle of immunotherapy is that the tumor microenvrionment renders tumor-infiltrating DCs, and DLN DCs dysfunctional in their ability to mount an effective antitumor response. Interestingly, we found that IL-33 therapy increased DC number, and upregulated costimulatory molecule (CD40, CD80, CD86, or MHC-II) expression. In exvivo experiments, DCs from DLNs of IL-33 treated mice were far superior at priming T cells to produce IFN-γ than those from control groups. Also of note, IL-33 DCs were able to elicit this strong IFN-γ response without the addition of exogenous antigen, indicating that IL-33 treated DCs retain antigen presenting capabilities, as well as full T cell priming ability. Most clinically relevant was that we observed this effect in our BrafV600EPTEN tumors, which express the melanoma associated antigen gp100 that can serve as an immunotherapy target (49).
We have shown that dysfunctional mDCs from tumor-bearing mice can be rescued to cross-prime antitumor CD8+ T cells likely through a ST2-MyD88-STAT1 axis. In support of such a model, it has been reported that functional maturation of mDCs requires STAT1 activation and that STAT1 deficiency in DCs leads to imparied costimulatory molecule expression, antigen presentation and ability to mount a Th1 responses (50, 55). Moreover, DCs also require STAT-1 phosphorylation for the induction of peptide-specific CTL (51). Thus, our data suggest that IL-33-induced activation and maturation of mDCs can be regulated at least in part by ST2-MyD88-mediated activation of JAK/STAT1 signal transduction pathways.
Targeting CD40 with an agonist mAb has been tested in mice and patients with both hematological cancers and solid tumors (52, 53). Several humanized anti-CD40 mAb have completed phase I clinical trial and are currently being evaluated in phase II trials. As rIL-33 therapy resulted in increased expression of costimulation molecules including CD40 on tumor-associated DCs in vivo, we examined the antitumor effect of combination therapy of agonistic anti-CD40 and rIL-33 administration. The synergistic antitumor activity was observed particularly in the BPS1 model as anti-CD40 therapy alone failed to inhibit tumor growth. Certainly, whether the use of other immunotherapeutics, such as checkpoint inhibitors anti–PD-1 and/or anti–CTLA-4, requires further investigation. On the other hand, we found that this combination therapy did not trigger severe side effects including autoimmune diseases. Instead, considering the great ability of IL-33 to induce peripheral Treg and MDSC expansion (54), it is likely that combined IL-33 and mAb therapy would increase cancer immunity while reducing autoimmunity. Nevertheless, any sign of autoimmune activity if occured or the possibility of unintended tissue damage and/or abnormal tissue remodeling resulting from combination therapy also need careful investigation.
Collectively, our results provides new insights into the underlying cellular and molecular mechanisms by which IL-33 regulates antitumor CD8+ T cell immunity to inhibit tumor growth. The immune effects of IL-33 could be highly context-dependent, and vary in the presence of different cellular targets, phases of immune responses and given model systems. Importantly, we propose in a rational manner how to maximize therapeutic efficacy of IL-33 administration by mechanism-based combinatory approaches against established cancer.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health Tetramer Facility for providing the Db/gp100 tetramers. We thank Dr. Xue-Feng Bai for his insightful discussion.
This research was in part supported by National Institutes of Health grant CA149669, Northwestern Memorial Foundation-Friends of Prentice Grants Initiative, SPORE Pilot Award (P50 CA090386), Northwestern University RHLCCC Flow Cytometry Facility, a Cancer Center Support Grant (NCI CA060553).
References
- 1.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 2.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989;258:301–304. doi: 10.1016/0014-5793(89)81679-5. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 6.Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, Lukic ML. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52:89–99. doi: 10.1007/s12026-012-8283-9. [DOI] [PubMed] [Google Scholar]
- 7.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 8.Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M, Okayama Y, Akira S, Saito H, Galli SJ, Nakae S. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukoc Biol. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 9.Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369–26380. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 10.Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, Komai-Koma M, Pitman N, Li Y, Niedbala W, McKenzie AN, Teixeira MM, Liew FY, Xu D. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–4790. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 11.Savinko T, Matikainen S, Saarialho-Kere U, Lehto M, Wang G, Lehtimaki S, Karisola P, Reunala T, Wolff H, Lauerma A, Alenius H. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol. 2012;132:1392–1400. doi: 10.1038/jid.2011.446. [DOI] [PubMed] [Google Scholar]
- 12.Tordesillas L, Goswami R, Benede S, Grishina G, Dunkin D, Jarvinen KM, Maleki SJ, Sampson HA, Berin MC. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest. 2014;124:4965–4975. doi: 10.1172/JCI75660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 15.Jones LA, Roberts F, Nickdel MB, Brombacher F, McKenzie AN, Henriquez FL, Alexander J, Roberts CW. IL-33 receptor (T1/ST2) signalling is necessary to prevent the development of encephalitis in mice infected with Toxoplasma gondii. Eur J Immunol. 2010;40:426–436. doi: 10.1002/eji.200939705. [DOI] [PubMed] [Google Scholar]
- 16.Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nature reviews. Rheumatology. 2011;7:321–329. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- 17.Villarreal DO, Weiner DB. Interleukin 33: a switch-hitting cytokine. Curr Opin Immunol. 2014;28C:102–106. doi: 10.1016/j.coi.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumann C, Bonilla WV, Frohlich A, Helmstetter C, Peine M, Hegazy AN, Pinschewer DD, Lohning M. T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc Natl Acad Sci U S A. 2015;112:4056–4061. doi: 10.1073/pnas.1418549112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonilla WV, Frohlich A, Senn K, Kallert S, Fernandez M, Johnson S, Kreutzfeldt M, Hegazy AN, Schrick C, Fallon PG, Klemenz R, Nakae S, Adler H, Merkler D, Lohning M, Pinschewer DD. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Li G, Zhu Y, Liu L, Chen E, Turnquist H, Zhang X, Finn OJ, Chen X, Lu B. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells. Eur J Immunol. 2011;41:3351–3360. doi: 10.1002/eji.201141629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao K, Li X, Zhang L, Bai L, Dong W, Gao K, Shi G, Xia X, Wu L, Zhang L. Transgenic expression of IL-33 activates CD8(+) T cells and NK cells and inhibits tumor growth and metastasis in mice. Cancer Lett. 2013;335:463–471. doi: 10.1016/j.canlet.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Villarreal DO, Wise MC, Walters JN, Reuschel EL, Choi MJ, Obeng-Adjei N, Yan J, Morrow MP, Weiner DB. Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res. 2014;74:1789–1800. doi: 10.1158/0008-5472.CAN-13-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Wang X, Yang Q, Zhao X, Wen W, Li G, Lu J, Qin W, Qi Y, Xie F, Jiang J, Wu C, Zhang X, Chen X, Turnquist H, Zhu Y, Lu B. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J Immunol. 2015;194:438–445. doi: 10.4049/jimmunol.1401344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gajewski TF. Identifying and overcoming immune resistance mechanisms in the melanoma tumor microenvironment. Clin Cancer Res. 2006;12:2326s–2330s. doi: 10.1158/1078-0432.CCR-05-2517. [DOI] [PubMed] [Google Scholar]
- 25.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 26.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin A, Schildknecht A, Nguyen LT, Ohashi PS. Dendritic cells integrate signals from the tumor microenvironment to modulate immunity and tumor growth. Immunology letters. 2010;127:77–84. doi: 10.1016/j.imlet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 29.van Mierlo GJ, Boonman ZF, Dumortier HM, den Boer AT, Fransen MF, Nouta J, van der Voort EI, Offringa R, Toes RE, Melief CJ. Activation of dendritic cells that cross-present tumor-derived antigen licenses CD8+ CTL to cause tumor eradication. J Immunol. 2004;173:6753–6759. doi: 10.4049/jimmunol.173.11.6753. [DOI] [PubMed] [Google Scholar]
- 30.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayuzumi N, Matsushima H, Takashima A. IL-33 promotes DC development in BM culture by triggering GM-CSF production. Eur J Immunol. 2009;39:3331–3342. doi: 10.1002/eji.200939472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123:1047–1054. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011;41:1675–1686. doi: 10.1002/eji.201041033. [DOI] [PubMed] [Google Scholar]
- 34.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr., You MJ, DePinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hooijkaas AI, Gadiot J, van der Valk M, Mooi WJ, Blank CU. Targeting BRAFV600E in an inducible murine model of melanoma. Am J Pathol. 2012;181:785–794. doi: 10.1016/j.ajpath.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Chen S, Wang L, Fan J, Ye C, Dominguez D, Zhang Y, Curiel TJ, Fang D, Kuzel TM, Zhang B. Host miR155 promotes tumor growth through a myeloid-derived suppressor cell-dependent mechanism. Cancer Res. 2015;75:519–531. doi: 10.1158/0008-5472.CAN-14-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buhtoiarov IN, Sondel PM, Wigginton JM, Buhtoiarova TN, Yanke EM, Mahvi DA, Rakhmilevich AL. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology. 2011;132:226–239. doi: 10.1111/j.1365-2567.2010.03357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin MU. Special aspects of interleukin-33 and the IL-33 receptor complex. Semin Immunol. 2013;25:449–457. doi: 10.1016/j.smim.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Jovanovic IP, Pejnovic NN, Radosavljevic GD, Pantic JM, Milovanovic MZ, Arsenijevic NN, Lukic ML. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int J Cancer. 2014;134:1669–1682. doi: 10.1002/ijc.28481. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Razumilava N, Gores GJ, Walters S, Mizuochi T, Mourya R, Bessho K, Wang YH, Glaser SS, Shivakumar P, Bezerra JA. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J Clin Invest. 2014;124:3241–3251. doi: 10.1172/JCI73742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada D, Rizvi S, Razumilava N, Bronk SF, Davila JI, Champion MD, Borad MJ, Bezerra JA, Chen X, Gores GJ. IL-33 facilitates oncogene induced cholangiocarcinoma in mice by an IL-6 sensitive mechanism. Hepatology. 2015 doi: 10.1002/hep.27687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui G, Qi H, Gundersen MD, Yang H, Christiansen I, Sorbye SW, Goll R, Florholmen J. Dynamics of the IL-33/ST2 network in the progression of human colorectal adenoma to sporadic colorectal cancer. Cancer Immunol Immunother. 2015;64:181–190. doi: 10.1007/s00262-014-1624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun P, Ben Q, Tu S, Dong W, Qi X, Wu Y. Serum interleukin-33 levels in patients with gastric cancer. Digestive diseases and sciences. 2011;56:3596–3601. doi: 10.1007/s10620-011-1760-5. [DOI] [PubMed] [Google Scholar]
- 44.Gillibert-Duplantier J, Duthey B, Sisirak V, Salaun D, Gargi T, Tredan O, Finetti P, Bertucci F, Birnbaum D, Bendriss-Vermare N, Badache A. Gene expression profiling identifies sST2 as an effector of ErbB2-driven breast carcinoma cell motility, associated with metastasis. Oncogene. 2012;31:3516–3524. doi: 10.1038/onc.2011.525. [DOI] [PubMed] [Google Scholar]
- 45.Bergis D, Kassis V, Ranglack A, Koeberle V, Piiper A, Kronenberger B, Zeuzem S, Waidmann O, Radeke HH. High Serum Levels of the Interleukin-33 Receptor Soluble ST2 as a Negative Prognostic Factor in Hepatocellular Carcinoma. Translational oncology. 2013;6:311–318. doi: 10.1593/tlo.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jovanovic I, Radosavljevic G, Mitrovic M, Juranic VL, McKenzie AN, Arsenijevic N, Jonjic S, Lukic ML. ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma. Eur J Immunol. 2011;41:1902–1912. doi: 10.1002/eji.201141417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JY, Lim SC, Kim G, Yun HJ, Ahn SG, Choi HS. Interleukin-33/ST2 axis promotes epithelial cell transformation and breast tumorigenesis via upregulation of COT activity. Oncogene. 2014 doi: 10.1038/onc.2014.418. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Zhu L, Lu X, Bian H, Wu X, Yang W, Qin Q. IL-33/ST2 pathway contributes to metastasis of human colorectal cancer. Biochem Biophys Res Commun. 2014;453:486–492. doi: 10.1016/j.bbrc.2014.09.106. [DOI] [PubMed] [Google Scholar]
- 49.Steinberg SM, Zhang P, Malik BT, Boni A, Shabaneh TB, Byrne KT, Mullins DW, Brinckerhoff CE, Ernstoff MS, Bosenberg MW, Turk MJ. BRAF inhibition alleviates immune suppression in murine autochthonous melanoma. Cancer immunology research. 2014;2:1044–1050. doi: 10.1158/2326-6066.CIR-14-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson SH, Yu CR, Mahdi RM, Ebong S, Egwuagu CE. Dendritic cell maturation requires STAT1 and is under feedback regulation by suppressors of cytokine signaling. J Immunol. 2004;172:2307–2315. doi: 10.4049/jimmunol.172.4.2307. [DOI] [PubMed] [Google Scholar]
- 51.Pilz A, Kratky W, Stockinger S, Simma O, Kalinke U, Lingnau K, von Gabain A, Stoiber D, Sexl V, Kolbe T, Rulicke T, Muller M, Decker T. Dendritic cells require STAT-1 phosphorylated at its transactivating domain for the induction of peptide-specific CTL. J Immunol. 2009;183:2286–2293. doi: 10.4049/jimmunol.0901383. [DOI] [PubMed] [Google Scholar]
- 52.Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol. 2013;25:230–237. doi: 10.1016/j.coi.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khong A, Nelson DJ, Nowak AK, Lake RA, Robinson BW. The use of agonistic anti-CD40 therapy in treatments for cancer. Int Rev Immunol. 2012;31:246–266. doi: 10.3109/08830185.2012.698338. [DOI] [PubMed] [Google Scholar]
- 54.Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, Wang Z, Lang M, Stolz DB, Zheng XX, Demetris AJ, Liew FY, Wood KJ, Thomson AW. IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J Immunol. 2011;187:4598–4610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson LM, Scott P. STAT1 expression in dendritic cells, but not T cells, is required for immunity to Leishmania major. J Immunol. 2007;178:7259–66. doi: 10.4049/jimmunol.178.11.7259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.