Abstract
NLRP3 inflammasome is a critical player in innate immunity. Neutrophil recruitment to tissues and effective function of neutrophils are critical innate immune function to bacterial clearance. However, the role of NLRP3 in neutrophil-dependent bacterial clearance in polymicrobial sepsis is unclear. Herein, we evaluated the role of NLRP3 in polymicrobial sepsis induced by cecal ligation and puncture (CLP). Our results exhibit protection from death in NLRP3-deficient (Nlrp3−/−) and NLRP3 inhibitor-treated wild-type (C57Bl/6) mice. Both Nlrp3−/− and NLRP3 inhibitor-treated mice displayed lower bacterial load albeit no impairment in neutrophil recruitment to peritoneum. However, neutrophil depletion abrogated protection from death of nd NLRP3-deficient (Nlrp3−/−) mice in response to CLP. Intriguingly, Nlrp3−/− peritoneal cells (primarily neutrophils) following CLP demonstrate decreased autophagy, augmented phagocytosis and enhanced scavenger receptor (MARCO) and mannose binding leptin (MBL) expression. These findings enhance our understanding of the critical role of NLRP3 in modulating autophagy and phagocytosis in neutrophils and suggest that therapies should be targeted to modulate both autophagy and phagocytosis in neutrophils to control bacterial burden in tissues during CLP-induced polymicrobial sepsis.
Keywords: Host Defense, Neutrophil, Scavenger Receptors, Inflammasome, sepsis
INTRODUCTION
Despite extensive scientific and clinical care management, sepsis continues to be one of the leading causes of infectious deaths in the U.S. (1–3). Events which follow sepsis represent the stages of progression; therefore, relationship between the innate immune cascades and sepsis have long been under scrutiny (4, 5). Neutrophils are critical component of the host innate immune response and play a central role in sepsis, resulting in the release of cytokines, chemokines and antimicrobial proteins, and phagocytose microbial pathogens (6, 7). However, neutrophil recruitment to the site of infection has been shown to be reduced during severe sepsis, which is associated with reduction in adherence (7), chemotaxis (8), phagocytosis (9), and production of reactive oxygen intermediates (10, 11) although the molecular and cellular mechanisms have not been explored.
NOD-Like Receptors (NLRs) are important in the context of sepsis because these pattern recognition receptors recognize pathogen associated molecular patterns, as well as a variety of damage associated molecular patterns (12, 13). Of these, NLRP3 is of particular interest as it forms a caspase-1 activating molecular complex termed “inflammasome” (14, 15). Caspase-1 activation by the inflammasome promotes the maturation of IL-1β and IL-18 (14, 15). Animal and human studies have highlighted the importance of the inflammasome pathways in the innate immune response to sepsis (16, 17). For example, caspase-1−/− mice are protected from endotoxic shock and E. coli induced sepsis (18). In humans, NLRP3 the protein mutated in familial Mediterranean fever has been shown to regulate production of mature IL-1β by complexing with procaspase-1 and apoptosis-associated speck-like protein containing a CARD domain (19). However, the role of NLRP3 in polymicrobial sepsis has not been delineated in detail.
Autophagy is a conserved intracellular process which contributes to degradation and recycling of cellular proteins and organells to maintain cellular homeostasis (20, 21). Autophagy is shown to contribute to innate immune responses and inflammation (21). Phagocytosis is a related process to that of autophagy which is associated with host defense against microbial infection (22). Although both of these processes are involved in host defense, unlike autophagy, phagocytosis deals with the ingestion of extracellular agents (22, 23). It is now however clear that both of these processes are stimulated by the signaling cascades originating from pattern recognition receptors (24). Intriguingly, recent investigations have indicated that autophagy can modulate phagocytosis in murine macrophages (25). However, it is unclear whether NLRP3 modulates cellular processes, such as autophagy or phagocytosis in the setting of CLP in order to enhance bacterial clearance.
In initial studies using a sepsis-model, it has been demonstrated that gene silencing of NLRP3 exhibits reduced hepatic cytokines, neutrophil recruitment and macrophages pyroptosis (26). Similarly, lung pathology was decreased along with attenuated accumulation of inflammatory cells as well as cytokine and chemokine levels in Nlrp3−/− mice in a hyperoxia model of lung injury (27). In a separate study, Nlrp3−/− mice were more vulnerable to dextran sodium sulfate-induced colitis associated with higher leukocyte counts and increased chemokine production in the colon (28). These conflicting results in different mucosal organs warrant future studies related to the role of NLRP3 in polymicrobial sepsis.
The present report assessed the effect of NLRP3 in during CLP on survival of mice deficient in NLRP3 (Nlrp3−/−) and wild-type (C57Bl/6) mice treated with NLRP3 inhibitor. The corresponding bacterial load in the lungs and extrapulmonary organs were determined. Both neutrophil recruitment and function were also determined. Peritoneal cells and intraperitoneal organs isolated from Nlrp3−/− mice following CLP were used to measure autophagy, phagocytosis and scavenger receptor expression. Results demonstrated improved survival in Nlrp3−/− mice undergoing CLP which was associated with decreased bacterial burden in organs. Additionally, cellular recruitment was not affected while autophagy in neutrophils was attenuated while phagocytosis was augmented. Furthermore, the expression of MARCO and MBL expression was up-regulated upon deletion of NLRP3 while caspase-1 was attenuated. These findings together suggest a protective effect upon NLRP3 deletion in CLP-induced polymicrobial sepsis.
METHODS
Ethics Statement
Animal experiments were conducted in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocol was approved by the Institutional Animal Care and Use Committee at Louisiana State University. Mice were monitored after any manipulations and all efforts were made to minimize pain and distress.
Mice
Eight- to ten-week-old Nlrp3−/− male mice were back-crossed 10 times with C57BL/6 mice for this study (29) and C57BL/6 (WT) male mice were used as age- and gender-matched controls. Animals were handled in accordance with Louisiana State University Animal Welfare Committee’s approved protocol.
Cecal ligation and puncture
In this experiment, cecal ligation and puncture (CLP) was used as moderate polymicrobial sepsis model as described in previous publication (30). Animals with sham operation underwent the same protocol without CLP. Mice undergoing CLP were given 1 ml of subcutaneous warm pyrogen-free saline for fluid resuscitation. Mice were given subcutaneous buprenorphin (0.05 mg/kg body weight) for postoperative analgesia at every 6 h for the entire duration of experiment. For E. coli-induced peritonitis, mice were injected i.p. with E. coli at a dose of 5× 108CFU/kg body weight. The survival of mice was monitored at every 6 h after injection. In a parallel experiment, a NLRP3 inhibitor glibenclamide (aka glyburite; Invivogen, San Diego, CA) was prepared in DMSO to a final concentration of 25 mg/mL. In WT mice, A total of 1mg/mouse was administered intraperitoneally (i.p.) immediatedly after CLP. In control group, the same amount of DMSO (40 μl) was used instead of glibenclamide (31). Survival was monitored every 12 h up to 10 days. In another set of experiments, neutrophils were depleted by i.p. injection of 50 μg of anti-Ly6G mAb (clone IA8; BD Biosciences) or isotype control Ab (clone R35–95; BD Biosciences) at 12 and 2 h before CLP as described earlier (32–34).
Determination of bacterial CFU
Bacterial count was assessed as previously described (33). The lungs and spleens of control and infected mice were weighed and homogenized in 1 ml of 0.9% saline using a tissue homogenizer. The solid tissue was allowed to sediment for 10 min at room temperature and the supernatants were serially diluted. Twenty milliliter aliquots of each sample were plated on tryptic soy agar plates. The number of colonies was enumerated after incubation at 37°C overnight.
Peritoneal lavage
Peritoneal exudate cells were recovered by peritoneal lavage with 4 ml of sterile, warm, heparinized RPMI 1640 medium (GIBCO/BRL, Bethesda, Md.) and PMN percentage were counted manually by a hemocytometer (32, 33).
Phagocytosis and bacterial killing activity of neutrophils
The phagocytic activity of peritoneal neutrophils was measured by the uptake of red fluorescent pHrodo Escherichia coli bioparticles (35, 36) as described. Briefly, 1× 106 neutrophils were suspended in 100 uL of HBSS containing 20 mM HEPES, pH 7.4, and mixed with 5uL of pHrodo E. coli bioparticles. The mixture was incubated for 30 minutes at 37°C for uptake activity. After incubation, neutrophils were washed twice with component C (Invitrogen), then resuspended in 100 uL of component C. Absorbance change at different time points was recorded with a spectrophotometer (U-2001; Hitachi, Tokyo, Japan). The neutrophil mediated killing was performed as previously described with slight modification (32). Neutrophils isolated from bone marrow (1×106) were suspended in RPMI 1640 with 10%v/v FBS and were incubated with 1×106 (1 MOI) opsonized bacteria in a shaking water bath at 37°C for 120 min with continuous agitation. Samples were harvested at 0, 30, 60 or 120 min, and a portion of the sample was spun at 100× g for 10 min to collect the bacteria in media. The neutrophil pellet was resuspended in 1ml cold PBS, and the debris was broken up by using a homogenizer to rate engulfed bacteria. Colony counting of viable bacteria was conducted by plating 20-ul aliquots of each sample on trypticase soy agar plates. The number of colonies was countered after incubation at 37°C overnight.
To determine whether autophagy regulates phagocytosis, neutrophils (105 cells) in Opti-MEM® medium are pre-treated with 10 μM cytochalasin D (phagocytosis inhibitor) or 10 mM 3-methyladenine (autophagy inhibitor) for one hour, followed by addition of pHrodo™ BioParticles® (35, 36). The degree of phagocytosis was measured.
Western Blotting
An assessment of Histone Citrullination and PAD-4 activation in the isolated neutrophils from peritoneal lavage fluid and human neutrophils post CLP was done by extracting cellular proteins using Urea/CHAPS/Tris (lysis) buffer (32). In brief, the harvested cells were lysed using lysis buffer containing and complete protease and phosphatase inhibitor cocktail (Roche Co, Indianapolis, IN) for 15 mins in cold conditions. Samples were spun at maximum speed in eppendorf centrifuge to remove cellular debris. To ensure equal loading of protein onto the gel, a Bradford protein assay was performed (Bio-Rad, Herculus, CA). Proteins were fractionated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred electrophoretically to Immobilon-P transfer membrane (Millipore UK, Watford, United Kingdom) by conventional wet blotting. Membranes were incubated in blocking buffer (1×TBS, 5% [wt/vol] non-fat dry milk, 0.1% Tween-20) for 1 hour followed by overnight incubation with primary antibodies viz. citrullinated histone and PAD-4 (at 1: 1000 dilution) in blocking buffer at 4°C. Antibody to GAPDH was added to the concentration of 1:5000. Incubation with species-specific horseradish peroxidase (HRP)-conjugated secondary antibodies at a 1 in 2000 dilution was performed in blocking buffer for 1 h. Labeling was detected using the enhanced chemiluminescence (ECL) reagent (Amersham Biosciences, United Kingdom). Densitometric analysis of protein bands were perfomed as described in previous publications (37, 38). In brief, densitometry was determined by normalizing protein expression to GAPDH. Data were then transformed for sham mice to have a reference value of one (average) for each group and the fold change in sepsis group was determined by the relative fold difference. Thereafter, the fold difference between WT and KO mice was analyzed for significance.
Scanning electron microscopy
Neutrophils isolated from peritoneal cavity of CLP induced or Sham mice were cultured for 8h in DMEM containing 1 mM HEPES. Cells were fixed with 2.5% glutaraldehyde and 1% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) overnight at 4 degrees. In some experiments The fixed cells were washed twice with sodium cacodylate and postfixed with 1% osmium tetroxide and 1% tannic acid, dehydrated with a graded series of ethanol, and then dried in hexamethyldisalizane (39). The samples were subsequently mounted on colloidal graphite, sputter-coated, and visualized using a scanning electron microscope (S2460 N; Hitachi, San Jose, CA).
Flow cytometry
Immediately after mice were euthanized, 6 ml of physiological saline was injected into peritoneal cavity and was lavaged repeatedly. Peritoneal cells were collected, and subsequently stained for flow cytometry using anti-Gr1-PerCP, anti-CD11b-PE, and anti-F4/80-APC (40). All flow cytometry data was analyzed using FlowJo VX software.
Statistics
Data are expressed as means ± SEM. Statistical analyses were performed using GraphPad Prism® (v.5.03) (GraphPad, San Diego, CA). Data were tested for normality using Shapiro-Wilk normality test. Data with normal (Gaussian) distribution were compared using Student’s t-test (between two groups) or with one-way ANOVA (more than 2 groups) with Tukey’s multiple comparison test. Survival curves were analyzed by the log rank test. Differences in values were defined as significant at a p value of less than 0.05, 0.01 or 0.001.
RESULTS
Nlrp3−/− mice were resistant to polymicrobial sepsis and E. coli challenge
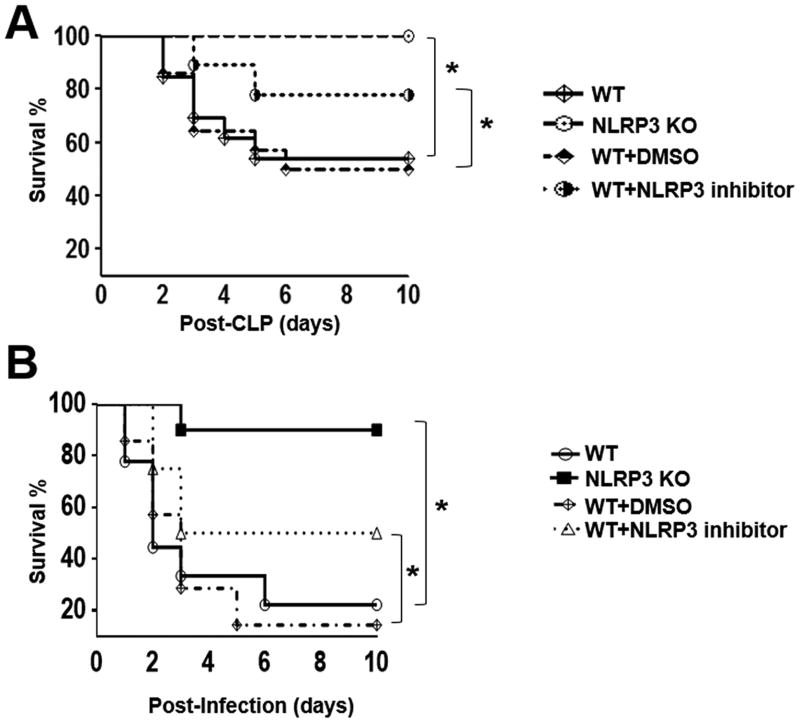
To investigate the role of NLRP3 in host protection against death from polymicrobial sepsis, Nlrp3−/− and wild-type (C57Bl/6) mice underwent CLP and the survival was monitored every 6 hours for first 48 hours and every 12 hours thereafter for up to 10 days. Interestingly, we observed that all Nlrp3−/− mice survived up to 10 days whereas only 56% of C57Bl/6 survived (Fig 1A). On the other hand, all sham (control) mice from both Nlrp3−/− and WT mice show no mortality (data not shown). Next, WT mice were administrated a single i.p. dose of 1 mg NLRP3 inhibitor/mouse immediately after CLP. Pharmacological inhibition of NLRP3 after sepsis resulted in increased survival compared to CLP induced WT or WT treated DMSO (Fig 1A). To determine the role of NLRP3 in E. coli peritonitis, we injected 5×108 CFU/Kg E. coli i.p. and found that Nlrp3−/− mice were protected as compared with WT controls (Fig. 1B). In parallel experiments, WT mice were administrated a single i.p. dose of 1 mg NLRP3 inhibitor/mouse immediately after E. coli challenge. NLRP3 inhibitor enhanced survival as compared with DMSO administered (control) mice after E. coli infection (Fig. 1B).
FIGURE 1. Survival in Nlrp3−/− (KO) mice or mice treated with NLRP3 inhibitor following CLP.
(A) Survival of Nlrp3−/− mice and WT mice treated with NLRP3 inhibitor following CLP. The mortality rates of WT and Nlrp3−/− (KO) mice, and mice treated with NLRP3 inhibitor were monitored for 10 days after CLP. A total of 1 mg NLRP3 inhibitor/mouse or the same volume of DMSO was administered intraperitoneally (i.p.) immediately after CLP. *p<0.05 between WT and Nlrp3−/− mice or inhibitor treated WT and DMSO treated WT mice [n=20 mice/group]. (B) Survival of Nlrp3−/− mice and WT mice treated with NLRP3 inhibitor after E. coli-induced sepsis. WT and Nlrp3−/− (KO) mice, and WT mice treated with DMSO (control) and NLRP3 inhibitor were subjected to E. coli (5×108 CFU/kg of body weight) challenge as described in material and methods. Mice were monitored for 10 days for survival. WT=18 mice/group; Nlrp3−/− (KO) mice=20 mice/group; WT mice treated with DMSO=14 mice/group; and WT mice treated with NLRP3 inhibiitor=12 mice/group. p<0.05 between groups.
NLRP3 deficiency augments bacterial clearance following CLP
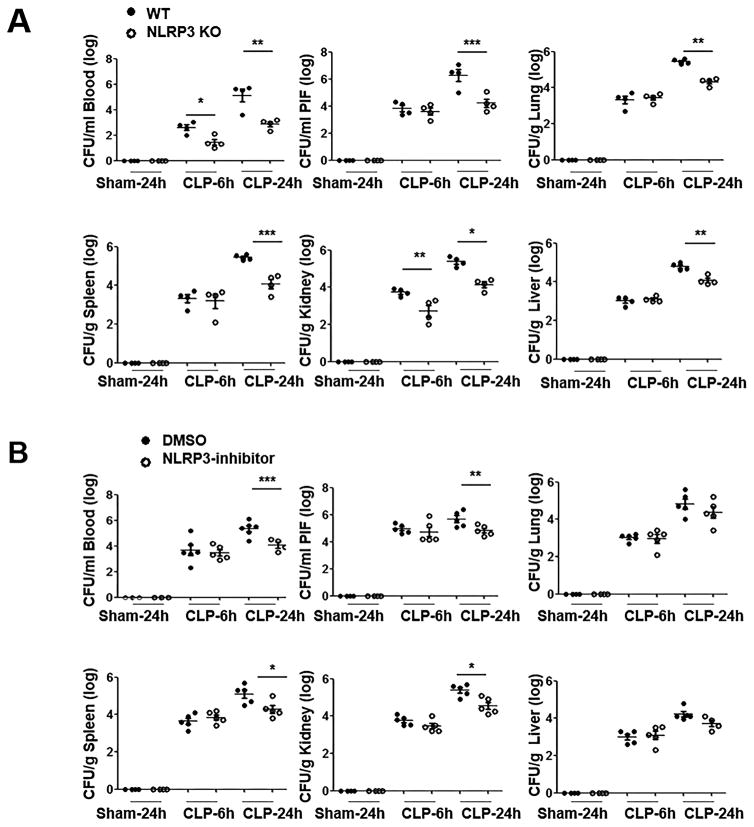
We next investigated if host resistance to CLP-induced sepsis in Nlrp3−/− mice resulted from augmented bacterial clearance. To accomplish this, CLP was performed on Nlrp3−/− and WT mice and bacterial burden was determined at 6 h and 24 h post-CLP. As shown in Fig. 2A, bacterial clearance in peritoneal lavage fluid and extra-peritoneal tissues, including the blood, lung, liver, spleen and kidney of Nlrp3−/− mice was augmented at 24 h. In fact, bacterial clearance in blood and kidney was augmented even at an early time-point (6 h) (Fig. 2A). WT mice treated with NLRP3 inhibitor showed significantly reduced bacterial burden in peritoneal lavage fluid, blood, spleen and kidney at 24 h post-CLP (Fig. 2B).
FIGURE 2. Bacterial clearance in peritoneum and extraperitoneal tissues in Nlrp3−/− (KO) mice and NLRP3 inhibitor treated mice following CLP.
(A) Bacterial burden in Nlrp3−/− and WT (control) mice in peritoneum and extra-peritoneal organs during CLP-induced sepsis. Bacterial colonies were enumerated and the results are expressed either as CFU/ml of blood and peritoneal lavage fluid or CFU/g of liver, lung, spleen and kidney. [n=4 mice/group] (B) Bacterial burden in peritoneum and extra-peritoneal tissues in WT mice treated with the NLRP3 inhibitor as compared to WT mice treated with DMSO (control). At 6 h and 24 h post-CLP, mice were euthanized to obtain blood, peritoneal lavage fluid, and extraperitoneal organs. [n=5 mice/group] *p<0.05, **p<0.01 and ***p<0.001 between WT and KO mice.
NLRP3 is dispensible for neutrophil recruitment to the peritoneum
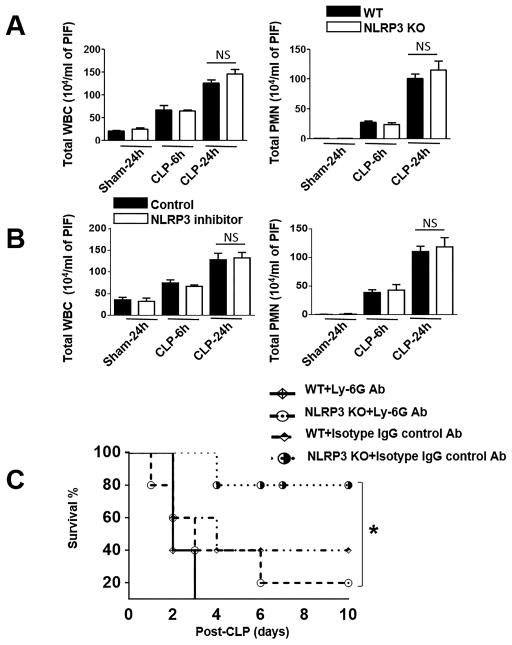
To determine the recruitment of myeloid cells to the peritoneal cavity of Nlrp3−/− and WT mice following CLP, peritoneal lavage was collected at 6 h and 24 h post-CLP and recruited cells were enumerated. In Nlrp3−/− mice, total white blood cell and neutrophil recruitment to the peritoneal cavity was similar to that of WT at both 6 h and 24 h following CLP (Fig. 3A). In sham (control) groups, however no significant cellular influx in the peritoneal cavity was observed in both Nlrp3−/− and WT mice (Fig. 3A). In a similar manner, neutrophil recruitment to the peritoneum was similar in NLRP3 inhibitor (in DMSO) administered and DMSO administered WT mice (Fig. 3B).
Figure 3. Neutrophil recruitment to peritoneum in Nlrp3−/− (KO) mice and NLRP3 inhibitor-treated mice after CLP.
(A) Total WBC and PMN counts in peritoneal lavage fluid of WT and Nlrp3−/− (KO) mice that were subjected to sham or CLP-induced sepsis [n=6–8 mice/group]. (B) Total WBC and PMN numbers in peritoneal lavage fluid of groups of WT mice that were treated with either NLRP3 inhibitor or DMSO (control) followed by CLP-induced sepsis. The peritoneum was lavaged at 6 h and 24 h after CLP in groups of mice and the WBC and PMN in the peritoneal lavage were enumerated [n=6–8 mice/group]. (C) Survival of Nlrp3−/− and WT mice after neutrophil depletion and CLP. Groups of Nlrp3−/− and WT mice were treated with anti-Ly6G (neutrophil depleted) or isotype (control) Ab at 12 and 2 h prior to CLP. Mortality was monitored over 10 days and the results were analyzed by log rank test. n=10 mice/group; *p<0.05 between WT and Nlrp3−/− mice or WT mice treated with NLRP3 inhibitor and DMSO treated (control) mice.
Neutrophils are critical for survival and controlling bacterial burden in WT and Nlrp3−/− mice following CLP
Neutrophil recruitment and proper function of neutrophils in tissues/organs are critical events for bacterial clearance after CLP (6). Therefore, we next examined whether neutrophils are critical to control survival in CLP. To accomplish this, we depleted neutrophils at 12 hour and 2 hour prior to CLP using Ly6G-specific mAb, 1A8. Although all of the neutrophil-depleted WT mice were found dead within 3 days following CLP, 60% of isotype-matched control mAb-treated animals were alive up to 10 days after CLP (Fig. 3C). In a similar manner, Most of the neutrophil-depleted Nlrp3−/− mice (90%) were found dead within 6 days following CLP, 20% of isotype-matched control mAb-treated animals were found dead up to 10 days following CLP (Fig. 3C). Hence, both neutrophil depleted groups, Nlrp3−/− and WT mice showed reduced survival as compared with their controls at 6 h and 24 h post-infection (Fig. 3C), suggesting that neutrophils are indeed a major player in NLRP3-induced host protection in polymicrobial sepsis.
Nlrp3−/− peritoneal cells and tissues exhibit decreased autophagy
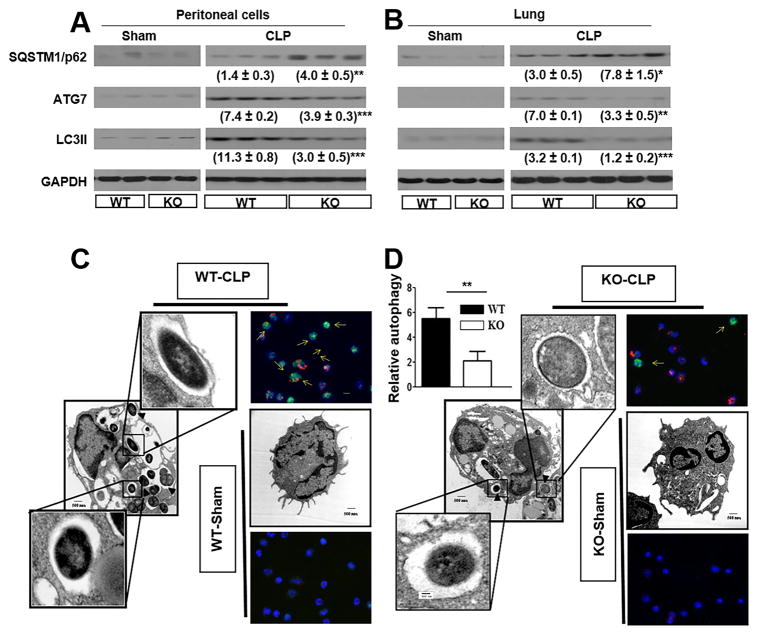
Autophagy (macroautophagy) is a critical event in bacterial clearance which involves multiple albeit complex cascades (41, 42). The autophagy genes (Atg) regulate the formation of autophagosomes formation via Atg5-Atg12 and LC3-II complexes. Atg5 is conjugated to Atg12 in a ubiquitination reaction that needs both Atg10 and Atg7. Afterwards, the Atg5-Atg12 complex noncovalently interacts with Atg16 to form a protein complex. The lipidated LC3, termed as LC3II, is eventually affixed to the autophagosome membrane until it becomes autolysosomes. Because of these reasons, Atg7 and LC3II are considered as key autophagy markers (43). As compared with thioglycollate-induced peritoneal cells (>93% neutrophils), peritoneal cells (>94% neutrophils) isolated from Nlrp3−/− mice at 24 h post-CLPdisplayed reduction in Atg7, impairment of LC3II formation and enhanced sequestome/p63 (SQSTM1), a polyubiquitin chain binding protein associated with ubiquitin proteasome degradation (Fig. 4A). In a similar manner, lung tissues showed reduction in Atg7, inhibition of LC3II formation and enhanced sequestosome 1 (SQSTM1) (Fig. 4B). In addition, we also enumerated the number of autophagasomes in neutrophils using scanning electron microscopy and found that the autophagosmes are decreased in Nlrp3−/− neutrophils (Fig. 4C–D).
Figure 4. Autophagy in neutrophils of Nlrp3−/− (KO) mice following CLP.
Expression of autophagy markers (ATG7 and LC3II) and expression of SQSTM1 (sequestosome) Nlrp3−/− mice that underwent CLP as observed in Peritoneal cells (A), and lung (B). The protein expression was visualized and quantitated using Western Blotting as described in Methods section. Each lane contains a lysate from a single mouse. This is a representative blot of 3 independent experiments with identical results. (C–D) Electron microscopy images of neutrophils showing autophagosomes in Nlrp3−/− mice. Groups of Nlrp3−/− and WT mice underwent CLP and peritoneal cells were collected after 24 h. Relative autophagy was calculated by enumerating the number of autophagic structure per cell. *p<0.05, **p<0.01 and ***p<0.001 between peritoneal cells from WT and Nlrp3−/− mice.
Nlrp3−/− neutrophils show augmented phagocytosis
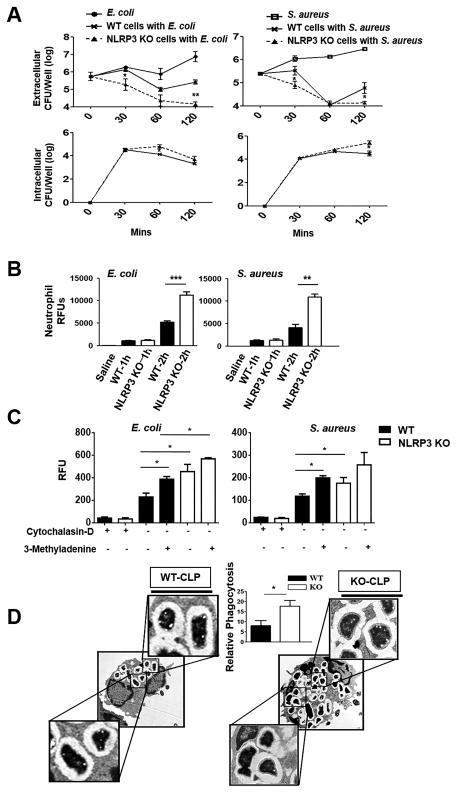
We next infected bone marrow-derived neutrophils with a Gram-negative bacterium, E. coli and a Gram-positive bacterium, Staphylococcus aureus to determine extracellular and intracellular CFU following infection. As compared with WT neutrophils, Nlrp3−/− neutrophils exhibit improved bacterial clearance at 120 mins post-infection (Fig. 5A), suggesting that this may be due to enhanced uptake by Nlrp3−/− neutrophils. To address this issue, we determined the uptake of pHrodo™ Red E. coli and S. aureus BioParticles® by purified peritoneal neutrophils from Nlrp3−/− and WT mice at both 1 and 2 h post-infection. We found that at 1 h, phagocytosis of E. coli and S. aureus was no different in both sets of neutrophils whereas at 2 h, Nlrp3−/− neutrophils showed higher phagocytosis as compared to WT groups (Fig. 5B). In order to understand the link between autophagy and phagocytosis, neutrophils were treated with cytochalasin D (a phagocytosis inhibitor) or 3-Methyladenine (an autophagy inhibitor), and the phagocytosis activity was measured in neutrophils at 1.5 h post-infection. As expected, phagocytosis was suppressed in the presence of cytochalasan D in neutrophils obtained from both WT and Nlrp3−/− mice, whereas, phagocytosis activity was significantly enhanced in presence of 3-methyladenine in both WT and Nlrp3−/− mice (Fig. 5C). Phagocytosis activity in Nlrp3−/− neutrophils however remained higher than WT mice regardless the presence of 3-methyladenine (Fig. 5C). The electron microscopy of neutrophils from Nlrp3−/− mice subjected to CLP also showed more internalization of bacteria (phagocytosis) and supports enhanced phagocytosis observed in neutrophils isolated from Nlrp3−/− mice (Fig 5D).
Figure 5. Phagocytosis in neutrophils of Nlrp3−/− (KO) mice.
(A) Bacterial clearance of E. coli or S. aureus from bone marrow-derived neutrophils of Nlrp3−/− and WT mice up to 120 min. Bone marrow neutrophils from WT and Nlrp3−/− mice were infected with E. coli (MOI of 1) and S. aureus (MOI of 10) and assessed for bacterial killing capacity by estimating extracellular and intracellular CFUs at 30, 60 and 120 min after infection. These experiments performed in triplicates. (B) Uptake of pHrodo™ Red E. coli and S. aureus BioParticles® by purified peritoneal neutrophils from Nlrp3−/− and WT mice. Nlrp3−/− and WT peritoneal neutrophils were incubated with pHrodo™ Red E. coli BioParticles® or S. aureus BioParticles® for up to 2 h (MOI of 10). Phagocytosis was measured as relative fluorescent units (RFUs). These experiments performed in triplicates. (C) Phagocytosis activity in Nlrp3−/− and WT neutrophils subjected to autophagy inhibitor (3-Methyladenine) or phagocytosis inhibitor (Cytochalasin D) 1 h prior to infection with either E. coli or S. aureus (MOI of 10). These experiments performed in triplicates. (D) Electron microscopy images of neutrophils showing internalization of bacteria in neutrophils of Nlrp3−/− and WT mice following CLP. Relative phagocytosis was calculated by counting the number of phagosomes per cell (n=6 mice/group). *p<0.05, **p<0.01 and ***p<0.001 between WT and Nlrp3−/− neutrophils.
Nlrp3−/− neurophils display higher exression of MARCO and MBL
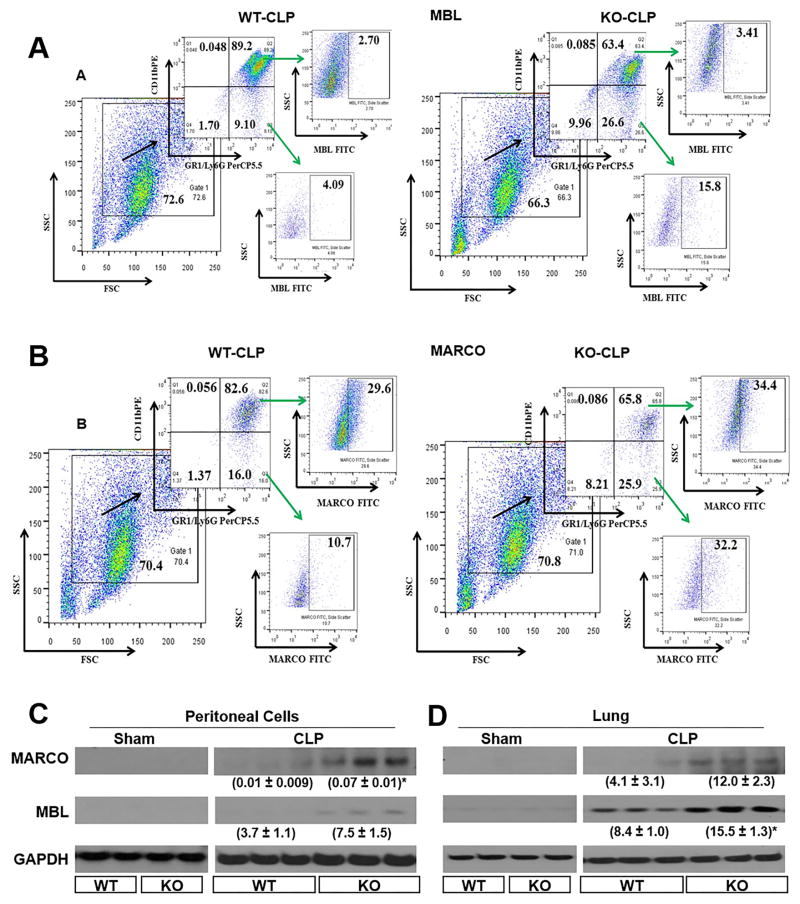
Scavenger and pattern recognition receptors are expressed by both myeloid cells and endothelial cells and these receptors contribute to uptake and clearance of host molecules and microbes (44). Regarding bacteria, these host molecules bind and internalize bacteria and their products, such as lipoteichoic acid from Gram-positive bacteria and lipopolysaccharide from Gram-negative bacteria (44), Since we found that Nlrp3−/− neurophils exhibit augmented phagocysosis, we determined the cell surface expression of MARCO and MBL because prior studies have shown to increase the uptake of microbes, including Gram-positive and Gram-negative bacteria (25). We therefore studied whether MARCO and MBL surface expression was enhanced in Nlrp3−/− neurophils obtained from CLP mice. Using flow cytometry and westen blotting, there was upregulation of MARCO and MBL expression in peritoneum and lungs in Nlrp3−/− mice as compared to WT controls (Fig. 6 A-D).
Figure 6. Expression of collagenous structure (MARCO) and mannose binding leptin (MBL) in Nlrp3−/− neutrophils in CLP.
(A) Representative flow cytometric dot plot images for MARCO in peritoneal cells of Nlrp3−/− and WT mice following CLP. (B) Representative flow cytometric dot plot images for MBL in peritoneal cells of Nlrp3−/− and WT mice after CLP. Groups of Nlrp3−/− and WT mice underwent CLP and peritoneal cells and organs were collected at 24 h post-CLP. Peritoneal cells were analyzed by flow cytometry as described in methods section for MARCO and MBL. This dot plot is from peritoneal cells obtained from 4 mice/group (C–D) MARCO and MBL of sham (elicited with thioglycollate) and CLP treated Nlrp3−/− and WT mice in peritoneal cells (C), and lung (D). Peritoneal cells (C), and lung homogenates (D) were visualize by western blotting for MARCO and MBL expression as described in Materials and Methods section. Each lane contains a lysate from a single mouse. This is a representative blot of 3 independent experiments with identical results. Neutrophils were induced in sham groups by thioglycollate injection into peritoneum as described in Methods. *p<0.05, **p<0.01 and ***p<0.001 between WT and Nlrp3−/− mice.
Caspase-1 activation is reduced in Nlrp3−/− neutrophils
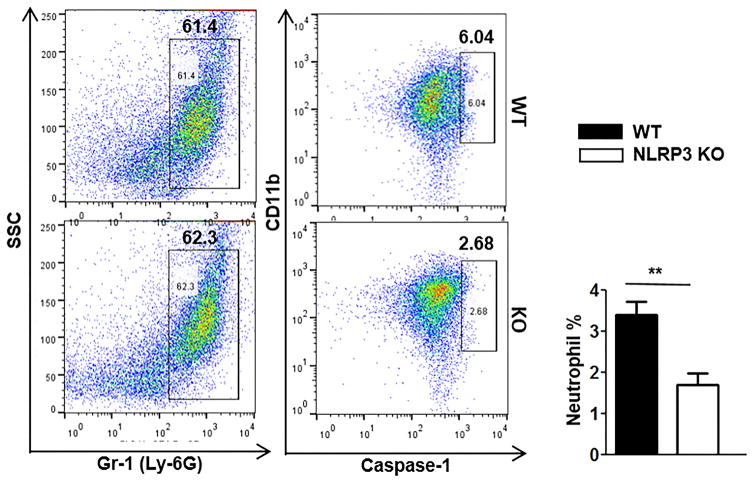
Caspase-1 activation is a hallmark of inflammasome activation (15). Therefore, we examined whether NLRP3-mediated downstratem signaling involves caspase-1 activation, we examined caspase-1 activation in both WT and Nlrp3−/− neutrophils. We observed that WT peritoneal neutrophils showed higher percentage of caspase-1-positive cells as compared to Nlrp3−/− peritoneal neutrophils cells at 24 h post-CLP (Fig. 7).
Figure 7. Effect of NLRP3 in caspase-1 activation in polymicrobial sepsis.
Representative flow cytometric dot plot images of caspase-1 in peritoneal neutrophils of Nlrp3−/− and WT mice after CLP-induced sepsis. Cells were washed from peritoneum at 24h after CLP, then stained with CD11b, Gr-1 and cleaved caspase-1 Ab. The data are expressed as means ± SEM and the bar chart shows the results from 5 mice/group. **p<0.01 between Nlrp3−/− and WT mice.
DISCUSSION
NLRP3 is known to be activated by bacterial/pathogen stimulation (45) and in sepsis, NLRP3 over activation can result in tissue injury and susceptibility to infections (8). Since all Nlrp3−/− mice and most of the glibenclamide (NLRP3 inhibitor) treated WT mice survived following CLP, the presence of NLRP3 is deleterious in mice undergoing CLP. We validated the findings of CLP using intraperitoneal E. coli infection. As compared with Nlrp3−/− mice, survival was lower with the inhibitor treated mice. This could be due to incomplete inhibition of the NLRP3 inhibitor at this concentration. In this context, different concentrations of NLRP3 inhibitor in blocking NLRP3 inflammasome following bacterial infection in mice are required in future studies.
It is known that neutrophils is responsible for preventing infection by killing of invading pathogens and can be recruited in large numbers to combat attack by pathogens (45). Accumulation of neutrophils in the circulatory system, in particular, the capillaries, and their movement into the lung parenchymatic cells involves multiple steps including stiffening of the neutrophils, retention in blood vessels and capillaries, endothelium attachment and diapedesis to the lung alveoli (46, 47). Several adhesion molecules expressed by endothelial cells are known to associate with neutrophil migration, such as ICAM-1, E-selectin and VCAM-1. The significance of these is well documented in infection/inflammation, where TNFα results in their upregulation, ICAM-1 and VCAM-1 in particular (48, 49). Our previous study has shown that infection with K. pneumoniae leads to a TRIF signaling which upregulates TNFα and subsequently increases the expression of ICAM-1(50). Additionally, TRIF and MyD88 also upregulates VCAM-1. However, our findings suggest that NLRP3 is dispensable for neutrophil recruitment in CLP although neutrophils are critical for survival and bacterial clearance. The similar levels of neutrophil recruitment to the peritoneum but improved survival in Nlrp3−/− mice parts led to speculation that there could be a difference in the neutrophil function between WT and Nlrp3−/− mice.
Autophagy is an important function by which pathogen clearance is propagated primarily in macrophages and neutrophils (25). In agreement with the results of a previous study (51), in this investigation, polymicrobial sepsis induced by CLP resulted in regulation of autophagy and phagocytosis in NLRP3-dependent manner. Additional experiments with autophagy and phagocytosis inhibitors demonstrated that when autophagy was decreased, there was a marked increase in the phagocytosis in neutrophils. The increased phagocytosis in the Nlrp3−/− neutrophils was further associated with increased expression of receptors of innate immunity or scavenger receptors, such as MARCO and MBL, that are known to upregulate the uptake and clearance of host molecules and microbes (25, 52), as along with increased SQSTM1 expression. A similar observation was reported by Bonilla et al (25) where accumulation of SQSTM1 was associated with increase in activity of scavenger receptors, MARCO and macrophage scavenger receptor 1 (MSR-1), in Atg7−/− mice and they have suggested that increased phagocytosis in Atg7−/− cells is autophagy dependent. Moreover, Lima et al (53) have reported that autophagy and phagocytosis are interdependent and complementary to each other. A decrease in phagocytosis by neutrophils was observed as a result of E. coli-induced sepsis in humans by Wenisch et al (54). In another study, Krasnodembskaya et al (55) have reported an increase in phagocytosis as a result of intervention with mesenchymal stem cells in mice with sepsis. In a separate study, Wu et al (8) have studied the effect of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) deletion in mice with polymicrobial sepsis and reported improved bacterial clearance and survival. However, contrary to our findings, Wu et al (8) have observed no change in the phagocytosis activity of the neutrophils. Because of this, they have attributed the improved survival in LOX-1−/− mice to increased migration of neutrophils and suggested impaired neutrophil chemotaxis by LOX-1 as a mechanism.
Activation of NLRP3 inflammasome results in the activation of caspase-1 which subsequently can convert the inactive pro IL-1β and pro IL-18 to their active forms, IL-1β and IL-18 (45). Gogos & Drosou (56) have reported increased concentrations of pro and anti-inflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-10, sTNFR type-I and -II and IL-1ra during sepsis in human subjects. In our study, however, there was a significant reduction in the secretion of cytokines and chemokines such as IL-1β, IL-17A, IL-6, IL-12p40, TNF-α, and IL-10 in the peritoneal fluid and serum of Nlrp3−/− mice (data not shown). Similar to the cytokines, the active (cleaved) caspase -1 was detected at a higher level in WT mice as compared to the Nlrp3−/− mice, in the present investigation, demonstrating the NLRP3 dependent activation of caspase -1 and subsequent effect on secretion of proinflammatory cytokines, including IL-1β. A study by Sarkar et al (18) showed that caspase-1 knockout mice could resist bacterial challenge by E. coli and these results were replicated by inhibiting caspase-1 function in WT mice, administered with synthetic pancaspase inhibitor. A major cause for sepsis related death is tissue injury and multiple organ dysfunctions due to excessive activation and secretion of proinflammatory cytokines (8, 18) and their down regulation would render protection and improve survival outcomes. Luo et al (57) have used hemin for inhibition of NLRP3 activation in CLP mice and reported decreased levels of IL-1β, IL-18 and caspase-1 activation, resulting in decreased histopathological lung injury.
Although CLP model is shown to be useful in order to understand the mechanisms of the host response to pathogens, numerous technical variations can affect the severity of outcome and degree of inflammation induced by the CLP model. The size of the needle puncture and number of punctures will modulate outcome of polymicrobial sepsis (58, 59). A limitation in the present investigation is that we only used a single sized needle with defined number of punctures. Another limitation is that we did not use antibiotics in mice because antibiotics can enhance bacterial clearance and reduce the dissemination of bacteria to multiple organs, severity of sepsis is dependent on the use of antibiotics (60, 61). The other limitation is that we used only male mice in our experiments. In general, male mice are more susceptible to CLP than are female mice (62, 63).
In conclusion, we identified a critical effect of NLRP3 on bacterial clearance. NLRP3 deficiency plays a protective role by decreasing autophagy in order to increase phagocytosis, primarily in neutrophils. Depletion of neutrophils in NLRP3-deficient mice reduces host survival. Taken together, these observations represent the multifaceted role of NLRP3 in neutrophils during polymicrobial sepsis in a murine model.
Acknowledgments
Supported by Scientist Awards from the Flight Attendant Medical Research Institute (CIA and YCSA to SJ; YCSA to SB); and grants from the NIH (R01 HL-091958 and R01 AI-113720 to SJ and R15ES023151-01 to SB.
The authors thank Millennium Pharmaceuticals for providing NLRP3 knockout mice and Sangeetha Ravi Kumar for helpful discussion and editing the manuscript.
ABBREVIATIONS
- CLP
Cecal ligation and puncture
- KO
Knockout
- MARCO
Macrophage receptor with collageneous structure
- MBL
Mannose binding lectin
- NLR
NOD-like receptor
- WT
Wild-type
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.King EG, Bauza GJ, Mella JR, Remick DG. Pathophysiologic mechanisms in septic shock. Lab Invest. 2014;94:4–12. doi: 10.1038/labinvest.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170:1435–1444. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossaint J, Zarbock A. Pathogenesis of Multiple Organ Failure in Sepsis. Crit Rev Immunol. 2015;35:277–291. doi: 10.1615/critrevimmunol.2015015461. [DOI] [PubMed] [Google Scholar]
- 6.Craciun FL, Schuller ER, Remick DG. Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J Immunol. 2010;185:6930–6938. doi: 10.4049/jimmunol.1002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lerman YV, Kim M. Neutrophil migration under normal and sepsis conditions. Cardiovasc Hematol Disord Drug Targets. 2015;15:19–28. doi: 10.2174/1871529x15666150108113236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, Sawamura T, Kurdowska AK, Ji HL, Idell S, Fu J. LOX-1 deletion improves neutrophil responses, enhances bacterial clearance, and reduces lung injury in a murine polymicrobial sepsis model. Infect Immun. 2011;79:2865–2870. doi: 10.1128/IAI.01317-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alves-Filho JC, Spiller F, Cunha FQ. Neutrophil paralysis in sepsis. Shock. 2010;34(Suppl 1):15–21. doi: 10.1097/SHK.0b013e3181e7e61b. [DOI] [PubMed] [Google Scholar]
- 10.Maier RV. Pathogenesis of multiple organ dysfunction syndrome--endotoxin, inflammatory cells, and their mediators: cytokines and reactive oxygen species. Surg Infect (Larchmt) 2000;1:197–204. doi: 10.1089/109629600750018123. discussion 204–195. [DOI] [PubMed] [Google Scholar]
- 11.Martins PS, Kallas EG, Neto MC, Dalboni MA, Blecher S, Salomao R. Upregulation of reactive oxygen species generation and phagocytosis, and increased apoptosis in human neutrophils during severe sepsis and septic shock. Shock. 2003;20:208–212. doi: 10.1097/01.shk.0000079425.52617.db. [DOI] [PubMed] [Google Scholar]
- 12.Crouser E, Exline M, Knoell D, Wewers MD. Sepsis: links between pathogen sensing and organ damage. Curr Pharm Des. 2008;14:1840–1852. doi: 10.2174/138161208784980572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opitz B, Eitel J, Meixenberger K, Suttorp N. Role of Toll-like receptors, NOD-like receptors and RIG-I-like receptors in endothelial cells and systemic infections. Thromb Haemost. 2009;102:1103–1109. doi: 10.1160/TH09-05-0323. [DOI] [PubMed] [Google Scholar]
- 14.Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015;265:35–52. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 17.Wiersinga WJ, Leopold SJ, Cranendonk DR, van der Poll T. Host innate immune responses to sepsis. Virulence. 2014;5:36–44. doi: 10.4161/viru.25436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson NT, Wewers MD. Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1beta and interleukin-18. Am J Respir Crit Care Med. 2006;174:1003–1010. doi: 10.1164/rccm.200604-546OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Repa A, Bertsias GK, Petraki E, Choulaki C, Vassou D, Kambas K, Boumpas DT, Goulielmos G, Sidiropoulos P. Dysregulated production of interleukin-1beta upon activation of the NLRP3 inflammasome in patients with familial Mediterranean fever. Hum Immunol. 2015;76:488–495. doi: 10.1016/j.humimm.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Abdelaziz DH, Khalil H, Cormet-Boyaka E, Amer AO. The cooperation between the autophagy machinery and the inflammasome to implement an appropriate innate immune response: do they regulate each other? Immunol Rev. 2015;265:194–204. doi: 10.1111/imr.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibutani ST, Saitoh T, Nowag H, Munz C, Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat Immunol. 2015;16:1014–1024. doi: 10.1038/ni.3273. [DOI] [PubMed] [Google Scholar]
- 22.Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev. 2015;264:182–203. doi: 10.1111/imr.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kagan JC, Iwasaki A. Phagosome as the organelle linking innate and adaptive immunity. Traffic. 2012;13:1053–1061. doi: 10.1111/j.1600-0854.2012.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuk JM, Jo EK. Crosstalk between autophagy and inflammasomes. Mol Cells. 2013;36:393–399. doi: 10.1007/s10059-013-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonilla DL, Bhattacharya A, Sha Y, Xu Y, Xiang Q, Kan A, Jagannath C, Komatsu M, Eissa NT. Autophagy regulates phagocytosis by modulating the expression of scavenger receptors. Immunity. 2013;39:537–547. doi: 10.1016/j.immuni.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Ren J, Zhou B, Ding C, Chen J, Wang G, Gu G, Wu X, Liu S, Hu D, Li J. Gene silencing of non-obese diabetic receptor family (NLRP3) protects against the sepsis-induced hyper-bile acidaemia in a rat model. Clin Exp Immunol. 2015;179:277–293. doi: 10.1111/cei.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukumoto J, Fukumoto I, Parthasarathy PT, Cox R, Huynh B, Ramanathan GK, Venugopal RB, Allen-Gipson DS, Lockey RF, Kolliputi N. NLRP3 deletion protects from hyperoxia-induced acute lung injury. Am J Physiol Cell Physiol. 2013;305:C182–189. doi: 10.1152/ajpcell.00086.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sordi R, Fernandes D, Heckert BT, Assreuy J. Early potassium channel blockade improves sepsis-induced organ damage and cardiovascular dysfunction. Br J Pharmacol. 2011;163:1289–1301. doi: 10.1111/j.1476-5381.2011.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batra S, Cai S, Balamayooran G, Jeyaseelan S. Intrapulmonary administration of leukotriene B(4) augments neutrophil accumulation and responses in the lung to Klebsiella infection in CXCL1 knockout mice. J Immunol. 2012;188:3458–3468. doi: 10.4049/jimmunol.1101985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai S, Batra S, Lira SA, Kolls JK, Jeyaseelan S. CXCL1 regulates pulmonary host defense to Klebsiella Infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. J Immunol. 2010;185:6214–6225. doi: 10.4049/jimmunol.0903843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai S, Batra S, Wakamatsu N, Pacher P, Jeyaseelan S. NLRC4 inflammasome-mediated production of IL-1beta modulates mucosal immunity in the lung against gram-negative bacterial infection. J Immunol. 2012;188:5623–5635. doi: 10.4049/jimmunol.1200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez LG, Zolla-Pazner S, Montefiori DC. Antibody-DEPENDENT, FcgammaRI-mediated neutralization of HIV-1 in TZM-bl cells occurs independently of phagocytosis. J Virol. 2013;87:5287–5290. doi: 10.1128/JVI.00278-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson Remen KM, Lerner UH, Gustafsson JA, Andersson G. Activation of the liver X receptor-beta potently inhibits osteoclastogenesis from lipopolysaccharide-exposed bone marrow-derived macrophages. J Leukoc Biol. 2013;93:71–82. doi: 10.1189/jlb.0712339. [DOI] [PubMed] [Google Scholar]
- 37.Yoseph BP, Klingensmith NJ, Liang Z, Breed ER, Burd EM, Mittal R, Dominguez JA, Petrie B, Ford ML, Coopersmith CM. Mechanisms of Intestinal Barrier Dysfunction in Sepsis. Shock. 2016;46:52–59. doi: 10.1097/SHK.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dominguez JA, Vithayathil PJ, Khailova L, Lawrance CP, Samocha AJ, Jung E, Leathersich AM, Dunne WM, Coopersmith CM. Epidermal growth factor improves survival and prevents intestinal injury in a murine model of pseudomonas aeruginosa pneumonia. Shock. 2011;36:381–389. doi: 10.1097/SHK.0b013e31822793c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douda DN, Jackson R, Grasemann H, Palaniyar N. Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. J Immunol. 2011;187:1856–1865. doi: 10.4049/jimmunol.1004201. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jo EK, Yuk JM, Shin DM, Sasakawa C. Roles of autophagy in elimination of intracellular bacterial pathogens. Front Immunol. 2013;4:97. doi: 10.3389/fimmu.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pareja ME, Colombo MI. Autophagic clearance of bacterial pathogens: molecular recognition of intracellular microorganisms. Front Cell Infect Microbiol. 2013;3:54. doi: 10.3389/fcimb.2013.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klionsky DJ, Codogno P. The mechanism and physiological function of macroautophagy. J Innate Immun. 2013;5:427–433. doi: 10.1159/000351979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balamayooran T, Balamayooran G, Jeyaseelan S. Review: Toll-like receptors and NOD-like receptors in pulmonary antibacterial immunity. Innate Immun. 2010;16:201–210. doi: 10.1177/1753425910366058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 46.Bazan-Socha S, Bukiej A, Marcinkiewicz C, Musial J. Integrins in pulmonary inflammatory diseases. Curr Pharm Des. 2005;11:893–901. doi: 10.2174/1381612053381710. [DOI] [PubMed] [Google Scholar]
- 47.Worthen GS, Schwab B, 3rd, Elson EL, Downey GP. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989;245:183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- 48.Andonegui G, Goyert SM, Kubes P. Lipopolysaccharide-induced leukocyte-endothelial cell interactions: a role for CD14 versus toll-like receptor 4 within microvessels. J Immunol. 2002;169:2111–2119. doi: 10.4049/jimmunol.169.4.2111. [DOI] [PubMed] [Google Scholar]
- 49.Skerrett SJ, Liggitt HD, Hajjar AM, Ernst RK, Miller SI, Wilson CB. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004;287:L143–152. doi: 10.1152/ajplung.00030.2004. [DOI] [PubMed] [Google Scholar]
- 50.Cai S, Batra S, Shen L, Wakamatsu N, Jeyaseelan S. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol. 2009;183:6629–6638. doi: 10.4049/jimmunol.0901033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi W, Watanabe E, Fujimura L, Watanabe-Takano H, Yoshidome H, Swanson PE, Tokuhisa T, Oda S, Hatano M. Kinetics and protective role of autophagy in a mouse cecal ligation and puncture-induced sepsis. Crit Care. 2013;17:R160. doi: 10.1186/cc12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ono K, Nishitani C, Mitsuzawa H, Shimizu T, Sano H, Suzuki H, Kodama T, Fujii N, Fukase K, Hirata K, Kuroki Y. Mannose-Binding Lectin Augments the Uptake of Lipid A, Staphylococcus aureus, and Escherichia coli by Kupffer Cells through Increased Cell Surface Expression of Scavenger Receptor A. The Journal of Immunology. 2006;177:5517–5523. doi: 10.4049/jimmunol.177.8.5517. [DOI] [PubMed] [Google Scholar]
- 53.Lima JG, de Freitas Vinhas C, Gomes IN, Azevedo CM, dos Santos RR, Vannier-Santos MA, Veras PS. Phagocytosis is inhibited by autophagic induction in murine macrophages. Biochem Biophys Res Commun. 2011;405:604–609. doi: 10.1016/j.bbrc.2011.01.076. [DOI] [PubMed] [Google Scholar]
- 54.Wenisch C, Parschalk B, Patruta S, Brustbauer R, Graninger W. Effect of polyclonal immunoglobulins on neutrophil phagocytic capacity and reactive oxygen production in patients with gram-negative septicemia. Infection. 1999;27:183–186. doi: 10.1007/BF02561525. [DOI] [PubMed] [Google Scholar]
- 55.Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. AJP: Lung Cellular and Molecular Physiology. 2012;302:L1003–L1013. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drosou G. Pro- versus Anti-inflammatory Cytokine Profile in Patients with Severe Sepsis: A Marker for Prognosis and Future Therapeutic Options. The Journal of Infectious Diseases. 2000 doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 57.Luo YP, Jiang L, Kang K, Fei DS, Meng XL, Nan CC, Pan SH, Zhao MR, Zhao MY. Hemin inhibits NLRP3 inflammasome activation in sepsis-induced acute lung injury, involving heme oxygenase-1. Int Immunopharmacol. 2014;20:24–32. doi: 10.1016/j.intimp.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 58.Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun. 1999;67:6603–6610. doi: 10.1128/iai.67.12.6603-6610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ebong SJ, Call DR, Bolgos G, Newcomb DE, Granger JI, O’Reilly M, Remick DG. Immunopathologic responses to non-lethal sepsis. Shock. 1999;12:118–126. doi: 10.1097/00024382-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Enoh VT, Fairchild CD, Lin CY, Varma TK, Sherwood ER. Differential effect of imipenem treatment on wild-type and NK cell-deficient CD8 knockout mice during acute intra-abdominal injury. Am J Physiol Regul Integr Comp Physiol. 2006;290:R685–693. doi: 10.1152/ajpregu.00678.2005. [DOI] [PubMed] [Google Scholar]
- 61.Newcomb D, Bolgos G, Green L, Remick DG. Antibiotic treatment influences outcome in murine sepsis: mediators of increased morbidity. Shock. 1998;10:110–117. doi: 10.1097/00024382-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Dienstknecht T, Schwacha MG, Kang SC, Rue LW, Bland KI, Chaudry IH. Sex steroid-mediated regulation of macrophage/monocyte function in a two-hit model of trauma-hemorrhage and sepsis. Cytokine. 2004;25:110–118. doi: 10.1016/j.cyto.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Zellweger R, Zhu XH, Wichmann MW, Ayala A, DeMaso CM, Chaudry IH. Prolactin administration following hemorrhagic shock improves macrophage cytokine release capacity and decreases mortality from subsequent sepsis. J Immunol. 1996;157:5748–5754. [PubMed] [Google Scholar]