Abstract
The thalamus is implicated in the neuropathology of schizophrenia, and multiple modalities of noninvasive neuroimaging provide converging evidence for altered thalamocortical dynamics in the disorder, such as functional connectivity and oscillatory power. However, it remains a challenge to link these neuroimaging biomarkers to underlying neural circuit mechanisms. One potential path forward is a “Computational Psychiatry” approach that leverages computational models of neural circuits to make predictions for the dynamical impact dynamical impact on specific thalamic disruptions hypothesized to occur in the pathophysiology of schizophrenia. Here we review biophysically-based computational models of neural circuit dynamics for large-scale resting-state networks which have been applied to schizophrenia, and for thalamic oscillations. As a key aspect of thalamocortical dysconnectivity in schizophrenia is its regional specificity, it is important to consider potential sources of intrinsic heterogeneity of cellular and circuit properties across cortical and thalamic structures.
Keywords: computational model, functional connectivity, neural oscillations, thalamus, thalamic reticular nucleus
1. Introduction
A key research challenge in biological psychiatry is to link systems-level biomarkers of a disorder to underlying alterations in neural circuits hypothesized to play a role in the pathophysiology. Schizophrenia (SCZ) is a disorder for which clinical neuroscience has leveraged a range of experimental modalities to discover systems-level neuroimaging markers based on the dynamics of neural activity in large-scale brain networks. These biomarkers can be observed noninvasively in patients using modalities such as functional magnetic resonance imaging (fMRI), electrocorticography (EEG), and magnetocorticography (MEG). Yet the mechanistic links between these emergent systems-level dynamics and underlying synaptic and neuronal processes are poorly understood. Mechanistic understanding of how synaptic or cellular pathologies can give rise to neuroimaging findings and clinical symptoms is a key step toward rational design of therapeutics acting at the synaptic level. This knowledge gap between mechanisms and biomarkers arises because of the need for ways to bridge the fine-grained neurophysiology of brain microcircuits and the properties of macrocircuits studied in clinical research using noninvasive neuroimaging.
One emerging approach to bridging this gap is to leverage advances in computational neuroscience to generate rigorous and testable hypotheses related to the neural bases of psychiatric pathophysiology (Anticevic et al., 2015c; Wang and Krystal, 2014). More specifically, this “Computational Psychiatry” approach can harness computational models of neural systems that incorporate key neuronal and synaptic details, allowing mechanistic characterization of how cellular-level disruptions may propagate upward to produce systems-level dysfunction. Computational models of neural circuits models can therefore yield dissociable systems-level predictions for distinct synaptic-level perturbations, thereby allowing macroscopic measures in humans or animals to support inferences about microscopic pathophysiologies. This approach has translational potential, as it stands to inform the one-to-many mapping problem in neuroimaging research, i.e., the difficulty to map a statistical map from neuroimaging onto a given upstream cellular-level mechanism that may be ‘driving’ such an effect. Circuit models are developed and constrained by experiments in pre-clinical animal models at different levels, such as synaptic kinetics and spiking activity. By then examining which changes in the biologically interpretable model parameters map onto clinical findings, we can develop testable mechanistic hypotheses for specific circuit disruptions in the disease.
There is mounting evidence that SCZ can be characterized by widespread abnormalities of connectivity and interactions in brain circuits across a range of spatial scales, from microcircuits to large-scale networks. The thalamus is a critical node in large-scale brain networks, and thalamocortical interactions are emerging as a key component of “dysconnectivity” in SCZ. In this piece, we review recent experimental functional neuroimaging findings of altered dynamics of thalamocortical circuits in SCZ during task-free, spontaneous activity at rest or during sleep. We then describe biophysically-based computational modeling approaches that can be readily extended to study altered thalamocortical dynamics in SCZ. These computational approaches include models of large-scale resting-state networks, which are well suited to resting-state fMRI measurements, as well as biophysically-detailed models of neurons and microcircuits in the thalamus, which generate oscillatory activity that can be related to EEG/MEG measurements at timescales that fMRI cannot resolve. One salient aspect of thalamocortical dysconnectivity observed with fMRI is its regional specificity, implicating bidirectional changes in thalamic interactions with different cortical regions and preferentially more severe alterations in specific thalamic nuclei (e.g., the prefrontal-projecting thalamic nuclei). It is therefore critical to consider the heterogeneity of circuit properties across cortical areas and thalamic nuclei, which may contribute to the observed preferential disturbances arising from disease-related perturbations. Finally, we suggest future directions for computational and experimental research to probe the mechanistic basis of thalamocortical dysfunction in SCZ.
2. Recent experimental findings of thalamocortical dynamics in schizophrenia
Given the highly convergent and divergent connectivity between thalamus and cortex, it is important to characterize the topography of connectivity alterations across large-scale networks in SCZ. FMRI allows whole-brain measurement of the low-frequency blood oxygen level-dependent (BOLD) signal. Resting-state functional connectivity MRI (rs-fcMRI) has emerged as a powerful tool for characterizing the intrinsic functional architecture of brain network dynamics at rest. Its emerging use is built upon the hypothesis that neuropsychiatric conditions, such as SCZ, are brain disorders that affect computations across large-scale neural networks. Complementing fMRI, other noninvasive methods such as electroencephalography (EEG) and magnetoencephalography (MEG) can provide greater temporal resolution of neural dynamics. Because the thalamus is implicated in specific dynamical modes, such as oscillatory sleep spindles, these measures can relate specifically to thalamic function. In this section, we describe some recent studies revealing biomarkers of altered neural dynamics in SCZ, using fMRI, EEG, and MEG, which are particularly well suited to computational modeling.
2.1. Resting-state BOLD functional connectivity
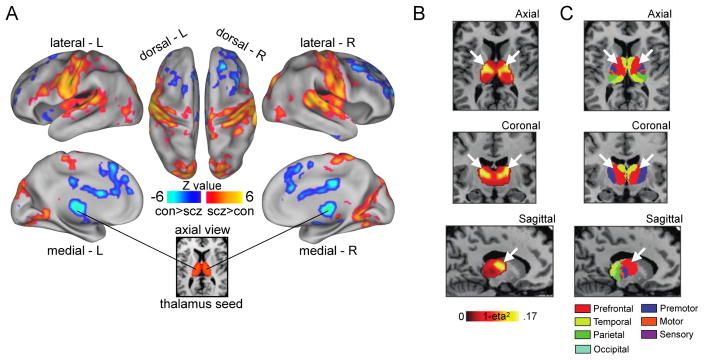
In recent years, a number of independent rs-fcMRI studies have characterized thalamocortical connectivity in SCZ, converging on a robust set of alterations (Welsh et al., 2010; Woodward et al., 2012; Anticevic et al., 2014a,b, 2015a; Woodward and Heckers, 2015). Thalamocortical connectivity alterations in SCZ are bidirectional and regionally specific (Fig. 1A). Relative to controls, patients with SCZ exhibit lower connectivity between the thalamus and regions of prefrontal cortex, striatum, and cerebellum (Anticevic et al., 2014a). In contrast, in SCZ the thalamus is over-connected with sensory-motor cortex. There is a strong relationship between thalamic over- and under-connectivity across subjects. Specifically, there is a strong negative correlation between them, suggesting a common mechanism may underlie both the over- and under-connectivity patterns (Anticevic et al., 2014a, 2015a; Woodward and Heckers, 2015).
Figure 1. Thalamic dysconnectivity in SCZ.
(A) Significant whole-brain between-group differences in thalamic connectivity between healthy controls (CON) and individuals with schizophrenia (SCZ). Red-orange (blue) foci mark areas where patients exhibited stronger (reduced) thalamic coupling. (B) Intrinsic thalamic dysconnectivity pattern based on group dissimilarity. The brightest voxels are associated with highest between-group differences. (C) Thalamus subdivisions based on the FSL thalamic atlas, defined by their connectivity to different regions of cortex. White arrows indicate the correspondence between thalamic regions with greatest dysconnectivity in SCZ and thalamic subdivisions strongly connected with prefrontal cortex (red). Adapted from Anticevic et al. (2014a).
Regional specificity of dysconnectivity extends to the thalamus as well. Thalamus is divided into multiple distinct nuclei with different input and output synaptic connection patterns with cortex. Of particular interest here is the mediodorsal (MD) nucleus, which is strongly interconnected with prefrontal cortex. The MD nucleus appears to preferentially drive the thalamocortical dysconnectivity pattern described above (Welsh et al., 2010; Anticevic et al., 2014a,b) (Fig. 1B,C).
SCZ is a neurodevelopmental disorder with a complex illness progression. Neural biomarkers can alter dramatically across prodrome, early-stage, and chronic stages. Interestingly, this pattern—thalamic under-connectivity with prefrontal cortex and over-connectivity with sensorimotor cortex—appears to be present across illness stages. Woodward and Heckers (2015) found it to be present in both chronic and early-stage individuals with psychosis (SCZ/schizoaffective disorder and bipolar I disorder with psychotic features). Anticevic et al. (2015a) found this pattern to be present in young help-seeking individuals at clinically high risk for psychosis, and particularly strong for the subset of patients who converted to full-blown illness at a later time. These recent findings collectively indicate that the observed thalamic dysconnectivity is not consistent with typical confounds that emerge as a function of chronic illness—namely long-term medication effects and additional co-morbid clinical factors. Therefore, this effect may present a viable translational target that can be studied using complementary electrophysiological techniques.
2.2. Electrophysiological measures of oscillatory deficits
Relative to fMRI, EEG and MEG are more limited in their ability to measure and localize neural activity in deep subcortical structures. Where MEG measures can be localized to thalamus, it lacks spatial resolution to resolve specific thalamic nuclei (Roux et al., 2013). Despite their spatial limitations, these modalities can provide important complementary data because they can resolve activity with much finer temporal resolution than fMRI. This temporal resolution can be especially leveraged to probe thalamocortical activity because there are specific spatiotemporal dynamics associated with thalamus, building upon a large body of in vitro and in vivo physiological studies in animal models, which occur at timescales faster than can be resolved with fMRI. Here we focus on two alterations which implicate the thalamus and for which biophysically-based neural models have shed light on the neuronal and synaptic mechanisms underlying them: delta-band (1–4 Hz) oscillatory activity, and sleep spindles. These findings have built upon physiological studies of the cellular and synaptic properties of thalamic neurons, in particular the thalamocortical cells in relay and association thalamic nuclei (TC cells) which project to cortex, and GABAergic cells in the thalamic reticular nucleus (TRN) (RE cells) with are interconnected with TC cells.
2.2.1. Delta-band alterations
As described below, physiological studies have found that isolated TC or RE cells can generate delta-band (1–4 Hz) oscillations through the interplay of their intrinsic active membrane conductances (Jones, 2012). Alterations in delta-band activity may therefore reflect thalamic disturbances. However, it is important to note that there are likely multiple generators of delta-band activity, including mechanisms in cortex that do not involve the thalamus. Multiple EEG and MEG studies have found elevated delta-band power at rest in patients with SCZ relative to controls (Galderisi et al., 2009; Siekmeier and Stufflebeam, 2010), in addition to elevated theta power. This elevated resting-state delta power appears mostly over frontal brain areas (Venables et al., 2009).
2.2.2. Spindle oscillations
Spindles are patterns of 8–15 Hz oscillations within a bout that waxes and wanes over a timescale of roughly one second. Spindle activity is a signature of rapid-eye-movement (REM) sleep. As described below, a large body of physiological and computational modeling work has characterized the mechanisms underlying spindles through the interplay between cellular and synaptic processes in RE-TC circuits (Jones, 2012). Using EEG, Ferrarelli et al. (2007, 2010) found reduced spindle activity in patients with SCZ. Moreover, spindle number was negatively correlated with severity of both positive and negative symptoms (Ferrarelli et al., 2010). Given the strong evidence of thalamic circuits in spindle generation, these findings strongly implicate deficits in thalamus and TRN in particular (Ferrarelli and Tononi, 2011). (See also the review by Ferrarelli in this issue.)
3. Computational models of thalamocortical dynamics
As noted, one approach to Computational Psychiatry uses biophysically-based models of neural circuits to make dissociable predictions for systems-level phenomena arising from distinct synaptic-level perturbations. This approach has been applied to a range of questions in schizophrenia (for a review, see Anticevic et al., 2015c). For example, models of local cortical microcircuits comprising recurrently connected excitatory and inhibitory neurons can generate oscillatory activity in the gamma (30–80 Hz) range. Such models have been applied test how gamma oscillations are impacted by schizophrenia-related synaptic alterations, which can be compared to gamma-related biomarkers observed in schizophrenia. Microcircuit models have also been applied to study the persistent neural activity underlying working memory in prefrontal cortical circuits and its potential dysfunction in schizophrenia. In particular, “attractor network” models have been applied to study how distinct synaptic alterations in excitation-inhibition balance can produce dissociable patterns of errors in working memory tasks, thereby linking synaptic dysfunction to cognitive deficits (Murray et al., 2014).
Here we describe two computational modeling frameworks that can be related to the experimental measurements described above and thereby potentially inform understanding of the mechanisms underlying neuroimaging biomarkers at the neural system level, such as thalamocortical dysconnectivity.
3.1. Computational models of large-scale resting-state networks
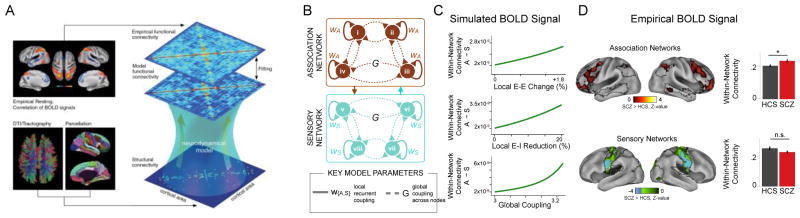
In recent years, a class of computational models have been developed to explicitly relate to neural dynamics measured in rs-fcMRI experiments, to address a range of questions such as how structural connectivity and physiological dynamics interact to shape functional connectivity (Deco et al., 2011; Deco and Kringelbach, 2014). These models represent the brain at rest as a network of interconnected nodes (Fig. 2A). Each node represents a brain region or parcel, and simulates neural activity of the local circuit at a simplified level of biophysical fidelity. For instance, a node representing a cortical area can simulate mean-field ensembles of excitatory pyramidal neurons and inhibitory interneurons (Deco et al., 2014). Model nodes are connected by long-range inter-areal projections. The structure of these long-range inter-areal projections can be constrained by known neuroanatomical connectivity as measured by tractography through diffusion MRI (Deco et al., 2013). The net synaptic input to each node therefore contains contributions from local recurrent connections, long-range connections from other nodes, fluctuating background input, and external inputs.
Figure 2. Computational models of large-scale resting-state dynamics.
(A) Modeling framework. Diffusion MRI-derived tractography provides the underlying structural connectivity among nodes in the model. Nodes simulate activity of local neural circuits, which interact through long-range connections. The model produces correlated spatiotemporal patterns in the simulated BOLD signal, which can be compared to empirical fMRI data, to optimize fitting of the biologically interpretable model parameters. Adapted from Deco et al. (2013). (B) Expansion of the model to incorporate hierarchical heterogeneity of local circuit properties, specifically stronger recurrent excitation (w) in association cortical networks compared to sensory cortical networks. (C) Elevated excitation-inhibition ratio increases preferential dysconnectivity in association networks in the model. Plotted is the difference between association and sensory measures (A–S) of within-network connectivity (covariance). Connectivity increases with three model parameters that all elevate the net E/I ratio. (D) Empirical measures of within-network connectivity in SCZ reveal preferential increase in connectivity in association networks, in line with model predictions. Adapted from Yang et al. (2016).
As nodes respond to their inputs, their activity fluctuates in time. Due to the long-range coupling, fluctuations are correlated across nodes, creating patterns of functional connectivity. The resultant functional connectivity pattern is thereby shaped by the underlying structural connectivity pattern, but also by physiological properties of the local cortical microcircuits. Neural activity in each node can be converted into a simulated BOLD signal via a hemodynamic model, enabling more direct comparison to fMRI measures. The biologically interpretable parameters of the model can then be fitted to achieve the best match between simulated and empirical functional connectivity.
These resting-state models have been related to rs-fcMRI findings in psychiatric disorders such as SCZ (Deco and Kringelbach, 2014; Anticevic et al., 2015c). Thus far these models have primarily applied to cortex only, without inclusion of thalamus or other subcortical structures. In a series of studies, we applied these models to study the large-scale impact of alterations in the excitation-inhibition (E/I) ratio in cortical circuits, relating these effects to rs-fcMRI biomarkers in SCZ (Yang et al., 2014; Anticevic et al., 2015b; Yang et al., 2016). Yang et al. (2014) found that increasing the effective strength of connectivity at either the local or long-range level, resulting in an elevated E/I ratio, can capture the elevated local and global neural variability observed in SCZ. These models can be readily extended to thalamocortical interactions, by simulating activity in multiple thalamic nuclei as nodes, constrained by nucleus-specific patterns of thalamocortical structural connectivity. Thalamic nodes in the model should account for the distinct local microcircuit properties of thalamus; for instance, TC cells lack the strong local recurrent excitatory connectivity found in cortical microcircuits (Jones, 2012).
As noted, a salient aspect of the clinical findings is regional specificity and bidirectionally of functional connectivity alterations (Anticevic et al., 2014a). These suggest that to capture empirical findings, an important model extension will be to incorporate heterogeneity of local properties across cortical and/or thalamic nodes. As a step in this direction, such an extension has been explored in heterogeneity of local recurrent connectivity across cortical hierarchy. Yang et al. (2016) found that elevated E/I ratio in a homogenous model yielded elevated mean functional connectivity, as measured by covariance of the BOLD signal. In line with this model prediction, analysis of resting-state fMRI revealed that this connectivity is increased in SCZ, but that this increase is preferential in association cortex relative to sensory cortex (Fig. 2D). This finding of regional specificity motivated extension of the model to incorporate stronger recurrent connectivity in association areas, which is consistent with known neurobiology of cortical microcircuitry (as discussed below). The extension of cortical heterogeneity in the model was sufficient to produce preferential elevation of connectivity in association networks, under the same global increase in E/I ratio (Fig. 2C). This study provides proof-of-principle synthesis of proposals for localized versus global neural deficits in SCZ. Put differently, there is a longstanding tension between two competing neurobiological frameworks of SCZ: pervasive deficits that are widespread across the brain, versus focal dysfunction in a few key ‘hotspots’. The described modeling framework helps to reconcile this tension. It also potentially serves as an example for future modeling studies incorporating thalamocortical heterogeneity to capture the regionally specific and bidirectional connectivity alterations observed in SCZ, which remain mechanistically uncharacterized.
3.2. Computational models of oscillations in thalamic circuits
Biophysically-based circuit models have been developed to probe the generation of oscillations in thalamocortical systems. These oscillations arise through the interplay between ionic cellular and synaptic processes.
3.2.1. Delta oscillations in thalamic neurons
Active voltage-dependent conductances of TC and RE cells strongly shape their firing activity. Both TC and RE cells strongly express T-type calcium channels, mediating the associated current IT, which endows the cell with ability to operate in two modes: a tonic-firing mode when IT is not active, and a burst-firing mode that relies on IT (McCormick and Huguenard, 1992; Destexhe et al., 1993a,b, 1996b). When starting at a relatively depolarized baseline potential (> 60 mV), IT inactivates, and the cell fires in a tonic mode characterized by regular firing of single spikes. In contrast, if the cell is held at a hyperpolarized potential for 50–100 ms, then the cell can enter a burst mode upon stimulation. At these hyperpolarized potentials, IT de-inactivates. If the cell is then depolarized, the IT can be activated, generating a low-threshold Ca2+ spike and a burst of action potentials. This provides a complex cellular-level regulatory mechanism for specific cellular states in thalamic neurons, which may be dysfunctional in SCZ and can be observed as altered oscillations in a given frequency range.
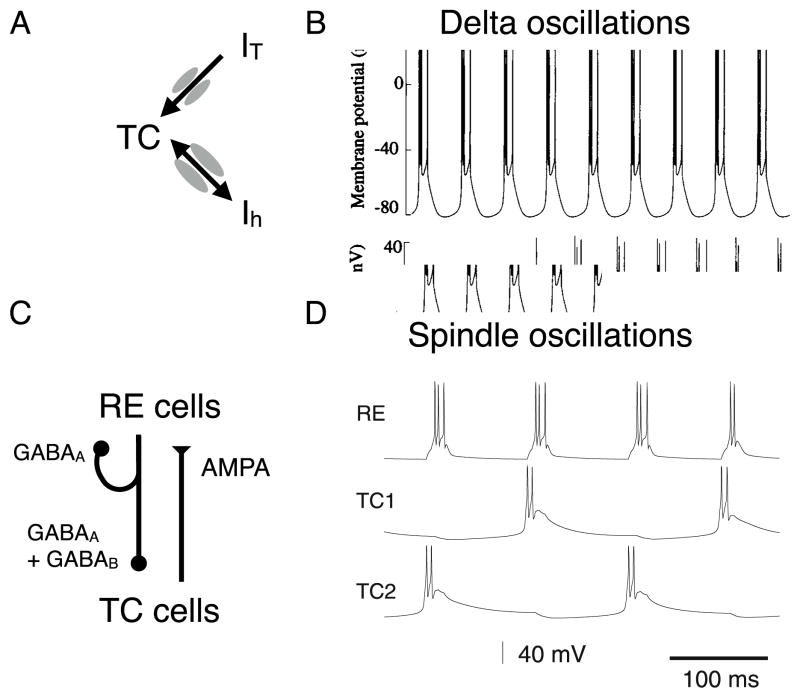
For TC cells, delta (1–4 Hz) oscillations can be modeled through an interplay between IT and a hyperpolarization-activated current (IH) (Destexhe et al., 1993b) (Fig. 1A, B). This cycle begins with hyperpolarization of the TC cells, which de-inactivates IT and activates IH. The IH depolarizes the cell, which then activates IT. Depolarization by IT causes a burst of Na-K action potentials. During the high-voltage burst, IT inactivates and IH deactivates, causing the cell to then become hyperpolarized, and the burst can begin again. Similarly, RE neurons can robustly generate delta oscillations through single-cell mechanisms, in this case through the interplay of IT and Ca2+-dependent K+ current (IKCa). IT generates a burst, and the accumulating calcium then activates IKCa. IKCa then hyperpolarizes the cell, and the cycle can begin again.
3.2.2. Spindle oscillations in thalamo-reticular circuits
Spindle oscillations are thought to arise through recurrent synaptic interactions in thalamic circuits (Destexhe et al., 1996a; Jones, 2012). The dominant model for spindles describes a reciprocally connected RE-TC microcircuit (Destexhe et al., 1996a) (Fig. 3C). In these models, RE neurons are interconnected via GABAA synapses and project to TC neurons with GABAA and GABAB synapses. TC neurons, in turn, project back onto RE cells with glutamatergic AMPA synapses. In the cycle of the spindle oscillation, TC neurons excite hyperpolarized RE neurons, triggering an IT-mediated burst. RE neurons then strongly inhibit both TC neurons and RE neurons. This inhibition-related hyperpolarization activates IT in both, causing a rebound burst in TC neurons, beginning the cycle again. This leads to an offset spiking pattern between the populations. RE neurons typically burst in every cycle, whereas TC neurons tend to skip cycles (Fig. 3D). The waxing-and-waning envelope of the bout of spindles can be achieved through dependence of IH on intracellular Ca2+ that accumulates during the spindles. Modeling studies have shown importance of feedback from cortex to TRN in mediating coherence of spindles across the thalamus (Destexhe et al., 1998).
Figure 3. Cellular-level models of oscillations in thalamic-reticular systems.
(A) Among other currents, thalamocortical (TC) cells possess T-type calcium current (IT) and a hyperpolarization-activated cation current (IH). (B) The interplay between IT and IH can generate delta (1–4 Hz) oscillations in isolated TC model cells. Adapted from Destexhe et al. (1993b). (C) Schematic of interconnected networks of TC cells and reticular (RE) cells in the TRN. (D) Synaptic interactions between TC and RE cells can generate spindle (8–15 Hz) oscillations in a circuit model. Adapted from Destexhe et al. (1996a).
3.3. Implications for cellular and synaptic dysfunction in SCZ
Both types of described oscillatory activity can be recorded in SCZ patients and captured computationally via biophysically-based models. These biophysically-based circuit models can therefore serve as productive platforms to test the effects of various cellular and synaptic perturbations on system-level dynamical properties. As discussed, both the delta and spindle oscillations in thalamic circuits rely on T-type calcium channels. Recently, a large genome-wide association study (GWAS) has identified genes encoding calcium channels as major sites associated with SCZ. Interestingly, one gene is CACNA1I (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014), which encodes the Cav3.3 a T-type calcium channel expressed strongly in the TRN. (See also the review by Richard & Lisman in this special issue discussing the link between the GWAS work and thalamic abnormalities in SCZ.) Computational models can probe how changing the strength and kinetics of the T-type calcium conductance alters neuronal firing.
N-methyl-D-aspartate receptor (NMDAR) hypofunction is a leading synaptic dysfunction implicated in SCZ. This hypothesis has been explored using pharmacological NMDAR antagonists, such as ketamine, which are leading pharmacological models of SCZ (Krystal et al., 2003). One consequence of NMDAR hypofunction may be disinhibition, which can occur through preferential antagonism of NMDARs on inhibitory neurons relative to excitatory neurons (Lisman et al., 2008; Nakazawa et al., 2012). This preferential impact may arise from differential expression of NMDAR subunits in different cell types. In cortex, pyramidal neurons express relatively high levels of the NR2B subunit (Wang et al., 2008; Xi et al., 2009), whereas inhibitory interneurons may preferentially express the NR2C subunit (Xi et al., 2009). Similarly, the inhibitory RE cells in the TRN also express NR2C receptors (Zhang et al., 2012a). Interestingly, NR2C receptors show a greater sensitivity to the NMDAR antagonist ketamine, compared to NR2A/B/D receptors (Kotermanski and Johnson, 2009), which potentially underlies the net disinhibition observed under subanesthetic administration of NMDAR antagonists (Krystal et al., 2003; Homayoun and Moghaddam, 2007). This effect can be parsimoniously modeled in a biophysically-based framework as a small net reduction of NMDAR conductance from inhibitory onto excitatory cells, which has been related to experimental effects of ketamine in humans (Anticevic et al., 2012).
Physiological studies provide support that thalamus may be a key site vulnerable of NMDAR hypofunction. Physiological experiments found that NMDAR antagonist can switch an RE neuron from the tonic firing mode to a delta-frequency bursting mode (Zhang et al., 2009). An in vivo experiment, in rats under urethane anesthesia, found that injection of the NMDAR antagonist MK-801 into MD thalamus can strongly alter spontaneous delta oscillations in medial prefrontal cortex (Kiss et al., 2011). Another experiment, in awake rats, found that injection of ketamine into thalamus increased firing rates and delta power in both thalamus and hippocampus (Zhang et al., 2012b).
Disruptions within cortex could also alter the nature of corticothalamic interactions. For instance, Crandall et al. (2015) found that at low cortical firing rates, the net impact of L6 cortex on TC cells is inhibitory, due to feedforward inhibition mediated by RE cells. However, at high cortical firing rates, the corticothalamic impact switches from net inhibitory to net excitatory, due to short-term synaptic plasticity. One intriguing potential consequence of this is that if cortex were disinhibited and exhibited elevated activity (Homayoun and Moghaddam, 2007; Yang et al., 2016), then corticothalamic interactions could switch from a mode of negative feedback, enabling inhibitory control and gating, to a mode of positive feedback. This would possibly result in dysregulation of thalamic functions, such as sensory gating (Wimmer et al., 2015), which are implicated in SCZ (Braff and Geyer, 1990).
4. Potential sources of regional thalamocortical heterogeneity in SCZ
As described, SCZ is associated with striking dissociation across brain networks in terms of patterns of thalamic dysconnectivity. Sensorimotor cortex and associated thalamic nuclei exhibit over-connectivity, whereas prefrontal cortex and associated thalamic nuclei exhibit under-connectivity, and this effect in thalamus appears preferentially elevated in the MD nucleus. Broadly, this pattern could be driven by distinct disruptions to specific brain areas involved. Alternatively, a widespread common disruption could give rise to preferential deficits due to heterogeneity in the underlying circuitry of the areas involved (Yang et al., 2016), as noted above. Here we discuss some heterogeneity in the microcircuitry of thalamocortical systems, which may potentially contribute to the observed regional specificity in thalamocortical dysconnectivity.
4.1. Intrinsic thalamic heterogeneity
There is evidence that thalamic nuclei may vary in their intrinsic physiological properties. In particular, studies have found that both in vitro and in vivo, TC cells in higher-order thalamic nuclei exhibit a higher propensity for burstiness than those in sensory relay nuclei. Wei et al. (2011) compared firing properties of lateral geniculate nucleus (LGN) compared to pulvinar nucleus, and found that pulvinar neurons show a higher propensity for bursting, and express more T-type calcium channels (Cav3.2). Ramcharan et al. (2005) analyzed single-neuron spike trains from alert monkeys recorded across multiple thalamic nuclei. They found higher likelihood of bursting activity in higher-order nuclei, such as MD and pulvinar, compared to early relay nuclei such as LGN. These higher-order nuclei had lower spontaneous activity, which may allow more deinactivation of IT thereby facilitating bursting. These intrinsic differences in thalamic physiology may render cells in specific thalamic subdivisions differentially sensitive to an underlying perturbation (e.g. stemming from NMDAR hypofunction).
4.2. Intrinsic cortical heterogeneity
Cortex is similarly heterogeneous in its microcircuitry, and these inter-areal differences may contribute to differential alterations under a global, cortex-wide perturbation (Yang et al., 2016). There is evidence suggesting differences in recurrent connectivity across the cortical hierarchy, with marked differences between sensory and association cortices. At the cellular level, Elston and colleagues have systematically quantified areal differences in the synaptic morphology of pyramidal cells in the monkey (Elston, 2003; Elston et al., 2005). They measured the number of spines, sites of excitatory synapses, on pyramidal cells, and found hierarchical increases from primary sensory to prefrontal areas. The increase in spine counts was driven by increases both in both dendritic length and in synapse density. In addition to the number of synapses, the net physiological recurrent strength, which relates to model findings, would also depend on other factors, such as the average conductance of a single synapse. Physiological measurements, e.g. using paired recordings in slice preparations, could potentially provide more direct evidence related to this mechanism.
There are also important inter-areal differences in the dominant timescale of cellular and synaptic properties. Morphological differences between pyramidal cells in dorsolateral prefrontal cortex and primary visual cortex can drive physiological differences (Amatrudo et al., 2012). Areas differ in their neuromodulatory inputs, and their receptor expression (Bernard et al., 2012). Inter-areal differences in neuromodulatory tone could therefore shape inter-areal differences in cellular physiology. Inter-areal differences in synaptic properties could also contribute. At recurrent excitatory synapses, the timescales of NMDAR-mediated synaptic currents are twice as long in prefrontal cortex compared to primary visual cortex, due to differential expression of NMDAR subunits (Wang et al., 2008). One approach for more systematic analysis of neuronal and synaptic properties comes from mapping receptor and gene expression patterns across the brain. For instance, expression of NMDA receptors is higher in prefrontal cortex compared to visual cortex (Scherzer et al., 1998). Recent high-throughput studies have revealed areal differences in mRNA expression, including differential expression related to synaptic and neuromodulatory properties (Bernard et al., 2012; Hawrylycz et al., 2012). However, at present it is not straightforward to map these expression levels to their effects on physiological dynamics.
4.3. Unifying intrinsic heterogeneity into computational modeling studies
As noted, a key uncharacterized effect in SCZ pertains to the bidirectional and functionally related hyper- and hypo-connectivity between the thalamus and sensory versus associative cortices. The sections above highlight some physiologically plausible ways in which preexisting intrinsic features of thalamic and cortical neurons may produce a distinct pattern of effects across different thalamocortical loops. The forthcoming expansion of existing computational models stands to play a vital role in deepening our intuition regarding how such a spatially-specific effect may occur and how it may operate across distinct timescales through alterations of oscillatory activity.
5. Future directions: toward mechanistic mapping of thalamic dysconnectivity in SCZ
In this review, we have described key experimental findings of thalamocortical dysfunction in SCZ, as well as computational modeling frameworks for studying neural dynamics which can be extended to study these phenomena. A Computational Psychiatry approach to understanding dynamical biomarkers in SCZ has the potential to link across levels of analysis to inform mechanisms, and facilitate translational research from pharmacological and animal studies to clinical observations. Future experimental and modeling studies are needed to further refine our characterization of these biomarkers, and to probe their underlying synaptic and cellular mechanisms.
Future experimental studies should examine how the fMRI and electrophysiological biomarkers described above may be related. EEG experiments can test whether spindle deficits during sleep correlate, across subjects, with abnormal delta activity during awake rest, to test for a shared mechanism. It is also important to leverage multi-modal analyses to understand the relationship between large-scale dysconnectivity and the oscillatory disturbances. For instance, one could probe whether the pattern of BOLD functional connectivity observed by fMRI is correlated across subjects or states with spindle deficits observed in EEG/MEG. Moreover, simultaneous fMRI/EEG could reveal the spectral signatures that accompany resting-state thalamic dysconnectivity.
Future computational modeling studies should leverage the modeling advances described above to directly capture these thalamocortical dynamical alterations, to test sites of specific perturbations. It will be important to explore what set of cellular and/or synaptic alterations can give rise to both diminished spindle activity during sleep-like conditions, yet the increased delta-band oscillations during rest-like conditions, along with the bidirectional spatial pattern observed using BOLD fMRI. Another computational challenges lies in integration of a multi-scale approach to combine the oscillatory activity, which relies on modeling detailed cellular processes, with the large-scale network dynamics, which has thus far primarily been modeled using simplified, mean-field neural activity.
Another important direction for the interplay between computational and experimental studies will be in the integration of the approaches described above with other analytical techniques yielding complementary perspectives on large-scale network dynamics in human neuroimaging data. Analyses based on graph theory can characterize the topological features of large-scale brain networks, which can be applied to both structural connectivity data derived from diffusion-weighted imaging as well as functional connectivity derived from BOLD (Bullmore & Sporns, 2009). Although such analyses have primarily focused on cortico-cortical connections only, changes in thalamocortical connectivity could greatly affect topological measures due to the highly convergent and divergent nature of thalamo-cortical projections. Furthermore, new analytic methods have also shed light on the dynamics of functional connectivity patterns, which may be altered in schizophrenia (Damaraju et al., 2014). Computational model of network dynamics can potentially shed light on the mechanistic basis of these more abstract measures (Cabral et al., 2012; Hansen et al., 2015).
Another important combined experimental and modeling direction will be in relating these task-free biomarkers to thalamocortical function during sensory processing and cognition, which critically rely on proper thalamocortical interactions. Thalamocortical circuits are believed to play an important role in core cognitive functions such as attention and gating (Wimmer et al., 2015), and potentially play a role in predictive coding, all of which are implicated in schizophrenia. Computational models can test the impact on these functions of the perturbations constrained by the SCZ-related biomarker dynamics. It will be important for models to make dissociable predictions from competing synaptic-level hypotheses, which can be tested with neural and behavioral data in humans (Murray et al., 2014). In turn, these effects can then be reverse-engineered in animal models in disease states or under pharmacological manipulation (Wells et al., 2016). In that sense, computational modeling stands at an important intersection of translation between human and animal research. Furthermore, use of such models stands to help resolve emerging controversies surrounding physiological artifact, which challenge the validity of clinical neuroimaging effects in case-control designs. Put simply, neural circuit models do not move, breathe or have a heart beat, yet they can generate testable and competing predictions regarding expected neuroimaging effects (Yang et al., 2014). In summary, a Computational Psychiatry approach to understanding dynamical biomarkers in SCZ, such as thalamic dysconnectivity, has the potential to link across levels of analysis to inform mechanisms, and facilitate translational research from pharmacological and animal studies to clinical observations.
Acknowledgments
Funding was provided by CTSA Grant Number TL1 TR000141 (JDM); NIH grants 1DP5-OD012109 and R01MH108590, and NARSAD Independent Investigator Award (AA).
Footnotes
Conflict of Interest
Dr. Anticevic consults and holds options with BlackThorn Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RA, Stephan KE, Brown HR, Frith CD, Friston KJ. The computational anatomy of psychosis. Front Psychiatry. 2013;4:47. doi: 10.3389/fpsyt.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatrudo JM, Weaver CM, Crimins JL, Hof PR, Rosene DL, Luebke JI. Influence of highly distinctive structural properties on the excitability of pyramidal neurons in monkey visual and prefrontal cortices. J Neurosci. 2012;32(40):13644–60. doi: 10.1523/JNEUROSCI.2581-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, Savic A, Krystal JH, Pearlson GD, Glahn DC. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014a;24(12):3116–30. doi: 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, Niciu MJ, Morgan PT, Surti TS, Bloch MH, Ramani R, Smith MA, Wang XJ, Krystal JH, Corlett PR. Nmda receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A. 2012;109(41):16720–5. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, McEwen SC, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt BA, Olvet D, Mathalon DH, McGlashan TH, Perkins DO, Belger A, Seidman LJ, Tsuang MT, van Erp TGM, Walker EF, Hamann S, Woods SW, Qiu M, Cannon TD. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015a;72(9):882–91. doi: 10.1001/jamapsychiatry.2015.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Hu X, Xiao Y, Hu J, Li F, Bi F, Cole MW, Savic A, Yang GJ, Repovs G, Murray JD, Wang XJ, Huang X, Lui S, Krystal JH, Gong Q. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci. 2015b;35(1):267–86. doi: 10.1523/JNEUROSCI.2310-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Murray JD, Barch DM. Bridging levels of understanding in schizophrenia through computational modeling. Clin Psychol Sci. 2015c;3(3):433–459. doi: 10.1177/2167702614562041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Yang G, Savic A, Murray JD, Cole MW, Repovs G, Pearlson GD, Glahn DC. Mediodorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophr Bull. 2014b;40(6):1227–43. doi: 10.1093/schbul/sbu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A, Lubbers LS, Tanis KQ, Luo R, Podtelezhnikov AA, Finney EM, McWhorter MME, Serikawa K, Lemon T, Morgan R, Copeland C, Smith K, Cullen V, Davis-Turak J, Lee CK, Sunkin SM, Loboda AP, Levine DM, Stone DJ, Hawrylycz MJ, Roberts CJ, Jones AR, Geschwind DH, Lein ES. Transcriptional architecture of the primate neocortex. Neuron. 2012;73(6):1083–99. doi: 10.1016/j.neuron.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. human and animal model studies. Arch Gen Psychiatry. 1990;47(2):181–8. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cabral J, Hugues E, Kringelbach ML, Deco G. Modeling the outcome of structural disconnection on resting-state functional connectivity. Neuroimage. 2012 Sep 30;62(3):1342–53. doi: 10.1016/j.neuroimage.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Crandall SR, Cruikshank SJ, Connors BW. A corticothalamic switch: controlling the thalamus with dynamic synapses. Neuron. 2015;86(3):768–82. doi: 10.1016/j.neuron.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, Mueller BA, Pearlson GD, Potkin SG, Preda A, Turner JA. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage Clin. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci. 2011;12(1):43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Deco G, Kringelbach ML. Great expectations: using whole-brain computational connectomics for understanding neuropsychiatric disorders. Neuron. 2014;84(5):892–905. doi: 10.1016/j.neuron.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Deco G, Ponce-Alvarez A, Hagmann P, Romani GL, Mantini D, Corbetta M. How local excitation-inhibition ratio impacts the whole brain dynamics. J Neurosci. 2014;34(23):7886–98. doi: 10.1523/JNEUROSCI.5068-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Ponce-Alvarez A, Mantini D, Romani GL, Hagmann P, Corbetta M. Resting-state functional connectivity emerges from structurally and dynamically shaped slow linear fluctuations. J Neurosci. 2013;33(27):11239–11252. doi: 10.1523/JNEUROSCI.1091-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Babloyantz A, Sejnowski TJ. Ionic mechanisms for intrinsic slow oscillations in thalamic relay neurons. Biophys J. 1993a;65(4):1538–52. doi: 10.1016/S0006-3495(93)81190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Bal T, McCormick DA, Sejnowski TJ. Ionic mechanisms underlying synchronized oscillations and propagating waves in a model of ferret thalamic slices. J Neurophysiol. 1996a;76(3):2049–70. doi: 10.1152/jn.1996.76.3.2049. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Mechanisms underlying the synchronizing action of corticothalamic feedback through inhibition of thalamic relay cells. J Neurophysiol. 1998;79(2):999–1016. doi: 10.1152/jn.1998.79.2.999. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M, Sejnowski TJ, Huguenard JR. In vivo, in vitro, and computational analysis of dendritic calcium currents in thalamic reticular neurons. J Neurosci. 1996b;16(1):169–85. doi: 10.1523/JNEUROSCI.16-01-00169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, McCormick DA, Sejnowski TJ. A model for 8–10 hz spindling in interconnected thalamic relay and reticularis neurons. Biophys J. 1993b;65(6):2473–7. doi: 10.1016/S0006-3495(93)81297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN. Cortex, cognition and the cell: new insights into the pyramidal neuron and prefrontal function. Cereb Cortex. 2003;13(11):1124–38. doi: 10.1093/cercor/bhg093. [DOI] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, Defelipe J. A study of pyramidal cell structure in the cingulate cortex of the macaque monkey with comparative notes on inferotemporal and primary visual cortex. Cereb Cortex. 2005;15(1):64–73. doi: 10.1093/cercor/bhh109. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164(3):483–92. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, Bria P, Kalin NH, Tononi G. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167(11):1339–48. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophr Bull. 2011;37(2):306–15. doi: 10.1093/schbul/sbq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi S, Mucci A, Volpe U, Boutros N. Evidence-based medicine and electrophysiology in schizophrenia. Clin EEG Neurosci. 2009;40(2):62–77. doi: 10.1177/155005940904000206. [DOI] [PubMed] [Google Scholar]
- Hawrylycz MJ, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489(7416):391–9. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen EC, Battaglia D, Spiegler A, Deco G, Jirsa VK. Functional connectivity dynamics: modeling the switching behavior of the resting state. Neuroimage. 2015 Jan 15;105:525–35. doi: 10.1016/j.neuroimage.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Nmda receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27(43):11496–500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The thalamus. Springer Science & Business Media; 2012. [Google Scholar]
- Kiss T, Hoffmann WE, Scott L, Kawabe TT, Milici AJ, Nilsen EA, Hajós M. Role of thalamic projection in nmda receptor-induced disruption of cortical slow oscillation and short-term plasticity. Front Psychiatry. 2011;2:14. doi: 10.3389/fpsyt.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J Neurosci. 2009;29(9):2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169(3–4):215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992;68(4):1384–400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- Murray JD, Anticevic A, Gancsos M, Ichinose M, Corlett PR, Krystal JH, Wang XJ. Linking microcircuit dysfunction to cognitive impairment: effects of disinhibition associated with schizophrenia in a cortical working memory model. Cereb Cortex. 2014;24(4):859–72. doi: 10.1093/cercor/bhs370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62(3):1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramcharan EJ, Gnadt JW, Sherman SM. Higher-order thalamic relays burst more than first-order relays. Proc Natl Acad Sci U S A. 2005;102(34):12236–41. doi: 10.1073/pnas.0502843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, Wibral M, Singer W, Aru J, Uhlhaas PJ. The phase of thalamic alpha activity modulates cortical gamma-band activity: evidence from resting-state meg recordings. J Neurosci. 2013;33(45):17827–35. doi: 10.1523/JNEUROSCI.5778-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer CR, Landwehrmeyer GB, Kerner JA, Counihan TJ, Kosinski CM, Standaert DG, Daggett LP, Veliçelebi G, Penney JB, Young AB. Expression of n-methyl-d-aspartate receptor subunit mrnas in the human brain: hippocampus and cortex. J Comp Neurol. 1998;390(1):75–90. doi: 10.1002/(sici)1096-9861(19980105)390:1<75::aid-cne7>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmeier PJ, Stufflebeam SM. Patterns of spontaneous magnetoencephalographic activity in patients with schizophrenia. J Clin Neurophysiol. 2010;27(3):179–90. doi: 10.1097/WNP.0b013e3181e0b20a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature Rev Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Venables NC, Bernat EM, Sponheim SR. Genetic and disorder-specific aspects of resting state eeg abnormalities in schizophrenia. Schizophr Bull. 2009;35(4):826–39. doi: 10.1093/schbul/sbn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Stradtman GG, 3rd, Wang XJ, Gao WJ. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc Natl Acad Sci U S A. 2008;105(43):16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Krystal JH. Computational psychiatry. Neuron. 2014;84(3):638–54. doi: 10.1016/j.neuron.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Bonjean M, Petry HM, Sejnowski TJ, Bickford ME. Thalamic burst firing propensity: a comparison of the dorsal lateral geniculate and pulvinar nuclei in the tree shrew. J Neurosci. 2011;31(47):17287–99. doi: 10.1523/JNEUROSCI.6431-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MF, Wimmer RD, Schmitt LI, Feng G, Halassa MM. Thalamic reticular impairment underlies attention deficit in Ptchd1(Y/-) mice. Nature. 2016;532(7597):58–63. doi: 10.1038/nature17427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RC, Chen AC, Taylor SF. Low-frequency bold fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36(4):713–22. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM. Thalamic control of sensory selection in divided attention. Nature. 2015;526(7575):705–9. doi: 10.1038/nature15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Heckers S. Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169(10):1092–9. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi D, Zhang W, Wang HX, Stradtman GG, Gao WJ. Dizocilpine (MK-801) induces distinct changes of N-methyl-D-aspartic acid receptor subunits in parvalbumin-containing interneurons in young adult rat prefrontal cortex. Int J Neuropsychopharmacol. 2009;12(10):1395–1408. doi: 10.1017/S146114570900042X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GJ, Murray JD, Repovs G, Cole MW, Savic A, Glasser MF, Pittenger C, Krystal JH, Wang XJ, Pearlson GD, Glahn DC, Anticevic A. Altered global brain signal in schizophrenia. Proc Natl Acad Sci U S A. 2014;111(20):7438–43. doi: 10.1073/pnas.1405289111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GJ, Murray JD, Wang XJ, Glahn DC, Pearlson GD, Repovs G, Krystal JH, Anticevic A. Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc Natl Acad Sci U S A. 2016;113(2):E219–28. doi: 10.1073/pnas.1508436113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Buonanno A, Vertes RP, Hoover WB, Lisman JE. NR2C in the thalamic reticular nucleus; effects of the nr2c knockout. PLoS One. 2012a;7(7):e41908. doi: 10.1371/journal.pone.0041908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Llinas RR, Lisman JE. Inhibition of nmdars in the nucleus reticularis of the thalamus produces delta frequency bursting. Front Neural Circuits. 2009;3:20. doi: 10.3389/neuro.04.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yoshida T, Katz DB, Lisman JE. NMDAR antagonist action in thalamus imposes δ oscillations on the hippocampus. J Neurophysiol. 2012b;107(11):3181–9. doi: 10.1152/jn.00072.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]