Abstract
Tumor hypoxia is recognized as a limiting factor for the efficacy of radiotherapy, because it enhances tumor radioresistance. It is strongly suggested that assessing tumor oxygenation could help to predict the outcome of cancer patients undergoing radiation therapy. Strategies have also been developed to alleviate tumor hypoxia in order to radiosensitize tumors. In addition, oxygen mapping is critically needed for intensity modulated radiation therapy (IMRT), in which the most hypoxic regions require higher radiation doses and the most oxygenated regions require lower radiation doses. However, the assessment of tumor oxygenation is not yet included in day-to-day clinical practice. This is due to the lack of a method for the quantitative and non-invasive mapping of tumor oxygenation. To fully integrate tumor hypoxia parameters into effective improvements of the individually tailored radiation therapy protocols in cancer patients, methods allowing non-invasively repeated, safe, and robust mapping of changes in tissue oxygenation are required. In this review, non-invasive methods dedicated to assessing tumor oxygenation with the ultimate goal of predicting outcome in radiation oncology are presented, including positron emission tomography used with nitroimidazole tracers, magnetic resonance methods using endogenous contrasts (R1 and -based methods), and electron paramagnetic resonance oximetry; the goal is to highlight results of studies establishing correlations between tumor hypoxic status and patients’ outcome in the preclinical and clinical settings.
Keywords: tumor oxygenation, oximetry, tumor hypoxia, hypoxia imaging, radiotherapy outcome
Introduction
The effects of chemotherapy and radiotherapy have long been known to be affected by hypoxia (1, 2). Irradiation of normoxic tissues induces water ionization and the formation of radicals such as reactive oxygen species which are able to react with DNA and form DNA radicals. In the absence of oxygen, these radicals can easily be stabilized by cell “scavengers” in order to protect DNA. However, when oxygen is present, the DNA radicals react with oxygen and the damage is fixed. This reinforcement of the X-rays’ efficiency in the presence of oxygen is known as the “oxygen enhancing effect” (3). The “oxygen enhancement ratio” is the ratio of doses required to obtain the same cell survival under hypoxic and aerobic conditions. This value for mammalian cells varies from 2.5 to 3.0 (1, 4), indicating that hypoxic tumor cells will require a dose 2.5–3 times higher to be killed than normoxic cells. Radioresistance is considered maximal at 0.2 mmHg (corresponding to anoxia) and decreases progressively to 20 mmHg, which is the oxygen concentration at which hypoxia-induced resistance is almost nil (4). There are therefore two possible strategies for improving the curative effect of radiotherapy on hypoxic cells: alleviating hypoxia by increasing oxygen availability and increasing the dose of irradiation on hypoxic tumors. From a meta-analysis gathering 10,108 patients with solid tumors and observation of clinical practice, Overgaard concluded that “Ample data exist to support a high level of evidence for the benefit of hypoxic modification. However, hypoxic modification still has no impact on general clinical practice” (5). The unavailability of biomarkers as well as the lack of an ideal method for assessing tumor hypoxia, and for monitoring tumor response to radiosensitizers alleviating hypoxia, are issues that prevent the selection of patients who could benefit from increasing the pO2 level. The ideal method for patient stratification should be non-invasive, available in both preclinical and clinical settings, repeatable over a short period of time in order to monitor both chronic and acute hypoxia before and during the course of radiotherapy, quantitative from 0 to at least 40 mmHg, widely available in imaging centers, and predictive of the radiotherapy outcome. Finally, this method should provide a parametric value which is easily convertible into a dose of irradiation. Up to now, despite the efforts of scientists, no technique has met all these criteria. Indirect exogenous and endogenous markers for immunohistochemical detection of tumor hypoxia as biomarkers for personalized radiation oncology have recently been reviewed (6), following a previous large-scale review of hypoxia imaging methods in 2012 (7). Reviews with a special focus on preclinical assessment or the imaging of hypoxia have also provided a full description and technical details regarding each methodology (8, 9). Finally, a recent review addresses functional MRI (fMRI) methods in the field of radiation therapy of head and neck tumors (10). This article reviews the results of preclinical and clinical studies acquired using non-invasive imaging methods to assess tumor oxygenation in an attempt to establish correlations with patients’ outcome (according to the oxygen level in their tumors), with special emphasis on preclinical quantitative methods, such as electron paramagnetic resonance (EPR) oximetry and clinically translatable endogenous contrast magnetic resonance (MR)-based methods, which have so far been less validated than positron emission tomography (PET)-based methods (Table 1). Cross-validation studies between methods and with quantitative methods are also presented in order to better establish the relevance of each oximetric method. A first section is dedicated to polarographic electrodes that have pioneered in vivo oxygen measurements and provided the first human demonstration of the occurrence of hypoxia in human tumors. This article summarizes and assesses the value of MR and non-MR methods used to assess tumor oxygenation in order to predict the outcome of radiation therapy (Figure 1).
Table 1.
Oxymetric studies linking hypoxia and radiation therapy outcome.
| Oxymetric technique | Animal studies | Reference | Clinical studies | Reference | Cross-validation with quantitative oxymetric methods? | Reference |
|---|---|---|---|---|---|---|
| Eppendorf electrodes | C3H mammary tumors: significant difference in local tumor control between the fraction of hypoxic values (<2.5 mmHg) and less hypoxic tumors | (36) | Prostate cancer study (n = 57): 8-year survival is 78% for moderately hypoxic tumors and 46% for severe hypoxic tumors | (16) | n.a. | |
| Head and neck cancer study (n = 35): 2-year locoregional control is two times lower for hypoxic tumors (i.e., with 15% of readings <2.5 mmHg) | (15) | |||||
| PET 18F-MISO | FaDu hSCC xenografts: prognostic value of pretreatment 18F-MISO hypoxic volume; SUVmax was not associated with local control | (25) | 5 head and neck studies (n = 45; 73; 12; 17; 15) | (21, 24, 26, 27, 29) | Mixed results | (23) |
|
Lack of correlation with Eppendorf measurements in head and neck tumors | |||||
| PET 18F-FAZA | Rhabdomyosarcoma: lower uptake linked to better local tumor control at 90 days post-irradiation | (36) | Head and neck cancer study: DAHANCA trial (n = 40), high tumor uptake is correlated to lower disease-free survival | (38) | Positive results | (38) |
| Validated with EPR oximetry in the preclinical setting (rat rhabdomyosarcomas) | ||||||
| 9L glioma and rhabdomyosarcoma: significant correlation between 18F-FAZA T/B and tumor growth delay | (37) | |||||
| PET 18F-FETNIM | 1 head and neck cancer study (n = 21) | (21) | NO (but compared with other nitroimidazoles) | (44, 45) | ||
| 2 lung cancer studies (n = 26; 32) |
|
|||||
| 1 cervical cancer study (n = 16) | ||||||
| 1 esophageal cancer study (n = 28) | ||||||
| High fractional hypoxic volumes, uptake, or baseline SUVmax correlated with PFS, OS, or clinical response | ||||||
| PET 60CU-ATSM | Canine sinonasal tumors: lack of correlation between Cu-ATSM uptake and outcome | (51) | 3 cervical cancer studies (n = 14; 15; 38) | (21, 48–50) | Mixed results | (40, 57–63) |
| 2 head and neck cancer studies (n = 15; 11) |
|
|||||
| 3 lung cancer studies (n = 19; 22; 7) | Potential link with tumor redox status | |||||
| 1 rectal cancer study (n = 19) | ||||||
|
||||||
| Dynamic contrast-enhanced magnetic resonance imaging | Melanoma xenografts: low ktrans is correlated with increased radioresistance | (78) | Cervical cancer study: ktrans and ABrix parameters correlated with poor outcome | (80) | Mixed results | (82) |
| ||||||
| Cervical cancer xenografts: basal ktrans correlated to the outcome of RT; skewness (heterogeneity) in ktrans distribution correlated with poorer outcome | (79) |
|
(83, 84) | |||
| Mouse fibrosarcoma: none of the tested DCE parameters (ktrans, vp, Kep, % of perfused voxels) were related to RT outcome | (69) | |||||
| G3H prolactinomas (rats) | (100) | Cervical cancer study: basal was predictive for RT response | 90 | Mixed results | (96–98) | |
| RIF-1 fibrosarcomas (mice) |
|
|||||
| was predictive for a transient reduction in tumor size; low baseline was linked to a small reduction in tumor size | ||||||
| R1–T1 of water protons | Dunning R3327-AT1 rat prostate | (92) | Mixed results | |||
| A large increase in R1 response to hyperoxic challenge was linked to a longer tumor growth delay after radiation therapy | No study addressing potential correlations between R1–T1 and quantitative pO2 measurements | |||||
| R1–T1 of lipid protons | 9L glioma | (101) | Mixed results | (101, 123) | ||
| Water and lipids T1 are less predictive of RT outcome than in this model |
|
|||||
| Combined R1 and MRI | Dunning rat prostate tumors | (121) | ||||
| Useful factors to predict tumor response to hypofractionation | ||||||
| EPR oximetry | C6 and 9L glioma | (151) | ||||
| pO2 assessed after a first course of RT was a prognostic indicator of differential response to RT between the two glioma models | ||||||
| TLT and FSaII syngeneic tumors | (69, 76, 130, 132, 136–146, 148) | |||||
| pO2 assessed during/after administration of treatments able to alleviate tumor oxygenation was predictive of the outcome of RT when administered during this window of reoxygenation | ||||||
Figure 1.
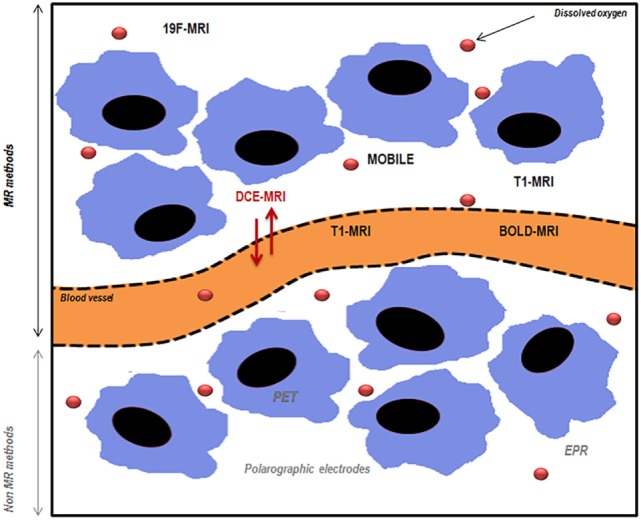
Schematic representation of magnetic resonance (MR) and non-MR methods used to assess tumor oxygenation. PET, positron emission tomography; EPR, electron paramagnetic resonance; MRI, magnetic resonance imaging; DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; BOLD-MRI, blood oxygen level-dependent imaging; MOBILE, mapping of oxygen by imaging lipids relaxation enhancement. Adapted from Price et al. (11).
Polarographic Oxygen Electrodes
Polarographic electrodes are probes that can be introduced directly into the tissue of interest. The reduction of oxygen at the cathode extremity will generate a detectable current proportional to the pO2. The electrodes’ measurements provide histograms of pO2, describing the frequency of pO2 measurements registered during a defined period of time and corresponding to the mean oxygen level for 50–100 cells that are located around the polarographic electrode (12). Since this technique requires the insertion of the probe inside the tumor, the tissue itself is damaged and a delay is necessary before measurement to allow for stabilization. This also prevents repeated-measurements experiments on the same site and limits the application of the electrodes to accessible tumors. Moreover, as the operation of the polarographic electrodes requires oxygen, the signal-to-noise ratio will obviously decrease with the oxygen concentration, making measurements difficult under severe hypoxia. Since they are invasive and since their function is oxygen consuming, they cannot be chosen as the ideal method for tumor oxygenation measures. The Eppendorf electrode system (which was commercially available) has been developed to limit this consumption effect: the electrode is moved through the tissue of interest and an oxygen measurement is registered every 0.4 mm (after a 0.7-mm step forward and a 0.3-mm step backward). Reducing the delay after the back-step to a minimum helps to ensure negligible consumption of oxygen by the electrode and to decrease the tissue compression artifact (13). The polarographic electrodes have the advantage of providing real-time measurements that can be static or moving (in the case of Eppendorf electrodes). Despite their limitations, the polarographic electrodes have been widely used as a “gold standard” in preclinical and clinical experiments. With regard to clinical use, data from more than 125 clinical studies are available (14). It was shown in 2005 in a head and neck study involving 397 patients that tumor hypoxia assessed using Eppendorf electrodes was associated with a poor prognosis (15). Eppendorf electrodes have also highlighted that the outcome of patients with prostate cancer is linked to the level of tumor hypoxia. The 8-year survival was found to be 78% for patients with moderate hypoxia but just 46% for patients with severe hypoxic tumors; these results were independent of well-established risk factors such as tumor stage, Gleason score (defining the prostate tumor grades), prostate-specific antigen, perineural invasion, serum hemoglobin level, and hormonal therapy use (16). The prognostic value of tumor pO2 Eppendorf measurements was less clear in a multicenter human cervix carcinoma study involving 127 patients (17). Finally, as a “gold standard” method, the polarographic electrodes have often been used to validate new techniques aimed at assessing tumor hypoxia (18). However, this technique remains invasive and cannot be used to map tumor heterogeneity or to repeat measurements on the same site for a long time. Alternative methods are therefore required.
Positron Emission Tomography
Hypoxia PET imaging is a non-invasive technique widely used in preclinical and clinical studies. This method requires the intravenous injection of a radiotracer (e.g., nitroimidazole) that will diffuse into cells and will be reduced intracellularly. This is reversible under normoxic conditions; but under hypoxia, the radiolabeled molecules will be trapped and will react with cellular macromolecules such as nucleic acids and proteins. The reduction requires the activity of reductases that are only present in viable hypoxic cells. As a consequence, the accumulation and detection of radiotracers will be enhanced in hypoxic regions, whereas the necrotic cells will not be visible to PET imaging. The quantification of the tracer uptake is generally expressed as the tumor-to-background (TBR) ratio at a given time after the tracer injection. 2-Nitroimidazoles have been developed as radiosensitizers (19). Because they have a nitro (NO2) group linked to the imidazole structure, they can undergo up to six electron reductions, eventually resulting in an amino group (NH2) (20). For PET imaging, these tracers are labeled with radioisotopes: fluorine-18 (18F) or carbon-11 (11C). The most important compounds designed to image hypoxia are described below.
18F-Fluoromisonidazole
18F-FMISO is a commonly used hypoxia tracer in preclinical and clinical studies. Due to its lipophilicity, this molecule easily crosses the cell membranes and is then trapped if intracellular hypoxia remains below a threshold of 10 mmHg. The cellular clearance of 18F-FMISO is quite low in normoxic tissues, thereby hampering the contrast between normoxic tissues and moderate hypoxic tumor tissues. As a result, a TBR ratio of 1.2 is usually used to delineate regions of hypoxia after a minimum delay of 2 h (20, 21). The best signal-to-noise ratio has been observed 4 h after tracer injection (22). In a study of Mortensen et al. (23), it was not possible to correlate 18F-FMISO with Eppendorf electrodes in the clinical setting in head and neck tumors (23).
In preclinical and clinical studies, the level of hypoxia highlighted by 18F-FMISO has been correlated with the response to therapy and outcome (24). Non-hypoxic volume estimated using 18F-FMISO uptake showed significantly better local control after single-dose irradiation than hypoxic tumors in FaDu hSCC xenografts (25). In a recent review on PET imaging, Fleming and colleagues listed all the applications of 18F-FMISO in clinical trials (21). This tracer has been successfully used to image hypoxia in gliomas, head and neck, and breast and renal tumors. However, the use of 18F-FMISO in sarcomas, pancreatic cancers, or rectal cancers was compromised because of the non-specific accumulation of 18F-FMISO in normoxic surrounding tissues or because of insufficient tracer uptake. Four head and neck tumor studies were able to correlate one 18F-FMISO-related tumor parameter (T:Bmax, SUVmax, or T:Mmax) with disease-free survival or locoregional failure (21), whereas one study was not able to establish any correlation (26). To date, different TBRmax thresholds for stratification have been reported; therefore, standardized methods still need to be determined in multicenter studies (27). Tumor mapping of hypoxia with 18F-FMISO could be useful for planning intensity modulated radiation therapy (IMRT) on patients with head and neck cancers, since when the hypoxic regions are well delineated, it is possible to boost the dose delivered to those areas. 18F-FMISO maps were used for this purpose on two patients in a study from Lee and colleagues in 2008. With the knowledge of hypoxic areas that they gained, these authors were able to escalate the dose to 84 Gy for 10 patients. Moreover, they raised the delivered irradiation doses up to 100 and 105 Gy for two patients in hypoxic areas (28). A single-center trial combining multimodal hypoxia imaging, including 18F-FMISO, and IMRT in patients with inoperable stage III non-small cell lung carcinoma (NSCLC) tumors was started in 2012 (29). Recent data also suggest that selective dose painting to hypoxic tumor subvolumes requires adaptation during treatment (30). 18F-FMISO has also been used to monitor reoxygenation of the tumors during the course of radiotherapy: in 10 patients, a decrease in the uptake of 18F-FMISO was observed in eight tumors after the delivery of 20 Gy (31). The reoxygenation process has also been observed in patients with glioblastoma treated by fractionated radiotherapy and concomitant temozolomide administration (32). The results showed a significant decrease in tumor hypoxia attributed to the radiotherapy effect. Finally, it has been suggested that the hypoxic areas of the tumors are correlated with neovascularization and with the tumor metabolism rate in glioblastoma multiform (33). This conclusion comes from a preliminary study involving 10 patients who underwent magnetic resonance imaging (MRI) to evaluate tumor perfusion after the injection of a gadolinium-based contrast agent and several PET imaging protocols: the first of these was 18F-FMISO as a reporter of hypoxia and the second was l-methyl-11C-methionine (11C-MET), an amino acid whose uptake reflects tumor activity and which is currently used for glioma detection and grading (34).
18F-Fluoroazomycin-Arabinofuranoside and 18F-Flortanidazole
18F-fluoroazomycin-arabinofuranoside (18F-FAZA) is a more hydrophilic nitroimidazole that displays faster clearance from blood and normal tissues than 18F-FMISO. As a result, imaging tumor hypoxia with this radiotracer improves the signal-to-noise ratio. In a preclinical study on rhabdomyosarcoma, a correlation between 18F-FAZA uptake and actual values of pO2 measured by EPR has been established, reflecting quantitative aspects of the method (35). Moreover, 18F-FAZA seems to be predictive of the response to radiotherapy: less hypoxic rhabdomyosarcoma tumors (defined by a lower uptake of 18F-FAZA) demonstrated better local tumor control 90 days after radiotherapy than more hypoxic tumors (36). Similarly, a significant correlation between 18F-FAZA T/B ratio and tumor growth delay was found in 9L glioma (37). With regard to clinical applications, 18F-FAZA imaging has been successfully performed in gliomas, lymphomas, lung, head and neck, and cervical and rectal tumors (21). The results of the DAHANCA 24 trial on head and neck cancers have proven that 18F-FAZA uptake is a good prognostic factor of tumor response to radiotherapeutic treatment (38). Finally, 18F-FAZA-PET images have been successfully exploited to delineate radiotherapy planning for head and neck squamous cell carcinoma, with 86 Gy being the dose to deliver in hypoxic areas. The treatment protocol included three phases and was based on 18F-FAZA-PET images acquired before irradiation and after the 7th and 17th fractions (39). 18F-flortanidazole (18F-HX4) is a hydrophilic nitroimidazole which quickly clears from normoxic tissues, allowing imaging 90 min after tracer administration; its low accumulation in the brain, heart, and gastrointestinal tract enables these body parts to be imaged (20). In a comparative study looking at several markers of hypoxia in an in vivo model (head and neck carcinoma cells SQ20b), 18F-FAZA, 18F-HX4, and 18F-FMISO uptakes were correlated with hypoxia, despite a relatively low accumulation of 18F-FAZA in muscles and tumors (40). In a second comparative study, 18F-HX4 and 18F-FAZA were found to be sensitive to an increase of hypoxia, induced by the breathing of a gas mixture containing 7% O2, when the tumor-to-blood ratio was used. However, when only the tumor-to-muscle was used, only 18F-FAZA revealed a significant decrease in tumor oxygenation (41).
18F-Fluoroerythronitroimidazole
18F-labeled fluoroerythronitroimidazole (FETNIM) was suggested as another marker of tumor hypoxia for use with PET in 1995 (42). Initial data suggested that 18F-FETNIM shows low peripheral metabolism, little defluorination, and possible metabolic trapping in hypoxic tumor tissue (43). 18F-FETNIM distribution has been positively correlated with 18F-FAZA in murine mammary tumors under normoxic and hyperoxic conditions (44). It has been tested in head and neck, lung, cervical, and esophageal clinical cancer studies, with significant correlations between patient outcome and either high fractional hypoxic volumes, F-FETNIM uptake, or baseline SUVmax (21). Cross-validation studies with other quantitative oxymetric markers are lacking. However, F-FETNIM has been compared to F-FAZA in the preclinical and clinical settings, with positive correlations (21, 44, 45).
Copper (II) Diacetyl-Bis (N4-Methylthiosemicarbazone)
Copper (II) diacetyl-bis (N4-methylthiosemicarbazone) (Cu-ATSM) can be used as a radiotracer with 60–64Cu with variable half-times (46). This agent displays high lipophilicity and rapid clearance from normoxic tissues, thereby enabling imaging 30 min after its administration (47). In the absence of oxygen, the Cu(II) is irreversibly reduced to Cu(I) in viable mitochondria and therefore becomes trapped in hypoxic cells. In the study by Carlin and colleagues, the 64Cu-ATSM molecule displayed a better uptake in tumor than 18F-FMISO, 18F-FAZA, and 18F-HX4. However, its distribution within the tumor was not similar to the other tracers: the accumulation of 64Cu-ATSM was greater at the tumor periphery and the uptake was lower in the tumor center where perfusion was also reduced (40). The low accumulation of 60Cu-ATSM in the urinary tract makes it an ideal candidate for imaging pelvic organs. For example, the uptake of this radiotracer has been inversely correlated with the patient outcome (in terms of progression-free survival) for 38 patients with cervical cancer (48). Similar observations have been performed in head and neck, and rectal and lung tumors, as reviewed in (16) and in more recent studies in NSCLC and head and neck tumors (49, 50). Few preclinical studies have attempted to link Cu-ATSM uptake and outcome; one study of canine tumors was not able to establish any correlation (51). Planning of dose painting can also be achieved using Cu-ATSM, which detects the hypoxic regions in preclinical and clinical models (52–54). However, Cu-ATSM uptake does not only reflect hypoxia: in a study of six tumor cell lines, the maximum uptake was cell line dependent and was linked to the redox status of tumor cells. The retention of Cu was higher in cells with an abnormally reduced status (55). Moreover, the in vitro results demonstrated that hypoxia selectivity was optimal 30–60 min after the administration of Cu-ATSM, but this is a limiting factor for in vivo applications, since the distribution of the tracer during the first hour after its administration is limited by a reduced tumor blood flow. The latter imaging is suggested to be rather linked to the active transport of Cu alone that has been dissociated from the Cu-ATSM complex, although these observations are again cell line dependent (55). The fact that copper metabolism may also play a role in the uptake mechanism of 64Cu-ATSM was confirmed in a more recent publication showing similar contributions between 64Cu-ATSM and 64Cu-acetate (56). Further studies have demonstrated that Cu-ATSM uptake is not correlated with an increase in hypoxia (57, 58) or that Cu-ATSM uptake is not co-localized with hypoxia marked with immunohistochemistry (40, 59, 60). Only one recent study has concluded in favor of a positive correlation between tracer accumulation and hypoxia but not in both tumor models under study (61). It seems that Cu-ATSM is not a specific marker of tumor hypoxia, but it has been successfully correlated with the NADH and NADPH levels: Cu-ATSM uptake is rather observed in tumors with abnormally reduced status, which may or may not be linked to hypoxia (62, 63). Finally, another study by Vavere and Lewis investigated the link between Cu-ATSM uptake and the fatty acid synthesis pathway, which consumes NADPH, and correlated the level of fatty acid synthase with the Cu-ATSM uptake (64). Consequently, Cu-ATSM images cannot be interpreted in terms of oxygenation only and, although Cu-ATSM is predictive of radiotherapy outcome, it is unclear whether this is linked to tumor hypoxia.
MRI Methods
19F-MRI
19F-MRI is a non-invasive method able to map tumor hypoxia quantitatively, after the injection of a perfluorocarbon emulsion. Calibration curves relating the longitudinal relaxation rate to pO2 can be acquired for a given temperature and a given perfluorocarbon (65) and used to map tumor oxygenation quantitatively. A major advantage of this calibration is the independent property of the absolute 19F signal intensity, linked to perfluorocarbon uptake. The fluorocarbon (PFC) relaxometry using echo planar imaging for dynamic oxygen mapping method developed by Mason and colleagues has been successfully used to monitor positive and negative changes in tumor oxygenation (65–68) as well as to map the heterogeneity of response to hyperoxic challenges within each tumor: it appears that well-oxygenated areas at baseline will display an increase in oxygenation earlier than hypoxic areas (65). Similarly, 19F-MRI mapping was able to monitor the effect of a radiosensitizer, S-nitrosocaptopril, which induces a significant increase in tumor pO2 from 20 to 60 min after its administration (69). Due to an acquisition time reduced to 1.5 min, Jordan and colleagues were able to monitor spontaneous oxygenation fluctuations in the range of 5–30 mmHg in transplantable mouse liver tumors and to identify hypoxia cycles in this model (70, 71). PFC can remain in the tumor and enables repeated measurements. The injection of perfluoro-15-Crown-Ether in Shionogi tumors, a murine mammary carcinoma which has acquired a dependence on androgens, has shown that 19F-MRI is able to distinguish three hormone-dependent oxygenation statuses (72). Tumor tissue heterogeneity can be assessed by the diffusion-based multispectral technique in order to distinguish tumor necrosis from viable tumor tissue and to detect subcutaneous adipose tissue. In a recent study, Shi and colleagues monitored the tumor response to hyperoxic and hypoxic challenges by considering the tumor as a whole or by considering each tissue type separately. The pO2 increased significantly when the authors considered the tumor as a whole, and this response was enhanced further when they focused on the viable tumor tissue (73). However, in a study comparing 18F-PET imaging and 19F-MRI, it was shown that fluorine mapping with MRI was less sensitive to small pO2 changes (from 3 to 5 mmHg) in some tumors (35). Moreover, before performing 19F-MRI, the toxicity of the chosen PFC needs to be taken into account since it has been observed, for example, that the early toxicities (thrombosis and tissue necrosis) observed with HFB could be avoided by using 15C5 (74). Despite this, approval is being awaited from the FDA for the investigation in a clinical trial of PFCs as a biomarker of tumor response to radiotherapy.
Dynamic Contrast-Enhanced Magnetic Resonance Imaging (DCE-MRI)
Dynamic contrast-enhanced MRI is a method widely used in preclinical and clinical research to assess information on tumor hemodynamics. A bolus of gadolinium-based contrast agent is injected, and its distribution within the tissue of interest is analyzed through signal enhancement, thereby providing information on perfusion and permeability. This technique is regularly combined with oximetric methods such as PET imaging, blood oxygen level-dependent (BOLD)-MRI, or EPR oximetry to assess tumor hemodynamic parameters and their impact on therapy (75–77). Two parameters are regularly assessed by DCE-MRI, using the Tofts model: ktrans, representing the volume transfer constant between blood plasma and extravascular extracellular space, and vp, defining the blood plasma volume per unit volume of tissue. Low-perfused tumor areas suffer from an insufficient supply of oxygen, thereby leading to hypoxia. It has therefore been suggested that DCE-MRI could be used as an indirect method to detect hypoxic areas in tumors. In a recent preclinical study by Øvrebø and colleagues, ktrans was found to be predictive of tumor response to radiotherapy: low ktrans was associated with an increased radioresistance in hypoxic melanoma xenografts, suggesting that DCE-MRI is a biomarker of tumor radioresistance in hypoxic tumors (78). Further studies on two cervical cancer xenografts have confirmed those results, indicating that the radiotherapy outcome can be correlated with ktrans values measured before the treatment taking each tumor model separately (79). Furthermore, skewness in the distribution of the ktrans parameter was also correlated with poorer patient outcome, highlighting the heterogeneity of perfusion within these tumors (80). Conversely, a study on fibrosarcoma was not able to show any correlation between DCE-related parameters (ktrans, vp, Kep, % of perfused voxels) and the outcome of radiation therapy (81). With respect to validation of the technique with other oximetric methods, attempts have been made in preliminary clinical studies to assess correlations between the level of hypoxia and permeability, with mixed results, in head and neck cancer and gliomas using pimonidazole staining (an immunohistological staining aimed at detecting tumor hypoxia) and in cervical cancer using polarographic electrodes (82–84). Søvik and colleagues have also demonstrated that DCE-MRI could be used to monitor the changes in tumor oxygenation during the course of radiotherapy in order to adapt IMRT to changes in hypoxia distribution within the tumor after several doses of irradiation (85). The Brix model can also be used for the analysis of DCE-MRI images. Perfusion or permeability is then assessed by the parameter ABrix, also known to measure the extravascular extracellular space (86). In order to compare the Brix and Tofts models and their ability to predict the outcome of patients, Andersen and colleagues tested both models on patients with cervical cancers. They concluded that low values of ktrans and ABrix can be associated with poor outcome (87). A recent study also demonstrated that low ABrix could be correlated with an upregulation of genes involved in the response to hypoxia (88).
Nevertheless, numerous precautions have to be taken when interpreting DCE-MRI images in terms of oxygenation because perfusion is not the only feature influencing pO2. Moreover, despite the establishment of relations between ktrans and oxygen tensions or immunohistochemical measurements in some studies, estimates of perfusion remain indirect estimates of hypoxia and, in some circumstances, do not relate to hypoxic status (89). It is also important to mention that the contrast agent distribution can also be altered by perfusion and extracellular volume, leading to misestimation of oxygenation in necrotic areas (85).
Blood Oxygen Level-Dependent Magnetic Resonance Imaging
Blood oxygen level-dependent MRI, or fMRI, uses endogenous contrast and is sensitive to the effective transversal relaxation rate of protons . This measurement is sensitive to the ratio of oxyhemoglobin and deoxyhemoglobin, the latter being a paramagnetic agent that shortens .
In preclinical studies, BOLD-MRI has proven its ability to monitor changes in oxygenation levels during hyperoxic challenges (90–92). Our group has compared the changes in BOLD signal following the administration of carbogen or isosorbide dinitrate and has observed that the magnitude of the changes is stronger during the carbogen challenge (affecting the hemoglobin saturation) than after the administration of the NO donor (93). The oxygenation concentration is not the only parameter that affects : changes in tumor blood flow, blood volume, blood pH, or metabolic status can also influence the measurements (94, 95). Changes in should therefore be carefully considered when they are treated as an indicator of changes in tumor oxygenation. Moreover, no correlation has been established between measurements and absolute values of pO2 (96). In comparisons with pimonidazole staining, both correlation and inverse correlation have been observed in prostate and mammary tumors, respectively (97, 98). BOLD-MRI is therefore used to monitor tumor oxygenation changes rather than to map tumor hypoxia quantitatively. Spontaneous fluctuations have been successfully monitored by BOLD-MRI, which has led to the identification of several cycles of hypoxia, with periods ranging from 3 min to 1 h (99). Moreover, the tumor regions in which tumor oxygenation fluctuates have been related to areas with functional vasculature. The prognostic value of was investigated in a preclinical study. The authors subjected rats with GH3-prolactinomas and mice with RIF-1 fibrosarcomas to carbogen breathing before radiation therapy. The GH3 prolactinomas displayed large and a large response to the hyperoxic challenge (), and this was predictive of a transient reduction in the tumor size after irradiation. However, the inhibition of tumor growth exhibited with the RIF-1 fibrosarcoma was smaller, and this was related to a low at baseline and to a poor response to the hyperoxic challenge (100). Recently, our group observed that was predictive of radiation therapy outcome in rat 9L-glioma tumors but not in rhabdomyosarcoma tumors (101). In a study by Kim and colleagues, the results from a small sample of cancer cervical patients suggested that the values are predictive of the radiotherapeutic response (102).
It is important to remember that the BOLD signal is related to the amount of deoxyhemoglobin and therefore linked to the blood pO2 and blood saturation of oxygen (SO2). There is therefore a real interest in quantifying the BOLD signal (103). A technique known as multiparametric quantitative BOLD has been recently developed to achieve a quantitative mapping of tissue oxygenation. The method is based on the acquisition of several images, with standard sequences, aimed at measuring the blood volume fraction, field inhomogeneities (by mapping B0), and the tissue T2 before and after the administration of a contrast agent. These three values are then integrated in a model describing the signal in order to calculate average oxygen saturation in each voxel (95). This method has been successfully applied to map tumor hypoxia in the brain in stroke or gliomas (104). This approach allows a quantitative measurement of the blood oxygen saturation and represents a major improvement in the use of BOLD imaging to map tumor hypoxia and to monitor tumor oxygenation changes.
1H Relaxation Imaging
Oxygen is a paramagnetic agent that shortens the longitudinal relaxation time (T1) of surrounding protons. Consequently, T1 mapping appears as a possibility for mapping tumor hypoxia. This method does not require any contrast agent and is widely available in medical imaging centers. Moreover, a correlation can be established between pO2 values and relaxation rates, as was done, for example, between relaxation rates and arterial blood oxygen pressure in a pig (105), revealing the quantitative aspect of such measurements. However, since T1 relaxation is also influenced by temperature, tissue of interest, blood flow, and basal blood oxygen saturation, a calibration between T1 values and pO2 cannot be established so easily. Nevertheless, assessing T1 measurements has been a useful method for monitoring changes in tissue oxygenation.
In a recent study by Muir and colleagues reporting the use of a hyperbaric chamber for rodents, T1 values measured in the brain were found to be significantly reduced (with an increase in related R1 values) when successive switches were made from normobaric air to hyperbaric air and then to hyperbaric oxygen (106). On the basis of a comparison between the changes in T1 values in liver, kidney, and muscle of healthy rats obtained during transitions from air to pure oxygen, to carbogen (10% CO2 and 90% O2), or to a mixture of ambient air with 10% CO2, it was concluded that these measurements were sensitive to oxygen dissolved in tissues when there was no concomitant change in blood flow (107). However, in the same study, the authors highlighted the lack of sensitivity of T1 measurements to a decrease in oxygenation during a switch from pure oxygen to carbogen or from air to a mixture of air and 10% CO2. The explanation may lie in the vasodilatation induced by CO2, which offsets the decrease in tissue oxygenation, resulting in unexpected positive R1 changes (107). However, this issue is controversial: in a preclinical study aimed at evaluating brain oxygenation during a hyperoxic challenge, the R1 (R1 = 1/T1) values were similarly increased in the cerebral cortex and in the pituitary gland during both carbogen and pure oxygen breathing, despite a decrease in brain perfusion induced by pure oxygen breathing (indeed, in the absence of CO2, vasoconstriction occurs) (108).
T1 measurements have been assessed together with for monitoring tumor oxygenation in murine prolactinoma models and prostate tumor xenografts undergoing a hyperoxic challenge. The changes in relaxation rates were found to be related to the basal oxygenation status: the most hypoxic tumors exhibited significantly reduced R1 values and significantly higher values (109).
T1 measurements have recently been used in conjunction with perfusion MRI to quantify the hypoxic fraction in multiple models with differing hypoxic and vascular phenotypes (110).
In a preliminary human study on healthy volunteers, this technique was successfully used to monitor the increase in oxygenation in normal myocardium, spleen, and arterial blood. However, there was no significant change observed in liver, skeletal muscle, or subcutaneous fat (111). These discrepancies in the results were attributed to a lack of sensitivity, to the uncontrolled motion of organs, and to differences in blood flow, blood volume, and regional oxygen consumption. Two years later, a study by Noseworthy and colleagues also failed to monitor the oxygenation in skeletal muscles subjected to a hyperoxic challenge involving pure oxygen with T1 measurement despite a significant change in T2 (112). Nevertheless, further work demonstrated the effect of oxygen in shortening the longitudinal relaxation time in healthy volunteers’ muscles, spleen, renal cortex, subcutaneous fat, placenta, and liver (113–116). Furthermore, in another study by O’Connor and colleagues, the authors observed significant differences of response induced by either pure oxygen or carbogen depending on the tissue of interest: carbogen induced a lower T1-shortening effect than pure oxygen in the spleen, whereas the opposite phenomenon was observed in the liver. Ten patients with abdominal tumors were then subjected to pure oxygen breathing. A significant increase in R1 values was observed in eight of these patients with ovarian, cervical, or gastrointestinal malignancies (117). The effect of hyperoxia has also been investigated in the brain using a dynamic T1-weighted sequence called tissue oxygen level dependent (TOLD) (118). This method allows reduced acquisition times and is more suited for a dynamic assessment of changes induced by a hyperoxic challenge. Haddock and colleagues (119) monitored the oxygenation of brain tissue during two protocols: the first was composed of a twice-repeated switch from ambient air to pure oxygen with two 2-min intervals, while the second began with a normoxic phase followed by a switch to pure oxygen breathing during 7 min followed by a final breathing of air. Due to the T1 effect, they could observe changes in signal intensity whose magnitude was related to the changes in brain oxygenation monitored with BOLD-MRI. Furthermore, TOLD measurements have been demonstrated to be sensitive to dynamic hyperoxic challenges (120) and seem to be predictive of the radiotherapy outcome (92). Prostate tumors that are well reoxygenated during pure oxygen breathing before radiotherapy display a significantly larger regrowth delay than low responders to hyperoxia (92). Combined BOLD and TOLD contrasts were also recently assessed in Dunning rat prostate tumors and were shown to be useful prognostic factors for predicting tumor response to hypofractionation (121). However, these results remain preliminary and need to be further investigated. The study by Burrell and colleagues highlights the complementary character of concomitant T1 and measurements: although the mean response was an increase in R1 values and a decrease in values in both models, they observed that the magnitude of R1 and changes was dependent on the basal oxygenation status. The explanation may be linked to hemoglobin saturation: in well-oxygenated tumors, the increase in oxygen supply will raise the amount of dissolved oxygen rather than increase the already well-saturated hemoglobin. This results in higher amplitude changes of positive ΔR1 rather than of negative (109). It is also important to remember that the measurement is influenced by the hemoglobin and deoxyhemoglobin ratio and is therefore sensitive to vascular oxygenation, whereas the T1 measurement is rather sensitive to oxygen dissolved in tissues. As there is a real interest in combining the measurement of R1 and , Ding and colleagues have recently proposed a new sequence enabling simultaneous acquisition of and T1 measurement. With this new method, they monitored the dynamic changes in oxygenation of abdominal organs (spleen, medulla, and renal cortex) (118).
From the foregoing, we can conclude that there is real value in measuring the 1H relaxation time to obtain information on tissue oxygenation non-invasively. By comparing the sensitivity of 1H-MRI oximetry with 19F-MRI using perfluorocarbons, Tadamura and colleagues observed that: “T1 shortening effect with oxygenation observed using 19F MR system with PFCs is much greater than that observed with 1H MRI because of large oxygen solubility of PFC compared with that of aqueous media. Therefore, the PFC method is more sensitive to tissue oxygenation state.” (111). In all the techniques described above, the signal was mainly influenced by protons of the water molecules. As oxygen solubility is higher in lipids than in water, focusing on the relaxation of protons belonging to lipid molecules would improve the sensitivity of the techniques described above. Consequently, a new technique called mapping of oxygen by imaging lipids relaxation enhancement (MOBILE) aimed at selectively measuring the T1 of the lipid component has been proposed (122). This method has demonstrated its ability to distinguish the oxygenation levels in tumor tissue homogenates submitted to different oxygen concentrations. Moreover, the MOBILE technique can be used to map local pO2 in several tissues such as liver, muscles, brain (infarcted or not), and mammary tumors. Both positive and negative changes in tumor oxygenation can be monitored with the MOBILE technique. Its quantitative aspect has also been demonstrated on mammary tumor models presenting high lipid content (123). However, the method was not applicable to tumors with low lipid content. An alternative method based on the deconvolution of global T1 in fat and water components was recently developed. However, a quantitative aspect could not be demonstrated in rhabdomyosarcomas and glioma models, and lipids T1 and global T1 turned out to be less predictive of the outcome of radiation therapy than (101). Considering the clinical application of MOBILE, the method has demonstrated its ability to identify hypoxia in stroke areas (124). The method is currently being investigated in human gliomas, with a pilot study showing that global R1 and lipid R1 values are significantly lower in tumors than in the “normal appearing white matter” of patients or the healthy brains of volunteers and that lipid R1 measurements enable discrimination between tumor areas and peritumoral edema (125).
Using the exogenous source of contrast hexamethyldisiloxane (HMDSO), R. P. Mason’s group identified a source of oxygen-sensitive contrast, called “proton imaging of siloxanes to map tissue oxygenation levels,” which was validated using hyperoxic breathing challenge in Dunning prostate R3327 MAT-Lu tumor-implanted rats but was not assessed as a predictive marker of the outcome of radiation therapy (126).
Electron Paramagnetic Resonance
Quantitative assessments of tumor partial pressure of oxygen can be obtained with EPR. This magnetic resonance technique is sensitive to paramagnetic species (molecules presenting unpaired electrons). Because of the insufficient amount of radical species in viable tissues, EPR oximetry requires the injection of a paramagnetic probe into the site of interest. Particulate probes can be injected in the tumor once and used for repeated measurements during several months, with a high sensitivity (changes lower than 0.2 mmHg can be detected with this method) (8). The interactions between the two unpaired electrons of oxygen and the paramagnetic probe will lead to a change in T2 that can be observed by a change in the linewidth of the EPR spectrum acquired. The measurements themselves are non-invasive and enable the real-time monitoring of oxygenation changes over several hours or more. However, these measurements are restricted to surface tissues: in vivo EPR is performed with “L-band” spectrometers, operating at 1 GHz or less, allowing the penetration of microwaves up to a 10-mm maximum depth into the tissue (127). Although EPR spectrometry provides no anatomical information on tumor hypoxia, it has been successfully used in several tumor models to monitor changes in oxygenation levels induced by an increase in oxygen delivery (128–136), or by an inhibition of tumor consumption (76, 81, 137–143), or both (69, 144–149). As a quantitative technique, EPR oximetry is also predictive of tumor response to radiotherapy and can also be applied to monitor tumor reoxygenation after the administration of a radiosensitizer in order to determine the best therapeutic window in which radiotherapy should be performed (81). In 2010, Khan et al. showed that carbogen-induced reoxygenation of F98 glioma, assessed using EPR oximetry, significantly increased the tumor growth delay after radiation therapy (150). Also, in a study on C6 glioma, a first irradiation was applied and the changes in tumor oxygenation were assessed by EPR oximetry. Some tumors grew up to 150% of their basal oxygenation level. After a second irradiation, the well-reoxygenated tumors had a significant tumor growth delay compared to tumors whose response to the first irradiation was less than 50% from the baseline. In the same study, a second tumor model (9L glioma) did not exhibit an increase in tumor oxygenation after the first irradiation and remained radioresistant (151). Finally, in a study assessing the effect of benzyl nicotinate, EPR oximetry provided dynamic information on the changes in tumor pO2, which could be used to identify responders and non-responders and schedule therapy during the experiments (152). For the moment, the clinical application of EPR spectroscopy is just starting and restricted to three centers owning prototypes of human EPR equipment (153).
Electron paramagnetic resonance imaging is more challenging. Because of the fast relaxation of paramagnetic species (a matter of nanoseconds), most of the EPR experiments use EPR in a “Continuous Wave” mode: unlike in MRI measurements, the sample is submitted to a constant electromagnetic radiation and the measurement is performed by sweeping the magnetic field in order to reach the resonance condition. This increases the acquisition time. However, EPRI has demonstrated its ability to image oxygenation levels quantitatively in vitro and to map tumor hypoxia in preclinical models using a triarylmethyl probe (154, 155). A few researchers have developed home-made “Pulsed” EPR systems that work in the same way as MRI scanners available nowadays.
This makes shorter acquisition times possible and allows the repeated mapping of tumor hypoxia during spontaneous fluctuations or during hyperoxic challenges (156). In order to correlate the pO2 maps with anatomical information and perfusion measurements, researchers have developed a coil enabling EPR and MR imaging (157). By imaging tumor hypoxia with pulsed EPRI, Matsumoto and colleagues were able to follow tumor reoxygenation after the administration of sunitinib (a multi-tyrosine kinase inhibitor) after 4 days of treatment. They combined this chemotherapy with irradiation and concluded that the tumors subjected to a combination of these two therapies had a longer growth delay than tumors in mice receiving one of the therapies separately (158). The EPR measurements are mainly based on the transversal relaxation rate of the spin probe, but they can also be influenced by the probe concentration. A new method was successfully developed to address this issue: Epel and colleagues recently published a paper in which they measured the spin probe longitudinal relaxation rate with pulsed EPRI and a triarylmethyl probe which improves pO2 images by virtually eliminating the sensitivity to triarylmethyl concentration (159).
Finally, progress has been made in improving the sensitivity of MRI by exploiting the paramagnetic resonance: the use of Overhauser-enhanced MRI (OMRI) can increase the sensitivity of 1H measurement. OMRI is based on a double resonance principle: a paramagnetic agent (an EPR sensor) is first hyperpolarized and then a transfer of electron polarization occurs toward the surrounding water’s protons. As a result, the image intensity is enhanced. For oximetric measurements, Oxo63 (a soluble paramagnetic sensor also used in EPRI) can be used and the signal enhancement can be interpreted in terms of oxygenation. The Overhauser enhancement corresponds to the amplitude of the enhanced signal; it depends on the linewidth of the paramagnetic agent, which in turn depends on the oxygen concentration (160). Consequently, OMRI is a quantitative method that is able to monitor tumor oxygenation: it enables the detection of tumor reoxygenation during a carbogen challenge, and the pO2 values assessed at baseline as well as during the carbogen breathing are in agreement with pO2 obtained with Eppendorf electrodes in the same tumors (161). More recently, Oxo63 has been proposed as a marker of both hypoxia and permeability: since the molecular mass of Oxo63 is three times higher than that of gadolinium complexes (usually used as perfusion markers), its blood-to-tissue transfer will reflect permeability rather than perfusion. By analyzing the dynamic enhancement of 1H-MRI after Oxo63 administration to squamous cell carcinoma tumor-bearing mice, the authors concomitantly assessed tumor perfusion (the image’s increased contrast being proportional to the contrast agent concentration) and oxygenation (the image’s enhancement being inversely correlated with the oxygen concentration) (162). Further developments need to be achieved before this technique can be implemented in a clinical setting. In addition, the current research involving OMRI is restricted to a small number of laboratories, since the equipment is not common. For the moment, the major limitation is the undesired heating of the sample due to the saturation pulse.
Conclusion
Several techniques are available to estimate tumor hypoxia. The pO2 measurements assessed with polarographic electrodes have been correlated with treatment outcome in both preclinical and clinical studies. However, this invasive technique is unable to provide maps of tumor hypoxia to plan radiotherapy. PET imaging is the most widespread method used in preclinical and clinical studies. It is involved in the delineation of the targeted volumes for radiotherapy planning. However, this method requires the injection of a radiotracer, and the imaging can only be achieved after a delay to allow tracer accumulation in hypoxic areas. Pulsed EPR imaging is of great interest in assessing tumor hypoxia in preclinical models. However, the instrumentation for pulsed EPR in preclinical conditions is restricted to a few imaging laboratories and the lack of clinical EPR imagers as well as the injection of an EPR sensor limit its applications. MR methods such as measurements and T1 measurement are promising, since they use oxygen as an endogenous contrast agent and can be easily implemented on all MRI scanners. Nevertheless, further studies are needed to investigate whether the relaxation times can be established as biomarkers of hypoxia and, more importantly, as predictive markers of radiotherapy outcome.
Author Contributions
FC contributed to the redaction of the main text and drew the figure; BG revised the manuscript; BJ contributed to the redaction, revision of the main text, and computed data for the table.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
FC is a Televie Fellow, and BJ is a Senior Research Associate of the F.R.S. - FNRS.
References
- 1.Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol (1953) 26(312):638–48. 10.1259/0007-1285-26-312-638 [DOI] [PubMed] [Google Scholar]
- 2.Moulder JE, Rockwell S. Tumor hypoxia: its impact on cancer therapy. Cancer Metastasis Rev (1987) 5(4):313–41. 10.1007/BF00055376 [DOI] [PubMed] [Google Scholar]
- 3.Churchill-Davidson I. Oxygenation in radiotherapy of malignant disease of the upper air passages. The oxygen effect of radiotherapy. Proc R Soc Med (1964) 57:635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JM. Tumor hypoxia in cancer therapy. Methods Enzymol (2007) 435:297–321. 10.1016/S0076-6879(07)35015-5 [DOI] [PubMed] [Google Scholar]
- 5.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol (2007) 25(26):4066–74. 10.1200/JCO.2007.12.7878 [DOI] [PubMed] [Google Scholar]
- 6.Vordermark D, Horsman MR. Hypoxia as a biomarker and for personalized radiation oncology. Recent Results Cancer Res (2016) 198:123–42. 10.1007/978-3-662-49651-0_6 [DOI] [PubMed] [Google Scholar]
- 7.Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol (2012) 9(12):674–87. 10.1038/nrclinonc.2012.171 [DOI] [PubMed] [Google Scholar]
- 8.Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol (2006) 82(10):699–757. 10.1080/09553000601002324 [DOI] [PubMed] [Google Scholar]
- 9.Mason RP, Zhao D, Pacheco-Torres J, Cui W, Kodibagkar VD, Gulaka PK, et al. Multimodality imaging of hypoxia in preclinical settings. Q J Nucl Med Mol Imaging (2010) 54(3):259–80. [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan J, Lo G, King AD. Functional magnetic resonance imaging techniques and their development for radiation therapy planning and monitoring in the head and neck cancers. Quant Imaging Med Surg (2016) 6(4):430–48. 10.21037/qims.2016.06.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price JM, Robinson SP, Koh DM. Imaging hypoxia in tumors with advanced MRI. Q J Nucl Med Mol Imaging (2013) 57:257–70. [PubMed] [Google Scholar]
- 12.Stone HB, Brown JM, Phillips TL, Sutherland RM. Oxygen in human tumors: correlations between methods of measurement and response to therapy. Summary of a workshop held November 19-20, 1992, at the National Cancer Institute, Bethesda, Maryland. Radiat Res (1993) 136(3):422–34. 10.2307/3578556 [DOI] [PubMed] [Google Scholar]
- 13.Dewhirst MW, Klitzman B, Braun RD, Brizel DM, Haroon ZA, Secomb TW. Review of methods used to study oxygen transport at the microcirculatory level. Int J Cancer (2000) 90(5):237–55. [DOI] [PubMed] [Google Scholar]
- 14.Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal (2007) 9(8):1221–35. 10.1089/ars.2007.1628 [DOI] [PubMed] [Google Scholar]
- 15.Nordsmark M, Bentzen SM, Rudat V, Brizel D, Lartigau E, Stadler P, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol (2005) 77(1):18–24. 10.1016/j.radonc.2005.06.038 [DOI] [PubMed] [Google Scholar]
- 16.Turaka A, Buyyounouski MK, Hanlon AL, Horwitz EM, Greenberg RE, Movsas B. Hypoxic prostate/muscle pO2 ratio predicts for outcome in patients with localized prostate cancer: long-term results. Int J Radiat Oncol Biol Phys (2012) 82(3):e433–9. 10.1016/j.ijrobp.2011.05.037 [DOI] [PubMed] [Google Scholar]
- 17.Nordsmark M, Loncaster J, Aquino-Parsons C, Chou SC, Gebski V, West C, et al. The prognostic value of pimonidazole and tumour pO2 in human cervix carcinomas after radiation therapy: a prospective international multi-center study. Radiother Oncol (2006) 80(2):123–31. 10.1016/j.radonc.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 18.Gagel B, Reinartz P, Dimartino E, Zimny M, Pinkawa M, Maneschi P, et al. pO(2) Polarography versus positron emission tomography ([(18)F] fluoromisonidazole, [(18)F]-2-fluoro-2’-deoxyglucose). An appraisal of radiotherapeutically relevant hypoxia. Strahlenther Onkol (2004) 180(10):616–22. 10.1007/s00066-004-1229-y [DOI] [PubMed] [Google Scholar]
- 19.Wardman P. Chemical radiosensitizers for use in radiotherapy. Clin Oncol (R Coll Radiol) (2007) 19(6):397–417. 10.1016/j.clon.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 20.Walsh JC, Lebedev A, Aten E, Madsen K, Marciano L, Kolb HC. The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal (2014) 21(10):1516–54. 10.1089/ars.2013.5378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming IN, Manavaki R, Blower PJ, West C, Williams KJ, Harris AL, et al. Imaging tumour hypoxia with positron emission tomography. Br J Cancer (2015) 112(2):238–50. 10.1038/bjc.2014.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henriques de Figueiredo B, Merlin T, de Clermont-Gallerande H, Hatt M, Vimont D, Fernandez P, et al. Potential of [18F]-fluoromisonidazole positron-emission tomography for radiotherapy planning in head and neck squamous cell carcinomas. Strahlenther Onkol (2013) 189(12):1015–9. 10.1007/s00066-013-0454-7 [DOI] [PubMed] [Google Scholar]
- 23.Mortensen LS, Buus S, Nordsmark M, Bentzen L, Munk OL, Keiding S, et al. Identifying hypoxia in human tumors: a correlation study between 18F-FMISO PET and the Eppendorf oxygen-sensitive electrode. Acta Oncol (2010) 49(7):934–40. 10.3109/0284186X.2010.516274 [DOI] [PubMed] [Google Scholar]
- 24.Rajendran JG, Schwartz DL, O’Sullivan J, Peterson LM, Ng P, Scharnhorst J, et al. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res (2006) 12(18):5435–41. 10.1158/1078-0432.CCR-05-1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schutze C, Bergmann R, Bruchner K, Mosch B, Yaromina A, Zips D, et al. Effect of [(18)F]FMISO stratified dose-escalation on local control in FaDu hSCC in nude mice. Radiother Oncol (2014) 111(1):81–7. 10.1016/j.radonc.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 26.Lee N, Nehmeh S, Schoder H, Fury M, Chan K, Ling CC, et al. Prospective trial incorporating pre-/mid-treatment [18F]-misonidazole positron emission tomography for head-and-neck cancer patients undergoing concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys (2009) 75(1):101–8. 10.1016/j.ijrobp.2008.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monnich D, Welz S, Thorwarth D, Pfannenberg C, Reischl G, Mauz PS, et al. Robustness of quantitative hypoxia PET image analysis for predicting local tumor control. Acta Oncol (2015) 54(9):1364–9. 10.3109/0284186X.2015.1071496 [DOI] [PubMed] [Google Scholar]
- 28.Lee NY, Mechalakos JG, Nehmeh S, Lin Z, Squire OD, Cai S, et al. Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys (2008) 70(1):2–13. 10.1016/j.ijrobp.2007.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Askoxylakis V, Dinkel J, Eichinger M, Stieltjes B, Sommer G, Strauss LG, et al. Multimodal hypoxia imaging and intensity modulated radiation therapy for unresectable non-small-cell lung cancer: the HIL trial. Radiat Oncol (2012) 7:157. 10.1186/1748-717X-7-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zschaeck S, Haase R, Abolmaali N, Perrin R, Stutzer K, Appold S, et al. Spatial distribution of FMISO in head and neck squamous cell carcinomas during radio-chemotherapy and its correlation to pattern of failure. Acta Oncol (2015) 54(9):1355–63. 10.3109/0284186X.2015.1074720 [DOI] [PubMed] [Google Scholar]
- 31.Tachibana I, Nishimura Y, Shibata T, Kanamori S, Nakamatsu K, Koike R, et al. A prospective clinical trial of tumor hypoxia imaging with 18F-fluoromisonidazole positron emission tomography and computed tomography (F-MISO PET/CT) before and during radiation therapy. J Radiat Res (2013) 54(6):1078–84. 10.1093/jrr/rrt033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narita T, Aoyama H, Hirata K, Onodera S, Shiga T, Kobayashi H, et al. Reoxygenation of glioblastoma multiforme treated with fractionated radiotherapy concomitant with temozolomide: changes defined by 18F-fluoromisonidazole positron emission tomography: two case reports. Jpn J Clin Oncol (2012) 42(2):120–3. 10.1093/jjco/hyr181 [DOI] [PubMed] [Google Scholar]
- 33.Kawai N, Maeda Y, Kudomi N, Miyake K, Okada M, Yamamoto Y, et al. Correlation of biological aggressiveness assessed by 11C-methionine PET and hypoxic burden assessed by 18F-fluoromisonidazole PET in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging (2011) 38(3):441–50. 10.1007/s00259-010-1645-4 [DOI] [PubMed] [Google Scholar]
- 34.Farwell MD, Pryma DA, Mankoff DA. PET/CT imaging in cancer: current applications and future directions. Cancer (2014) 120(22):3433–45. 10.1002/cncr.28860 [DOI] [PubMed] [Google Scholar]
- 35.Tran LB, Bol A, Labar D, Jordan B, Magat J, Mignion L, et al. Hypoxia imaging with the nitroimidazole 18F-FAZA PET tracer: a comparison with OxyLite, EPR oximetry and 19F-MRI relaxometry. Radiother Oncol (2012) 105(1):29–35. 10.1016/j.radonc.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 36.Mortensen LS, Busk M, Nordsmark M, Jakobsen S, Theil J, Overgaard J, et al. Accessing radiation response using hypoxia PET imaging and oxygen sensitive electrodes: a preclinical study. Radiother Oncol (2011) 99(3):418–23. 10.1016/j.radonc.2011.06.034 [DOI] [PubMed] [Google Scholar]
- 37.Tran LB, Bol A, Labar D, Karroum O, Bol V, Jordan B, et al. Potential role of hypoxia imaging using (18)F-FAZA PET to guide hypoxia-driven interventions (carbogen breathing or dose escalation) in radiation therapy. Radiother Oncol (2014) 113(2):204–9. 10.1016/j.radonc.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 38.Mortensen LS, Johansen J, Kallehauge J, Primdahl H, Busk M, Lassen P, et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol (2012) 105(1):14–20. 10.1016/j.radonc.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 39.Servagi-Vernat S, Differding S, Sterpin E, Hanin FX, Labar D, Bol A, et al. Hypoxia-guided adaptive radiation dose escalation in head and neck carcinoma: a planning study. Acta Oncol (2015) 54(7):1008–16. 10.3109/0284186X.2014.990109 [DOI] [PubMed] [Google Scholar]
- 40.Carlin S, Zhang H, Reese M, Ramos NN, Chen Q, Ricketts SA. A comparison of the imaging characteristics and microregional distribution of 4 hypoxia PET tracers. J Nucl Med (2014) 55(3):515–21. 10.2967/jnumed.113.126615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peeters SG, Zegers CM, Lieuwes NG, van Elmpt W, Eriksson J, van Dongen GA, et al. A comparative study of the hypoxia PET tracers [(1)(8)F]HX4, [(1)(8)F]FAZA, and [(1)(8)F]FMISO in a preclinical tumor model. Int J Radiat Oncol Biol Phys (2015) 91(2):351–9. 10.1016/j.ijrobp.2014.09.045 [DOI] [PubMed] [Google Scholar]
- 42.Yang DJ, Wallace S, Cherif A, Li C, Gretzer MB, Kim EE, et al. Development of F-18-labeled fluoroerythronitroimidazole as a PET agent for imaging tumor hypoxia. Radiology (1995) 194(3):795–800. 10.1148/radiology.194.3.7862981 [DOI] [PubMed] [Google Scholar]
- 43.Gronroos T, Eskola O, Lehtio K, Minn H, Marjamaki P, Bergman J, et al. Pharmacokinetics of [18F]FETNIM: a potential marker for PET. J Nucl Med (2001) 42(9):1397–404. [PubMed] [Google Scholar]
- 44.Gronroos T, Bentzen L, Marjamaki P, Murata R, Horsman MR, Keiding S, et al. Comparison of the biodistribution of two hypoxia markers [18F]FETNIM and [18F]FMISO in an experimental mammary carcinoma. Eur J Nucl Med Mol Imaging (2004) 31(4):513–20. 10.1007/s00259-003-1404-x [DOI] [PubMed] [Google Scholar]
- 45.Wei Y, Zhao W, Huang Y, Yu Q, Zhu S, Wang S, et al. A comparative study of noninvasive hypoxia imaging with 18F-fluoroerythronitroimidazole and 18F-fluoromisonidazole PET/CT in patients with lung cancer. PLoS One (2016) 11(6):e0157606. 10.1371/journal.pone.0157606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wuest M, Wuest F. Positron emission tomography radiotracers for imaging hypoxia. J Labelled Comp Radiopharm (2013) 56(3–4):244–50. 10.1002/jlcr.2997 [DOI] [PubMed] [Google Scholar]
- 47.Grosu AL, Piert M, Weber WA, Jeremic B, Picchio M, Schratzenstaller U, et al. Positron emission tomography for radiation treatment planning. Strahlenther Onkol (2005) 181(8):483–99. 10.1007/s00066-005-1422-7 [DOI] [PubMed] [Google Scholar]
- 48.Dehdashti F, Grigsby PW, Lewis JS, Laforest R, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by PET with 60Cu-labeled diacetyl-bis(N4-methylthiosemicarbazone). J Nucl Med (2008) 49(2):201–5. 10.2967/jnumed.107.048520 [DOI] [PubMed] [Google Scholar]
- 49.Kinoshita T, Fujii H, Hayashi Y, Kamiyama I, Ohtsuka T, Asamura H. Prognostic significance of hypoxic PET using (18)F-FAZA and (62)Cu-ATSM in non-small-cell lung cancer. Lung Cancer (2016) 91:56–66. 10.1016/j.lungcan.2015.11.020 [DOI] [PubMed] [Google Scholar]
- 50.Lopci E, Grassi I, Rubello D, Colletti PM, Cambioli S, Gamboni A, et al. Prognostic evaluation of disease outcome in solid tumors investigated with 64Cu-ATSM PET/CT. Clin Nucl Med (2016) 41(2):e87–92. 10.1097/RLU.0000000000001017 [DOI] [PubMed] [Google Scholar]
- 51.Bradshaw TJ, Bowen SR, Deveau MA, Kubicek L, White P, Bentzen SM, et al. Molecular imaging biomarkers of resistance to radiation therapy for spontaneous nasal tumors in canines. Int J Radiat Oncol Biol Phys (2015) 91(4):787–95. 10.1016/j.ijrobp.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clausen MM, Hansen AE, Af Rosenschold PM, Kjaer A, Kristensen AT, McEvoy FJ, et al. Dose escalation to high-risk sub-volumes based on non-invasive imaging of hypoxia and glycolytic activity in canine solid tumors: a feasibility study. Radiat Oncol (2013) 8:262. 10.1186/1748-717X-8-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chao KS, Bosch WR, Mutic S, Lewis JS, Dehdashti F, Mintun MA, et al. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys (2001) 49(4):1171–82. 10.1016/S0360-3016(00)01433-4 [DOI] [PubMed] [Google Scholar]
- 54.Nyflot MJ, Harari PM, Yip S, Perlman SB, Jeraj R. Correlation of PET images of metabolism, proliferation and hypoxia to characterize tumor phenotype in patients with cancer of the oropharynx. Radiother Oncol (2012) 105(1):36–40. 10.1016/j.radonc.2012.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burgman P, O’Donoghue JA, Lewis JS, Welch MJ, Humm JL, Ling CC. Cell line-dependent differences in uptake and retention of the hypoxia-selective nuclear imaging agent Cu-ATSM. Nucl Med Biol (2005) 32(6):623–30. 10.1016/j.nucmedbio.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 56.Hueting R, Kersemans V, Cornelissen B, Tredwell M, Hussien K, Christlieb M, et al. A comparison of the behavior of (64)Cu-acetate and (64)Cu-ATSM in vitro and in vivo. J Nucl Med (2014) 55(1):128–34. 10.2967/jnumed.113.119917 [DOI] [PubMed] [Google Scholar]
- 57.Yuan H, Schroeder T, Bowsher JE, Hedlund LW, Wong T, Dewhirst MW. Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone). J Nucl Med (2006) 47(6):989–98. [PubMed] [Google Scholar]
- 58.Matsumoto K, Szajek L, Krishna MC, Cook JA, Seidel J, Grimes K, et al. The influence of tumor oxygenation on hypoxia imaging in murine squamous cell carcinoma using [64Cu]Cu-ATSM or [18F]Fluoromisonidazole positron emission tomography. Int J Oncol (2007) 30(4):873–81. [PubMed] [Google Scholar]
- 59.O’Donoghue JA, Zanzonico P, Pugachev A, Wen B, Smith-Jones P, Cai S, et al. Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) positron emission tomography: comparative study featuring microPET imaging, pO2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models. Int J Radiat Oncol Biol Phys (2005) 61(5):1493–502. 10.1016/j.ijrobp.2004.12.057 [DOI] [PubMed] [Google Scholar]
- 60.Hansen AE, Kristensen AT, Jorgensen JT, McEvoy FJ, Busk M, van der Kogel AJ, et al. (64)Cu-ATSM and (18)FDG PET uptake and (64)Cu-ATSM autoradiography in spontaneous canine tumors: comparison with pimonidazole hypoxia immunohistochemistry. Radiat Oncol (2012) 7:89. 10.1186/1748-717X-7-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li F, Jorgensen JT, Forman J, Hansen AE, Kjaer A. 64Cu-ATSM reflects pO2 levels in human head and neck cancer xenografts but not in colorectal cancer xenografts: comparison with 64CuCl2. J Nucl Med (2016) 57(3):437–43. 10.2967/jnumed.115.155663 [DOI] [PubMed] [Google Scholar]
- 62.Yoshii Y, Yoneda M, Ikawa M, Furukawa T, Kiyono Y, Mori T, et al. Radiolabeled Cu-ATSM as a novel indicator of overreduced intracellular state due to mitochondrial dysfunction: studies with mitochondrial DNA-less rho0 cells and cybrids carrying MELAS mitochondrial DNA mutation. Nucl Med Biol (2012) 39(2):177–85. 10.1016/j.nucmedbio.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 63.Colombie M, Gouard S, Frindel M, Vidal A, Cherel M, Kraeber-Bodere F, et al. Focus on the controversial aspects of (64)Cu-ATSM in tumoral hypoxia mapping by PET imaging. Front Med (2015) 2:58. 10.3389/fmed.2015.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vavere AL, Lewis JS. Examining the relationship between Cu-ATSM hypoxia selectivity and fatty acid synthase expression in human prostate cancer cell lines. Nucl Med Biol (2008) 35(3):273–9. 10.1016/j.nucmedbio.2007.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hunjan S, Zhao D, Constantinescu A, Hahn EW, Antich PP, Mason RP. Tumor oximetry: demonstration of an enhanced dynamic mapping procedure using fluorine-19 echo planar magnetic resonance imaging in the Dunning prostate R3327-AT1 rat tumor. Int J Radiat Oncol Biol Phys (2001) 49(4):1097–108. 10.1016/S0360-3016(00)01460-7 [DOI] [PubMed] [Google Scholar]
- 66.Zhao D, Jiang L, Hahn EW, Mason RP. Tumor physiologic response to combretastatin A4 phosphate assessed by MRI. Int J Radiat Oncol Biol Phys (2005) 62(3):872–80. 10.1016/j.ijrobp.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 67.Zhao D, Jiang L, Hahn EW, Mason RP. Comparison of 1H blood oxygen level-dependent (BOLD) and 19F MRI to investigate tumor oxygenation. Magn Reson Med (2009) 62(2):357–64. 10.1002/mrm.22020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mason RP, Zhao D, Liu L, Trawick ML, Pinney KG. A perspective on vascular disrupting agents that interact with tubulin: preclinical tumor imaging and biological assessment. Integr Biol (Camb) (2011) 3(4):375–87. 10.1039/c0ib00135j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jordan BF, Peeterbroeck J, Karroum O, Diepart C, Magat J, Gregoire V, et al. Captopril and S-nitrosocaptopril as potent radiosensitizers: comparative study and underlying mechanisms. Cancer Lett (2010) 293(2):213–9. 10.1016/j.canlet.2010.01.016 [DOI] [PubMed] [Google Scholar]
- 70.Jordan BF, Cron GO, Gallez B. Rapid monitoring of oxygenation by 19F magnetic resonance imaging: simultaneous comparison with fluorescence quenching. Magn Reson Med (2009) 61(3):634–8. 10.1002/mrm.21594 [DOI] [PubMed] [Google Scholar]
- 71.Magat J, Jordan BF, Cron GO, Gallez B. Noninvasive mapping of spontaneous fluctuations in tumor oxygenation using 19F MRI. Med Phys (2010) 37(10):5434–41. 10.1118/1.3484056 [DOI] [PubMed] [Google Scholar]
- 72.McNab JA, Yung AC, Kozlowski P. Tissue oxygen tension measurements in the Shionogi model of prostate cancer using 19F MRS and MRI. MAGMA (2004) 17(3–6):288–95. 10.1007/s10334-004-0083-3 [DOI] [PubMed] [Google Scholar]
- 73.Shi Y, Oeh J, Eastham-Anderson J, Yee S, Finkle D, Peale FV, Jr, et al. Mapping in vivo tumor oxygenation within viable tumor by 19F-MRI and multispectral analysis. Neoplasia (2013) 15(11):1241–50. 10.1593/neo.131468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mignion L, Magat J, Schakman O, Marbaix E, Gallez B, Jordan BF. Hexafluorobenzene in comparison with perfluoro-15-crown-5-ether for repeated monitoring of oxygenation using 19F MRI in a mouse model. Magn Reson Med (2013) 69(1):248–54. 10.1002/mrm.24245 [DOI] [PubMed] [Google Scholar]
- 75.McMillan KM, Rogers BP, Field AS, Laird AR, Fine JP, Meyerand ME. Physiologic characterisation of glioblastoma multiforme using MRI-based hypoxia mapping, chemical shift imaging, perfusion and diffusion maps. J Clin Neurosci (2006) 13(8):811–7. 10.1016/j.jocn.2005.12.025 [DOI] [PubMed] [Google Scholar]
- 76.Ansiaux R, Dewever J, Gregoire V, Feron O, Jordan BF, Gallez B. Decrease in tumor cell oxygen consumption after treatment with vandetanib (ZACTIMA; ZD6474) and its effect on response to radiotherapy. Radiat Res (2009) 172(5):584–91. 10.1667/RR1744.1 [DOI] [PubMed] [Google Scholar]
- 77.Astner ST, Shi K, Vaupel P, Molls M. Imaging of tumor physiology: impacts on clinical radiation oncology. Exp Oncol (2010) 32(3):149–52. [PubMed] [Google Scholar]
- 78.Øvrebø KM, Gulliksrud K, Mathiesen B, Rofstad EK. Assessment of tumor radioresponsiveness and metastatic potential by dynamic contrast-enhanced magnetic resonance imaging. Int J Radiat Oncol Biol Phys (2011) 81(1):255–61. 10.1016/j.ijrobp.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 79.Ellingsen C, Hompland T, Galappathi K, Mathiesen B, Rofstad EK. DCE-MRI of the hypoxic fraction, radioresponsiveness, and metastatic propensity of cervical carcinoma xenografts. Radiother Oncol (2014) 110(2):335–41. 10.1016/j.radonc.2013.10.018 [DOI] [PubMed] [Google Scholar]
- 80.Shukla-Dave A, Lee NY, Jansen JF, Thaler HT, Stambuk HE, Fury MG, et al. Dynamic contrast-enhanced magnetic resonance imaging as a predictor of outcome in head-and-neck squamous cell carcinoma patients with nodal metastases. Int J Radiat Oncol Biol Phys (2012) 82(5):1837–44. 10.1016/j.ijrobp.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jordan BF, Gallez B. Surrogate MR markers of response to chemo- or radiotherapy in association with co-treatments: a retrospective analysis of multi-modal studies. Contrast Media Mol Imaging (2010) 5(6):323–32. 10.1002/cmmi.397 [DOI] [PubMed] [Google Scholar]
- 82.Cooper RA, Carrington BM, Loncaster JA, Todd SM, Davidson SE, Logue JP, et al. Tumour oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother Oncol (2000) 57(1):53–9. 10.1016/S0167-8140(00)00259-0 [DOI] [PubMed] [Google Scholar]
- 83.Donaldson SB, Betts G, Bonington SC, Homer JJ, Slevin NJ, Kershaw LE, et al. Perfusion estimated with rapid dynamic contrast-enhanced magnetic resonance imaging correlates inversely with vascular endothelial growth factor expression and pimonidazole staining in head-and-neck cancer: a pilot study. Int J Radiat Oncol Biol Phys (2011) 81(4):1176–83. 10.1016/j.ijrobp.2010.09.039 [DOI] [PubMed] [Google Scholar]
- 84.Linnik IV, Scott ML, Holliday KF, Woodhouse N, Waterton JC, O’Connor JP, et al. Noninvasive tumor hypoxia measurement using magnetic resonance imaging in murine U87 glioma xenografts and in patients with glioblastoma. Magn Reson Med (2014) 71(5):1854–62. 10.1002/mrm.24826 [DOI] [PubMed] [Google Scholar]
- 85.Søvik A, Malinen E, Skogmo HK, Bentzen SM, Bruland OS, Olsen DR. Radiotherapy adapted to spatial and temporal variability in tumor hypoxia. Int J Radiat Oncol Biol Phys (2007) 68(5):1496–504. 10.1016/j.ijrobp.2007.04.027 [DOI] [PubMed] [Google Scholar]
- 86.Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging (1997) 7(1):91–101. 10.1002/jmri.1880070113 [DOI] [PubMed] [Google Scholar]
- 87.Andersen EK, Hole KH, Lund KV, Sundfor K, Kristensen GB, Lyng H, et al. Pharmacokinetic parameters derived from dynamic contrast enhanced MRI of cervical cancers predict chemoradiotherapy outcome. Radiother Oncol (2013) 107(1):117–22. 10.1016/j.radonc.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 88.Halle C, Andersen E, Lando M, Aarnes EK, Hasvold G, Holden M, et al. Hypoxia-induced gene expression in chemoradioresistant cervical cancer revealed by dynamic contrast-enhanced MRI. Cancer Res (2012) 72(20):5285–95. 10.1158/0008-5472.CAN-12-1085 [DOI] [PubMed] [Google Scholar]
- 89.Hammond EM, Asselin MC, Forster D, O’Connor JP, Senra JM, Williams KJ. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin Oncol (R Coll Radiol) (2014) 26(5):277–88. 10.1016/j.clon.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 90.Karczmar GS, River JN, Li J, Vijayakumar S, Goldman Z, Lewis MZ. Effects of hyperoxia on T2* and resonance frequency weighted magnetic resonance images of rodent tumours. NMR Biomed (1994) 7(1–2):3–11. 10.1002/nbm.1940070103 [DOI] [PubMed] [Google Scholar]
- 91.Robinson SP, Howe FA, Griffiths JR. Noninvasive monitoring of carbogen-induced changes in tumor blood flow and oxygenation by functional magnetic resonance imaging. Int J Radiat Oncol Biol Phys (1995) 33(4):855–9. 10.1016/0360-3016(95)00072-1 [DOI] [PubMed] [Google Scholar]
- 92.Hallac RR, Zhou H, Pidikiti R, Song K, Stojadinovic S, Zhao D, et al. Correlations of noninvasive BOLD and TOLD MRI with pO2 and relevance to tumor radiation response. Magn Reson Med (2014) 71(5):1863–73. 10.1002/mrm.24846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jordan BF, Misson P, Demeure R, Baudelet C, Beghein N, Gallez B. Changes in tumor oxygenation/perfusion induced by the no donor, isosorbide dinitrate, in comparison with carbogen: monitoring by EPR and MRI. Int J Radiat Oncol Biol Phys (2000) 48(2):565–70. 10.1016/S0360-3016(00)00694-5 [DOI] [PubMed] [Google Scholar]
- 94.Jordan BF, Crokart N, Baudelet C, Cron GO, Ansiaux R, Gallez B. Complex relationship between changes in oxygenation status and changes in R*2: the case of insulin and NS-398, two inhibitors of oxygen consumption. Magn Reson Med (2006) 56(3):637–43. 10.1002/mrm.20963 [DOI] [PubMed] [Google Scholar]
- 95.Christen T, Lemasson B, Pannetier N, Farion R, Remy C, Zaharchuk G, et al. Is T2* enough to assess oxygenation? Quantitative blood oxygen level-dependent analysis in brain tumor. Radiology (2012) 262(2):495–502. 10.1148/radiol.11110518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baudelet C, Gallez B. How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn Reson Med (2002) 48(6):980–6. 10.1002/mrm.10318 [DOI] [PubMed] [Google Scholar]
- 97.Hoskin PJ, Carnell DM, Taylor NJ, Smith RE, Stirling JJ, Daley FM, et al. Hypoxia in prostate cancer: correlation of BOLD-MRI with pimonidazole immunohistochemistry-initial observations. Int J Radiat Oncol Biol Phys (2007) 68(4):1065–71. 10.1016/j.ijrobp.2007.01.018 [DOI] [PubMed] [Google Scholar]
- 98.McPhail LD, Robinson SP. Intrinsic susceptibility MR imaging of chemically induced rat mammary tumors: relationship to histologic assessment of hypoxia and fibrosis. Radiology (2010) 254(1):110–8. 10.1148/radiol.2541090395 [DOI] [PubMed] [Google Scholar]
- 99.Baudelet C, Ansiaux R, Jordan BF, Havaux X, Macq B, Gallez B. Physiological noise in murine solid tumours using T2*-weighted gradient-echo imaging: a marker of tumour acute hypoxia? Phys Med Biol (2004) 49(15):3389–411. 10.1088/0031-9155/49/15/006 [DOI] [PubMed] [Google Scholar]
- 100.Rodrigues LM, Howe FA, Griffiths JR, Robinson SP. Tumor R2* is a prognostic indicator of acute radiotherapeutic response in rodent tumors. J Magn Reson Imaging (2004) 19(4):482–8. 10.1002/jmri.20024 [DOI] [PubMed] [Google Scholar]
- 101.Cao-Pham TT, Tran LB, Colliez F, Joudiou N, El Bachiri S, Gregoire V, et al. Monitoring tumor response to carbogen breathing by oxygen-sensitive magnetic resonance parameters to predict the outcome of radiation therapy: a preclinical study. Int J Radiat Oncol Biol Phys (2016) 96(1):149–60. 10.1016/j.ijrobp.2016.04.029 [DOI] [PubMed] [Google Scholar]
- 102.Kim CK, Park SY, Park BK, Park W, Huh SJ. Blood oxygenation level-dependent MR imaging as a predictor of therapeutic response to concurrent chemoradiotherapy in cervical cancer: a preliminary experience. Eur Radiol (2014) 24(7):1514–20. 10.1007/s00330-014-3167-0 [DOI] [PubMed] [Google Scholar]
- 103.Yablonskiy DA, Sukstanskii AL, He X. Blood oxygenation level-dependent (BOLD)-based techniques for the quantification of brain hemodynamic and metabolic properties – theoretical models and experimental approaches. NMR Biomed (2013) 26(8):963–86. 10.1002/nbm.2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Christen T, Bouzat P, Pannetier N, Coquery N, Moisan A, Lemasson B, et al. Tissue oxygen saturation mapping with magnetic resonance imaging. J Cereb Blood Flow Metab (2014) 34(9):1550–7. 10.1038/jcbfm.2014.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hatabu H, Tadamura E, Chen Q, Stock KW, Li W, Prasad PV, et al. Pulmonary ventilation: dynamic MRI with inhalation of molecular oxygen. Eur J Radiol (2001) 37(3):172–8. 10.1016/S0720-048X(00)00298-9 [DOI] [PubMed] [Google Scholar]
- 106.Muir ER, Cardenas D, Huang S, Roby J, Li G, Duong TQ. MRI under hyperbaric air and oxygen: effects on local magnetic field and relaxation times. Magn Reson Med (2014) 72(4):1176–81. 10.1002/mrm.25027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Winter JD, Estrada M, Cheng HL. Normal tissue quantitative T1 and T2* MRI relaxation time responses to hypercapnic and hyperoxic gases. Acad Radiol (2011) 18(9):1159–67. 10.1016/j.acra.2011.04.016 [DOI] [PubMed] [Google Scholar]
- 108.Uematsu H, Takahashi M, Hatabu H, Chin CL, Wehrli SL, Wehrli FW, et al. Changes in T1 and T2 observed in brain magnetic resonance imaging with delivery of high concentrations of oxygen. J Comput Assist Tomogr (2007) 31(5):662–5. 10.1097/rct.0b013e3180319114 [DOI] [PubMed] [Google Scholar]
- 109.Burrell JS, Walker-Samuel S, Baker LC, Boult JK, Jamin Y, Halliday J, et al. Exploring DeltaR(2) * and DeltaR(1) as imaging biomarkers of tumor oxygenation. J Magn Reson Imaging (2013) 38(2):429–34. 10.1002/jmri.23987 [DOI] [PubMed] [Google Scholar]
- 110.O’Connor JP, Rose CJ, Waterton JC, Carano RA, Parker GJ, Jackson A. Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res (2015) 21(2):249–57. 10.1158/1078-0432.CCR-14-0990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tadamura E, Hatabu H, Li W, Prasad PV, Edelman RR. Effect of oxygen inhalation on relaxation times in various tissues. J Magn Reson Imaging (1997) 7(1):220–5. 10.1002/jmri.1880070134 [DOI] [PubMed] [Google Scholar]
- 112.Noseworthy MD, Kim JK, Stainsby JA, Stanisz GJ, Wright GA. Tracking oxygen effects on MR signal in blood and skeletal muscle during hyperoxia exposure. J Magn Reson Imaging (1999) 9(6):814–20. [DOI] [PubMed] [Google Scholar]
- 113.McGrath DM, Naish JH, O’Connor JP, Hutchinson CE, Waterton JC, Taylor CJ, et al. Oxygen-induced changes in longitudinal relaxation times in skeletal muscle. Magn Reson Imaging (2008) 26(2):221–7. 10.1016/j.mri.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 114.O’Connor JP, Jackson A, Buonaccorsi GA, Buckley DL, Roberts C, Watson Y, et al. Organ-specific effects of oxygen and carbogen gas inhalation on tissue longitudinal relaxation times. Magn Reson Med (2007) 58(3):490–6. 10.1002/mrm.21357 [DOI] [PubMed] [Google Scholar]
- 115.Kim KA, Park MS, Kim IS, Kiefer B, Chung WS, Kim MJ, et al. Quantitative evaluation of liver cirrhosis using T1 relaxation time with 3 Tesla MRI before and after oxygen inhalation. J Magn Reson Imaging (2012) 36(2):405–10. 10.1002/jmri.23620 [DOI] [PubMed] [Google Scholar]
- 116.Huen I, Morris DM, Wright C, Parker GJ, Sibley CP, Johnstone ED, et al. R1 and R2 * changes in the human placenta in response to maternal oxygen challenge. Magn Reson Med (2013) 70(5):1427–33. 10.1002/mrm.24581 [DOI] [PubMed] [Google Scholar]
- 117.O’Connor JP, Naish JH, Parker GJ, Waterton JC, Watson Y, Jayson GC, et al. Preliminary study of oxygen-enhanced longitudinal relaxation in MRI: a potential novel biomarker of oxygenation changes in solid tumors. Int J Radiat Oncol Biol Phys (2009) 75(4):1209–15. 10.1016/j.ijrobp.2008.12.040 [DOI] [PubMed] [Google Scholar]
- 118.Ding Y, Mason RP, McColl RW, Yuan Q, Hallac RR, Sims RD, et al. Simultaneous measurement of tissue oxygen level-dependent (TOLD) and blood oxygenation level-dependent (BOLD) effects in abdominal tissue oxygenation level studies. J Magn Reson Imaging (2013) 38(5):1230–6. 10.1002/jmri.24006 [DOI] [PubMed] [Google Scholar]
- 119.Haddock B, Larsson HB, Hansen AE, Rostrup E. Measurement of brain oxygenation changes using dynamic T(1)-weighted imaging. Neuroimage (2013) 78:7–15. 10.1016/j.neuroimage.2013.03.068 [DOI] [PubMed] [Google Scholar]
- 120.Zhao D, Pacheco-Torres J, Hallac RR, White D, Peschke P, Cerdan S, et al. Dynamic oxygen challenge evaluated by NMR T1 and T2* – insights into tumor oxygenation. NMR Biomed (2015) 28(8):937–47. 10.1002/nbm.3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.White DA, Zhang Z, Li L, Gerberich J, Stojadinovic S, Peschke P, et al. Developing oxygen-enhanced magnetic resonance imaging as a prognostic biomarker of radiation response. Cancer Lett (2016) 380(1):69–77. 10.1016/j.canlet.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jordan BF, Magat J, Colliez F, Ozel E, Fruytier AC, Marchand V, et al. Mapping of oxygen by imaging lipids relaxation enhancement: a potential sensitive endogenous MRI contrast to map variations in tissue oxygenation. Magn Reson Med (2013) 70(3):732–44. 10.1002/mrm.24511 [DOI] [PubMed] [Google Scholar]
- 123.Colliez F, Neveu MA, Magat J, Cao Pham TT, Gallez B, Jordan BF. Qualification of a noninvasive magnetic resonance imaging biomarker to assess tumor oxygenation. Clin Cancer Res (2014) 20(21):5403–11. 10.1158/1078-0432.CCR-13-3434 [DOI] [PubMed] [Google Scholar]
- 124.Colliez F, Safronova MM, Magat J, Joudiou N, Peeters AP, Jordan BF, et al. Oxygen mapping within healthy and acutely infarcted brain tissue in humans using the NMR relaxation of lipids: a proof-of-concept translational study. PLoS One (2015) 10(8):e0135248. 10.1371/journal.pone.0135248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Safronova MM, Colliez F, Magat J, Joudiou N, Jordan BF, Raftopoulos C, et al. Mapping of global R1 and R2* values versus lipids R1 values as potential markers of hypoxia in human glial tumors: a feasibility study. Magn Reson Imaging (2016) 34(2):105–13. 10.1016/j.mri.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 126.Kodibagkar VD, Wang X, Pacheco-Torres J, Gulaka P, Mason RP. Proton imaging of siloxanes to map tissue oxygenation levels (PISTOL): a tool for quantitative tissue oximetry. NMR Biomed (2008) 21(8):899–907. 10.1002/nbm.1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gallez B, Baudelet C, Jordan BF. Assessment of tumor oxygenation by electron paramagnetic resonance: principles and applications. NMR Biomed (2004) 17(5):240–62. 10.1002/nbm.900 [DOI] [PubMed] [Google Scholar]
- 128.Gallez B, Jordan BF, Baudelet C, Misson PD. Pharmacological modifications of the partial pressure of oxygen in murine tumors: evaluation using in vivo EPR oximetry. Magn Reson Med (1999) 42(4):627–30. [DOI] [PubMed] [Google Scholar]
- 129.Ansiaux R, Baudelet C, Jordan BF, Beghein N, Sonveaux P, De Wever J, et al. Thalidomide radiosensitizes tumors through early changes in the tumor microenvironment. Clin Cancer Res (2005) 11(2 Pt 1):743–50. [PubMed] [Google Scholar]
- 130.Crokart N, Jordan BF, Baudelet C, Ansiaux R, Sonveaux P, Gregoire V, et al. Early reoxygenation in tumors after irradiation: determining factors and consequences for radiotherapy regimens using daily multiple fractions. Int J Radiat Oncol Biol Phys (2005) 63(3):901–10. 10.1016/j.ijrobp.2005.02.038 [DOI] [PubMed] [Google Scholar]
- 131.Cron GO, Beghein N, Crokart N, Chavee E, Bernard S, Vynckier S, et al. Changes in the tumor microenvironment during low-dose-rate permanent seed implantation iodine-125 brachytherapy. Int J Radiat Oncol Biol Phys (2005) 63(4):1245–51. 10.1016/j.ijrobp.2005.07.971 [DOI] [PubMed] [Google Scholar]
- 132.Ansiaux R, Baudelet C, Cron GO, Segers J, Dessy C, Martinive P, et al. Botulinum toxin potentiates cancer radiotherapy and chemotherapy. Clin Cancer Res (2006) 12(4):1276–83. 10.1158/1078-0432.CCR-05-1222 [DOI] [PubMed] [Google Scholar]
- 133.Cron GO, Beghein N, Ansiaux R, Martinive P, Feron O, Gallez B. 19F NMR in vivo spectroscopy reflects the effectiveness of perfusion-enhancing vascular modifiers for improving gemcitabine chemotherapy. Magn Reson Med (2008) 59(1):19–27. 10.1002/mrm.21469 [DOI] [PubMed] [Google Scholar]
- 134.Khan N, Li H, Hou H, Lariviere JP, Gladstone DJ, Demidenko E, et al. Tissue pO2 of orthotopic 9L and C6 gliomas and tumor-specific response to radiotherapy and hyperoxygenation. Int J Radiat Oncol Biol Phys (2009) 73(3):878–85. 10.1016/j.ijrobp.2008.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Segers J, Crokart N, Danhier P, Gregoire V, Jordan BF, Gallez B. Use of xanthinol nicotinate as a co-treatment for radio- and chemo-therapy in experimental tumors. Int J Cancer (2010) 126(2):583–8. 10.1002/ijc.24724 [DOI] [PubMed] [Google Scholar]
- 136.Crokart N, Danhier F, Daugimont L, Goncalves N, Jordan BF, Gregoire V, et al. Potentiation of radiotherapy by a localized antiangiogenic gene therapy. Radiother Oncol (2013) 107(2):252–8. 10.1016/j.radonc.2013.03.018 [DOI] [PubMed] [Google Scholar]
- 137.Jordan BF, Gregoire V, Demeure RJ, Sonveaux P, Feron O, O’Hara J, et al. Insulin increases the sensitivity of tumors to irradiation: involvement of an increase in tumor oxygenation mediated by a nitric oxide-dependent decrease of the tumor cells oxygen consumption. Cancer Res (2002) 62(12):3555–61. [PubMed] [Google Scholar]
- 138.Crokart N, Radermacher K, Jordan BF, Baudelet C, Cron GO, Gregoire V, et al. Tumor radiosensitization by antiinflammatory drugs: evidence for a new mechanism involving the oxygen effect. Cancer Res (2005) 65(17):7911–6. 10.1158/0008-5472.CAN-05-1288 [DOI] [PubMed] [Google Scholar]
- 139.Ansiaux R, Baudelet C, Jordan BF, Crokart N, Martinive P, DeWever J, et al. Mechanism of reoxygenation after antiangiogenic therapy using SU5416 and its importance for guiding combined antitumor therapy. Cancer Res (2006) 66(19):9698–704. 10.1158/0008-5472.CAN-06-1854 [DOI] [PubMed] [Google Scholar]
- 140.Crokart N, Jordan BF, Baudelet C, Cron GO, Hotton J, Radermacher K, et al. Glucocorticoids modulate tumor radiation response through a decrease in tumor oxygen consumption. Clin Cancer Res (2007) 13(2 Pt 1):630–5. 10.1158/1078-0432.CCR-06-0802 [DOI] [PubMed] [Google Scholar]
- 141.Jordan BF, Christian N, Crokart N, Gregoire V, Feron O, Gallez B. Thyroid status is a key modulator of tumor oxygenation: implication for radiation therapy. Radiat Res (2007) 168(4):428–32. 10.1667/RR0931.1 [DOI] [PubMed] [Google Scholar]
- 142.Diepart C, Karroum O, Magat J, Feron O, Verrax J, Calderon PB, et al. Arsenic trioxide treatment decreases the oxygen consumption rate of tumor cells and radiosensitizes solid tumors. Cancer Res (2012) 72(2):482–90. 10.1158/0008-5472.CAN-11-1755 [DOI] [PubMed] [Google Scholar]
- 143.De Preter G, Deriemaeker C, Danhier P, Brisson L, Cao Pham TT, Gregoire V, et al. A fast hydrogen sulfide-releasing donor increases the tumor response to radiotherapy. Mol Cancer Ther (2016) 15(1):154–61. 10.1158/1535-7163.MCT-15-0691-T [DOI] [PubMed] [Google Scholar]
- 144.Jordan BF, Beghein N, Aubry M, Gregoire V, Gallez B. Potentiation of radiation-induced regrowth delay by isosorbide dinitrate in FSaII murine tumors. Int J Cancer (2003) 103(1):138–41. 10.1002/ijc.10786 [DOI] [PubMed] [Google Scholar]
- 145.Jordan BF, Sonveaux P, Feron O, Gregoire V, Beghein N, Gallez B. Nitric oxide-mediated increase in tumor blood flow and oxygenation of tumors implanted in muscles stimulated by electric pulses. Int J Radiat Oncol Biol Phys (2003) 55(4):1066–73. 10.1016/S0360-3016(02)04505-4 [DOI] [PubMed] [Google Scholar]
- 146.Jordan BF, Sonveaux P, Feron O, Gregoire V, Beghein N, Dessy C, et al. Nitric oxide as a radiosensitizer: evidence for an intrinsic role in addition to its effect on oxygen delivery and consumption. Int J Cancer (2004) 109(5):768–73. 10.1002/ijc.20046 [DOI] [PubMed] [Google Scholar]
- 147.Sonveaux P, Jordan BF, Gallez B, Feron O. Nitric oxide delivery to cancer: why and how? Eur J Cancer (2009) 45(8):1352–69. 10.1016/j.ejca.2008.12.018 [DOI] [PubMed] [Google Scholar]
- 148.Karroum O, Kengen J, Danhier P, Magat J, Mignion L, Bouzin C, et al. Tumor reoxygenation following administration of mitogen-activated protein kinase inhibitors: a rationale for combination with radiation therapy. Radiother Oncol (2012) 105(1):64–71. 10.1016/j.radonc.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 149.Karroum O, Kengen J, Gregoire V, Gallez B, Jordan BF. Tumor reoxygenation following administration of the EGFR inhibitor, gefitinib, in experimental tumors. Adv Exp Med Biol (2013) 789:265–71. 10.1007/978-1-4614-7411-1_36 [DOI] [PubMed] [Google Scholar]
- 150.Khan N, Mupparaju S, Hekmatyar SK, Hou H, Lariviere JP, Demidenko E, et al. Effect of hyperoxygenation on tissue pO2 and its effect on radiotherapeutic efficacy of orthotopic F98 gliomas. Int J Radiat Oncol Biol Phys (2010) 78(4):1193–200. 10.1016/j.ijrobp.2010.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hou H, Mupparaju SP, Lariviere JP, Hodge S, Gui J, Swartz HM, et al. Assessment of the changes in 9L and C6 glioma pO2 by EPR oximetry as a prognostic indicator of differential response to radiotherapy. Radiat Res (2013) 179(3):343–51. 10.1667/RR2811.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hou H, Abramovic Z, Lariviere JP, Sentjurc M, Swartz H, Khan N. Effect of a topical vasodilator on tumor hypoxia and tumor oxygen guided radiotherapy using EPR oximetry. Radiat Res (2010) 173(5):651–8. 10.1667/RR1947.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jarvis LA, Williams BB, Schaner PE, Chen EY, Angeles CV, Hou H, et al. Phase 1 clinical trial of OxyChip, an implantable absolute pO2 sensor for tumor oximetry. Int J Radiat Oncol Biol Phys (2016) 96(2S):S109–10. 10.1016/j.ijrobp.2016.06.268 [DOI] [Google Scholar]
- 154.Velan SS, Spencer RG, Zweier JL, Kuppusamy P. Electron paramagnetic resonance oxygen mapping (EPROM): direct visualization of oxygen concentration in tissue. Magn Reson Med (2000) 43(6):804–9. [DOI] [PubMed] [Google Scholar]
- 155.Subramanian S, Koscielniak JW, Devasahayam N, Pursley RH, Pohida TJ, Krishna MC. A new strategy for fast radiofrequency CW EPR imaging: direct detection with rapid scan and rotating gradients. J Magn Reson (2007) 186(2):212–9. 10.1016/j.jmr.2007.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yasui H, Matsumoto S, Devasahayam N, Munasinghe JP, Choudhuri R, Saito K, et al. Low-field magnetic resonance imaging to visualize chronic and cycling hypoxia in tumor-bearing mice. Cancer Res (2010) 70(16):6427–36. 10.1158/0008-5472.CAN-10-1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Krishna MC, Matsumoto S, Yasui H, Saito K, Devasahayam N, Subramanian S, et al. Electron paramagnetic resonance imaging of tumor pO(2). Radiat Res (2012) 177(4):376–86. 10.1667/RR2622.1 [DOI] [PubMed] [Google Scholar]
- 158.Matsumoto S, Saito K, Takakusagi Y, Matsuo M, Munasinghe JP, Morris HD, et al. In vivo imaging of tumor physiological, metabolic, and redox changes in response to the anti-angiogenic agent sunitinib: longitudinal assessment to identify transient vascular renormalization. Antioxid Redox Signal (2014) 21(8):1145–55. 10.1089/ars.2013.5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Epel B, Bowman MK, Mailer C, Halpern HJ. Absolute oxygen R1e imaging in vivo with pulse electron paramagnetic resonance. Magn Reson Med (2014) 72(2):362–8. 10.1002/mrm.24926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Subramanian S, Matsumoto K, Mitchell JB, Krishna MC. Radio frequency continuous-wave and time-domain EPR imaging and Overhauser-enhanced magnetic resonance imaging of small animals: instrumental developments and comparison of relative merits for functional imaging. NMR Biomed (2004) 17(5):263–94. 10.1002/nbm.897 [DOI] [PubMed] [Google Scholar]
- 161.Krishna MC, English S, Yamada K, Yoo J, Murugesan R, Devasahayam N, et al. Overhauser enhanced magnetic resonance imaging for tumor oximetry: coregistration of tumor anatomy and tissue oxygen concentration. Proc Natl Acad Sci U S A (2002) 99(4):2216–21. 10.1073/pnas.042671399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Matsumoto S, Yasui H, Batra S, Kinoshita Y, Bernardo M, Munasinghe JP, et al. Simultaneous imaging of tumor oxygenation and microvascular permeability using Overhauser enhanced MRI. Proc Natl Acad Sci U S A (2009) 106(42):17898–903. 10.1073/pnas.0908447106 [DOI] [PMC free article] [PubMed] [Google Scholar]


