Abstract
Background
Angiogenesis, a hallmark of glioblastoma, can potentially be targeted by inhibiting the VEGF pathway using bevacizumab, a humanized monoclonal antibody against VEGF-A. This study was designed to determine the efficacy and safety of these regimens in the cooperative group setting.
Methods
Eligibility included age ≥ 18, recurrent or progressive GBM after standard chemoradiation. Treatment was intravenous bevacizumab 10 mg/kg and either irinotecan (CPT) 125 mg/m2 every 2 weeks or temozolomide (TMZ) 75–100 mg/m2 day 1–21 of 28 day cycle. Accrual goal was 57 eligible patients per arm. Primary endpoint was 6 month progression-free survival (6-m PFS); a predetermined rate of ≥ 35% to declare efficacy.
Results
60 eligible patients were enrolled on TMZ arm and 57 patients on CPT arm. Median age was 56, median KPS was 80. For TMZ arm, the 6-m-PFS rate was 39%(23/59); for the CPT arm, the 6-m-PFS rate was 38.6% (22/57). Objective responses: TMZ arm had 2(3%) CR, 9(16%) PR; CPT arm had 2(4%) CR, 13(24%) PR. Overall there was moderate toxicity: TMZ arm with 33(55%) grade 3, 11(18%) grade 4, and 1(2%) grade 5(fatal) toxicities; CPT arm had 22(39%) grade 3, 7(12%) grade 4, and 3(5%) grade 5 toxicities.
Conclusions
The 6-m-PFS surpassed the predetermined efficacy threshold for both arms, corroborating the efficacy of bevacizumab and CPT and confirming activity for bevacizumab and protracted TMZ for recurrent/progressive GBM, even after prior temozolomide exposure. Toxicities were within anticipated frequencies with a moderately high rate of venous thrombosis, moderate hypertension and one intracranial hemorrhage.
Keywords: glioblastoma, anti-angiogenic treatment, randomized trial, combination therapy
Introduction
The prognosis for patients with recurrent malignant glioma is poor. Patients with recurrent glioblastoma (GBM) have a median survival of 4 months, despite the administration of a variety of chemotherapy regimens including small molecule signal transduction modulators.1 In addition to the inherent treatment resistance often present in recurrent malignant gliomas, most available treatments have been limited by problems with delivery to the tumor because of widespread tumor infiltration into surrounding brain parenchyma, further accentuated by the inability to effectively cross the blood-brain barrier.2 Therefore, there has been great interest in targeting the angiogenesis that is a prominent feature of malignant gliomas, particularly GBM.3 Prior studies suggest that targeting the endothelial cells involved in tumor angiogenesis is not hampered by the development of resistance and that an early effect of anti-angiogenic treatment may be vascular normalization that improves regional blood flow and reduces edema and interstitial pressure potentially leading to better drug delivery.4,5 Additionally, studies suggest that prolonged exposure to lower doses of certain cytotoxic chemotherapy agents can provide an anti-angiogenesis effect, presumably by decreasing endothelial cell viability6 and further, there may be a selective effect on the stem cell niche.7,8
Several clinical trials have been published describing the use of bevacizumab in patients with recurrent malignant glioma. These trials describe the efficacy and toxicities of treatment both with bevacizumab as single agent or in combination with irinotecan, a combination with established efficacy in colon cancer.9,10 Despite concerns regarding the potential for intratumoral hemorrhage, the prior reports compiled in a recent review, suggest that this complication is infrequent in gliomas.11 The trials in recurrent glioma report a high objective response rate, as well as prolonged tumor control as determined by the 6-month progression free survival rate. On the basis of these results, bevacizumab as single agent treatment was given accelerated approval for recurrent GBM.12
Although single agent bevacizumab is approved for recurrent GBM, the combination of anti-angiogenic therapies with cytotoxic chemotherapy agents remains a commonly used strategy for many cancers including colon cancer, where bevacizumab as a single agent did not show efficacy.13 For malignant gliomas, the optimal bevacizumab treatment, either as a single agent or in a combination regimen, has not been established. A prior randomized phase II study evaluating bevacizumab alone and bevacizumab plus irinotecan was not designed to compare the two treatment regimens, but remains the largest multicenter effort in recurrent GBM to date, with a total of 167 patients accrued.10 The 6-month progression free survival rate in this study was 42.6% in the single agent bevacizumab arm and 50.3% with the bevacizumab and irinotecan combination. Objective response rates and median survival from study entry were 28.2% and 9.2 months for bevacizumab alone and 37.8% and 8.7 months for the combination. A high percentage of patients in both treatment arms developed grade 3 or higher toxicities with hypertension prominent in both arms, and fatigue and venous thromboembolic events more prevalent with the combination regimen.
The early efficacy data of bevacizumab with irinotecan, further supported by subsequent studies have generated interest in exploring the potential efficacy in combination with other cytotoxic agents. The spectrum of combination regimens with bevacizumab has recently been reviewed by Reardon et al.14 Recently, the results of two clinical trials evaluating the role of bevacizumab as a component of frontline therapy were reported.15,16 Both studies showed a prolongation of progression free survival, but overall survival was not improved.
Materials and Methods
Eligible patients had recurrent or progressive GBM or gliosarcoma. All patients were required to provide written informed consent. There were no limits placed on the number of prior treatment regimens, although patients with prior treatment with interstitial brachytherapy, stereotactic radiosurgery or Gliadel® wafers (polifeprosan 20 with carmustine implant) were required to have histologic evidence of recurrent tumor. Measurable tumor was not required if the patient underwent a repeat tumor resection prior to enrollment. Patients must have had completed radiation treatment more than 42 days prior to enrollment. Other important inclusion criteria included age ≥ 18 years, Karnofsky Performance status ≥ 70, systolic blood pressure ≤ 160 mg Hg or diastolic pressure ≤ 90 mg Hg, adequate hematologic function (white blood cell count (WBC) ≥ 3,000/µL, absolute neutrophil count (ANC) ≥ 1,500/µL, platelet count ≥ 100,000 cells/µL, and hemoglobin ≥ 10 gm/µL) renal and hepatic function. Patients must have been on a stable or decreasing dose of corticosteroids for the 5 days prior to study enrollment. Systemic anticoagulation with either warfarin or low molecular weight heparin was permitted.
Exclusion criteria included ongoing treatment with a hepatic enzyme-inducing anticonvulsant; an acute intratumoral hemorrhage on MR imaging; an active comorbid condition including recent (< 6 months) myocardial infarction, unstable angina, uncontrolled hypertension or history of recent (< 6 months) stroke or transient ischemic attack; major surgical procedure or history of abdominal abscess or fistula or gastrointestinal perforation within 28 days of study enrollment.
Study design
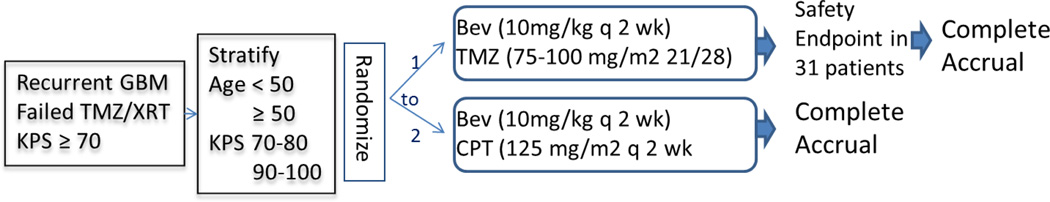
As outlined in Figure 1, patients were randomized to receive bevacizumab with irinotecan or temozolomide. The initial randomization was 2 to 1 to the irinotecan and temozolomide arms, respectively. This design allowed a 2 stage evaluation of the safety of the bevacizumab plus temozolomide arm, as the safety of this combination had not been previously evaluated. All patients received bevacizumab at a dose of 10 mg/kg every 2 weeks. Patients randomized to receive irinotecan received this agent at 125 mg/m2 every two weeks along with bevacizumab. Patients randomized to receive temozolomide were treated with a dose-dense schedule starting at 75 mg/m2 on days 1–21 of a 28-day cycle. Patients who did not develop grade 2 or higher myelotoxicity had the temozolomide dose increased to 100 mg/m2 for subsequent cycles. A cycle was defined by 4 weeks of treatment and patients were permitted to continue treatment for up to 24 cycles as long as the treatment was tolerated and there was no evidence of tumor progression. In case of toxicity, there were no dose modifications allowed for bevacizumab. If adverse events that required holding treatment with bevacizumab did not resolve within 8 weeks, bevacizumab treatment was discontinued. Specific toxicities such as intestinal perforation, central nervous system or pulmonary hemorrhage, congestive heart failure, myocardial ischemia, stroke or uncontrollable hypertension mandated permanent cessation of bevacizumab treatment. For irinotecan, grade 3 or 4 toxicities required holding treatment until these resolved to grade 1 or less. The dose was then reduced to 100 mg/m2. If grade 3 or 4 toxicities were noted at the lower dose, then a final dose reduction to 75 mg/m2 was permitted. Subsequent grade 3 or 4 toxicities mandated cessation of treatment. For temozolomide, grade 3 or 4 toxicities resulted in a dose reduction to 50 mg/m2 if the patient did not have the initial cycle 2 dose escalation or a dose reduction to 75 mg/m2 if the dose had previously been increased to 100mg/m2. An additional dose reduction to 35 mg/m2 was possible, but toxicity at this lowest dose level mandated treatment cessation. For both irinotecan and temozolomide, if treatment delays exceeded 4 weeks, the treatment was stopped.
Figure 1.
Study Schema
Assessments
Patients underwent a complete history and physical examination, baseline MR imaging, electrocardiogram and routine serum chemistries and hematologic studies within 14 days of initiating treatment. Subsequently, serum chemistries and complete blood counts were evaluated before each infusion of bevacizumab along with blood pressure measurements. Physical examination and urinary protein to creatinine (UPC) ratio was performed prior to each cycle of therapy. Repeat MR imaging was performed before initiation of odd numbered cycles. Objective responses included both partial and complete responses based on MR imaging and were determined using the MacDonald criteria.17 Partial responses and stable disease also required a confirmatory MR imaging at least one month later. All patients were followed until voluntary withdrawal of consent, loss to follow-up or death.
Statistical Considerations
Patients were stratified according to age (<50 years vs. ≥50 years) and Karnofsky performance status (70–80 vs. 90–100) then randomized in a 2:1 ratio between the bevacizumab and irinotecan arm and the bevacizumab and temozolomide arm according to the permuted block design as described by Zelen.18 The primary endpoint for the bevacizumab plus irinotecan arm was the 6-month progression-free survival rate (6-m PFS) which is measured as a proportion. The primary endpoint for the bevacizumab plus dose-dense temozolomide arm was safety and treatment-related toxicity. Secondary endpoints for the bevacizumab plus irinotecan arm included objective response rate, overall survival from study entry and assessment of treatment related toxicities. For the bevacizumab plus temozolomide arm, secondary endpoints included determination of the 6-m PFS, overall survival and objective response rates.
Sample size for efficacy for both arms was based on historical data reporting that 6-m PFS for 225 patients with GBM enrolled on 8 phase II studies for recurrent disease had an overall 6-m PFS of 15% with the 95% confidence interval of 10 to 19%. P0 was, therefore, set to 20% and p1 to 35%, looking for a 15% improvement. The type I error rate was set at 10% and power was 90%. Based on the above design parameters, accrual was set at 60 patients (assuming a 5% ineligible rate) to have the required 57 analyzable patients.
For determination of treatment tolerance of the bevacizumab and temozolomide arm, it was assumed there will be 15% discontinuation rate (a reduction from historical data of 35%). The type I error was set as 10% and the power was 90%. The two-stage design by Fleming is used to calculate the required sample size in each stage and corresponding rules for judging efficacy and toxicity.19 Twenty-nine analyzable patients are required for the first stage. If less than 7 patients experience drug discontinuation due to complication and 7–10 patients experience 6-m PFS among the first 29 patients, a total of 57 analyzable patients will be accrued for this arm.
Results
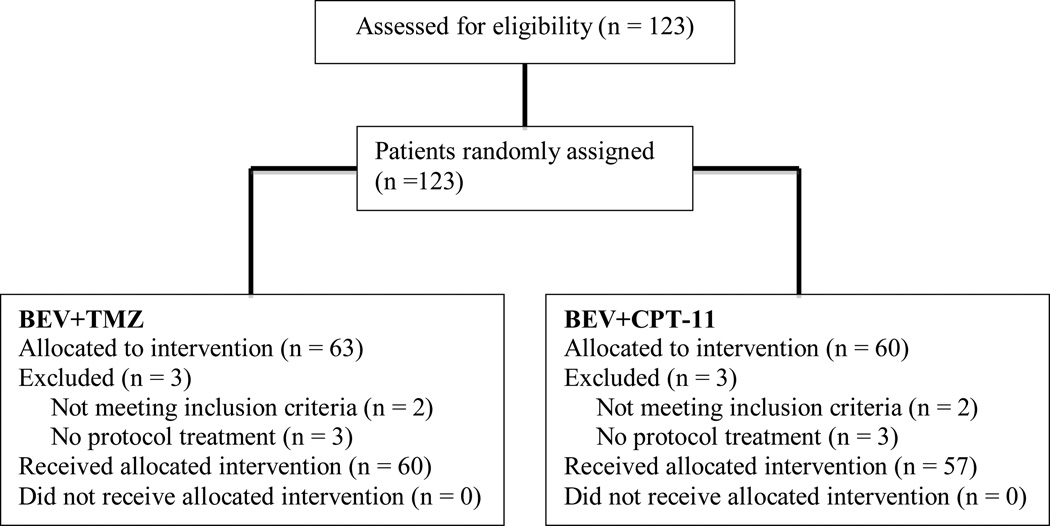
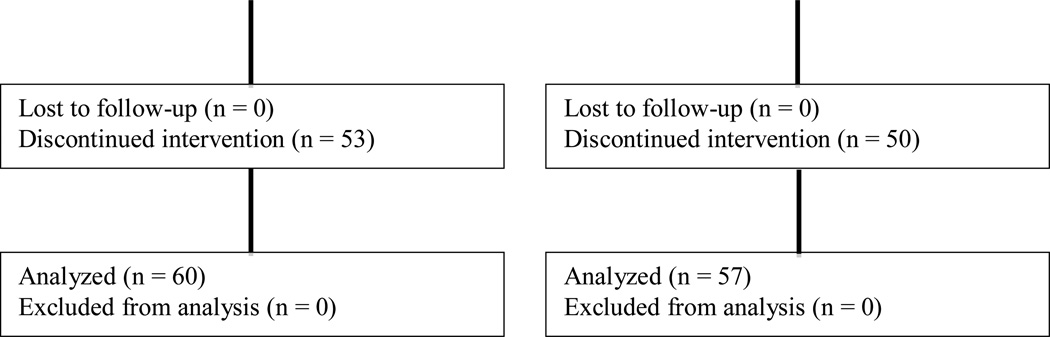
The study opened to accrual on March 1, 2007 with a target accrual of 121 patients. A total of 123 patients were enrolled with 63 randomized to bevacizumab plus irinotecan and 60 to bevacizumab plus temozolomide (see Figure 2 CONSORT diagram).
Figure 2.
CONSORT Diagram
Patient baseline characteristics are provided in Table 1. Six patients (3 patients in each arm) were either deemed ineligible (no central pathology review, n = 3; received an EIAED, n = 1) or never received treatment (n = 2). Adverse events were assessed using Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Table 1.
Patient Characteristics
| Characteristic | Bev + TMZ (n=60) |
Bev + Irinotecan (n=57) |
|---|---|---|
| Age: Median | 58 | 55 |
| Range | 24–82 | 23–78 |
| < 50 | 14 (23%) | 22 (39%) |
| ≥50 | 46 (77%) | 35 (61%) |
| Gender: | ||
| Male | 34 (57%) | 34 (60%) |
| Female | 26 (43%) | 23 (40%) |
| KPS: | ||
| 70–80 | 30 (50%) | 31 (54%) |
| 90–100 | 30 (50%) | 26 (46%) |
| Neurologic status: | ||
| No symptoms | 15 (25%) | 15 (26%) |
| Minor | 27 (45%) | 28 (49%) |
| Moderate | 18 (30%) | 14 (25%) |
| Initial surgery: | ||
| Biopsy | 3 (5%) | 8 (14%) |
| Subtotal | 21 (35%) | 18 (32%) |
| Gross total | 35 (58%) | 29 (51%) |
| Other | 1 (1%) | 2 (3%) |
| Additional surgical procedures: | ||
| None | 40 (67%) | 30 (53%) |
| Biopsy | 1 (2%) | 2 (4%) |
| Subtotal | 8 (13%) | 10 (18%) |
| Gross total | 10 (17%) | 14 (25%) |
| Other | 1 (2%) | 1 (2%) |
| Corticosteroids: | ||
| Yes | 19 (32%) | 17 (30%) |
| No | 41 (68%) | 40 (70%) |
|
Bev + TMZ (n = 60) Toxicity Grade |
Bev + Irinotecan (n = 57) Toxicity Grade |
|
Treatment Efficacy
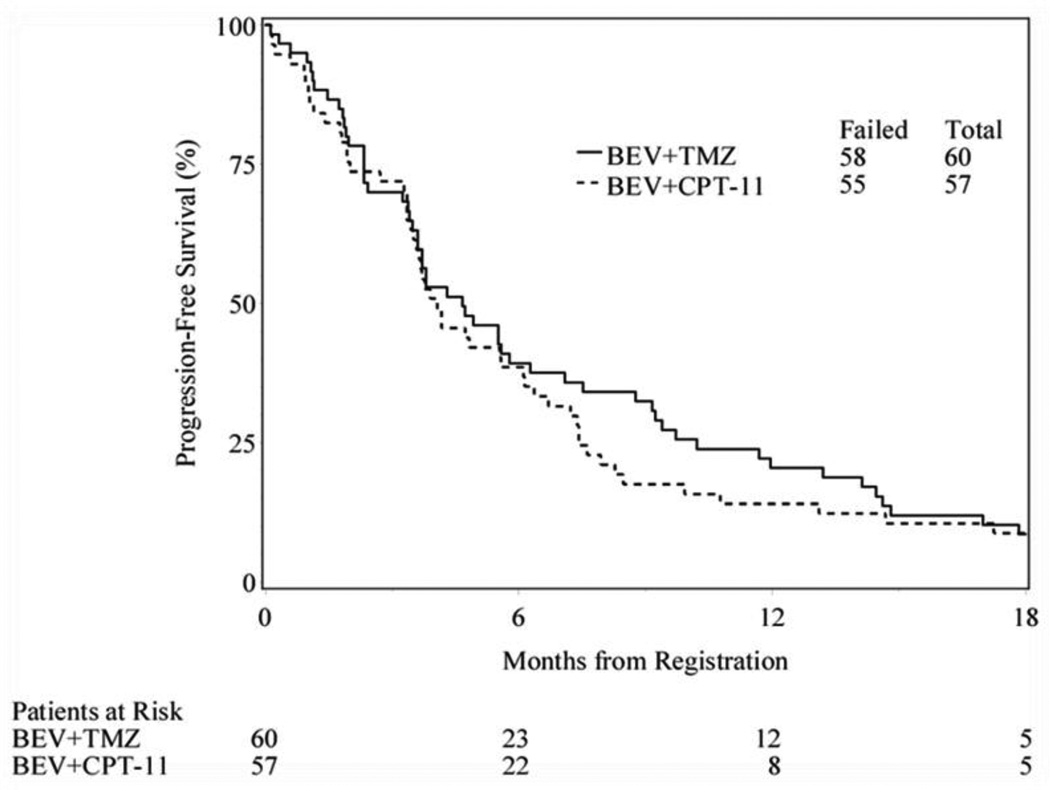
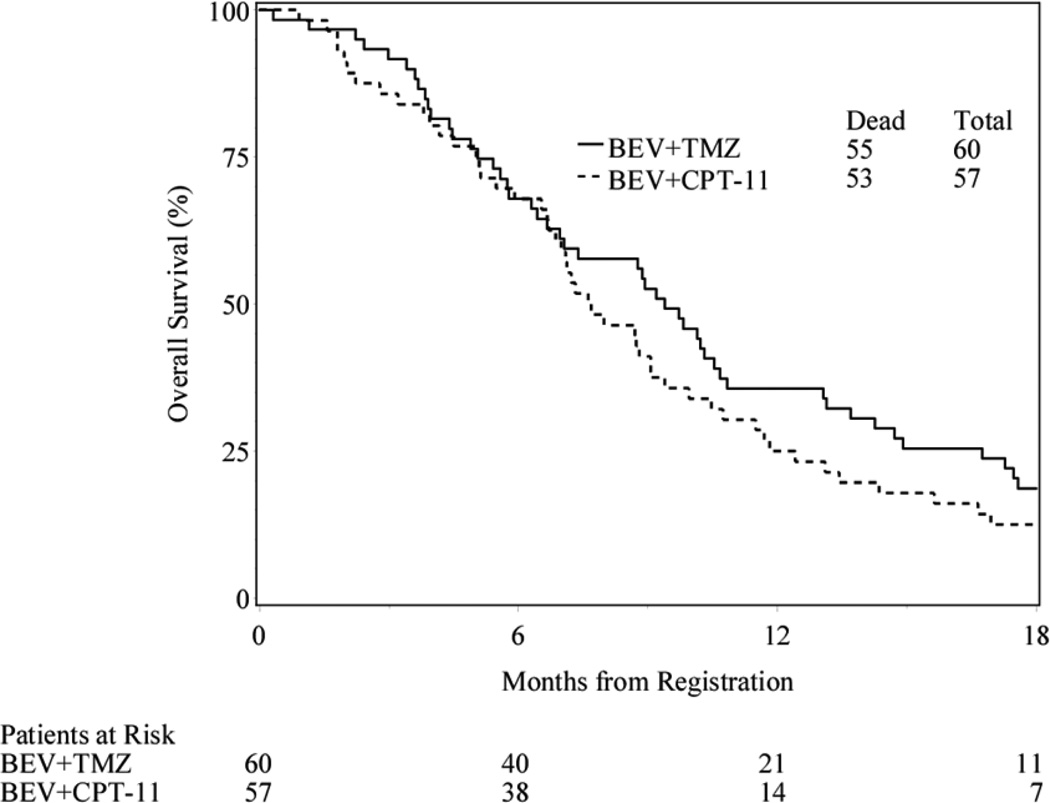
The primary efficacy endpoint was the 6-m PFS rate. Fifty-nine eligible patients (1 patient withdrew prior to 6 months) in the bevacizumab and temozolomide arm and all 57 eligible in the bevacizumab and irinotecan arm were evaluable for this endpoint. The 6-m PFS was 39% (23/59) for bevacizumab and temozolomide arm and 38.6% (22/57) in the bevacizumab and irinotecan arm. Median PFS and overall survival (OS) were 4.7 (95% confidence interval [CI]: 3.5, 6.3) and 9.4 months (95% CI: 6.7, 10.7) for the temozolomide containing arm. Median PFS and OS were 4.1 (95% CI: 3.4, 6.1) and 7.7 (95% CI: 6.7, 9.1) months for the irinotecan containing arm. (Figure 3)
Figure 3.
Progression Free and Overall Survival
Radiographic response was based on investigator assessment. Overall, 5 patients did not have measurable disease at study entry. The objective response rate (complete response + partial response) was 19.0% in the bevacizumab and temozolomide arm and 27.8% in the bevacizumab and irinotecan arm.
Safety and Toxicity
Overall, treatment was well tolerated. The adverse events are listed in Table 2 and are in accord with previous reports of bevacizumab treatment in this patient population. Toxicities likely related to temozolomide, such as myelotoxicity and irinotecan such as diarrhea, constipation and fatigue are consistent with the anticipated incidence and severity. There was one treatment-related grade 5 adverse event on the bevacizumab-temozolomide are: multi-organ failure. On the bevacizumab-irinotecan arm, there were three treatment-related grade 5 adverse events: death (that was otherwise not specified), hemorrhagic stroke, and infection.
Table 2.
Treatment-related toxicities
| CTCAE Toxicities of interest | 2 | 3 | 4 | 5 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|---|
| Allergy | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Hematologic | 5 | 21 | 9 | 0 | 14 | 13 | 3 | 0 |
| Cardiac | 0 | 4 | 1 | 0 | 4 | 3 | 0 | 0 |
| Thrombosis/Coagulopathy | 0 | 3 | 2 | 0 | 2 | 3 | 4 | 0 |
| Constitutional symptoms | 20 | 7 | 0 | 0 | 14 | 10 | 1 | 0 |
| Skin | 1 | 1 | 0 | 0 | 4 | 0 | 0 | 0 |
| Gastrointestinal | 16 | 2 | 2 * | 0 | 20 | 5 | 0 | 0 |
| Hemorrhage | 7 | 4 | 0 | 0 | 2 | 1 | 0 | 1 |
| Infection | 9 | 3 | 1 | 0 | 7 | 5 | 1 | 1 |
| Metabolic/electrolyte | 17 | 5 | 1 | 0 | 11 | 8 | 0 | 0 |
| Neurologic | 6 | 6 | 0 | 0 | 7 | 2 | 1 | 0 |
| Ocular/vision | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 |
| Pain | 7 | 3 | 0 | 0 | 6 | 0 | 0 | 0 |
| Respiratory | 2 | 1 | 0 | 0 | 3 | 1 | 0 | 0 |
| Renal | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Summary of Worst Adverse Event per Patient | ||||||||
| Worst non-hematologic toxicity |
21 35% |
23 38% |
5 8% |
1** 2% |
23 40% |
17 30% |
5 9% |
3** 5% |
| Worst overall toxicity | 9 15% |
33 55% |
11 18% |
1 2% |
20 35% |
22 39% |
7 12% |
3 5% |
Treatment discontinuation secondary to treatment-related toxicity was the primary endpoint of the first stage of the bevacizumab-temozolomide combination. From the initial 29 patients enrolled on this treatment arm, 5 patients required treatment cessation because of toxicity and 11 patients experienced 6-m PFS. Since less than 7 patients experienced treatment cessation and more than 7 experienced 6-m PFS as preset by the statistical plan, the second cohort of 31 patients was accrued to the study.
Discussion
One of the goals of this study was to confirm the efficacy that had been reported from clinical trials performed in single institutions, suggesting that the combination of bevacizumab and irinotecan had significant activity in patients with recurrent GBM. This study, launched before RANO criteria were established, was designed to confirm activity of this combination and utilized the established Macdonald Criteria17,20. The early literature, most notably a report from Vredenburgh et al described objective response rates of 60% and a 6-month PFS rate of 38% in patients using this treatment combination.21 Although not a randomized trial, these results compare very favorably with historical data which demonstrate a 5% objective response rate and 21% 6-m PFS rate for temozolomide as a single agent treatment for recurrent GBM.22 NRG Oncology RTOG 0625 was able to confirm the bevacizumab-irinotecan response and 6-m PFS data in a cooperative group setting, thereby providing important confirmation and support that the reported results were not from a patient selection bias that can occur at a tertiary care center. The other major goal of this clinical trial was to test the safety of combining bevacizumab with temozolomide which was confirmed as the treatment-related adverse event rate was lower than the preset threshold.
As described in the results section, the study achieved all of its pre-specified endpoints. The 6-m PFS for both the irinotecan and temozolomide containing arms exceeded the predetermined threshold of 35%. Furthermore, the toxicity profiles of both regimens were deemed to be within acceptable threshold. These results both confirmed the efficacy of bevacizumab combination regimens in recurrent GBM and provided the important safety data to permit the development and implementation of the randomized study in newly diagnosed GBM that was recently published.15
Interestingly, there was a recent study that suggests that the administration of bevacizumab as a single agent may not be as effective as a combination regimen in recurrent GBM. In contrast to the two trials that led to accelerated approval of single agent bevacizumab where 6-m PFS rates of 29% and 43% were reported, the recently reported Dutch BELOB study demonstrated only an 18% 6-m PFS rate for single-agent bevacizumab.23 This study was performed at a large number of sites in the Netherlands, with the majority of the accrual at non-academic centers. The 6-m PFS was 41% in the treatment group where bevacizumab was combined with lomustine. However, a subsequent randomized phase III trial comparing lomustine alone with the combination of lomustine and bevacizumab refutes these results.24 EORTC 26101 treated 149 patients with lomustine alone and 288 patients with the lomustine and bevacizumab combination. Although progression free survival assessed by imaging was prolonged with the combination (4.2 vs 1.5 months), overall survival showed no difference between the two arms (9.1 vs 8.6 months). Importantly, there was no difference in time to neurologic progression.
Despite these results, bevacizumab continues to be used as a salvage agent either alone or in combination regimens. Although the RTOG 0625 study described in this paper was not designed to determine comparative efficacy of the two combination regimens compared with single agent treatments, it does provide important safety data for treatment regimens combining bevacizumab with either dose-dense temozolomide or conventional dose irinotecan in patients with recurrent glioblastoma.
Acknowledgments
We would like to thank the NRG Oncology RTOG staff including Minhee Won, Kathryn Okrent, Sandrine Geinoz, Barbara Kaiser, and Denise Manfredi for their outstanding work on this clinical trial.
This project was supported by grants U10CA21661, U10CA180868, U10CA180822, U10CA37422, and U24CA180803 from the National Cancer Institute (NCI).
Footnotes
Conflicts of interest
Dr. Gilbert reports personal fees and non-financial support from Merck, personal fees from Genentech Roche, personal fees from Abbvie, personal fees from Wellcome Trust, and personal fees from Foundation Medicine, outside the submitted work. Dr. Sorensen reports employment by Siemens Healthcare, outside the submitted work. Dr. Mikkelsen has a consulting or advisory role with Roche Genentech and has received honoraria, travel and research funding from Roche Genentech, outside the submitted work. Dr. Penas-Prado has received research funding from Bayer, Genentech, Glaxo, and Novartis, outside the submitted work. Dr. Mehta has a leadership role with Pharmacyclics, stock or ownership interest in Pharmacyclics, consulting or advisory roles with Cavion, Elekta, Novartis and Novocure, and has received research funding from Novocure and Novellos, outside the submitted work.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17:2572. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. The New England journal of medicine. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nature reviews Neuroscience. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 4.Visted T, Lund-Johansen M. Progress and challenges for cell encapsulation in brain tumour therapy. Expert opinion on biological therapy. 2003;3:551–561. doi: 10.1517/14712598.3.4.551. [DOI] [PubMed] [Google Scholar]
- 5.Jain RK. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 6.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 7.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel R. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–3564. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 8.Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 9.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong TS, Wen PY, Gilbert MR, Schiff D. Management of treatment-associated toxicites of anti-angiogenic therapy in patients with brain tumors. Neuro-oncology. 2012;14:1203–1214. doi: 10.1093/neuonc/nor223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. The oncologist. 2009;14:1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 13.Tol J, Punt CJ. Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review. Clinical therapeutics. 2010;32:437–453. doi: 10.1016/j.clinthera.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Reardon DA, Turner S, Peters KB, et al. A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. Journal of the National Comprehensive Cancer Network : JNCCN. 2011;9:414–427. doi: 10.6004/jnccn.2011.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. The New England journal of medicine. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. The New England journal of medicine. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 18.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 19.Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- 20.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 21.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 22.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs procarbazine in patients with glioblastoma multiforme at first relapse. British journal of cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. The lancet oncology. 2014;15:943–953. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- 24.Wick W, Brandes AA, Gorlia T, et al. Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Proc Soc Neuro-Onc. 2015 [Google Scholar]