Abstract
Background
Small bowel obstruction (SBO) is a common diagnosis, however outcomes of and risk factors for SBO and malignant bowel obstruction (MBO) surgery are not well understood. We sought to characterize outcomes and risk factors for surgery for SBO and MBO.
Methods
A retrospective cohort study was performed utilizing prospectively-collected data from the Michigan Surgical Quality Collaborative (7/2012-3/2015). Cases included those with ICD9 diagnosis code of bowel obstruction and CPT codes for lysis of adhesions, intestinal bypass and small bowel resection. Cases were stratified by disseminated malignancy (MBO). Factors associated with complications and 30-day mortality were evaluated.
Results
2,233 patients underwent surgery for bowel obstruction, including 86 patients (3.9%) with MBO. MBO patients had an adjusted mortality rate of 14.5% (benign 5.0%); the adjusted complication rate was 32.2% (benign 27.0%). Factors independently associated with mortality included disseminated cancer, older age, American Society of Anesthesiologists IV/V, cirrhosis, ascites, urinary tract infection, sepsis, albumin <3.5, hematocrit <30, and bowel resection.
Conclusions
Surgery for bowel obstruction carries a relatively high risk for morbidity and mortality, particularly in patients with malignant bowel obstruction. Considering the identified risk factors for mortality may help clinicians make recommendations regarding surgery in the setting of MBO.
Keywords: Small bowel obstruction, malignant bowel obstruction, surgery, outcomes
Introduction
Small bowel obstruction results in more than 300,000 hospitalizations per year in the United States, and these cases are within the “bread & butter” diagnoses for the general surgeon [1,2]. A significant percentage of those patients (18-57%) require an operative intervention [3-6]. While the data varies depending on the patient population there appears to be a 4-37% rate of complications, and a mortality rate ranging from 2-6% for patients undergoing operative intervention for bowel obstruction [3-9]. However, most existing data come from single-institution studies or from studies using administrative databases which underestimate morbidity and mortality rates [3-5,7].
One group of small bowel obstruction patients who present a clinical challenge are those who present with an obstruction due to intra-abdominal malignancy, a “malignant bowel obstruction” (MBO). Patients presenting with MBO create a clinical dilemma, as they are often nearing the end-of-life; however, the intractable symptoms of pain, nausea and vomiting require management. There is a lack of prospective, clinical trials data to guide the care of these patients and data has often drawn from studies with small numbers of patients involved [10]. Existing studies have demonstrated that for patients undergoing operative intervention for MBO there is significant morbidity and mortality (7-67% and 6-32% respectively) [11-15]. The widely varying surgical morbidity and mortality rates in published studies leave clinicians with a lack of guidance when attempting to recommend treatment for patients with MBO.
While it is known that patients undergoing palliative surgery in the setting of metastatic or disseminated cancer have poor outcomes, we believe that there are subsets of these patients for whom palliative surgery is appropriate. A better knowledge of patient and surgical factors associated with morbidity and mortality for these patients would be helpful in clinical decision-making [16-20]. Some studies have attempted to identify patient factors associated with morbidity and mortality, but with inconsistencies between the results [11,16,20].
In this context, we sought to evaluate the surgical outcomes of patients with benign and malignant small bowel obstruction, utilizing a population-based, prospective surgical outcomes registry. The goals of our study were to: (1) compare patient characteristics and types of surgery performed for SBO patients with and without MBO; (2) Compare outcomes and resource utilization for these two cohorts; (3) determine risk factors for morbidity and mortality that might aid in clinicians’ decision making; and (4) determine whether the characteristics of hospitals treating MBO patients is associated with outcomes.
Materials and Methods
Data Source
We conducted a population-based, retrospective cohort study utilizing data prospectively collected by the Michigan Surgical Quality Collaborative (MSQC). MSQC is a consortium of community and academic hospitals throughout the state of Michigan that collects and disseminates information about surgical outcomes and conducts collaborative quality-improvement projects. Details regarding the MSQC data abstraction have been described before [21]. Briefly, specially trained data abstractors (nurses) conduct chart reviews to comprehensively collect rigorously defined patient demographics, preoperative risk factors, laboratory values, technical details of the operations, perioperative process of care, and the 30-day outcomes for patients undergoing specific surgical operations, using a sampling algorithm that minimizes selection bias. To ensure registry data validity, regular data audits are performed. This study was deemed “non-regulated” by the University of Michigan Institutional Review Board.
Patient Population
Patients aged 18 years and older undergoing surgery for small bowel obstruction based on the International Classification of Disease (ICD-9) code (560.x) from July 2012 to March 2015 at any of 51 MSQC community or academic hospitals were included in this study. The 51 hospitals were the hospitals with available data from the American Hospital Association (AHA) 2013 survey, out of the 66 total MSQC hospitals at the time of the study. Patients with Current Procedural and Terminology (CPT) codes suggesting colon surgery were excluded (e.g., any colectomy, Hartmann-type operation, etc.), to limit the cohort to patients with small bowel obstruction (SBO).
Main Exposure Variable
Having a diagnosis of disseminated cancer was the main exposure variable in this study. Disseminated cancer, is a comorbidity variable that is defined within MSQC as “cancer that has spread to one site or more sites in addition to the primary site AND in whom the presence of multiple metastases indicates the cancer is widespread, fulminant, or near terminal. Other terms describing disseminated cancer include “diffuse,” widely metastatic,” “widespread,” “carcinomatosis” or American Joint Committee on Cancer (AJCC) “Stage IV” cancer.” We utilized the diagnosis of disseminated cancer as a proxy for likely malignant small bowel obstruction. The patients were stratified into two groups for comparison: those with a likely MBO and those with a benign SBO.
Independent Variables
Demographic and clinical data analyzed for the main cohort included age, sex, race, American Society of Anesthesiologists (ASA) class and type of operation performed based on CPT codes. Comorbidities evaluated included preoperative congestive heart failure (CHF), arrhythmias, hypertension (HTN), smoking status, chronic obstructive pulmonary disease (COPD), ventilator use, dyspnea, pneumonia (PNA), diabetes, dialysis use, ascites, cirrhosis, peripheral vascular disease (PVD), deep vein thrombosis (DVT), bleeding disorder, Human Immune Deficiency Virus (HIV), chronic condition (requiring immunosuppression and/or steroid use), urinary tract infection (UTI) and pre-operative sepsis. Laboratory data collected included hematocrit (HCT), white blood cell count, albumin, and lactate level. Hospital characteristics included size (number of beds, <100, 100-399, ≥400), teaching status (non-teaching, minor teaching and major teaching), cancer designation by the American College of Surgeons (ACS), and setting (urban, rural) according to AHA definitions.
Outcomes Variables
The outcomes variables of interest were any morbidity, mortality within 30-days of operation, extended length of stay (LOS), hospital readmission, and reoperation within 30-days. Morbidity was defined as documentation of at least one postoperative complication, including intraoperative blood transfusion, surgical site infection, urinary tract infection, sepsis, Clostridium difficile infection, central line-associated bloodstream infection, pneumonia, unplanned intubation, acute renal failure, myocardial infarction, arrhythmia, stroke, pulmonary embolism, deep venous thrombosis, cardiac arrest, post-operative blood transfusion, and anastomotic leak. Extended LOS was defined as extending beyond the 75th percentile of length of stay for this cohort.
Statistical Analyses
Demographic and clinical variables for patients with and without disseminated cancer were compared using chi-square tests for categorical and 2-sided t-tests or Wilcoxon rank sum tests for continuous variables, as appropriate, with significance set at p < 0.05. Variables that had a p-value < 0.2 on the univariate analyses were included in multivariable logistic regression models in addition to the main exposure the patient’s sex and age. Models were evaluated for discrimination using the c-statistic and calibration using Hosmer-Lemeshow statistics. The c-statistic evaluates model discrimination and represents the area under the receiver operator characteristic curve. A value of 0.5 indicates that the model is equivalent to chance; a value of 1.0 indicates perfect discrimination. The Hosmer-Lemeshow statistic assesses whether predictive accuracy is consistent across deciles of predicted risk.
The β-coefficients of the covariates generated by the multivariable model allow for the calculation of an adjusted-rate for each outcome at each level of the main exposure. This is achieved by fixing the value of the exposure variable for each patient while keeping all other covariate values as observed. To measure the association between hospital characteristics and mortality in patients undergoing surgery for SBO with disseminated cancer, we stratified the risk-adjusted mortality rates by each hospital characteristic. The goal of this analysis was to evaluate the impact specific hospital characteristics had on outcomes.
All statistical analyses were conducted using STATA special edition (version 13.1, StataCorp, College Station, TX).
Results
The study population included 2,233 patients who underwent surgery for small bowel obstruction, of which 86 patients (3.9%) had MBO. The overall average age was 64.0 years (±16.6 years). Overall, patients in the MBO cohort were more likely to be male, and had more comorbidities as compared to the benign cohort. Comorbidities more common in the MBO cohort included: ascites, deep vein thrombosis, human immunodeficiency virus, urinary tract infection, chronic immunosuppression (steroid and/or chemotherapy use), anemia, and hypoalbuminemia (Table 1).
Table 1.
Clinical and Demographic Characteristics
| Patient Characteristics |
Benign (n=2,147, %) |
MBO (n=86, %) |
P value | |
|---|---|---|---|---|
| Age, y, mean ± SD | 63.9 ±16.7 | 65.2 ±12.8 | 0.455 | |
| Sex | Male | 811 (37.8) | 44 (51.2) | 0.012 |
| Female | 1336 (62.2) | 42 (48.8) | ||
| Race | Non-Hispanic White |
1651 (76.9) | 63 (73.3) | 0.433 |
| Other | 496 (23.1) | 23 (26.7) | ||
| ASA Class | 0.001 | |||
| I/II | 629 (29.3) | 10 (11.6) | ||
| III | 1182 (55.1) | 54 (62.8) | ||
| IV/V | 336 (15.6) | 22 (25.6) | ||
| Comorbidity Index | 0.002 | |||
| 0 | 406 (18.9) | 13 (15.1) | ||
| 1 | 611 (28.5) | 12 (14.0) | ||
| 2+ | 1130 (52.6) | 61 (70.9) | ||
| Hypertension | 1229 (57.2) | 47 (54.7) | 0.634 | |
| Dyspnea | 226 (10.5) | 14 (16.3) | 0.091 | |
| COPD | 300 (14.0) | 18 (20.9) | 0.070 | |
| Ascites | 101 (4.7) | 15 (17.4) | <0.001 | |
| Cirrhosis | 15 (0.7) | 2 (2.3) | 0.089 | |
| Diabetes | 295 (13.7) | 14 (16.3) | 0.504 | |
| Deep Vein Thrombosis | 167 (7.8) | 17 (19.8) | <0.001 | |
| HIV | 6 (0.3) | 3 (3.5) | <0.001 | |
| Urinary Tract Infection | 63 (2.9) | 6 (7.0) | 0.034 | |
| Pre-operative Sepsis | 187 (8.7) | 12 (14.0) | 0.094 | |
| Chronic Immunosuppression |
140 (6.5) | 12 (15.1) | 0.002 | |
| Laboratory Values | ||||
| Hematocrit | 0.001 | |||
| >30 | 1950 (90.8) | 68 (79.1) | ||
| 24-30 | 172 (8.0) | 15 (17.4) | ||
| <24 | 25 (1.2) | 3 (3.5) | ||
| Albumin | <0.001 | |||
| ≥ 3.5 | 1059 (49.3) | 32 (37.2) | ||
| <3.5 | 698 (32.5) | 46 (53.5) | ||
| Unknown | 390 (18.2) | 8 (9.3) | ||
Note: ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; HIV, Human Immunodeficiency Virus. Other variables tested but not statistically significant: congestive heart failure, arrhythmias, peripheral vascular disease, smoking, ventilator use, pneumonia, dialysis, bleeding disorder, white blood cell count and lactate level.
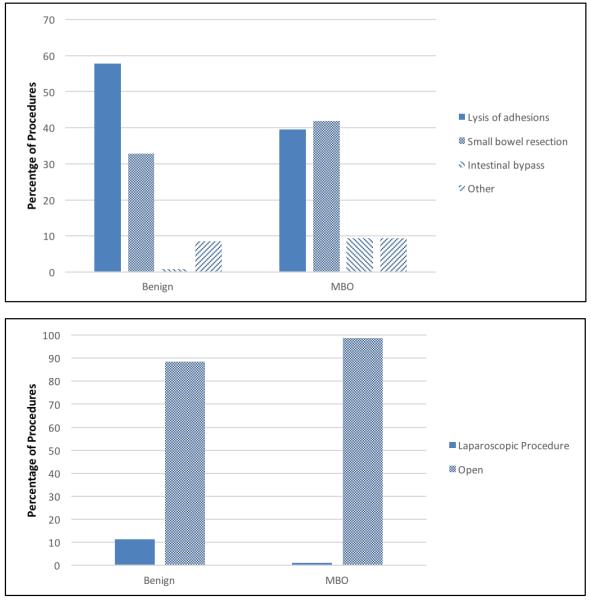
Operative interventions included lysis of adhesions (LOA), small bowel resection, intestinal bypass, and other (Figure 1). The patients with MBO underwent more extensive operative interventions, with 41.9% of the cohort undergoing small bowel resection (v. 32.8% benign) and 9.3% undergoing intestinal bypass (v. 0.9% benign). Very few patients with SBO underwent a laparoscopic procedure, 11% in the benign cohort and 1% in the MBO cohort (p-value 0.003).
Figure 1.
Percentage of Procedures by Diagnosis Category
Overall, the two cohorts of benign and MBO patients experienced many of the same post-operative complications. Significant differences observed between the two cohorts included the unadjusted percentage of patients with any complications (44.2% MBO cohort v. 26.6% benign cohort), superficial surgical site infection (11.6% vs 4.9%), and pneumonia (10.5% vs 4.6%). The unadjusted 30-day mortality rate for all patients was 5.6%, with 22.1% of the MBO patients dying within 30-days of their operation as compared to 4.9% of the benign patients. In terms of resource utilization, the MBO patients received significantly more intra-operative transfusions (16.3% vs 3.0%) and post-operative transfusions (12.8% vs 5.8%). They also had a significantly higher rate of extended LOS (36.0% vs 21.7%, Table 2).
Table 2.
Unadjusted Post-operative Complications, Mortality and Resource Utilization
| Benign (n, %) | MBO (n, %) | P Value | |
|---|---|---|---|
| Complications | |||
| Any Complication | 571 (26.6) | 38 (44.2) | <0.001 |
| Major Complication | 330 (15.4) | 17 (19.8) | 0.270 |
| Cardiac Arrest | 21 (1.0) | 1 (1.2) | 0.865 |
| Myocardial Infarction | 19 (0.9) | 0 (0.0) | 0.381 |
| Cardiac Arrhythmia | 80 (3.7) | 4 (4.7) | 0.658 |
| Stroke | 9 (0.4) | 0 (0.0) | 0.547 |
| Unplanned Intubation | 64 (3.0) | 5 (5.8) | 0.137 |
| Pulmonary Embolus | 18 (0.8) | 0 (0.0) | 0.394 |
| Acute Renal Failure | 46 (2.1) | 3 (3.5) | 0.404 |
| Deep Vein Thrombosis | 42 (2.0) | 2 (2.3) | 0.809 |
| Superficial SSI | 106 (4.9) | 10 (11.6) | 0.006 |
| Deep SSI | 32 (1.5) | 0 (0.0) | 0.254 |
| Organ Space SSI | 53 (2.5) | 2 (2.3) | 0.933 |
| Pneumonia | 98 (4.6) | 9 (10.5) | 0.012 |
| Urinary Tract Infection (Symptomatic) |
31 (1.4) | 0 (0.0) | 0.262 |
| Urinary Tract Infection (Catheter Associated) |
39 (1.8) | 0 (0.0) | 0.207 |
| Sepsis | 92 (4.3) | 4 (4.7) | 0.870 |
| Severe Sepsis | 86 (4.0) | 5 (5.8) | 0.406 |
| Clostridium difficile | 38 (1.8) | 1 (1.2) | 0.673 |
| CLABSI | 7 (0.3) | 0 (0.0) | 0.673 |
| Anastomotic Leak | 31 (1.4) | 0 (0.0) | 0.262 |
| Mortality | 105 (4.9) | 19 (22.1) | <0.001 |
| Resource Utilization | |||
| Intra-operative Transfusion |
65 (3.0) | 14 (16.3) | <0.001 |
| Post-operative Transfusion |
124 (5.8) | 11 (12.8) | 0.007 |
| Readmission 30 Days | 271 (12.6) | 16 (18.6) | 0.104 |
| Reoperation 30 Days | 180 (8.4) | 10 (11.6) | 0.290 |
| Extended LOS | 466 (21.7) | 31 (36.0) | 0.002 |
Note: SSI, surgical site infection; CLABSI, central line-associated blood stream infection; LOS, length of stay
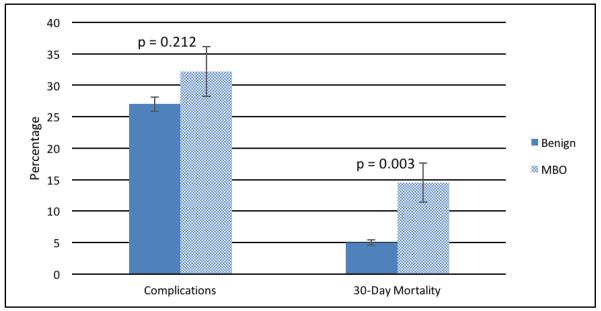
In the multivariable analysis, adjusted complication rates were similar for the two cohorts (32.2% MBO v. 27.0% benign, p-value 0.212, Figure 2). Independent risk factors for morbidity included: increasing age (adjusted odds ratio [95% Confidence Interval] - 1.01 (1.00-1.01)), male sex (1.39 [1.10-1.74]), increasing ASA score (III 1.60 [1.16-2.19] and IV/V 2.34 [1.58-3.46]), cirrhosis (3.24 [1.09-9.64]), sepsis (1.51 [1.07-2.14]), hypoalbuminemia (1.50 [1.20-1.89]), HCT <30 (1.45 [1.16-1.80]), and undergoing a small bowel resection (2.51 [2.07-3.06]). Having a laparoscopic procedure carried lower risk (0.48 [0.32-0.72], Table 3).
Figure 2.
Adjusted Complications Rate and 30 Day Mortality Rate per Diagnosis Category
Table 3.
Multivariable Analysis of Risk Factors for Complications and 30-Day Mortality
| Complications* | 30-Day Mortality** | |||
|---|---|---|---|---|
| Multivariable Analysis | Adjusted Odds Ratio (95% CI) |
P value | Adjusted Odds Ratio (95% CI) |
P value |
| Cancer | 1.33 (0.86-2.04) | 0.194 | 4.67 (2.27-9.61) | <0.001 |
| Age | 1.01 (1.00-1.01) | 0.014 | 1.07 (1.06-1.09) | <0.001 |
| Male Sex | 1.39 (1.10-1.74) | 0.005 | 1.47 (0.98-2.23) | 0.065 |
| ASA | ||||
| I/II | 1 (reference) | N/A | 1 (reference) | N/A |
| III | 1.60 (1.16-2.19) | 0.004 | 0.88 (0.39-2.02) | 0.771 |
| IV/V | 2.34 (1.58-3.46) | <0.001 | 3.06 (1.30-7.17) | 0.010 |
| Patient Characteristics | ||||
| Dyspnea | 1.19 (0.91-1.55) | 0.211 | 0.92 (0.45-1.87) | 0.819 |
| COPD | 1.11(0.86-1.45) | 0.420 | 1.49 (0.76-2.90) | 0.244 |
| Cirrhosis | 3.24 (1.09-9.64) | 0.034 | 6.71 (1.62-27.76) | 0.009 |
| Ascites | 1.20 (0.76-1.88) | 0.431 | 1.92 (1.13-3.24) | 0.015 |
| Deep Vein Thrombosis | 1.12 (0.76-1.65) | 0.555 | 1.56 (0.86-2.83) | 0.142 |
| Chronic Immunosuppression | 1.32 (0.88-1.99) | 0.180 | 1.97 (0.97-3.98) | 0.060 |
| Urinary Tract Infection | 1.51 (0.91-2.53) | 0.114 | 3.21 (1.76-5.83) | <0.001 |
| Sepsis | 1.51 (1.07-2.14) | 0.019 | 2.23 (1.21-4.12) | 0.010 |
| Laboratory Values | ||||
| Albumin < 3.5 | 1.50 (1.20-1.89) | 0.001 | 1.65 (1.07-2.54) | 0.024 |
| HCT < 30 | 1.45 (1.16-1.80) | 0.001 | 2.48 (1.52-4.06) | <0.001 |
| Procedure | ||||
| Lysis of Adhesions | 1 (reference) | N/A | 1 (reference) | N/A |
| Small Bowel Resection | 2.51 (2.07-3.06) | <0.001 | 1.96 (1.27-3.00) | 0.002 |
| Intestinal Bypass | 1.24 (0.46-3.33) | 0.668 | 1.51 (0.33-6.86) | 0.593 |
| Other | 1.14 (0.77-1.67) | 0.520 | 1.52 (0.83-2.77) | 0.171 |
| Laparoscopic | 0.48 (0.32-0.72) | <0.001 | 0.82 (0.28-2.40) | 0.720 |
Note: 95% CI, 95% confidence interval; N/A, not applicable; ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; HCT, hematocrit; LOA, lysis of adhesions
C-statistic=0.722, Hosmer-Lemeshow (HL) statistic=6.5 (p=0.595)
C-statistic=0.895, HL statistic=12.0 (p=0.149)
There was a significant difference in the adjusted 30-day mortality rate for MBO patients as compared to benign SBO patients (14.5% vs. 5.0%, p-value 0.003, Figure 2). Independent risk factors for 30-day mortality included: disseminated cancer (4.67 [2.27-9.61]), ascites (1.92 [1.13-3.24]), urinary tract infection (3.21 [1.76-5.83]), increasing age (1.07 [1.06-1.09]), ASA IV/V (3.06 [1.30-7.17]), cirrhosis (6.71 [1.62-27.76]), sepsis (2.23 [1.21-4.12]), hypoalbuminemia (1.65 [1.07-2.54]), HCT <30 (2.48 [1.52-4.06]) and undergoing a bowel resection (1.96 [1.27-3.00], Table 3).
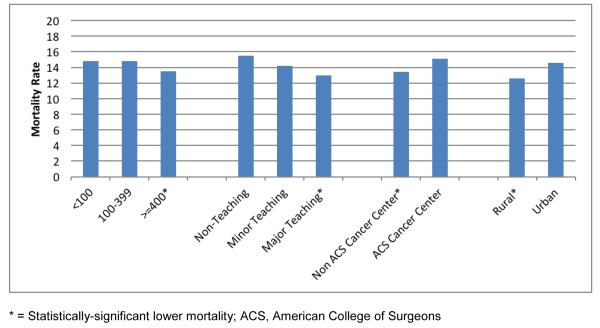
For the MBO patients, hospital characteristics were evaluated for association with adjusted mortality rates. The goal of this analysis was to determine if risk-adjusted outcomes for MBO surgery were better or worse when surgery was performed at particular types of institutions. For adjusted 30-day mortality, there were significantly higher mortality rates observed for MBO patients at <100 and 100-399 bed hospitals (compared to ≥400 bed hospitals), non-teaching and minor teaching hospitals (compared to major teaching), ACS cancer centers (compared to non-ACS cancer centers), and hospitals in an urban setting (compared to rural) (Figure 3). However, the differences in adjusted mortality are quite small, in the 1-2% range.
Figure 3.
Risk-Adjusted 30-Day Mortality Rate (%) for MBO Patients, by Hospital Characteristics
Discussion
The present study examined outcomes for a common surgical diagnosis, small bowel obstruction, and compared outcomes for patients undergoing surgery for benign versus malignant bowel obstruction. While SBO surgery is performed by almost all general surgeons, our study shows that it carries a higher risk for complications and mortality than may be commonly assumed. We observed risk-adjusted postoperative complication rates of 27.0% for benign SBO and 32.2% for MBO. Adjusted mortality rates were 5.0% and 14.5%, respectively.
Nationwide, SBO is associated with significant utilization of hospital and financial resources [1,2]. Consistent with other studies, we found that the majority of the procedures performed were LOA and small bowel resections [4,5,22]. However, our population-based study shows a few differences in outcomes, compared to prior studies [3,5,22]. We noted a 5.0% 30-day mortality for patients with benign bowel obstruction undergoing operative intervention which is higher than studies by Williams (2.1%), Chu (2.1 and 3.1%) and Schraufnagel (3%) [3,5,22]. In a study by Teixeira et al, data from the American College of Surgeons’ National Surgical Quality Improvement Program database was utilized and the overall mortality for patients undergoing operative intervention for small bowel obstruction was 5.1%, similar to our findings [23]. Many of the same risk factors for mortality were observed including increasing age, ASA score and sepsis [23].
This study also provides data to guide decision-making for patients presenting with a bowel obstruction and disseminated cancer. We found 14.5% adjusted risk of 30-day mortality for patients with MBO undergoing surgical intervention. While this is obviously a selected population that underwent surgery, this number still may be surprisingly low to many providers, who might consider surgery futile in any such cases. As clinical decision-making will be based on the individual patient presenting with bowel obstruction, additional risk factors for mortality identified in this study included increasing age, higher ASA classification, additional comorbidities (cirrhosis, ascites, UTI and sepsis), low HCT, low albumin, and the need to perform a small bowel resection. Thus, these factors may be useful to consider when counseling patients regarding specific treatment recommendations in the setting of MBO.
Palliative surgery has become an increasingly important topic within the surgical community, but due to the lack of randomized controlled trials, physicians are left with conflicting data on which to make challenging treatment decisions [10-15,27-31]. A systematic review of surgery for malignant bowel obstruction was recently performed by Olson and colleagues [13]. Overall, 17 studies were selected for inclusion, and the primary cancers included gastrointestinal and ovarian malignancies. The complication rate was 7-44% with a mortality rate ranging from 6-32%. A recent study utilized data from the American College of Surgeons’ National Surgical Quality Improvement Program to evaluate the outcomes of patients with disseminated cancer undergoing emergency surgery for perforation or obstruction. Of the 376 patients with obstruction there was a 41% complication rate and a 30-day mortality rate of 18%, similar to our findings [31].
While there is a relatively high risk for morbidity and mortality for surgical interventions for MBO, for some patients there is a real benefit [11-20,27-31]. In the process of navigating challenging treatment recommendations for MBO, the care of the patient should be multidisciplinary, with the support of palliative care services, who can assist with pain and symptom management and complex decision making [32]. While there are no official guidelines for the management of malignant bowel obstruction, there are treatment algorithms that can aid in pain and symptom management. The use of analgesics, anti-emetics and anti-secretory medications can offer a patient significant relief, even if the patient does not undergo surgery [33,34].
As much of the known data for outcomes in MBO is from retrospective studies and often many factors considered in the clinical decision making are unknown it can be challenging for surgeons to fully apply this data to current and future patients. Fortunately, there is a current multi-site SWOG clinical trial in progress to help answer the challenging questions of what patient factors and surgical interventions affect outcomes for MBO in a prospective manner [35]. The trial compares best medical management (anti-motility agents, anti-nausea agents and pain control) versus surgical intervention for patients with SBO and intra-abdominal malignancy (MBO) and will offer significant additional data to add to our understanding of how to manage this complex problem. In the meantime, data from our study can be added to the existing data to help guide the clinicians’ decision-making for patients presenting with a MBO.
This study has several limitations. First, this is a retrospective, observational study with inherent selection bias in the analysis of patient outcomes. Because the study involves only patients who had surgery, these individuals represent a highly selected group, likely with a better prognosis than other patients with MBO. Also, the diagnosis of MBO is assumed, based on the combination of SBO and disseminated malignancy. It is possible that some MBO patients actually had benign obstructions. Additional MBO patients may have been identified if they did not meet the definition of disseminated cancer, but did in fact have an obstruction due to malignancy. The nature of the obstruction - one location versus diffuse disease causing obstruction - was not able to be determined, as the definition of disseminated disease does not differentiate the disease severity. Lastly, there were a relatively small number of patients who met the criteria for MBO. However, our MBO cohort was larger than many published studies on this topic.
Conclusion
Surgery for small bowel obstruction is associated with a relatively high risk for morbidity and mortality, particularly in the setting of MBO. While many of the risk factors for mortality demonstrated in this study, including advanced age, higher ASA class, ascites, and malnutrition, are not able to be modified there remains clinical value in understanding the impact these factors can have on a patient’s mortality. In the setting of known disseminated malignancy, these results can help aid clinicians to make recommendations for or against surgery, by identifying patients at particularly high risk for 30-day mortality.
Acknowledgements
none
Dr. Wancata is supported by NIH 5T32CA009672-24. Dr. Hendren is supported by NIH/NCI 1K07 CA163665-22 and by the American Society of Colon and Rectal Surgeons Research Foundation.
Footnotes
Disclosures: No authors have disclosures related to the content of this study.
Presentations: This work was presented in part at the MSQC Conference 2015, September 24-25, 2015, Troy, MI.
Author Contributions: All authors meet the criteria for authorship including contributions to the conception of the work, data acquisition, analysis and interpretation of the data, drafting and revision of the manuscript, final approval of the version and agreement to be accountable for all aspects of the work.
Contributor Information
Lauren M Wancata, University of Michigan, Department of Surgery, 1500 East Medical Center Drive, Ann Arbor, MI 48109, United States.
Zaid M Abdelsattar, Mayo Clinic, Department of Surgery, 200 1st Street SW, Rochester, MN 55905, United States, Abdelsattar.Zaid@mayo.edu.
Pasithorn A Suwanabol, University of Michigan, Department of Surgery, 1500 East Medical Center Drive, Ann Arbor, MI 48109, United States, pasuwan@med.umich.edu.
Darrell A Campbell, Jr., Michigan Surgical Quality Collaborative, 2800 Plymouth Road, Ann Arbor, MI 48109, United States, darrellc@med.umich.edu.
Samantha Hendren, University of Michigan, Department of Surgery, 2124 Taubman Center, 1500 E. Medical Center Dr., SPC 5343, Ann Arbor, MI 48109, United States, hendren@med.umich.edu.
References
- 1.Ray NF, Denton WG, Thamer M, Henderson SC, Perry S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998;186:1–9. doi: 10.1016/s1072-7515(97)00127-0. [DOI] [PubMed] [Google Scholar]
- 2.Sikirica V, Bapat B, Candrilli SD, Davis KL, Wilson M, Johns A. The inpatient burden of abdominal and gynecological adhesiolysis in the US. BMC Surg. 2011 doi: 10.1186/1471-2482-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schraufnagel D, Rajaee S, Millham FH. How many sunsets? Timing of surgery in adhesive small bowel obstruction a study of the nationwide inpatient sample. J Trauma Acute Care Surg. 2013;74:181–187. doi: 10.1097/TA.0b013e31827891a1. [DOI] [PubMed] [Google Scholar]
- 4.Foster NM, McGory ML, Zingmond DS, KoCY Small bowel obstruction: a population based appraisal. J Am Coll Surg. 2006;203:170–176. doi: 10.1016/j.jamcollsurg.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Williams SB, Greenspoon J, Young HA, Orkin BA. Small bowel obstruction: conservative vs surgical management. Dis Colon Rectum. 2005;48:1140–1146. doi: 10.1007/s10350-004-0882-7. [DOI] [PubMed] [Google Scholar]
- 6.Jafari MD, Jafari F, Foe-Parker JE, Phelan MJ, Carmichael JC, Pigazzi A, Mills S, Stamos MJ. Adhesive small bowel obstruction in the United States: Has laparoscopy made an impact? Am Surg. 2015;81:1028–1033. [PubMed] [Google Scholar]
- 7.Fevang BT, Fevang J, Stangeland L, Søreide O, Svanes K, Viste A. Complications and death after surgical treatment of small bowel obstruction A 35-year institutional experience. Ann Surg. 2000;231:529–537. doi: 10.1097/00000658-200004000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kothari AN, Liles JL, Holmes CJ, Zapf MAC, Blackwell RH, Kliethermes S, Kuo PC, Luchette FA. “Right place at the right time” impacts outcomes for acute intestinal obstruction. Surgery. 2015;158:1116–1127. doi: 10.1016/j.surg.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ten Broek RPG, Issa Y, van Santbrink EJP, Bouvy ND, Kritwagen RFPM, Jeekel J, Bakkum EA, Rovers MM, van Goor H. Burden of adhesions in abdominal and pelvic surgery: Systematic review and met-analysis. BMJ. 2013;347 doi: 10.1136/bmj.f5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anthony T, Baron T, Mercandante S, Green S, Chi D, Cunningham J, Herbst A, Smart E, Krouse RS. Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manage. 2007;34:S49–59. doi: 10.1016/j.jpainsymman.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Alses OB, Kim S, Chen Z, Owonikoko TK, El-Rayes BF. Management patterns and predictors of mortality among US patients with cancer hospitalized for malignant bowel obstruction. Cancer. 2015;121:1772–1778. doi: 10.1002/cncr.29297. [DOI] [PubMed] [Google Scholar]
- 12.Dalal KM, Gollub MJ, Miner TJ, Wong WD, Geredes H, Schattner MA, Jaques DP, Temple LK. Management of patients with malignant bowel obstruction and stage IV colorectal cancer. J Palliat Med. 2011;14:822–828. doi: 10.1089/jpm.2010.0506. [DOI] [PubMed] [Google Scholar]
- 13.Paul Olson TJ, Pinkerton C, Brasel KJ, Schwarze ML. Palliative surgery for malignant bowel obstruction from carcinomatosis: a systematic review. JAMA Surg. 2014;149:383–392. doi: 10.1001/jamasurg.2013.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winner M, Mooney SJ, Hershman DL, Feingold DL, Allendorf JD, Wright JD, Neugut AI. Management and outcomes of bowel obstruction in patients with stage IV colon cancer: a population based cohort study. Dis Colon Rectum. 2013;56:834–843. doi: 10.1097/DCR.0b013e318294ed6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller G, Boman J, Shrier I, Gordon PH. Small-bowel obstruction secondary to malignant disease: an 11 year audit. Can J Surg. 2000;43:353–358. [PMC free article] [PubMed] [Google Scholar]
- 16.Bateni SB, Meyers FJ, Bold RJ, Canter RJ. Current perioperative outcomes for patients with disseminated cancer. J Surg Res. 2015;197:118–125. doi: 10.1016/j.jss.2015.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miner TJ, Brennan MF, Jaques DP. A prospective, symptom related, outcomes analysis of 1022 palliative procedures in advanced cancer. Ann Surg. 2004;204:719–726. doi: 10.1097/01.sla.0000141707.09312.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badgwell BD, Smith K, Liu P, Bruera E, Curley SA, Cormier JN. Indicators of surgery and survival in oncology inpatients requiring surgical evaluation for palliation. Support Care Cancer. 2009;17:727–734. doi: 10.1007/s00520-008-0554-6. [DOI] [PubMed] [Google Scholar]
- 19.Krouse RS, Nelson RA, Farrell BR, Grube B, Juarez G, Wagman LD, Chu DZ. Surgical palliation at a cancer center: incidence and outcomes. Arch Surg. 2001;136:773–778. doi: 10.1001/archsurg.136.7.773. [DOI] [PubMed] [Google Scholar]
- 20.Tseng WH, Yang X, Wang H, Martinez SR, Chen SL, Meyers FJ, Bold RJ, Canter RJ. Nomogram to predict risk of 30-day morbidity and mortality for patients with disseminated malignancy undergoing surgical intervention. Ann Surg. 2011;254:333–338. doi: 10.1097/SLA.0b013e31822513ed. [DOI] [PubMed] [Google Scholar]
- 21.Campbell DA, Jr, Englesbe MJ, Kubus JJ, Philips LR, Shanley CJ, Velanovich V, Lloyd LR, Hutton MC, Arneson WA, Share DA. Accelerating the pace of surgical quality improvement: the power of hospital collaboration. Arch Surg. 2010;145:985–991. doi: 10.1001/archsurg.2010.220. [DOI] [PubMed] [Google Scholar]
- 22.Chu DI, Gainsbury ML, Howard LA, Stucchi AF, Becker JM. Early versus late adhesiolysis for adhesive-related intestinal obstruction: A nationwide analysis of inpatient outcomes. J Gastrointest Surg. 2013;17:288–297. doi: 10.1007/s11605-012-1953-z. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira PG, Karamanos E, Talving P, Inaba K, Lam L, Demetriades D. Early operation is associated with survival benefit for patients with adhesive bowel obstruction. Ann Surg. 2013;258:459–465. doi: 10.1097/SLA.0b013e3182a1b100. [DOI] [PubMed] [Google Scholar]
- 24.Maung AA, Johnson DC, Piper GL, Barbosa RR, Rowell SE, Bokhari F, Collins JN, Gordon JR, Ra JH, Kerwin AJ, Eastern Association for the Surgery of Trauma Evaluation and management of small-bowel obstruction: an Eastern Association for the Surgery of Trauma practice management guideline. J Trauma Acute Care Surg. 2012;73:S362–369. doi: 10.1097/TA.0b013e31827019de. [DOI] [PubMed] [Google Scholar]
- 25.Di Saverio S, Coccolini F, Galati M, Smerieri N, Biffl WL, Ansaloni L, Tugnoli G, Velmahos GC, Sartelli M, Bendinelli C, Fraga GP, Kelly MD, Moore FA, Mandalá V, Mandalá S, Masetti M, Jovine E, Pinna AD, Peitzman AB, Leppaniemi A, Sugarbaker PH, Goor HV, Moore EE, Jeekel J, Catena F. Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2013 update of the evidence-based guidelines from the would society of emergency surgery ASBO working group. World J Emerg Surg. 2013;8 doi: 10.1186/1749-7922-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diaz JJ, Bokhari F, Mowery NT, Acosta JA, Block EFJ, Bromberg WJ, Collier BR, Cullinane DC, Dwyer KM, Griffen MM, Mayberry JC, Jerome R. Guidelines for management of small bowel obstruction. J Trauma. 2008;64:1651–1664. doi: 10.1097/TA.0b013e31816f709e. [DOI] [PubMed] [Google Scholar]
- 27.Chakraborty A, Selby D, Gardiner K, Myers J, Moravan V, Wright F. Malignant bowel obstruction: Natural history of a heterogeneous patient population followed prospectively over two years. J Pain Symptom Mange. 2011;41:412–420. doi: 10.1016/j.jpainsymman.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Shariat-Madar B, Jayakrishnan TT, Gamblin C, Turaga KK. Surgical Management of bowel obstruction in patients with peritoneal carcinomatosis. J Surg Oncol. 2014;110:666–669. doi: 10.1002/jso.23707. [DOI] [PubMed] [Google Scholar]
- 29.Amikura K, Sakamoto H, Yatsuoka T, Kawashima Y, Nishimura Y, Tanaka Y. Surgical management for a malignant bowel obstruction with recurrent gastrointestinal carcinoma. J Surg Oncol. 2010;101:228–232. doi: 10.1002/jso.21463. [DOI] [PubMed] [Google Scholar]
- 30.Henry JC, Pouly S, Sullivan R, Sharif S, Klemanski D, Abdel-Misih S, Arradaza N, Jarjoura D, Schmidt C, Bloomston M. A scoring system for the prognosis and treatment of malignant bowel obstruction. Surgery. 2012;152:747–757. doi: 10.1016/j.surg.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cauley CE, Panizales MT, Reznor G, Haynes AB, Havens JM, Kelley E, Mosenthal AC, Cooper Z. Outcomes after emergency abdominal surgery in patients with advanced cancer: Opportunities to reduce complications and improve palliative care. J Trauma Acute Care Surg. 2015;79:399–406. doi: 10.1097/TA.0000000000000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 33.Ripamonti CI, Easson AM, Gerdes H. Management of malignant bowel obstruction. Eur J Cancer. 2008;44:1105–1115. doi: 10.1016/j.ejca.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson HJ, Ferguson CI, Speakman J, Ismail T. Management of intestinal obstruction in advanced malignancy. Ann Med Surg (Lond) 2015;4:264–270. doi: 10.1016/j.amsu.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krouse RS, You YN. Prospective comparative effectiveness trial for malignant bowel obstruction: SWOG S1316. Bull Am Coll Surg. 2015;100:49–50. [PubMed] [Google Scholar]