PRESENTATION
An emerging molecular sequencing technique yielded the diagnosis of a rare infection with multi-systemic complications. A 53-year-old male with a history of remotely treated Hodgkin’s lymphoma, severe aortic stenosis and moderate mitral stenosis both attributed to chemoradiation, and recent diagnosis of culture-negative endocarditis, presented with fevers, left eye redness, and abdominal pain. Band-like abdominal pain began five days previously, wrapped around his back, and radiated into the groin with associated dysuria. He denied vision changes or headache, but acknowledged photophobia. He also had a six-year history of migratory polyarthritis, 100-pound unintentional weight loss, and declining short-term memory. Medical history also included hypertension and hypothyroidism for which he was prescribed aspirin, furosemide, levothyroxine, and metoprolol succinate. Long-term treatment with prednisone and methotrexate for presumed seronegative rheumatoid arthritis was stopped two months prior. He was a non-smoker with minimal alcohol consumption who lived in New England and denied recent travel or insect bites. As a retired wood worker, he described frequent exposure to untreated wood imported from South America and Sub-Saharan Africa.
One month previously, the patient developed fevers while undergoing workup for aortic valve replacement prompting transthoracic echocardiogram that revealed an aortic valve vegetation. Blood cultures obtained prior to antibiotics and serologies for Coxiella, Brucella, and Bartonella species were negative, as were HIV antibody, Treponemal IgG, and Whipple’s DNA whole blood polymerase chain reaction (PCR) tests. That hospitalization was complicated by recurrent fevers despite broad-spectrum antibiotics and bilateral uveitis. He was discharged on ceftriaxone and vancomycin for culture-negative endocarditis and had completed four weeks of a planned six-week course.
ASSESSMENT
On physical examination, he was febrile to 100.7 °F with a heart rate of 109 beats per minute and a blood pressure of 106/54 mmHg. Left-sided conjunctival injection and decreased visual acuity were noted. Auscultation revealed a harsh III/VI systolic murmur radiating to the carotid arteries, a II/VI diastolic murmur, and fine bibasilar crackles. Physical examination was notable for lower abdominal tenderness without peritoneal signs, scrotal erythema with associated warmth and tenderness, and bilateral inguinal lymphadenopathy.
Laboratories were significant for an elevated erythrocyte sedimentation rate of 111 mm/h, C-reactive protein of 143.7 mg/L, and leukocyte count of 16.12 K/uL. Blood cultures again returned negative. Lumbar spine magnetic resonance imaging (MRI) was negative for osteomyelitis or epidural abscess, but showed paraspinal and psoas muscle inflammation. Computed tomography (CT) scan of the abdomen and pelvis was negative apart from longstanding mesenteric lymphadenopathy. Scrotal ultrasound demonstrated bilateral epididymitis and mild orchitis. Transthoracic echocardiogram showed progression of the aortic valve vegetation from 1.2×0.8cm to 1.8×0.8cm.
DIAGNOSIS
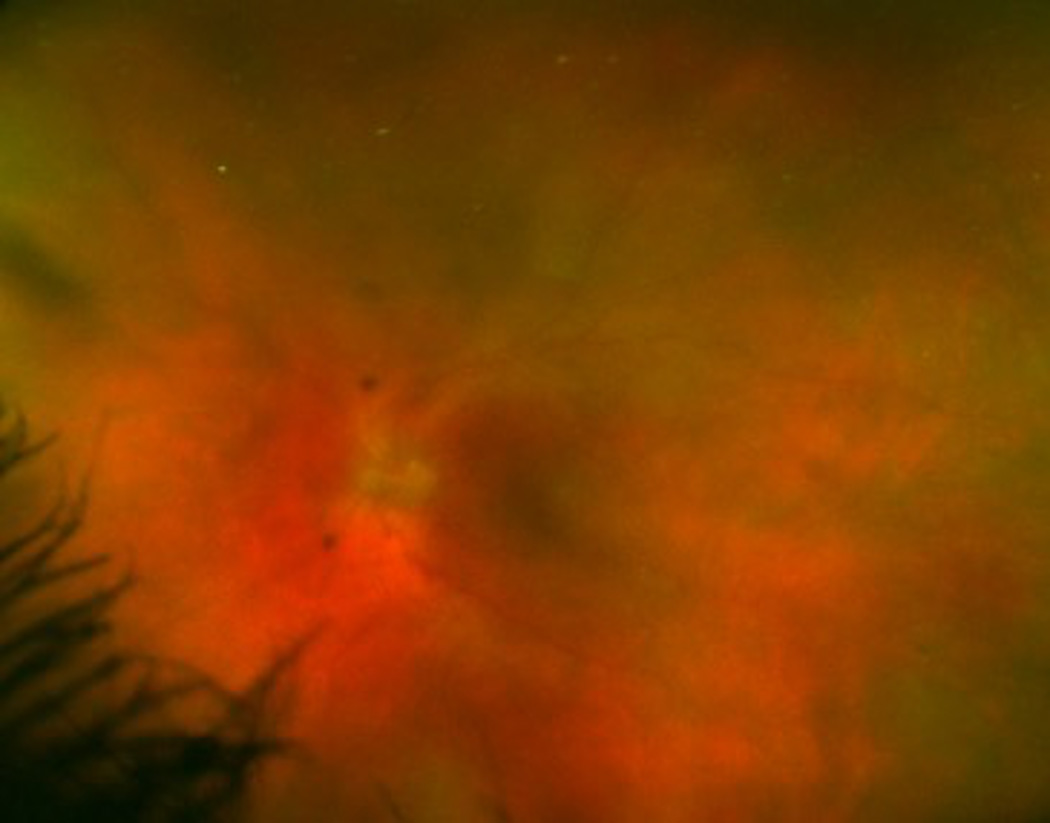
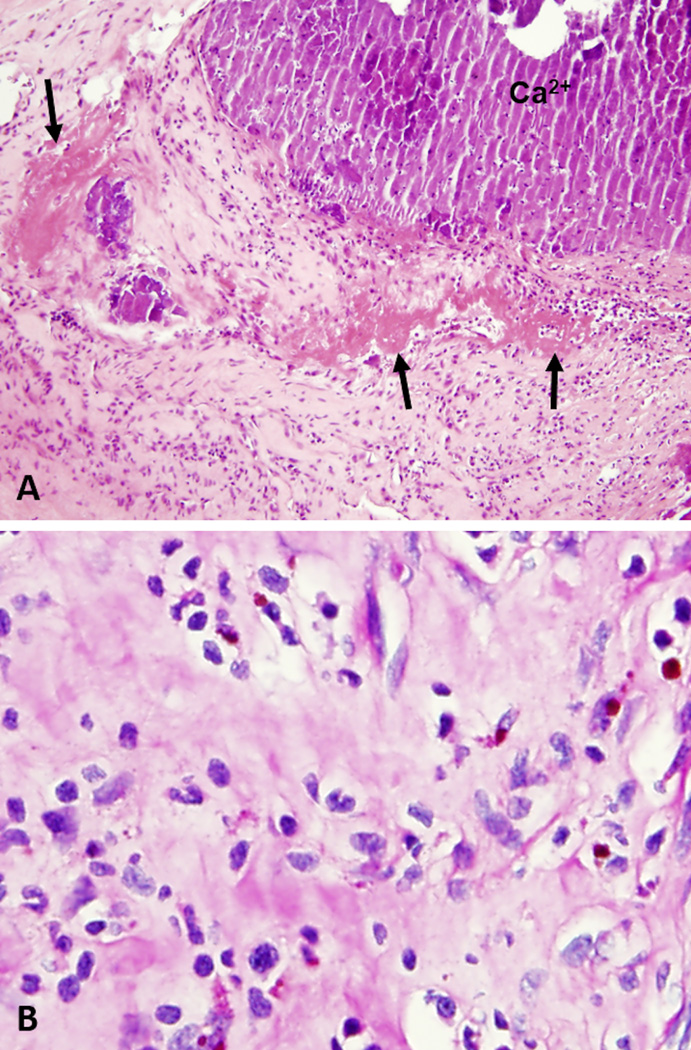
Fundoscopic exam revealed vitritis (Figure 1) as well as bilateral chorioretinal infiltrates, suspicious for endophalmitis. Vitreous fluid cultures were negative. In spite of intravitreal injection of ceftazidime, vancomycin, and amphotericin B, his vision deteriorated, prompting bilateral vitrectomy. He then underwent aortic and mitral valve replacement with tricuspid valve and aortic root repair for progressive valvular disease with culture-negative endocarditis thought not to be responding to empiric vancomycin and ceftriaxone. Histology of the aortic valve was consistent with healing endocarditis (Figure 2). Vitreal and aortic valve samples sent to the University of Washington for 16S ribosomal RNA (rRNA) sequencing returned positive for Tropheryma whipplei, providing critical confirmation of the diagnosis of Whipple’s disease with cardiac, ocular, genitourinary, musculoskeletal, and neurologic manifestations.
Figure 1.
Color photomicrograph with hazy view of the left retina due to significant vitritis.
Figure 2.
A) Histologic section of the aortic valve and vegetation demonstrating healing endocarditis with granulation tissue, fibrin deposition (arrows), and calcified debris admixed with acute inflammatory cells in the absence of identifiable microorganisms or foamy macrophages. (H&E, 40×). B) Periodic acid-Schiff (PAS) stain with diastase fails to demonstrate PAS-positive organisms within macrophages (400×).
MANAGEMENT
Given presentation four weeks into therapy with ceftriaxone, a first-line treatment for Whipple’s disease, his multi-system inflammatory symptoms were attributed to immune reconstitution inflammatory syndrome (IRIS). The migratory polyarthritis, myositis, epididymitis, and orchitis improved with a 14-day course of ceftriaxone post-operatively followed by trimethoprim-sulfamethoxazole (TMP-SMX) maintenance therapy. Eight months into TMP-SMX therapy, he developed progressive fatigue, malaise, and limited exercise capacity. Workup was notable for profound iron-deficiency anemia and hemolysis attributed to mechanical valve shear forces. Positron emission tomography (PET) CT was negative for evidence of Whipple’s recurrence, but increased inflammatory markers raised concern for relapsed infection, so ceftriaxone was reinitiated for three additional months with plans to resume oral maintenance therapy thereafter.
DISCUSSION
In 1907, George Whipple described a patient with weight loss, fatty stools, and arthritis, calling the illness an “intestinal lipodystrophy,” later coined Whipple’s disease.1 Nearly 100 years later, successful culture of the causative bacterium, Tropheryma whipplei, facilitated genomic sequencing, precipitating a new era of detection of Whipple’s disease.2 T. whipplei was previously thought to be a rare pathogen, primarily affecting middle-age Caucasian men, with estimated prevalence of less than one per million.2 However, general prevalence in asymptomatic carriers by stool PCR ranges from 1.5–7%, and is much higher in sewage treatment workers (12–25%), suggesting potential fecal-oral transmission.2–4
Cardinal features of Whipple’s disease include migratory polyarthritis, weight loss, and diarrhea, but it is also responsible for a wide spectrum of other cardiac, musculoskeletal, genitourinary, and neurologic findings.2 Molecular techniques demonstrate that T. whipplei is one of the most common causes of culture-negative endocarditis.5, 6 Cardiac involvement has been reported in 17–55% of Whipple’s disease cases, ranging from endocarditis, myocarditis, and pericarditis, to congestive heart failure.2 Ocular Whipple’s disease is rare and may cause decreased vision, floaters, eye redness, pain, and photophobia. Intraocular findings are typically bilateral, and commonly present as chronic uveitis with vitritis.7 As the yield of culture is low, 16S rRNA sequencing of vitreal fluid may help confirm ocular involvement.
Foamy macrophages are the histologic hallmark of Whipple’s disease. Small bowel biopsies of patients with gastrointestinal disease typically show sheets of foamy macrophages infiltrating and broadening the lamina propria with resultant blunting of the intestinal villi.8 The periodic acid-Schiff (PAS) stain reveals macrophages filled with coarsely granular, sickle-shaped, diastase-resistant, intracytoplasmic bacterial inclusions.8 These foamy macrophages are visible in any affected organ and may be present in valvular vegetations, though are not specific for T. whipplei infection.5, 8
Tissue-based broad-range 16S rRNA sequencing is a promising tool in the diagnosis of culture-negative bacterial endocarditis with an estimated sensitivity of 67%.6 In cases of suspected Whipple’s endocarditis, molecular testing may be particularly informative since the pathogen is difficult to culture and serologic testing is not routinely used.5 However, molecular techniques remain underutilized as they are not routinely available in many clinical laboratories, and they are not yet included among diagnostic criteria for infective endocarditis.
Current treatment recommendations are to begin with intravenous ceftriaxone or penicillin for central nervous system penetration, and then transition to oral maintenance therapy for a year with TMP-SMX.2 Doxycycline plus hydroxychloroquine is an alternative maintenance option for patients without neurologic involvement.2 Paradoxical worsening of symptoms soon after treatment initiation is suggestive of IRIS, which occurs in about 10% of patients on appropriate therapy.9 Treatment failure is common, highlighting the importance of close monitoring during and after therapy.10 PET-CT can help evaluate relapsed disease, but is not yet recommended for routine surveillance.11 Increased recognition of Whipple’s disease in the era of molecular diagnostics may lead to better insights about the best approach to treatment and monitoring of this protean disease.
Acknowledgments
Funding: This publication was supported by Grant Number T32 AI007433 from the National Institute of Allergy and Infectious Diseases. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Authors’ contributions:
All of the authors of this manuscript were directly involved in the care of this patient. CD1 conducted a literature review and compiled the first draft with contributions from SB, CD3, NW, and HR. SB, CD3, JS, EB, and HR performed significant revisions and GF provided photos and pathologic analysis of the case. All authors read and approved the final manuscript.
CD1 is an infectious disease fellow at Brigham & Women’s Hospital (BWH). SB and CD3 are internal medicine residents at BWH and University of San Francisco Medical Center respectively. NW is an ophthalmology fellow at Massachusetts Eye and Ear Infirmary and JS is an ophthalmology attending at the Joslin Diabetes Center. GF is a pathology resident at BWH. EB is an academic educator and the associate chief of Hospital Medicine and HR is an infectious disease attending, both at BWH.
Conflict of Interest: None
REFERENCES
- 1.Whipple GH. A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues. Johns Hopkins Hospital Bulletin. 1907 [Google Scholar]
- 2.Fenollar F, Puechal X, Raoult D. Medical progress - Whipple's disease. New England Journal of Medicine. 2007;356:55–66. doi: 10.1056/NEJMra062477. [DOI] [PubMed] [Google Scholar]
- 3.Fenollar F, Trani M, Davoust B, et al. Prevalence of asymptomatic Tropheryma whipplei carriage among humans and nonhuman primates. The Journal of infectious diseases. 2008;197:880–887. doi: 10.1086/528693. [DOI] [PubMed] [Google Scholar]
- 4.Schoniger-Hekele M, Petermann D, Weber B, Muller C. Tropheryma whipplei in the environment: survey of sewage plant influxes and sewage plant workers. Appl Environ Microbiol. 2007;73:2033–2035. doi: 10.1128/AEM.02335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geissdorfer W, Moos V, Moter A, et al. High frequency of Tropheryma whipplei in culture-negative endocarditis. Journal of clinical microbiology. 2012;50:216–222. doi: 10.1128/JCM.05531-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris KA, Yam T, Jalili S, et al. Service evaluation to establish the sensitivity, specificity and additional value of broad-range 16S rDNA PCR for the diagnosis of infective endocarditis from resected endocardial material in patients from eight UK and Ireland hospitals. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2014;33:2061–2066. doi: 10.1007/s10096-014-2145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Touitou V, Fenollar F, Cassoux N, et al. Ocular Whipple's disease: therapeutic strategy and long-term follow-up. Ophthalmology. 2012;119:1465–1469. doi: 10.1016/j.ophtha.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Dutly F, Altwegg M. Whipple's disease and "Tropheryma whippelii". Clinical microbiology reviews. 2001;14:561–583. doi: 10.1128/CMR.14.3.561-583.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feurle GE, Moos V, Schinnerling K, et al. The immune reconstitution inflammatory syndrome in whipple disease: a cohort study. Ann Intern Med. 2010;153:710–717. doi: 10.7326/0003-4819-153-11-201012070-00004. [DOI] [PubMed] [Google Scholar]
- 10.Lagier JC, Fenollar F, Lepidi H, Giorgi R, Million M, Raoult D. Treatment of classic Whipple's disease: from in vitro results to clinical outcome. J Antimicrob Chemother. 2014;69:219–227. doi: 10.1093/jac/dkt310. [DOI] [PubMed] [Google Scholar]
- 11.Lagier JC, Cammilleri S, Raoult D. Classic Whipple's disease diagnosed by (18)F-fluorodeoxyglucose PET. The Lancet. Infectious diseases. 2016;16:130. doi: 10.1016/S1473-3099(15)00503-4. [DOI] [PubMed] [Google Scholar]