Abstract
Only about 85% of men who have sex with men (MSM) with HIV have been tested for and diagnosed with HIV. Racial/ethnic disparities in HIV risk and HIV care outcomes exist within MSM. We examined racial/ethnic disparities in delayed HIV diagnosis MSM. Males aged ≥13 reported to the Florida Enhanced HIV/AIDS Reporting System 2000–2014 with a reported HIV transmission mode of MSM were analyzed. We defined delayed HIV diagnosis as an AIDS diagnosis within three months of the HIV diagnosis. Multilevel logistic regressions were used to estimate adjusted odds ratios (aOR). Of 39,301 MSM, 27% were diagnosed late. After controlling for individual factors, neighborhood socioeconomic status, and rural-urban residence, non-Latino Black MSM had higher odds of delayed diagnosis compared with non-Latino White MSM (aOR 1.15, 95% confidence interval [CI] 1.08–1.23). Foreign birth compared with US birth was a risk factor for Black MSM (aOR 1.27, 95% CI 1.12–1.44), but a protective factor for White MSM (aOR 0.77, 95% CI 0.68–0.87). Rural residence was a risk for Black MSM (aOR 1.79, 95% CI 1.36–2.35) and Latino MSM (aOR 1.87, 95% CI 1.24–2.84), but not for White MSM (aOR 1.26, 95% CI 0.99–1.60). HIV testing barriers particularly affect non-Latino Black MSM. Social and/or structural barriers to testing in rural communities may be significantly contributing to delayed HIV diagnosis among minority MSM.
Keywords: human immunodeficiency virus, men who have sex with men, late diagnosis, neighborhood
INTRODUCTION
The estimated number of new human immunodeficiency virus (HIV) infections among men who have sex with men (MSM) in the United States (US) in 2010 was 29,800 (Centers for Disease Control and Prevention [CDC], 2015). Black MSM accounted for the largest proportion of infections (38%) (CDC, 2016). Although the number of new HIV diagnoses among Black MSM increased 22% between 2005 and 2014, the upward trend appears to be slowing in recent years, increasing less than 1% between 2010 and 2014 (CDC, 2016). While estimating the rate of HIV among MSM has proven difficult, one study in New York City estimated that the case rate per 100,000 among non-Latino Black MSM was 8,781 between 2005–2008, compared with 3,221 among Latino MSM, and 1,241 among non-Latino White MSM (Pathela et al., 2011).
Unfortunately, of the estimated 647,700 MSM with HIV in the US at the end of 2011, only about 85% had been tested for and diagnosed with HIV (CDC, 2014a). This suggests that continued efforts to diagnose MSM with HIV promptly are needed to curb the HIV epidemic in this group. The general population of MSM with HIV experience better outcomes along the HIV care continuum when compared with other risk groups (CDC, 2014a).However, Black MSM with HIV experience the lowest rates of linkage to HIV care, retention in care, prescription of antiretroviral therapy (ART), and viral suppression compared with MSM from all other racial/ethnic groups (CDC, 2014b), and compared with their male heterosexual counterparts (CDC, 2014c).
While predictors such as Black race, homelessness, MSM disclosure (Nelson, et al., 2010), life stressors (Nelson, et al., 2014), and MSM-related stigma (Glick & Golden, 2010) have been associated with HIV testing and delayed diagnosis among MSM, less is known about predictors of delayed diagnosis among specific racial/ethnic groups of MSM, particularly those factors at the neighborhood level. Therefore, the objectives of this study were to (a) examine racial/ethnic disparities in delayed HIV diagnosis among MSM, and (b) identify specific individual- and neighborhood-level determinants of delayed HIV diagnosis for each MSM racial/ethnic group in Florida.
METHODS
Datasets
De-identified HIV surveillance records were obtained from the Florida Department of Health enhanced HIV/AIDS reporting system (eHARS). Cases age ≥13 who met the CDC HIV case definition (CDC, 2008) during the years 2000–2014 and had a reported HIV transmission mode of MSM were analyzed. Cases with missing or invalid data for ZIP code at time of HIV diagnosis and missing month and year of HIV diagnosis, and cases diagnosed in a correctional facility, were excluded. Cases diagnosed in a correctional facility were excluded because they are not representative of the HIV population in the neighborhood where the facility is located, and because they have different access to care than the general population with HIV infection. The 2009–2013 American Community Survey (ACS) was used to obtain neighborhood-level data using ZIP code tabulation areas (ZCTAs) (ACS, 2015). ZCTAs are used by the US Census Bureau to tabulate summary statistics and approximate US postal service ZIP codes (US Census Bureau, n.d.).
Individual-level variables
The following individual-level data were extracted from eHARS: ethnicity, race, HIV diagnosis year, sex at birth, age at HIV diagnosis, HIV transmission mode, birth country, HIV-to-AIDS interval in months (if case progressed to AIDS), residential ZIP code at time of HIV diagnosis, and whether the case was diagnosed at a correctional facility. Data on mode of HIV transmission were self-reported during HIV testing, reported by a health care provider, or extracted from medical chart reviews. Cases were coded as US-born if they were born in any of the 50 states, District of Columbia, Puerto Rico, or any US dependent territory. Delayed HIV diagnosis was defined as an AIDS diagnosis within 3 months of HIV diagnosis (CDC, 2013).
Neighborhood-level variables
Thirteen neighborhood-level socioeconomic status (SES) indicators were extracted from the ACS to develop an SES index of Florida neighborhoods (ZCTAs) (Niyonsenga et al., 2013): percent of households without access to a car, percent of households with ≥1 person per room, percent of population living below the poverty line, percent of owner-occupied homes worth ≥$300,000, median household income in 2013 percent of households with annual income <$15,000, percent of households with annual income ≥$150,000, income disparity (derived from percent of households with annual income <$10,000 and percent of households with annual income ≥$50,000), percent of population age ≥25 with less than a 12th grade education, percent of population age ≥25 with a graduate professional degree, percent of households living in rented housing, percent of population age ≥16 who were unemployed, and percent of population age ≥16 employed in high working class occupation (ACS occupation group: “managerial, business, science, and arts occupations”). Income disparity was calculated as the logarithm of 100 times the percent of households with annual income <$10,000 divided by the percent of households with annual income ≥$50,000 and was used as a proxy for the Gini-coefficient (Niyonsenga et al., 2013; Singh & Siahpush, 2002). All neighborhood-level indicators were coded so that higher scores corresponded with higher SES; they were then standardized (Niyonsenga et al., 2013).
To calculate the SES index, we started by conducting a reliability analysis. The Cronbach’s alpha for all 13 indicators was 0.93. We selected 7 indicators based on the correlation of the indicator with the total index (high correlation), and the Cronbach’s alpha if the item was deleted (low alpha). The 7 indicators selected were: percent below poverty, median household income, percent of households with annual income <$15,000, percent of households with annual income ≥$150,000, income disparity, percent of population age ≥25 with less than a 12th grade education, and high-class work. The resulting Cronbach’s alpha increased (0.94).
Second, we conducted a principal component analysis with and without varimax rotation, which revealed one factor with an eigenvalue greater than 1 (5.14). This factor accounted for 73.49% of the variance in the indicators. Because all the factor loadings were high (between 0.80 and 0.93), we retained all 7 indicators. Finally, we added the standardized scores for the 7 variables and categorized the scores into quartiles.
To categorize ZCTAs into rural or urban, we used Categorization C of Version 2.0 of the Rural-Urban Commuting Area (RUCA) codes, developed by the University of Washington WWAMI Rural Research Center (WWAMI Rural Health Research Center, n.d.).
Statistical analyses
Individual- and neighborhood-level data were merged by matching the ZIP code at time of HIV diagnosis of each case with the ZIP code’s corresponding ZCTA. We compared individual- and neighborhood-level characteristics by race/ethnicity. We used the Cochran-Mantel-Haenszel general association statistic for individual-level variables controlling for ZCTA, and the chi-square test for neighborhood-level variables. Multi-level (Level 1: individual; Level 2: neighborhood) logistic regression modeling was used to account for correlation among cases living in the same neighborhood through a random intercept using ZCTA. Crude and adjusted odds ratios and 95% confidence intervals for delayed diagnosis were calculated comparing cases by race/ethnicity. First, we estimated crude odds ratios (Model 1). Then we controlled for individual-level factors (Model 2). Finally, we controlled for individual- and neighborhood-level variables (Model 3). To identify unique predictors of delayed diagnosis for each group, separate models were estimated stratifying by race/ethnicity adjusting for year of HIV diagnosis, age, US/foreign-born status, injection drug use, socioeconomic status (index of 7 indicators), and rural/urban status. SAS software, version 9.4 (SAS Institute, Cary, NC 2002), was used to conduct analyses. Multivariate models were adjusted for year of HIV diagnosis to control for likely changes in HIV testing behaviors and HIV testing strategies over the 15-year study period. The Florida International University institutional review board approved this study, and the Florida Department of Health designated this study to be non-human subjects research.
RESULTS
Characteristics of participants
Of 91,867 HIV cases reported in Florida 2000–2014, 42,493 had MSM listed as a mode of HIV transmission. Of these, 1,311 were diagnosed in a correctional facility, 1,785 had missing data on ZIP code at time of HIV diagnosis, and 176 had missing data on month of HIV diagnosis (categories are not mutually exclusive). No cases under the age of 13 reported transmission mode as MSM. Of the remaining 39,301 cases analyzed in this study, 27.3% were diagnosed late (see Table 1). This represented a downward trend that started at 38.4% in 2000 and decreased to 18.5% by 2014.
Table 1.
Characteristics of men who have sex with men (MSM) age 13 years and older diagnosed with HIV in Florida, 2000—2014
| Total, n(%) | Non-Latino Black MSM, n(%) |
Latino MSM, n(%) |
Non-Latino White MSM, n(%) |
Other MSM, n(%) |
P-value | |
|---|---|---|---|---|---|---|
| Total | 39,301 | 11,183 | 10,747 | 16,446 | 925 | |
|
Individual-level variables |
||||||
| Year of HIV diagnosis | <0.0001 | |||||
| 2000–2004 | 13,520 (34.4) | 3,679 (32.9) | 3,399 (31.6) | 6,114 (37.2) | 328 (35.5) | |
| 2005–2009 | 12,576 (32.0) | 3,564 (31.9) | 3,309 (30.8) | 5,412 (32.9) | 291 (31.5) | |
| 2010–2014 | 13,205 (33.6) | 3,940 (35.2) | 4,039 (37.6) | 4,920 (29.9) | 306 (33.1) | |
| Age group at diagnosis |
<0.0001 | |||||
| 13–19 years | 1,314 (3.3) | 877 (7.8) | 241 (2.2) | 166 (1.0) | 30 (3.2) | |
| 20–39 years | 21,791 (55.5) | 7,140 (63.9) | 6,491 (60.4) | 7,601 (46.2) | 559 (60.4) | |
| 40–59 years | 14,779 (37.6) | 2,904 (26.0) | 3,720 (34.6) | 7,847 (47.7) | 308 (33.3) | |
| 60 years or older | 1,417 (3.6) | 262 (2.3) | 295 (2.7) | 832 (5.1) | 28 (3.0) | |
| US- vs. foreign-born | <0.0001 | |||||
| US-born | 29,097 (74.0) | 9,736 (87.1) | 4,058 (37.8) | 14,678 (89.3) | 625 (67.6) | |
| Foreign-born | 10,204 (26.0) | 1,447 (12.9) | 6,689 (62.3) | 1,768 (10.8) | 300 (32.4) | |
| Injection drug use | 0.0014 | |||||
| Yes | 2,096 (5.3) | 634 (5.7) | 442 (4.1) | 974 (5.9) | 46 (5.0) | |
| No | 37,205 (94.7) | 10,549 (94.3) | 10,305 (95.9) | 15,472 (94.1) | 879 (95.0) | |
| Delayed HIV diagnosis (AIDS within 3 months of HIV diagnosis) |
0.0603 | |||||
| Yes | 10,708 (27.3) | 3,089 (27.6) | 2,706 (25.2) | 4,656 (28.3) | 257 (27.8) | |
| No | 28,593 (72.8) | 8,094 (72.4) | 8,041 (74.8) | 11,790 (71.7) | 668 (72.2) | |
|
ZCTA-level variables |
||||||
| Socioeconomic status index, quartiles |
<0.0001 | |||||
| 1 (lowest SES) | 13,088 (33.3) | 5,796 (51.8) | 3,770 (35.1) | 3,269 (19.9) | 253 (27.4) | |
| 2 | 9,935 (25.3) | 2,847 (25.5) | 2,478 (23.1) | 4,364 (26.5) | 246 (26.6) | |
| 3 | 10,066 (25.6) | 1,656 (14.8) | 3,032 (28.2) | 5,128 (31.2) | 250 (27.0) | |
| 4 (highest SES) | 6,212 (15.8) | 884 (7.9) | 1,467 (13.7) | 3,685 (22.4) | 176 (19.0) | |
| RUCA classification | <0.0001 | |||||
| Rural | 917 (2.3) | 257 (2.3) | 122 (1.1) | 524 (3.2) | 14 (1.5) | |
| Urban | 38,384 (97.7) | 10,926 (97.7) | 10,625 (98.9) | 15,922 (96.8) | 911 (98.5) | |
MSM, men who have sex with men; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency virus, US, United States; ZCTA, ZIP code tabulation area; SES, socioeconomic status; RUCA, Rural-Urban Commuting Area. Percentage may not add up to 100 due to rounding.
Racial/ethnic disparities in delayed HIV diagnosis
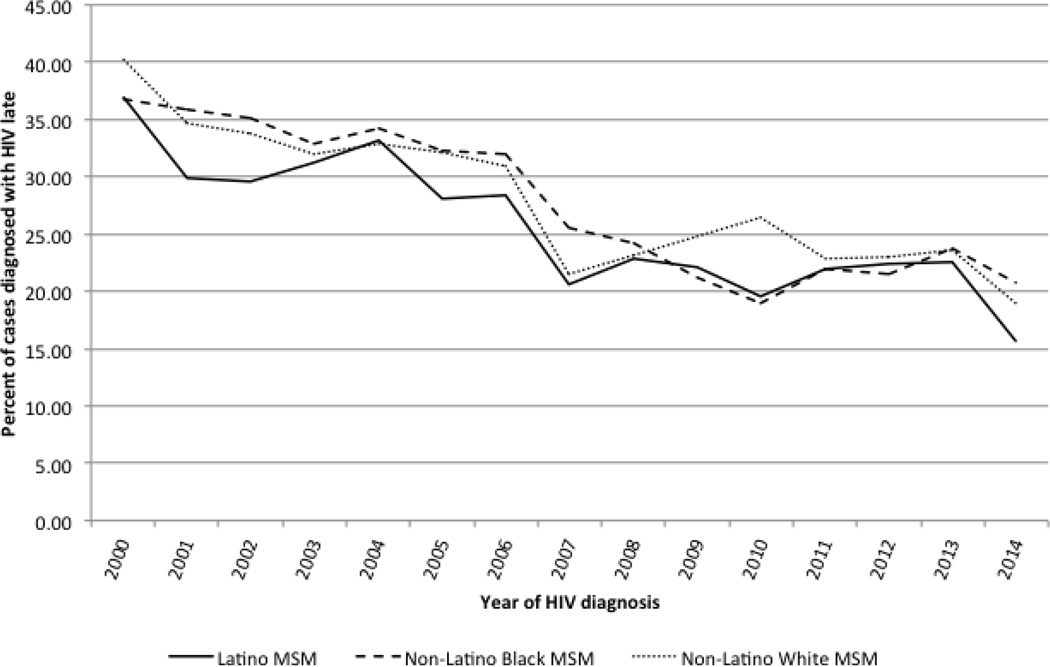
The proportion of cases diagnosed late decreased from 2000–2014 for all racial/ethnic groups (see Figure 1). In crude logistic regression models, Latino MSM had lower odds of delayed diagnosis compared with White MSM (see Table 2). After controlling for individual-level factors, Black MSM had higher odds of delayed diagnosis compared with White MSM, and the protective effect of Latino MSM disappeared. The higher odds for delayed diagnosis among Black MSM remained after controlling for neighborhood-level SES and rural/urban status.
Figure 1.
Delayed HIV diagnosis (AIDS diagnosis within 3 months of HIV diagnosis) among men who have sex with men by year of diagnosis and race/ethnicity, Florida, 2000–2014
TABLE 2.
Crude and adjusted odds ratios and 95% confidence intervals for delayed HIV diagnosis (AIDS diagnosis within 3 months of HIV diagnosis) among men who have sex with men (MSM) age 13 years and older, Florida, 2000–2014
| Model 1 Crude OR (95% CI) |
Model 2 Adjusted OR (95% CI) |
Model 3 Adjusted OR (95% CI) |
|
|---|---|---|---|
| Non-Latino Black MSM | 0.97 (0.92–1.02) | 1.21 (1.14–1.28) | 1.15 (1.08–1.23) |
| Latino MSM | 0.85 (0.81–0.90) | 0.98 (0.92–1.05) | 0.99 (0.93–1.07) |
| Non-Latino White MSM | Referent | Referent | Referent |
| Other MSM | 0.97 (0.84–1.13) | 1.11 (0.96–1.29) | 1.10 (0.94–1.28) |
MSM, men who have sex with men; OR, odds ratio for delayed HIV diagnosis; CI, confidence interval
Model 1: Crude rates
Model 2: Controlling for individual-level variables
Model 3: Controlling for individual-level variables and neighborhood-level variables
Predictors of delayed HIV diagnosis by race/ethnicity
HIV diagnosis during 2000–2009 compared with 2010–2014 and diagnosis at 20 years of age or older compared with 13–19 were predictors of delayed diagnosis for Black, Latino, and White MSM (see Table 3). Among Black MSM, being foreign-born compared with US-born, and living in a rural area compared with an urban area were additionally associated. Among Latino MSM, only residing in a rural area at time of HIV diagnosis was independently associated with delayed HIV diagnosis. Among White MSM, being foreign-born compared with US-born was protective.
Table 3.
Adjusted odds ratios and 95% confidence intervals for delayed HIV diagnosis (AIDS diagnosis within 3 months of HIV diagnosis) among men who have sex with men (MSM) age 13 years and older, stratified by race/ethnicity, Florida, 2000–2014
| Non-Latino Black MSM, AOR (95% CI) |
Latino MSM, AOR (95% CI) |
Non-Latino White MSM, AOR (95% CI) |
Other MSM, AOR (95% CI) |
|
|---|---|---|---|---|
| Individual-level variables | ||||
| Year of HIV diagnosis | ||||
| 2000–2004 | 1.78 (1.60–1.99) | 1.97 (1.77–2.20) | 1.94 (1.78–2.12) | 1.51 (1.03–2.22) |
| 2005–2009 | 1.29 (1.15–1.43) | 1.27 (1.13–1.42) | 1.22 (1.11–1.34) | 1.31 (0.89–1.94) |
| 2010–2014 | Referent | Referent | Referent | Referent |
| Age group at diagnosis | ||||
| 13–19 years | Referent | Referent | Referent | Referent |
| 20–39 years | 2.52 (2.02–3.14) | 2.47 (1.59–3.83) | 2.79 (1.65–4.71) | 4.01 (0.92–17.53) |
| 40–59 years | 4.65 (3.70–5.85) | 4.89 (3.14–7.60) | 5.00 (2.97–8.44) | 7.79 (1.77–34.25) |
| 60 years or older | 6.28 (4.53–8.72) | 5.60 (3.40–9.21) | 4.50 (2.61–7.73) | 7.75 (1.47–41.00) |
| US- vs. foreign-born | ||||
| US-born | Referent | Referent | Referent | Referent |
| Foreign-born | 1.27 (1.12–1.44) | 1.10 (0.99–1.20) | 0.77 (0.68–0.87) | 1.31 (0.94–1.83) |
| Injection drug use | ||||
| Yes | 0.85 (0.71–1.02) | 0.79 (0.63–1.00) | 0.86 (0.74–1.00) | 1.11 (0.54–2.27) |
| No | Referent | Referent | Referent | Referent |
| ZCTA-level variables | ||||
| Socioeconomic status index, quartiles |
||||
| 1 (lowest SES) | 1.03 (0.87–1.23) | 1.09 (0.92–1.31) | 0.97 (0.84–1.11) | 1.13 (0.71–1.82) |
| 2 | 0.95 (0.79–1.15) | 1.12 (0.92–1.35) | 1.07 (0.94–1.22) | 0.82 (0.51–1.32) |
| 3 | 0.87 (0.71–1.06) | 1.04 (0.86–1.26) | 1.12 (0.98–1.27) | 0.87 (0.53–1.41) |
| 4 (highest SES) | Referent | Referent | Referent | Referent |
| RUCA classification | ||||
| Rural | 1.79 (1.36–2.35) | 1.87 (1.24–2.84) | 1.26 (0.99–1.60) | 0.87 (0.25–3.04) |
| Urban | Referent | Referent | Referent | Referent |
MSM, men who have sex with men; US, United States; ZCTA, ZIP code tabulation area; SES, socioeconomic status; RUCA, Rural-Urban Commuting Area; AOR, adjusted odds ratio for delayed HIV diagnosis (adjusted for all variables in the column).
DISCUSSION
Twenty-seven percent of HIV diagnoses in Florida 2000–2014 with a reported mode of HIV transmission as MSM were diagnosed late. After adjusting for individual- and neighborhood-level factors, Black MSM were at increased odds of delayed diagnosis compared with White MSM. Among Black MSM, being foreign-born and residing in a rural area at the time of HIV diagnosis were risk factors. Rural residence was also a strong predictor of delayed diagnosis for Latino MSM. Neighborhood-level SES was not associated with delayed HIV diagnosis among any racial/ethnic MSM group in Florida.
The proportion of late HIV diagnoses among MSM in Florida for the years 2000–2014 was 27.3% (consistent with national estimates, [CDC, 2013)], decreased from 38.4% in 2000 to 18.5% in 2014. The decline may be partially due to revised recommendation for HIV testing such as the 2006 CDC (Branson, et al., 2006) and 2013 US Preventive Services Task Force (Moyer & US Preventive Services Task Force, 2013) guidelines for opt-out screening of adolescent and adult patients in healthcare settings. While several studies have examined racial/ethnic disparities in delayed HIV diagnosis among the general HIV infected population (Tang, Levy & Hernandez, 2011; Trepka et al., 2014; Yang et al., 2010), few studies have examined these disparities among MSM. One study of MSM diagnosed in 33 US states between 1996–2002 found significant differences in the proportion of Black MSM (23.1%, 95% CI 22.4–23.7) and Latino MSM (23.7%, 95% CI 22.6–24.7) who were diagnosed late compared with White MSM (18.4%, 95% CI 17.9–18.9) (Hall et al., 2007). However, the study included the earlier years of the epidemic and used AIDS diagnosis within 12 months of HIV diagnosis to define delayed diagnosis. In our study, Black MSM had higher odds of delayed diagnosis compared with White MSM after adjusting for individual-level factors. Black MSM tended to be younger, with over 70% diagnosed between the ages of 13 and 39, compared with 47% for White MSM. Differences in age, as well as year of diagnosis and nativity, appear to confound disparities in delayed diagnosis between Black and White MSM. Conversely, the apparent advantage among Latinos when compared with Whites in the crude model appears to be related to differences in individual-level factors.
It remains unclear why Black MSM are more likely to be diagnosed late with HIV. Previous studies and a meta-analysis suggest that Black MSM have higher rates of HIV testing (Pathela et al., 2011; Millet et al., 2007). However, a population-based study suggested that MSM-related stigma among Blacks (72%) and Black MSM (57%) is high, and higher than among Whites (52%) and White MSM (27%), and that unfavorable attitudes toward MSM are associated with no prior HIV testing (Glick & Golden, 2010). A quantitative study comparing MSM who tested late for HIV with those who did not test late found that being Black and homelessness, disclosing male-male sex to 50% or less of people in social circle, having 1 sexual partner versus more than 1 sexual partner in the past 6 months (Nelson et al., 2010), and experiencing multiple life stressors (Nelson et al., 2014) were associated with delayed HIV testing and diagnosis. Further, Black MSM experience more homelessness (Sullivan et al., 2014) and higher rates of depression than White MSM (Richardson et al., 1997), may be less likely to disclose their MSM status to others (Gates, 2010) and may have or perceive less social support (Stokes, Vanable & McKirnan, 1996).
Over 50% of Black MSM resided in neighborhoods in the lowest quartile of SES, compared with 35% of Latino MSM, and 20% of White MSM in our study. The disparity in delayed diagnosis between Black and White MSM decreased but remained after adjusting for neighborhood SES and rural/urban residence. Our results suggest that a comprehensive index of neighborhood SES and rural/urban status explain a portion of the observed disparities between Black MSM and White MSM and do not account for the disparity that remains after controlling for individual-level factors.
Being foreign-born was associated with delayed HIV diagnosis for Black MSM. Our results are similar to those from a national study of 33 US states that found a higher proportion of delayed HIV diagnosis (AIDS within 12 months of HIV diagnosis) among foreign-born Black MSM (44.1%) compared with US-born Black MSM (36.7%) (Johnson, Hu, & Dean, 2010). Our population of foreign-born Black MSM was primarily born in Haiti (49.1%), Jamaica (16.3%), and the Bahamas (5.6%). In the national study mentioned above by Johnson and colleagues, the proportion of Caribbean-born Blacks diagnosed late was 44.2%, higher than the proportion of African-born Blacks (42.1%). A study of 1,060 Blacks in Massachusetts found that foreign-born Blacks were less likely to report HIV testing compared with US-born Blacks (42% vs. 56%) (Ojikutu et al., 2013). Ojikutu et al. found that HIV-related stigma was higher, and knowledge was lower, among foreign-born Blacks compared with US-born Blacks, particularly among Caribbean-born participants than among sub-Saharan African participants (Ojikutu et al., 2013). They also found that over 50% of foreign-born Blacks reported that their most recent HIV test was part of an immigration requirement. The HIV testing requirement for immigrants was lifted in 2010 and has likely impacted testing patterns among immigrants (Winston & Beckwith, 2011).
After adjusting for individual-level factors and neighborhood SES, rural residence was a predictor of delayed diagnosis among Black and Latino MSM. Forty-one percent of both Black MSM and Latino MSM who resided in rural areas were diagnosed late, compared with 27% and 25% of their urban counterparts. A previous population-based cohort study of Florida HIV cases reported that 35% of Blacks in rural areas were diagnosed late, compared with 29% in urban areas (Trepka et al., 2014). This suggests that not only do Black MSM in rural areas have a higher risk of delayed HIV diagnosis when compared with Black MSM in urban areas, but also when compared with both the rural and urban general HIV infected Black population. It is possible that high levels of HIV- and MSM-related stigma, and higher risk of loss of confidentiality in rural areas compared with urban areas are preventing MSM from routine HIV testing, particularly for racial/ethnic minorities (Preston, et al., 2002). Fear of being the target of a violent crime due to hostility against MSM has been reported in a qualitative study of MSM in rural Wyoming (Williams, Bowen & Horvath, 2005). Of note, rural areas in Wyoming are likely very different and more isolated from larger cities than rural areas in Florida. A study in Europe found that MSM who resided in smaller cities reported higher internalized homonegativity compared to those who resided in larger cities, and that higher homonegativity was associated with decreased likelihood of HIV testing (Berg et al., 2011).
A limitation of this study is related to our definition of late diagnosis. It is possible that some individuals who had AIDS within three months of HIV diagnosis were not diagnosed with AIDS until after three months. However, we believe that the possibility of misclassification is small given that cases with AIDS likely had symptoms that encouraged prompt HIV care seeking behavior. Furthermore, HIV reporting was not mandated in Florida until 1997. It is possible that cases diagnosed prior to 1997 were later reported as new HIV diagnoses, and therefore, mistakenly appear to have a shorter HIV-to-AIDS time interval. Nevertheless, it is worth noting that our rate of delayed diagnosis for MSM was nearly identical to national estimates (CDC, 2013). Additionally, our dataset did not allow us to examine important variables, such as individual-level SES, access to health insurance, and HIV testing patterns and barriers. Finally, the small number of rural cases limited our ability to stratify racial/ethnic groups by rural/urban status to identify unique predictors of delayed diagnosis in rural areas.
Most cases of late HIV diagnosis can be prevented; it is estimated that only 3.6–13% of infections are due to accelerated disease progression (Sabharwal et al., 2011). Therefore, regular HIV testing, as per the current guidelines, offers an opportunity to diagnose individuals prior to developing AIDS. However, barriers to the implementation of routine testing exist, creating disparities across racial/ethnic and other groups. Our findings warrant future investigations on potential cultural barriers to HIV testing among foreign-born Black MSM, as well as on the contextual differences between rural and urban culture that appear to affect HIV testing among MSM. Strategies, such as using social networks to increase HIV testing, have shown promising results among Black MSM (Fuqua et al., 2012) and may also be effective among foreign-born and rural populations of Black MSM.
Acknowledgments
Research reported in this publication was supported by the National Institute on Drug Abuse (NIDA) under Award Number F31DA037790 and by the National Institute on Minority Health & Health Disparities (NIMHD) under Award Number 5R01MD004002. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDA, NIMHD, or the National Institutes of Health.
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
REFERENCES
- American Community Survey. 2009–2013 American Community Survey 5–Year Estimates. Washington, DC, USA: American Community Survey United States Census Bureau; 2015. [Google Scholar]
- Berg RC, Ross MW, Schmidt AJ EMIS Network. Structural inequalities are associated with internalized homonegativity among European MSM; Poster presented at: The Future of European Prevention among MSM conference; Stockholm, Sweden. 2011. [Google Scholar]
- Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, Clark JE, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. Morbidity & Mortality Weekly Report. 2006;55(RR14):1–4. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years–United States, 2008. [Accessed January 15, 2015];Morbidity & Mortality Weekly Report. 2008 57(RR10):1–8. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5710a1.htm. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2013. [Accessed September 1, 2014]. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data–United States and 6 US dependent areas–2011. HIV Surveillance Report. Vol.18 (No. 5). Retrieved from http://www.cdc.gov/hiv/pdf/2011_monitoring_hiv_indicators_hssr_final.pdf. [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV – United States, 2011. [Accessed November 2, 2015];Morbidity & Mortality Weekly Report. 2014a 63(47):1113–1117. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6347a5.htm. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Men living with diagnosed HIV who have sex with men: progress along the continuum of HIV care – United States, 2010. [Accessed November 2, 2015];Morbidity & Mortality Weekly Report. 2014b 63(38):829–833. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6338a2.htm. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Progress along the continuum of HIV care among blacks with diagnosed HIV– United States, 2010. [Accessed November 2, 2015];Morbidity & Mortality Weekly Report. 2014c 63(05):85–89. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6305a2.htm. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV in the United States: At a glance. [Accessed May 20, 2015];2015 Retrieved from http://www.cdc.gov/hiv/statistics/overview/ataglance.html.
- Centers for Disease Control and Prevention. HIV among African American Gay and Bisexual Men. [Accessed May 20, 2015];2016 Retrieved from http://www.cdc.gov/hiv/group/msm/bmsm.html.
- Fuqua V, Chen YH, Packet T, Dowling T, Ick TO, Nguyen B, Colifax GN, et al. Using social networks to reach Black MSM for HIV testing and linkage to care. AIDS & Behavior. 2012;16(2):256–265. doi: 10.1007/s10461-011-9918-x. [DOI] [PubMed] [Google Scholar]
- Gates GJ. Sexual minorities in the 2008 General Social Survey: Coming out and demographic characteristics. University of California; Los Angeles: The Williams Institute; 2010. [Google Scholar]
- Glick SN, Golden MR. Persistence of racial differences in attitudes toward homosexuality in the United States. Journal of Acquired Immune Deficiency Syndromes. 2010;55(4):516–523. doi: 10.1097/QAI.0b013e3181f275e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall HI, Byers RH, Ling Q, Espinoza L. Racial/ethnic and age disparities in HIV prevalence and disease progression among men who have sex with men in the United States. American Journal of Public Health. 2007;97(6):1060–1066. doi: 10.2105/AJPH.2006.087551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AS, Hu X, Dean HD. Epidemiologic differences between native-born and foreign-born black people diagnosed with HIV infection in 33 U.S. states, 2001–2007. Public Health Reports. 2010;125(Supplement 4):61–69. doi: 10.1177/00333549101250S410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet GA, Flores SA, Peterson JL, Bakeman R. Explaining disparities in HIV infection among black and white men who have sex with men: a meta–analysis of HIV risk behaviors. AIDS. 2007;21:2083–2091. doi: 10.1097/QAD.0b013e3282e9a64b. [DOI] [PubMed] [Google Scholar]
- Moyer VA US Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine. 2013;159(1):51–60. doi: 10.7326/0003-4819-159-1-201307020-00645. [DOI] [PubMed] [Google Scholar]
- Nelson KM, Thiede H, Hawes SE, Golden MR, Hutchenson R, Carey JW, Kurth A, et al. Why the wait? Delayed HIV diagnosis among men who have sex with men. Journal of Urban Health. 2010;87(4):642–655. doi: 10.1007/s11524-010-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KM, Thiede H, Jenkins RA, Carey JW, Hutchenson R, Golden MR. Personal and contextual factors related to delayed HIV diagnosis among men who have sex with men. AIDS Education & Prevention. 2014;26(2):122–133. doi: 10.1521/aeap.2014.26.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyonsenga T, Trepka MJ, Lieb S, Maddox LM. Measuring socioeconomic inequality in the incidence of AIDS: rural–urban considerations. AIDS & Behavior. 2013;17(2):700–709. doi: 10.1007/s10461-012-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojikutu B, Nnaji C, Sithole J, Schneider KL, Higgins-Biddle M, Cranston K, Earls F. All black people are not alike: differences in HIV testing patterns, knowledge, and experience of stigma between U.S.–born and non–U.S.–born blacks in Massachusetts. AIDS Patient Care & STDS. 2013;27(1):45–54. doi: 10.1089/apc.2012.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathela P, Braunstein SL, Schillinger JA, Shepard C, Sweeney M, Blank S. Men who have sex with men have a 140–fold higher risk for newly diagnosed HIV and syphilis compared with heterosexual men in New York City. Journal of Acquired Immune Deficiency Syndromes. 2011;58(4):408–416. doi: 10.1097/QAI.0b013e318230e1ca. [DOI] [PubMed] [Google Scholar]
- Preston DB, D’Augelli AR, Cain RE, Frederick WS. Issues in the development of HIV–preventive interventions for men who have sex with men (MSM) in rural areas. Journal of Primary Prevention. 2002;23(2):199–214. [Google Scholar]
- Richardson MA, Myers HF, Bing EG, Satz P. Substance use and psychopathology in African American men at risk for HIV infection. Journal of Community Psychology. 1997;25(4):353–370. [Google Scholar]
- Sabharwal CJ, Sepkowitz K, Mehta R, Shepard C, Bodach S, Torian L, Begier EM. Impact of accelerated progression to AIDS on public health monitoring of late HIV diagnosis. AIDS Patient Care & STDS. 2011;25(3):143–151. doi: 10.1089/apc.2010.0268. [DOI] [PubMed] [Google Scholar]
- Singh GK, Siahpush M. Increasing inequalities in all–cause and cardiovascular mortality among U.S. adults aged 25–64 years by area socioeconomic status, 1969–1998. International Journal of Epidemiology. 2002;31:600–613. doi: 10.1093/ije/31.3.600. [DOI] [PubMed] [Google Scholar]
- Stokes JP, Vanable PA, McKirnan DJ. Ethnic differences in sexual behavior, condom use, and psychosocial variables among black and white men who have sex with men. Journal of Sex Research. 1996;33(4):373–381. [Google Scholar]
- Sullivan PS, Peterson J, Rosenberg ES, Kelley CF, Cooper H, Vaughan A, Salazar LF, et al. Understanding Racial HIV/STI Disparities in Black and White Men Who Have Sex with Men: A Multilevel Approach. PLos One. 2014;9(3):e90514. doi: 10.1371/journal.pone.0090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JJ, Levy V, Hernandez MT. Who are California’s late HIV testers? An analysis of state AIDS surveillance data, 2000–2006. Public Health Reports. 2011;126:338–343. doi: 10.1177/003335491112600306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepka MJ, Fennie KP, Sheehan DM, Lutfi K, Maddox L, Lieb S. Late HIV diagnosis: differences by rural/urban residence, Florida, 2007–2011. AIDS Patient Care & STDS. 2014;28(4):188–197. doi: 10.1089/apc.2013.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. Geography: ZIP Code Tabulation Areas (ZCTAs) Washington, DC: USA: United States Census Bureau; (n.d.) [Google Scholar]
- Williams ML, Bowen AM, Horvath KJ. The social/sexual environment of gay men residing in a rural frontier state: implications for the development of HIV prevention programs. Journal of Rural Health. 2005;21(1):48–55. doi: 10.1111/j.1748-0361.2005.tb00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston SE, Beckwith CG. The impact of removing the immigration ban on HIV–infected persons. AIDS Patient Care & STDS. 2011;25(12):709–711. doi: 10.1089/apc.2011.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Chan SK, Mohammad N, Meyer JA, Risser J, Chronister KJ, Wolverton ML, et al. Late HIV diagnosis in Houston/Harris County, Texas, 2000–2007. AIDS Care. 2010;22:766–774. doi: 10.1080/09540120903431348. [DOI] [PubMed] [Google Scholar]