Abstract
Background and Purpose
For stroke prevention, patients with atrial fibrillation typically receive oral anticoagulation. The commonly used anticoagulant warfarin increases the risk of hemorrhagic transformation (HT) when a stroke occurs, however, and tissue plasminogen activator (tPA) treatment is restricted in these patients. The current study was designed to test the hypothesis that 12/15-LOX inhibition would reduce HT in warfarin-treated mice subjected to experimental stroke.
Methods
Warfarin was dosed orally in drinking water, and International Normalized Ratio (INR) values were determined using a Coaguchek device. C57BL6J mice or 12/15-LOX knockout mice were subjected to transient middle cerebral artery occlusion (MCAO) with 3 hours severe ischemia (Model A); or 2 h ischemia, and tPA infusion (Model B), with or without the 12/15-LOX inhibitor ML351. Hemoglobin was determined in brain homogenates, and hemorrhage areas on the brain surface and in brain sections were measured. 12/15-LOX expression was detected by immunohistochemistry.
Results
Warfarin treatment resulted in reproducible increased INR values and significant HT in both models. 12/15-LOX knockout mice suffered less HT following severe ischemia, and ML351 reduced HT in wild-type mice. When normalized to infarct size, ML351 still independently reduced hemorrhage. HT following tPA was similarly reduced by ML351.
Conclusions
In addition to its benefits in infarct size reduction, 12/15-LOX inhibition also may independently reduce HT in warfarin-treated mice. ML351 should be further evaluated as stroke treatment in anticoagulated patients suffering a stroke, either alone or in conjunction with tPA.
Keywords: 12/15-lipoxygenase, warfarin, hemorrhagic transformation, stroke, tPA
Introduction
Atrial fibrillation (AF) increases the risk of stroke, which is a leading cause of death and disability worldwide1. The prevalence of AF was between 2.7 and 6.1 million cases in 2010, and is estimated to rise up to 12.1 million by 20302. Administration of long-term oral anticoagulation reduces the risk of ischemic stroke in patients with AF3–5. However, the use of the vitamin K antagonist warfarin can be challenging because it significantly increases the risk of hemorrhagic transformation (HT) in patients with stroke6, 7. Warfarin-treated patients with effective anticoagulation, i.e. an international normalized ratio (INR) value greater than 1.7 are contraindicated for tPA treatment8, 9.
Accumulating data suggest that increased permeability of the blood brain barrier (BBB) after ischemia is one of the major causes of HT10. We have previously shown that following experimental stroke, 12/15-lipoxygenase (12/15-LOX) is increased in both neurons and vascular endothelial cells in the peri-infarct region11, 12. 12/15-LOX inhibition or knockout reduced the loss of endothelial tight junction protein claudin-5 and the leakage of Immunoglobulin G (Ig G) after transient ischemia12, both evidence for a role of 12/15-LOX in the disruption of the BBB seen after an ischemic event. We also demonstrated that the 12/15-LOX inhibitor LOXBlock-1 significantly reduced BBB leakage following tPA thrombolysis in a distal clot mouse model13.
We have recently introduced ML351 as a novel inhibitor of 12/15-LOX, which has shown effective neuroprotection both in vitro and in vivo14. Based on an established animal model of warfarin associated hemorrhagic transformation15, the present study was designed to investigate if inhibition of 12/15-LOX, or its gene knockout reduces warfarin-induced HT after experimental stroke. Furthermore, we evaluated whether 12/15-LOX inhibition reduces the risk of excess HT caused by thrombolytic treatment with tPA in warfarin-anticoagulated mice.
Materials and Methods
Mouse Model of Oral Anticoagulation
Male C57BL/6J mice (12 weeks old) from Jackson Laboratories and Alox15 knockout mice bred in our animal facility (in which the gene encoding 12/15-LOX is deleted) were used in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Following an established protocol15, 16, we administered warfarin by oral uptake through bottled drinking water. Briefly, different doses of Coumadin tablet (0, 1.25, 2.5, 5 mg) (Warfarin sodium, crystalline; Bristol Myers Squibb, Munich, Germany) were dissolved in 375 mL tap water, and the mice were fed for 24 hours with free access to the treated water. The international normalized ratio (INR) measurements were performed on venous blood sample drawn from the tail vein by a commercially available point of care coagumeter (Coaguchek XS®, Roche, Mannheim, Germany)17 immediately after warfarin withdrawal.
Transient Cerebral Focal Ischemia
A standard transient middle cerebral artery occlusion (MCAO) was performed as described previously12. Anesthesia was induced with 1.5% isoflurane in a mixture of 70% nitrous oxide and 30% oxygen delivered by face mask. Focal cerebral ischemia was induced by introducing a silicone-coated 6-0 monofilament until it occluded the ostium of the right MCA. The rectal temperature was maintained at 37±0.5 °C and the regional cerebral blood flow of the right front parietal cortex was continually monitored during the surgical procedure. The filament was withdrawn after two or three hours, as indicated, to allow reperfusion of the ischemic hemisphere. ML351 was delivered at 50 mg/kg body weight by intraperitoneal injection. The dose of the drug was based on our previous experiments14, and our findings using the filament model of MCAO shown in Supplementary Figure I. Control animals were injected with an equal volume of vehicle DMSO. To determine if ML351 would have any, possibly confounding effects on blood pressure, we monitored blood pressure in a separate group of mice before and after i.p. injection of 50 mg/kg ML351. We found no detectable effect on blood pressure (p=0.99, n=3 mice). To further investigate whether ML351 reduces the thrombolysis-associated HT in anticoagulated mice subjected to 2h of MCAO, 10 mg/kg tPA (Genentech Inc, San Francisco, CA) was given intravenously 3h after MCAO16 in re-anaesthetized mice and ML351 (50 mg/kg) was intraperitoneally injected directly prior to the administration of tPA. Mice that died during operation or prior to sacrifice were excluded from subsequent analyses. All animals were randomized. Investigators responsible for surgical procedures or drug treatments were blinded. End point assessments were performed by investigators blinded to the groups for which each animal was assigned.
Evaluation of HT and Infarct lesion
To ascertain hemorrhage in brain sections, mice were sacrificed following cardiac perfusion with phosphate-buffered saline (PBS) 24 hours after ischemia. The brain was sectioned into 1 mm slices and photographed. Red areas in the sections indicating hemorrhage were outlined and measured using the ImageJ program. The same sections were then stained with 2, 3, 5 -triphenyltetrazolium hydrochloride (TTC) to evaluate infarct size by the indirect method (contralateral hemisphere - uninjured ipsilateral hemisphere), again using ImageJ. For a quantitative analysis of HT, a spectrophotometric hemoglobin assay was used. In brief, mice were transcardially perfused with 30 mL PBS to remove intravascular blood under deep isoflurane anesthesia (5%) 24 h after ischemia. Brains were removed, separated into left and right hemispheres, and placed in glass tubes containing 1 mL PBS. After homogenization, ultrasound was applied to lyse erythrocytic membranes. After centrifugation for 30 minutes (13000 rpm, 4°C), 100 µl Drabkin’s reagent was added to 250 µL supernatant. Absorption was determined at 540 nm using a SpectraMax M5 photometer, and hemorrhage volumes were calculated based on a standard curve. Hemorrhage and hemoglobin volume were determined by an investigator blinded as to treatment groups.
Immunohistochemistry
Mice were treated with warfarin and subjected to MCAO as indicated. After 24 hours, anesthetized mice were perfused transcardially with ice-cold PBS (pH 7.4), followed with ice-cold 4% paraformaldehyde in PBS (pH 7.4). The brains were removed, immersed in the same fixative overnight at 4°C, and cryoprotected in 15% and 30% sucrose solutions in PBS at 4°C. Frozen coronal sections (20-µm-thick) were prepared using a cryostat. After blocking with PBS containing 0.2% Triton X-100 and 3% bovine serum albumin (BSA), sections were incubated at 4°C overnight in a PBS/0.2% Triton X-100/2% BSA solution with an affinity-purified rabbit polyclonal antibody directed towards the C-terminus of 12/15-LOX (characterized in18, diluted 1:100), followed by incubation with an Alexa-488 labeled secondary anti-rabbit antibody (Invitrogen, 1:200). Brain sections from 3 mice/group were imaged using a Nikon Eclipse Ti fluorescent microscope with NIS Elements software, keeping the settings constant. For each mouse, six images were taken from cortex and striatum, respectively, of both hemispheres, giving a total of 24 images per mouse. Signal intensity was measured for the ipsilateral brain sections using NIH ImageJ software, and normalized as the ratio of Max/Mean intensity.
Statistical analysis
The Wilcoxon-Mann-Whitney test was used to compare data in two groups. For tests with three or more groups, ANOVA with Dunnett's test was used. Data taken over time were analyzed with 2-way ANOVA. All data are expressed as mean ± S.E.M. Probability values <0.05 were considered significant.
Results
In a model of severe ischemia in conjunction with warfarin pretreatment, 12/15-LOX knockout mice show less hemorrhage than the corresponding wild type mice
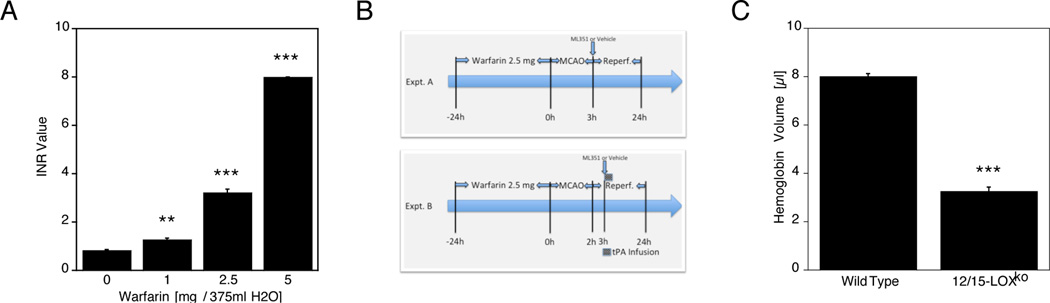
In a preliminary study, we determined a warfarin dose to provide effective and safe anticoagulation in C57Bl6 mice, according to Foerch et al19. The international normalized ratio (INR) values after 24 h of warfarin treatment increased in a dose-dependent manner (Fig. 1A). While 5 mg Warfarin added to the drinking water resulted in a very high INR value at the detection limit of the Coaguchek measuring device, the 2.5 mg dose of warfarin led to an INR value of 3.21±0.32 (mean ± S.E.M.), and we used this dosage for all subsequent experiments. We next established two models of warfarin-associated HT, characterized by severe (3h) focal ischemia (Expt. A in Fig. 1B), or by 2h MCAO followed by tPA thrombolysis (Exp. B). To determine whether or not the absence of 12/15-LOX reduces hemorrhage in an established model of anticoagulation followed by severe ischemia15, following pretreatment with warfarin we subjected 12/15-LOX ko mice (in which the ALOX15 gene has been deleted), and the corresponding wild-type mice to 3 hours of MCAO and 21 hours of reperfusion. Using a spectrophotometric hemoglobin assay, we observed high levels of hemorrhage in the ipsilateral hemispheres of wild-type mice (Fig. 1C). In contrast, the 12/15-LOX ko mice showed significantly less hemorrhage, suggesting that the absence of 12/15-LOX is protective. This was not due to any effect on the coagulation system, as the INR values were equal in both cohorts of mice (12/15-LOX ko 3.14±0.06; WT 3.20±0.06). Fewer knockouts died than wild type mice (Wild type 5/10, 12/15-LOX ko 3/10).
Figure 1.
A, Increasing amounts of warfarin added to the drinking water of mice led to increased INR values 24 hours later (**p<0.01, ***p<0.001 compared to no added warfarin; n=6/group). B, Experimental setup with 24 h warfarin pre-administration, followed by either 3h of MCAO (setup A) or 2h of MCAO, followed by tPA treatment 1h after reperfusion (setup B). Timeline not to scale. C, Photometrically determined hemoglobin levels in the ipsilateral brain hemisphere following warfarin pretreatment and 3h of MCAO were significantly lower in 12/15-LOX ko mice compared to matching wild type mice (*p<0.05; n=5/group).
Reduced hemorrhagic transformation in anticoagulated mice following administration of a 12/15-LOX inhibitor
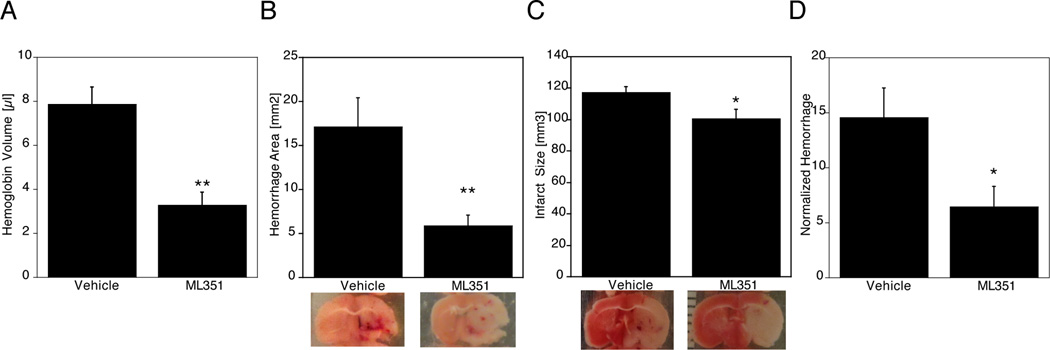
We next attempted to replicate these findings with pharmacological treatment. We have recently introduced a novel, highly selective inhibitor of 12/15-LOX. ML351 reduced infarct size in a permanent focal ischemia model, but had not been tested in a transient focal ischemia model at the time14. Using the classical filament model of transient focal ischemia with 2 hours of occlusion, we now found that ML351 reduced infarct size in a dose-dependent manner, with significant reductions at 25 and 50 mg/kg (see Supplementary Figure I). Because the 50 mg/kg dose was more effective, with an infarct size reduction of 43%, we used this dosage for subsequent experiments with warfarin anticoagulation. We administered ML351 to warfarin-pretreated mice at the time of reperfusion 3 hours after onset of ischemia. These mice developed significantly less hemorrhage on the ischemic side of the brain, compared to vehicle-treated mice (Figure 2A). In a separate study but using the same experimental parameters, we changed the work-up procedure to include imaging of the hemorrhage both on the surface of the brain, and in coronal brain sections. Mice with effective oral anticoagulation from the vehicle-treated group showed massive hemorrhages in brain sections after MCAO (Figure 2B). This was again significantly reduced in the mice treated with ML351. Subsequent TTC staining showed that this was accompanied by a slight but statistically significant reduction in infarct size (Figure 2C). Mortality was also lower in the inhibitor-treated mice, but the effect was not statistically significant (Vehicle 7/17, ML351 4/17; p=0.464).
Figure 2.
A, Significantly reduced hemoglobin levels in C57Bl6 mice treated with the 12/15-LOX inhibitor ML351 following warfarin and 3h MCAO, compared to vehicle treatment (*p<0.05). B, Less hemorrhage was detected in brain sections of mice treated with ML351 (*p<0.05). C, Infarct size comparison after TTC staining in mice subjected to warfarin plus 3h MCAO showed large infarcts in the vehicle treated mice, which were slightly but significantly reduced following ML351 treatment (*p<0.05). D, Following normalization for infarct size, hemorrhage remained significantly reduced by ML351 treatment (*p<0.05).
ML351 may be associated with reduced hemorrhagic transformation independently of its ability to reduce infarct size
One important issue arising here is whether or not the reduced hemorrhage is due to the reduction in infarct size, rather than a specific protective effect of ML351 on the brain vasculature. To address this question, we normalized the data by dividing the hemorrhage values by the infarct size determined for the same mouse. This normalized hemorrhage still showed a significantly reduced value in the ML351-treated mice (Figure 2D), suggesting that 12/15-LOX inhibition protects the vasculature against leakage in this anticoagulant model of severe ischemia, in addition to its neuroprotective effects.
Lipoxygenase inhibition reduces HT associated with tPA treatment in warfarin-treated mice
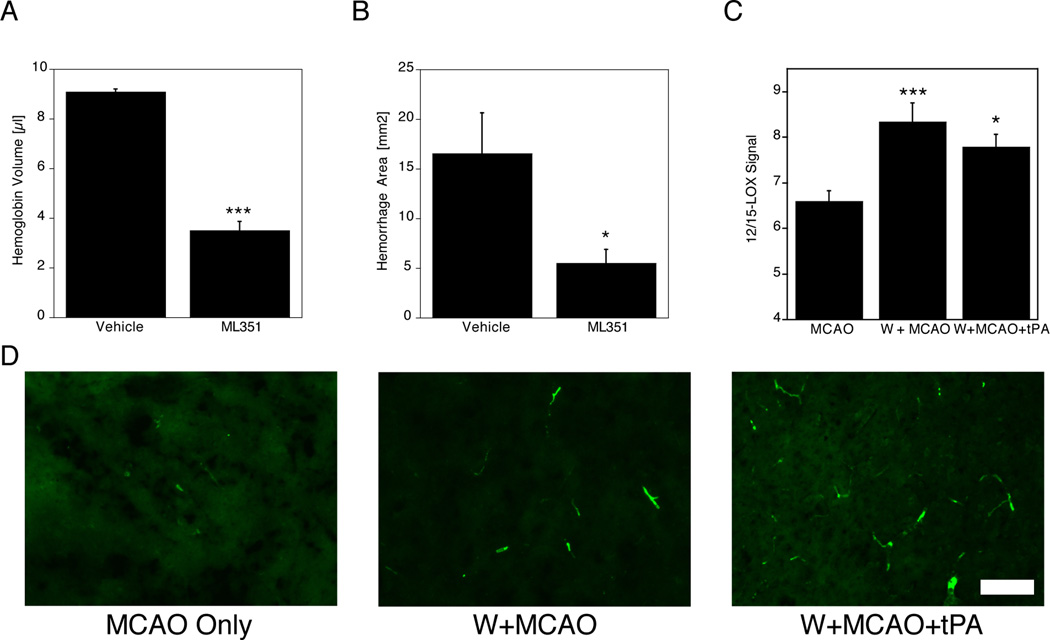
Compounding the increased risk of HT, another major factor leading to increased hemorrhage under warfarin anticoagulation is the use of thrombolytics, particularly tPA. To test if 12/15-LOX inhibition can provide a benefit under anticoagulation with thrombolysis16, we subjected mice to 24 hours of anticoagulant treatment, followed by 2 hours of MCAO and i.v. infusion of tPA one hour later. Treatment with either ML351 or vehicle was initiated by i.p. injection at the time of tPA infusion, and mice were sacrificed 24 hours after the onset of ischemia (see Figure 1B). In two separate cohorts of mice, we determined either the hemorrhage volume photometrically (Figure 3A), or the hemorrhages in brain sections (Figure 3B). In both measurements, treatment with ML351 led to a significant reduction in hemorrhage. In addition, fewer mice died in the ML351 group, but again the difference in mortality was not statistically significant (Vehicle 7/17, ML351 5/18; p=0.488). Taken together, these results indicated that ML351 reduced the hemorrhagic transformation that occurred subsequent to tPA infusion in warfarin-treated mice.
Figure 3.
A, Following warfarin pretreatment, then 2h of MCAO and tPA infusion 1h later, i.p. ML351 delivered concomitantly reduced hemoglobin levels in the ipsilateral side of the brain at 24h (*p<0.05). B, Hemorrhage in brain sections was significantly reduced by treatment with ML351 (*p<0.05; n=5 for vehicle, n=7 for ML351). C, 12/15-LOX protein, detected by immunohistochemistry, was increased in the brains of warfarin-pretreated mice (***p<0.001, *p<0.05 compared to MCAO only; n=3/group, 24 images/brain) following MCAO. D, Representative images show that most of the increased 12/15-LOX signal following warfarin treatment is vessel-derived (Scale Bar = 100 µm).
Moderate anticoagulation with warfarin leads to increased expression of 12/15-LOX following MCAO
To determine if warfarin pretreatment impacts the level of 12/15-LOX following experimental stroke, we compared 12/15-LOX signal in brain sections from mice pretreated with warfarin to mice without warfarin pretreatment, sacrificed after 2 hours of MCAO and 22 hours of recovery. A third cohort of mice was pretreated with warfarin, followed by 2h MCAO and subsequent infusion of tPA. As expected from our previous studies, immunohistochemistry showed increased 12/15-LOX protein on the ipsilateral side of the brains of mice subjected to MCAO under all three conditions (data not shown), but the 12/15-LOX signal was significantly higher in the warfarin model mice (Fig. 3C). Representative images taken from the ischemic hemisphere indicated that the signal is mostly increased in the vasculature, consistent with the hypothesis that increased vascular 12/15-LOX may contribute to HT (Fig. 3D).
Long term effects of 12/15-LOX inhibition under warfarin anticoagulation
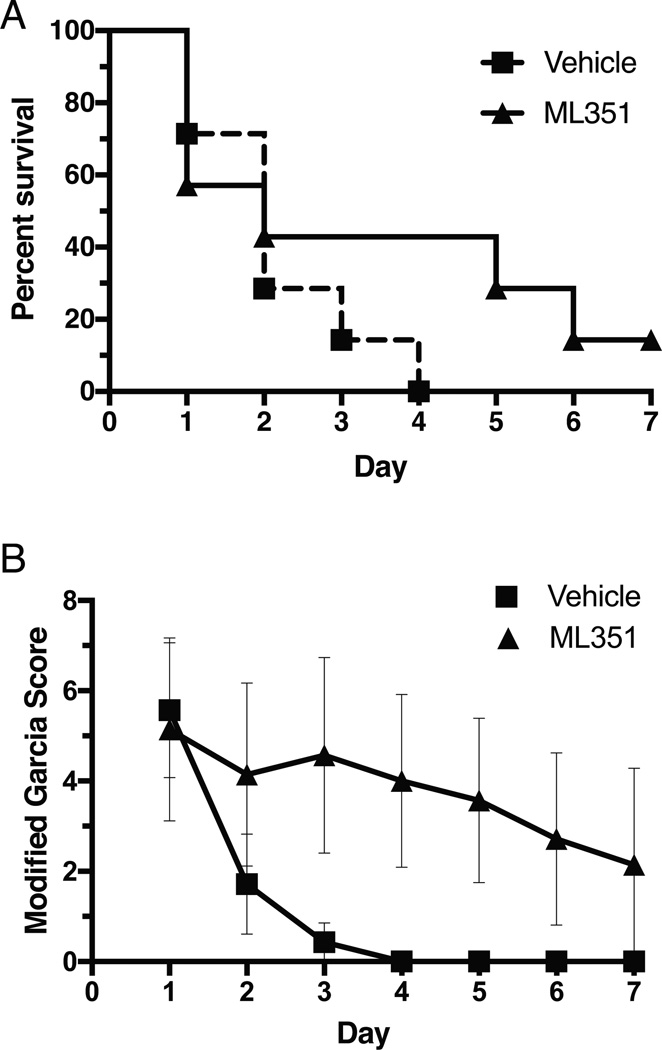
In a first attempt to gauge the effects of 12/15-LOX inhibition on extended outcome beyond the 24 hour time window, we subjected mice to warfarin pretreatment, followed by 3h of MCAO. Only 2 out of 22 mice lived past Day 2 and none longer than four days (data not shown), suggesting this model in our hands is too severe for long term outcome studies. We next reduced the MCAO period to 2 hours, allowing for improved survival. Unfortunately, using a new preparation of warfarin some of the mice developed unusually high INR values, and we excluded mice with INR>4 from the study, leaving 7 mice per group. With 2h MCAO following warfarin pretreatment, survival was better in the ML351 treatment group, although the difference was not statistically significant (Fig. 4A). The behavioral score using a modified Garcia Score according to Imai et al.20 showed a significant benefit for the ML351 treatment group (Fig. 4B).
Figure 4.
A, 7 days after warfarin plus 2h MCAO treatment, survival was better in ML351-treated animals, but the difference was not statistically significant (p=0.24; n=7/group). B, Behavioral outcome was significantly improved in the ML351 treatment group (p<0.05 by 2-way ANOVA).
Discussion
Although warfarin greatly reduces the risk of cardioembolic ischemic stroke, many patients with AF nonetheless experience a stroke while under anticoagulation21, 22. In addition, warfarin increases the risk of HT in case of stroke23. However, no effective treatment for this warfarin associated HT has been identified. Both our laboratory and others have previously shown that 12/15-LOX enzyme inhibition or gene knockout reduces ischemic injury in animal models of transient MCAO11, 12, 24. In the current study, we demonstrate for the first time that the 12/15-LOX inhibitor ML351 effectively protects against HT in two mouse models of warfarin-associated hemorrhage following ischemic stroke.
We have previously found that ML351 significantly reduced infarct size following permanent focal ischemia in a mouse model of ischemic stroke14. In the current study, we found that ML351 also has a protective effect on infarction in transient MCAO (see Supplementary Figure I). With 43% infarct size reduction when used at 50 mg/kg, the degree of protection afforded by ML351 is comparable to our previous findings with other 12/15-LOX inhibitors, as well as with infarct size reductions in the 12/15-LOX knockout mice (30–42% in MCAO studies with 90 minutes or 2h occlusion)11, 13, reinforcing the notion that 12/15-LOX is a valid target for neuroprotection. Given the severity of 3h occlusion under conditions of warfarin anticoagulation, we were somewhat surprised to see that ML351 could still reduce the infarct size, even with these very large infarcts (Figure 2C). In human stroke patients, an infarct size threshold around 70 cm3 is proposed where current stroke treatments are no longer capable of providing a benefit25. Our results suggest that a 12/15-LOX inhibitor may provide neuroprotection across a fairly broad range of stroke severity. Under warfarin anticoagulation, HT in the ML351 group was significantly lower than in the vehicle group (see Figure 2A and B), and this protection against HT remained significant even after adjusting for the different infarct sizes (Figure 2D). Our findings suggest that ML351 can reduce both the infarct lesion, as well as HT under effective anticoagulation, and these two effects may be independent of each other. Mortality in each experiment favored the group with reduced 12/15-LOX, although the differences did not reach statistical significance.
The safety of tPA in acute ischemic stroke patients treated with warfarin is a topic of controversy23, 26, 27. The current American Heart Association (AHA)/American Stroke Association (ASA) guidelines accept tPA treatment for patients with an INR≤ 1.7 as a measure of limited anticoagulant effect28. However, the reality of the situation is that tPA is the only potentially available therapy for patients under effective anticoagulation (with INR value 2 to 4) in case of a stroke. We have previously shown that another 12/15-LOX inhibitor, LOXBlock-1, reduced tPA associated hemorrhage in a thrombotic mouse model13. Therefore, 12/15-LOX might be a therapeutic target for HT associated with tPA treatment. In this study, we investigated the effect of ML351 when combined with tPA in a Warfarin-associated HT model. We found that ML351 administered at the same time as tPA at a clinically relevant time point can significantly reduce HT (see Figure 3). Several compounds, including a blocker of the prostaglandin receptor EP129, an activator of Nrf220, as well as recombinant annexin 230, have been shown to reduce BBB leakage in rodent models of ischemic stroke. Aside from blocking the activity of warfarin itself16, this is to our knowledge the first report of protection against tPA-induced HT in the context of warfarin anticoagulation.
It has long been considered that the main mechanism of HT subsequent to ischemic stroke is the leakage of blood by the disruption of BBB31, 32. Although the precise mechanisms that mediate BBB disruption and HT after ischemic insults are complex, there is likely to be a strong correlation with oxidative stress and 12/15-LOX might be a major contributor33, 34. We previously reported that the expression of 12/15-LOX was increased in endothelial cells following transient focal ischemia and its inhibitor baicalein reduced the loss of the endothelial tight junction protein claudin-5 after ischemia. We also demonstrated that 12/15-LOX knockout mice had less leakage of IgG into the brain parenchyma, as did animals treated with inhibitor12. In the current study, we measured hemispheric swelling as one manifestation of edema formation in a subset of mice with warfarin pretreatment and 3h MCAO. We found no statistically significant difference for the ratio of ipsi- to contralateral area in the mice treated with ML351 (1.13+−0.03 vs. 1.18+−0.04 for vehicle-treated mice, p=0.31; n=5/group). However, we agree with the reviewers that effects on edema can potentially be important as well, and future studies with larger N may be warranted. Warfarin pretreatment led to increased 12/15-LOX protein levels following stroke, especially in the brain vasculature (Figures 3C and 3D). This does not automatically mean that warfarin itself up-regulates 12/15-LOX; but it supports the idea that increased vascular 12/15-LOX contributes to BBB leakage leading to the increased HT, and provides a rationale for how 12/15-LOX inhibition may reduce HT.
There are several limitations to our study. One, we purposefully chose severe models of stroke and stroke with thrombolysis, in order to obtain high levels of hemorrhage. Whether or not a similar benefit would be apparent with less severe strokes remains to be seen. A first indication that this benefit is present can be taken from our experiment with 7 day survival where we combined warfarin with 2h of MCAO (Figure 4), but the relative contributions of infarct size reduction versus protection against HT in that setting need to be further studied. Two, we here used the classical filament model of MCAO, so directly thrombus-related effects on HT are not evaluated in this study. A thrombin injection model with subsequent tPA thrombolysis has been established35, but its use in conjunction with warfarin pretreatment may be challenging. We saw very similar protection against HT through 12/15-LOX inhibition in a distal MCAO thrombosis model, however, so the benefits of ML351 may be broadly applicable13. Three, the results shown here are specific to the use of warfarin as anticoagulant. Clinically, besides warfarin new oral anticoagulants including dabigatran, rivaroxaban, or apixaban are now in use. Further studies will be needed to evaluate the effects of 12/15-LOX inhibition in the context of these novel anticoagulants. Finally, while it appears likely that a reduction in HT provides a sustained benefit for warfarin-treated animals, this will have to be probed further in additional long-term outcome experiments with more extensive behavioral testing.
In summary, our results have shown that the novel inhibitor of 12/15-LOX, ML351 significantly reduced Warfarin-associated HT with and without tPA administration. These results suggest that ML351 could be a candidate for the treatment of anticoagulated patients suffering a stroke, either alone or in conjunction with tPA thrombolysis.
Supplementary Material
Acknowledgments
Sources of Funding: Support from the NIH (grants R01 NS069939 and R01 NS049430 to K.v.L.) is gratefully acknowledged.
Disclosures: Dr Foerch received speaker honoraria from Boehringer Ingelheim (modest). A patent for the use of ML351 to treat stroke has been applied for (PCT/US2014/052269).
References
- 1.Steinberg BA, Piccini JP. Anticoagulation in atrial fibrillation. BMJ. 2014;348:g2116. doi: 10.1136/bmj.g2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the u.S. Adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 3.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 4.Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke prevention in atrial fibrillation ii study. Lancet. 1994;343:687–691. [PubMed] [Google Scholar]
- 5.Design of a multicenter randomized trial for the stroke prevention in atrial fibrillation study. The stroke prevention in atrial fibrillation investigators. Stroke. 1990;21:538–545. doi: 10.1161/01.str.21.4.538. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: How well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–2692. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 7.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, et al. Thirty-day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke. 2012;43:1795–1799. doi: 10.1161/STROKEAHA.111.630731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazya MV, Lees KR, Markus R, Roine RO, Seet RC, Wahlgren N, et al. Safety of intravenous thrombolysis for ischemic stroke in patients treated with warfarin. Ann Neurol. 2013;74:266–274. doi: 10.1002/ana.23924. [DOI] [PubMed] [Google Scholar]
- 9.Alberts MJ, Naidech AM. Tpa and warfarin: Time to move forward. Neurology. 2013;80:514–515. doi: 10.1212/WNL.0b013e31827b1b7c. [DOI] [PubMed] [Google Scholar]
- 10.Lo EH. A new penumbra: Transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 11.van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37:3014–3018. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- 12.Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, et al. Protecting against cerebrovascular injury: Contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke. 2008;39:2538–2543. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yigitkanli K, Pekcec A, Karatas H, Pallast S, Mandeville E, Joshi N, et al. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Ann Neurol. 2013;73:129–135. doi: 10.1002/ana.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai G, Joshi N, Jung JE, Liu Y, Schultz L, Yasgar A, et al. Potent and selective inhibitors of human reticulocyte 12/15-lipoxygenase as anti-stroke therapies. J Med Chem. 2014;57:4035–4048. doi: 10.1021/jm401915r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeilschifter W, Spitzer D, Czech-Zechmeister B, Steinmetz H, Foerch C. Increased risk of hemorrhagic transformation in ischemic stroke occurring during warfarin anticoagulation: An experimental study in mice. Stroke. 2011;42:1116–1121. doi: 10.1161/STROKEAHA.110.604652. [DOI] [PubMed] [Google Scholar]
- 16.Pfeilschifter W, Spitzer D, Pfeilschifter J, Steinmetz H, Foerch C. Warfarin anticoagulation exacerbates the risk of hemorrhagic transformation after rt-pa treatment in experimental stroke: Therapeutic potential of pcc. PLoS One. 2011;6:e26087. doi: 10.1371/journal.pone.0026087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Illanes S, Zhou W, Heiland S, Markus Z, Veltkamp R. Kinetics of hematoma expansion in murine warfarin-associated intracerebral hemorrhage. Brain Res. 2010;1320:135–142. doi: 10.1016/j.brainres.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Pekcec A, Yigitkanli K, Jung JE, Pallast S, Xing C, Antipenko A, et al. Following experimental stroke, the recovering brain is vulnerable to lipoxygenase-dependent semaphorin signaling. FASEB J. 2013;27:437–445. doi: 10.1096/fj.12-206896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foerch C, Arai K, Jin G, Park KP, Pallast S, van Leyen K, et al. Experimental model of warfarin-associated intracerebral hemorrhage. Stroke. 2008;39:3397–3404. doi: 10.1161/STROKEAHA.108.517482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imai T, Takagi T, Kitashoji A, Yamauchi K, Shimazawa M, Hara H. Nrf2 activator ameliorates hemorrhagic transformation in focal cerebral ischemia under warfarin anticoagulation. Neurobiol Dis. 2016;89:136–146. doi: 10.1016/j.nbd.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–1026. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 22.Schwammenthal Y, Bornstein N, Schwammenthal E, Schwartz R, Goldbourt U, Tsabari R, et al. Relation of effective anticoagulation in patients with atrial fibrillation to stroke severity and survival (from the national acute stroke israeli survey [nasis]) Am J Cardiol. 2010;105:411–416. doi: 10.1016/j.amjcard.2009.09.050. [DOI] [PubMed] [Google Scholar]
- 23.Alberts MJ. Cerebral hemorrhage, warfarin, and intravenous tpa: The real risk is not treating. JAMA. 2012;307:2637–2639. doi: 10.1001/jama.2012.7265. [DOI] [PubMed] [Google Scholar]
- 24.Park HA, Kubicki N, Gnyawali S, Chan YC, Roy S, Khanna S, et al. Natural vitamin e alpha-tocotrienol protects against ischemic stroke by induction of multidrug resistance-associated protein 1. Stroke. 2011;42:2308–2314. doi: 10.1161/STROKEAHA.110.608547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. Mri-based selection for intra-arterial stroke therapy: Value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40:2046–2054. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xian Y, Liang L, Smith EE, Schwamm LH, Reeves MJ, Olson DM, et al. Risks of intracranial hemorrhage among patients with acute ischemic stroke receiving warfarin and treated with intravenous tissue plasminogen activator. JAMA. 2012;307:2600–2608. doi: 10.1001/jama.2012.6756. [DOI] [PubMed] [Google Scholar]
- 27.Ruecker M, Matosevic B, Willeit P, Kirchmayr M, Zangerle A, Knoflach M, et al. Subtherapeutic warfarin therapy entails an increased bleeding risk after stroke thrombolysis. Neurology. 2012;79:31–38. doi: 10.1212/WNL.0b013e31825dcdf0. [DOI] [PubMed] [Google Scholar]
- 28.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the american heart association/american stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council, and the atherosclerotic peripheral vascular disease and quality of care outcomes in research interdisciplinary working groups: The american academy of neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 29.Frankowski JC, DeMars KM, Ahmad AS, Hawkins KE, Yang C, Leclerc JL, et al. Detrimental role of the ep1 prostanoid receptor in blood-brain barrier damage following experimental ischemic stroke. Scientific reports. 2015;5:17956. doi: 10.1038/srep17956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Y, Fan X, Yu Z, Liao Z, Wang XS, van Leyen K, et al. Combination low-dose tissue-type plasminogen activator plus annexin a2 for improving thrombolytic stroke therapy. Frontiers in cellular neuroscience. 2015;9:397. doi: 10.3389/fncel.2015.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol. 2003;28:229–244. doi: 10.1385/MN:28:3:229. [DOI] [PubMed] [Google Scholar]
- 32.Lo EH, Moskowitz MA, Jacobs TP. Exciting, radical, suicidal: How brain cells die after stroke. Stroke. 2005;36:189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- 33.Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35:2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 34.van Leyen K. Lipoxygenase: An emerging target for stroke therapy. CNS Neurol Disord Drug Targets. 2013;12:191–199. doi: 10.2174/18715273112119990053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Yebenes I, Sobrado M, Zarruk JG, Castellanos M, Perez de la Ossa N, Davalos A, et al. A mouse model of hemorrhagic transformation by delayed tissue plasminogen activator administration after in situ thromboembolic stroke. Stroke. 2011;42:196–203. doi: 10.1161/STROKEAHA.110.600452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.