Abstract
The human sodium-dependent multivitamin transporter (hSMVT) is a product of the SLC5A6 gene and mediates biotin, pantothenic acid and lipoate uptake in a variety of cellular systems. We report here the identification of mutations R94X, a premature termination, and R123L, a dysfunctional amino acid change, both in exon 3 of the SLC5A6 gene in a child using whole genome-scanning. At 15 months of age, the child showed failure to thrive, microcephaly and brain changes on MRI, cerebral palsy and developmental delay, variable immunodeficiency, severe gastro-esophageal reflux requiring a gastrostomy tube/fundoplication, osteoporosis and pathologic bone fractures. After identification of the SLC5A6 mutations, he responded clinically to supplemental administration of excess biotin, pantothenic acid and lipoate with improvement in clinical findings. Functionality of the two mutants was examined by 3H-biotin uptake assay following expression of the mutants in human-derived intestinal HuTu-80 and brain U87 cells. The results showed severe impairment in biotin uptake in cells expressing the mutants compared to those expressing wild-type hSMVT. Live cell confocal imaging of cells expressing the mutants showed the R94X mutant to be poorly tolerated and localized in the cytoplasm, while the R123L mutant was predominantly retained in the endoplasmic reticulum. This is the first reporting of mutations in the SLC5A6 gene in man, and suggests that this gene is important for brain development and a wide variety of clinical functions.
Keywords: SLC5A6, mutations, SMVT, biotin, transporter
Introduction
Biotin is essential for normal cellular function, growth and development due to its involvement as a co-factor in multiple metabolic reactions (Bonjour 1984; Sweetman and Nyhan 1986). Deficiency of biotin leads to a variety of clinical abnormalities including growth retardation, neurological disorders [e.g., ataxia, developmental delay, hypotonia, seizures, and sensory and motor deficits (Ozand et al. 1998; Wolf 2001; Wolf 2013)], as well as dermatological disorders (Wolf 2001). Animal studies have shown that biotin deficiency during pregnancy leads to embryonic growth retardation, congenital malformation, and death (Watnabe et al. 2009; Quick and Shi 2015). Deficiency and sub-optimal levels of biotin have been reported in a variety of conditions including inborn errors of biotin metabolism, inflammatory bowel diseases, long-term therapy with anticonvulsant drugs, long-term parenteral nutrition, and in chronic alcoholism (Bonjour 1980; Bonjour 1984; Banares et al. 1989; Krause et al. 1985; Mock et al. 1997).
Humans cannot synthesize biotin, and must obtain it from exogenous sources via intestinal absorption. Intestinal absorption of biotin and transport of the vitamin into a variety of other cell-types occurs via a saturable, Na+-dependent, carrier-mediated mechanism that involves the human sodium-dependent multivitamin transporter, hSMVT, a product of the SLC5A6 gene (Balamurugan et al. 2003; Wang et al. 1999). This gene is located on chromosome 2, and the hSMVT protein is predicted to have 12 trans-membrane domains (TMD) with both the NH2 and COOH termini facing the cell interior (Wang et al. 1999). The hSMVT system also transports the water-soluble vitamin pantothenic acid (Prasad et al. 1997; Uchida et al. 2015) and the metabolically important lipoate (Prasad et al. 1997; Zehnfenning et al. 2015), each with their own deficiency states and metabolic effects. Here we show that the identification of 2 mutations in the hSMVT protein function can lead to dramatic clinical findings, and the administration of pharmacological dose of biotin, pantothenate and lipoic acid can improve the clinical presentation.
Materials and methods
Materials
EGFP-N3 and DsRed2-ER fluorescent proteins were from BD Biosciences (Palo Alto, CA). Cell lines (HuTu-80 and U87) were obtained from ATCC (Manassas, VA). DNA oligonucleotide primers were obtained from Sigma Genosys (Woodlands, TX). [3H]-Biotin (specific activity of 51Ci/mmol) was obtained from American Radiochemical Company (St. Louis, MO). The minimal essential medium (MEM) and Dulbecco's modified Eagle's medium (DMEM) were obtained from Invitrogen (Carlsbad, CA).
Generation of hSMVT-GFP clinical mutants
The Quik change™ site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used to introduce insertions or deletions of nucleotides into the open reading frame (ORF) of SLC5A6 gene as identified in the patient. Paired sense- and anti-sense primer oligonucleotides encompassing the specified underlined mutation sites (R94X; Forward 5’-TCAGAGATCTACTGATTTGGGACCCAA-3’; Reverse 5’-TTGGGTCCCAAATCAGTAGATCTCTGA-3’ and R123L Forward 5’-CCCGTTTTCTACCTCCTGCATCTCACC-3’; Reverse 5’-GGTGAGATGCAGGAGGTAGAAAACGGG-3’) and hSMVT-GFP (Subramanian et al. 2009) were used for PCR mutagenesis. Both mutated hSMVT constructs were verified for nucleotide change by DNA sequencing (Laragen, Los Angeles, CA).
Cell culture and transient transfection
Human duodenal-derived intestinal epithelial HuTu-80 cells were maintained in MEM and human brain-derived glioma U87 cells were maintained in DMEM. Both media were supplemented with 10% fetal bovine serum, penicillin (100,000 units/L), and streptomycin (10mg/L). For transient transfection, HuTu-80 and U87 cells were grown on sterile glass-bottomed Petri-dishes (MatTek, MA) and transfected at 90% confluency with 2μg plasmid DNA using 2μl LipofectAMINE 2000 (Invitrogen, CA). Forty eight hours later, cells were used for uptake and imaging analysis.
Uptake assay
HuTu-80 and U87 cells were transiently transfected with GFP, hSMVT-GFP(WT) and mutated constructs and 3H-biotin uptake assay was performed in Krebs-Ringer buffer [(K-R) in mM: 133 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine, 10 HEPES, and 10 MES, pH 7.4) at 37°C for 5 min [initial linear period (Balamurugan et al. 2003; Subramanian et al. 2009)]. 3H-biotin (5 nM) was added to the K-R buffer at the time of uptake assay, and after 5 min the reaction was terminated by ice-cold K-R buffer and the radioactive content of HuTu-80 and U87 cells were determined using a Beckman Coulter scintillation counter (Fullerton, CA). Protein content of cells was determined using a Bio-Rad DC Protein assay kit (Bio-Rad).
Confocal imaging of hSMVT mutants
Cell (HuTu-80 and U87) monolayers grown on glass bottomed petri-dishes were imaged for construct expression using a Nikon C-1 confocal scanner head attached to a Nikon inverted phase-contrast microscope. Fluorophores were excited using a 488-nm line from an argon ion-laser, and emitted fluorescence was monitored with a 515±30-nm band pass (GFP) or a 630 ± 60-nm long-pass filter (DsRed).
Data presentation and statistical analysis
All uptake data are means ± SE of at least 4 separate uptake determinations and are expressed in fmol/mg protein/5 min. The Student's t-test was used for statistical analysis and the statistically significance was being set at P < 0.05. Live cell confocal imaging was performed on at least three different occasions with three different batches of HuTu-80 and U87 cell lines.
Results
Case report
The infant was born full term, weighing 3.005 kg after an uneventful pregnancy. Feeding difficulties were noted shortly after birth and a naso-gastric (NG) tube was required for feeding. Weight gain and growth improved, but muscle tone increased and head circumference growth slowed, suggesting a diagnosis of cerebral palsy. Amino acids, organic acids, as well as microarray were normal, and polymicrogyria was suggested on a brain MRI. An initial genetics evaluation at another facility did not yield a clear diagnosis. He experienced multiple infections during the first months of life.
He was transferred to our hospital, and at 6 months of age underwent gastrostomy tube (GT) placement and fundoplication. Shortly thereafter he developed a GT leak, abdominal distention and gastrointestinal bleeding with ensuing hypovolemic shock. A respiratory syncytial virus (RSV) infection was found. Secondary thrombocytopenia was transient, but continued hypogammaglobinemia required ongoing immunoglobulin administrations. An electroencephalogram (EEG) was normal.
At 10 months of age, he had a muscle biopsy which demonstrated normal pathology by light and electron microscopy. At 11 months of age, he experienced an acute life-threatening event, with hyponatremia and transient acute kidney injury. He underwent a take-down of the fundoplication, and laparoscopic repair of a newly identified hiatal hernia, and required temporary parenteral nutrition via a Broviac catheter.
At 1 year of age, he could only roll over from front to back, and could not sit up. Polymicrogyria was not confirmed by a new MRI, which revealed a thin corpus callosum, atrophy/hypoplasia of the brain and hypoplasia of the pons (Figure 1). His development lagged further behind.
Fig.1. MRI Brain.
Generalized atrophy of the brain was found without hydrocephalus. There was a very thin corpus callosum.
At 15 months of age, he continued to have poor head control, and exhibited nystagmus. In addition, he did not sit unsupported, though he could stand with support. He demonstrated personality and he began to babble. On physical examination, he had a small head without dysmorphic features, moderate developmental delay with performance at about 6 months level, and spastic cerebral palsy.
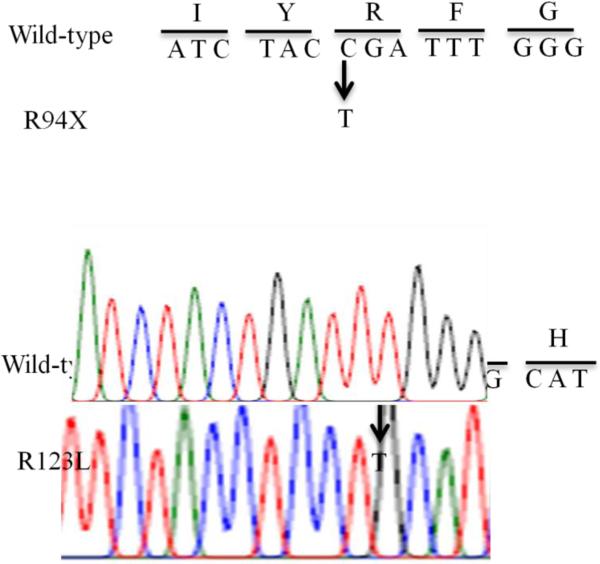
Whole Exome sequencing (GeneDx) (Retterer et al. 2016) demonstrated a p.Arg94Ter (CGA>TGA) c280C>T, R94X mutation and a p.Arg123Leu (CGC>CTC) c368 G>T R123L mutation, both in exon 3 in the SLC5A6 gene. Chromatograms of both mutations in the SLC5A6 gene are shown in Figure 2. Serum biotin concentration was normal, most likely because of both parenteral and GT nutrition.
Fig.2.
Chromatograms of the wild-type and the mutated (R94X and R123L) SLC5A6 gene.
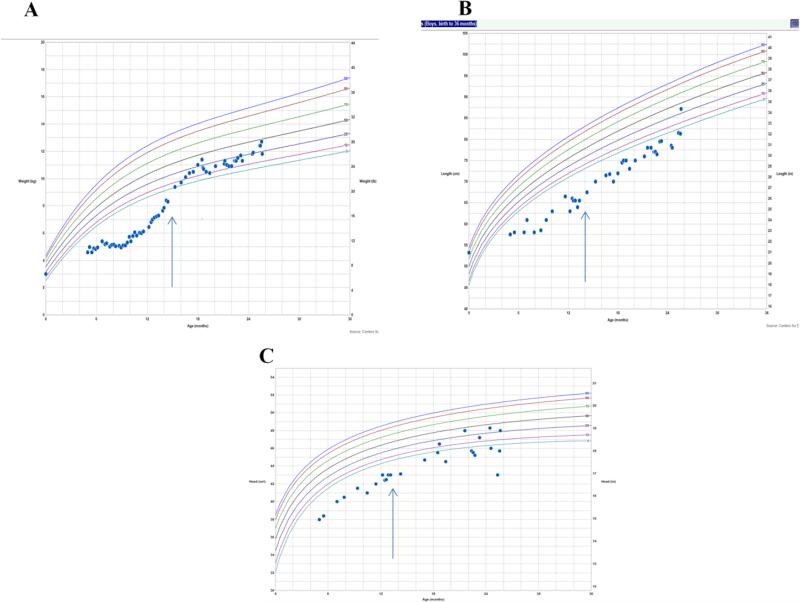
Following demonstration of the defect in hSMVT-encoding gene, large doses of biotin (10 mg/day, then 30 mg/day), pantothenic acid (250 mg/day, then 500 mg/day) and lipoic acid (150 mg/day, then 300 mg/day) were initiated at 19 months and increased at 24 months of age. The child exhibited improved development in motor and verbal skills with a few simple words. In addition, near-disappearance of nystagmus was noted, his immunoglobulin IgG and IgA concentrations normalized. His weight and height reached normal range, and his head growth improved over the next several months, as shown in Figure 3.
Fig.3. Growth curves.
Weight (A), growth (B) and head circumference (C) before and after biotin, pantothenic acid and lipoic acid were started at 15 months (arrow).
At 22 months of age, he became extremely irritable and was in pain with a swollen left lower extremity. He was diagnosed with fractures of his left distal tibia and fibula, along with osteopenia. Metabolic bone disease evaluation was significant for hypocalcemia, hypophosphatemia, normal urine calcium excretion, normal PTH concentration and lack of phosphaturia. The alkaline phosphatase was elevated and high 1,25-dihydroxy vitamin D (1,25(OH)2D) concentration was detected (Table 1). Of note, 3 months earlier, his 25-hydroxy vitamin D (25OHD) was 21 ng/mL and 1, 25(OH)2 D was 306 pg/mL. Follow-up biotin, lipoate, and pantothenic acid concentrations were not measured.
Table 1.
Biochemical parameters and trends
| Test \ Age | 22 mo | 25 mo | 27 mo | 29 mo | Normal Range |
|---|---|---|---|---|---|
| Calcium, serum | 8.2 | 9.1 | 9.1-10.3 mg/dL | ||
| Ionized calcium, venous | 1.26 | 1.2 | 1.12-1.23 mmol/L | ||
| Phosphorus, serum | 2.6 | 2.5 | 2.3 | 5.6 | 4.1-6.5 mg/dL |
| Alkaline phosphatase | 1208 | 765 | 142-336 Units/L | ||
| Manganese | 7.4 | 7-16 mcg/L | |||
| Zinc | 114 | 29-115 mcg/dL | |||
| PTH, intact | 71.6 | 13-85 pg/mL | |||
| 25-OHD | 21 | 20-100 ng/mL | |||
| 1,25-(OH)2D | 285 | 257 | 244 | 31-87 pg/mL | |
| Uca/Ucr | 0.13 | <0.2 | |||
| Uphos | <1 | TRP>85% |
PTH = parathyroid hormone, intact; Uca/Ucr = urine calcium-to-creatinine ratio; Uphos = urinary phosphorus; TRP = tubular reabsorption of phosphorus
Supplementation with calcium, phosphorus, as well as calcitriol was begun. Two weeks later, with continued pain, a bone survey revealed healing fractures of the right proximal radius and ulna, along with severe osteopenia. Although there was reassuring adequate callus formation documented, at 27 months of age he sustained a new fracture of the proximal right ulnar shaft, despite prescribed supplementation. At the time, ionized calcium was normal, and later, hypophosphatemia resolved, as tolerance to supplementation improved (Table 1).
At age 2 - 3/4 years, after 14 months of cofactor therapy, he was able to take steps holding on, had a 5-word vocabulary, good understanding and personality, and nystagmus was absent. No further immunoglobulin or bone problems were observed.
Experimental characterization of the identified clinical mutations
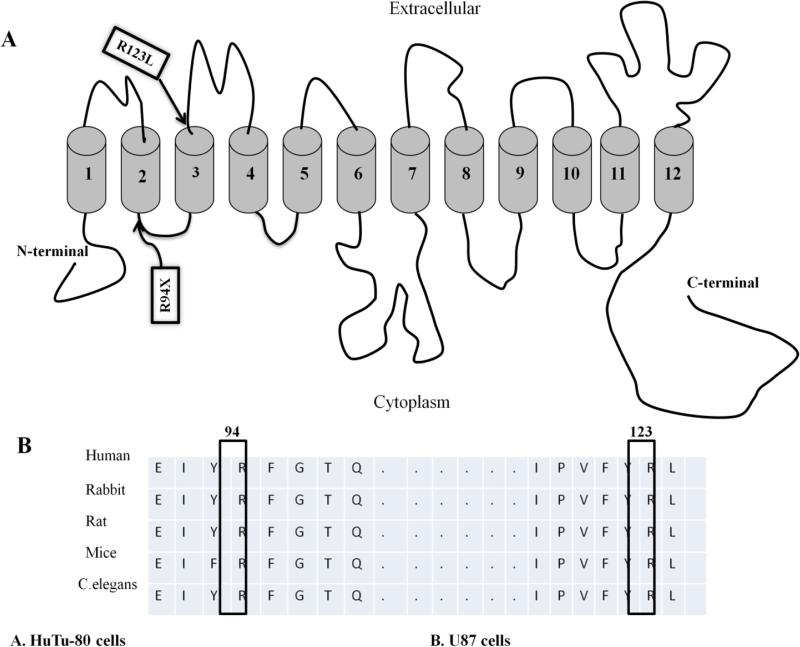
For the mutations identified in the SLC5A6 gene, the first mutation (R94X) is located in the intracellular loop between TMD2 and TMD3 of the predicted hSMVT secondary structure, while the second mutation (R123L) is located in the extracellular loop between TMD3 and TMD4 of the protein (Fig. 4A). These mutations affect amino acid residues that are conserved in the wild-type SMVT of different species (Fig. 4B).
Fig.4. Localization of clinical mutants in the hSMVT.
A: Predicted hSMVT secondary structures showing the clinical mutants. B: Amino acid sequence alignment by clustalW shows that both R94 and R123 are conserved across many species.
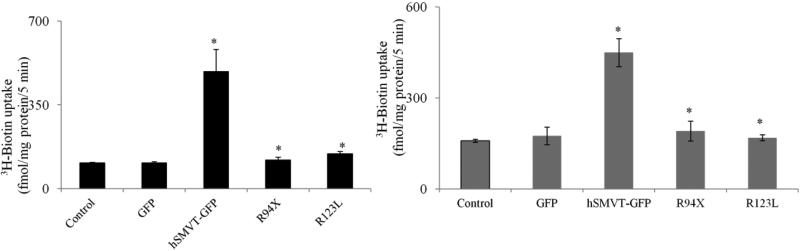
We examined the effect of the two identified hSMVT mutations on functionality of the transporter by performing a 3H-biotin uptake assay following expression of these mutants generated in appropriate human cell lines, human intestinal epithelial HuTu-80 and brain U87 cell lines. The HuTu-80 cells were chosen because our previous investigations have established their suitability in such type of studies (Subramanian et al. 2009), while the U87 cells were used since the patient displayed brain abnormalities. We transiently transfected these cells with GFP, hSMVT-GFP (WT), hSMVT [R94X]-GFP and hSMVT [R123L]-GFP then examined the initial rate of 3H-biotin uptake. As expected, a significant (P < 0.01) induction in biotin uptake was observed in both cell lines expressing the wild-type hSMVT compared to controls (Fig. 5A & B). On the other hand, cells expressing the hSMVT clinical mutants [R94X]-GFP and [R123L]-GFP showed no induction in biotin uptake (Fig. 5A & B). This shows that the identified hSMVT mutations that were constructed lead to impairment in functionality of the transport protein.
Fig.5. Effect of hSMVT clinical mutants on 3H-Biotin uptake.
HuTu-80 cells (A) and U87 cells (B) were transiently transfected with indicated constructs. After 48 hours of transfection 3H-biotin uptake (5 nM, pH 7.4; 5 min) was performed. Data are means ± SE of at least 4 separate experiments with multiple determinations. * P < 0.01.
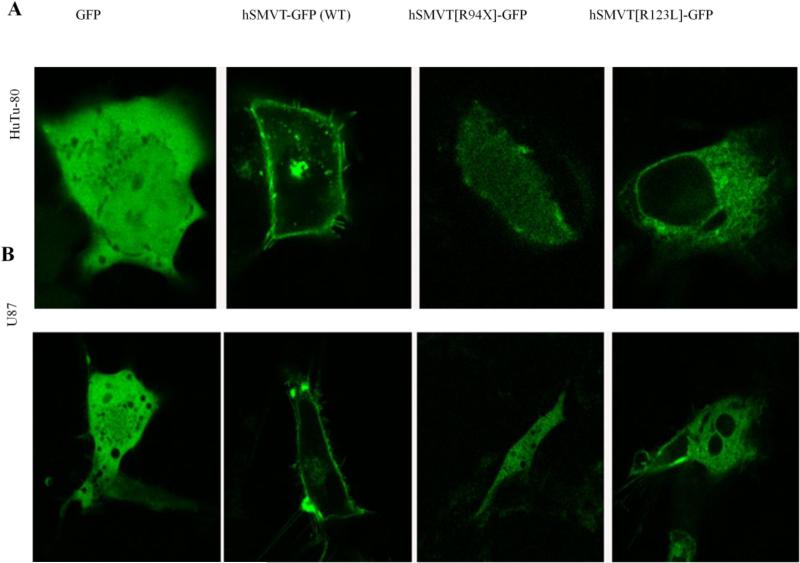
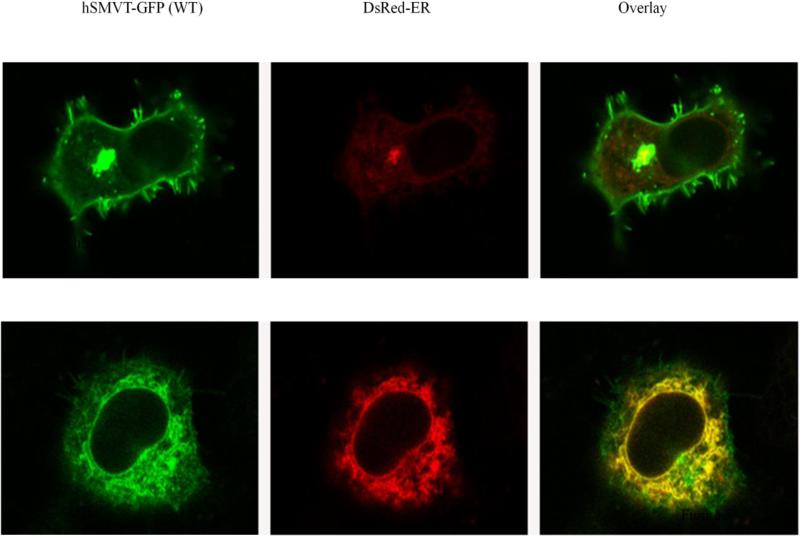
To determine the cellular distribution of the clinical mutants (R94X and R123L), we transfected the individual mutants into HuTu-80 and U87 cell lines followed by performing live cell confocal imaging. The results showed that while the GFP expressing cells display cytosolic localization, the hSMVT-GFP (WT) protein displayed its usual membrane expression in both cell lines (Fig. 6A & B) (Subramanian et al. 2009). In contrast, the hSMVT[R94X]-GFP construct was poorly expressed in cells and the expression was localized in the cytoplasm. As to the hSMVT[R123L]-GFP construct, both cell types appear to retain it predominantly intracellularly, most probably in the endoplasmic reticulum (ER) (Fig. 6A & B), with some expression at the cell surface. In these investigations we transfected cells with an equal amount of DNA and imaged cells with similar laser intensity for all construct. To confirm that the hSMVT[R123L]-GFP mutant is retained in the ER, we performed co-localization studies in HuTu-80 cells using an ER marker, DsRed-ER. The results showed a high-degree of co-localization between the hSMVT [R123L]-GFP mutant and the DsRed-ER marker (Fig. 7). Similar results were seen in co-localization studies with U87 cells; data not shown.
Fig.6. Cellular distribution of hSMVT clinical mutants.
A & B: Lateral (xy) confocal images of HuTu-80 and U87 cell lines expressing hSMVT-GFP (WT) and mutant constructs (an equal amount of DNA was transfected in these cells and imaged them with similar laser intensity for all indicated constructs).
Fig.7. Live-cell confocal imaging of HuTu-80 cells expressing hSMVT-GFP (WT), hSMVT[R123L]-GFP and DsRed-ER.
GFP (left) and DsRed (middle) channels were superimposed to generate a composite overlay (right) at the lateral (xy) confocal plane.
Discussion
Whole Exome analysis has aided in the identification of multiple disorders, usually by association of potentially causal mutations in a gene that explains the findings in patients with a similar clinical picture. Here we have a single case of a child with a novel clinical picture associated with a unique potentially important gene whose dysfunction might have wide spread clinical findings. To prove that mutations found in this gene were responsible for the disparate clinical picture, it was important to show that a diminished protein gene product led to altered substrate uptake results in vitro.
This study reports the identification of two mutations (R94X and R123L) in the SLC5A6 gene which encodes the hSMVT protein in a child using a genome-scanning approach. The hSMVT system transports three important nutrients: the water-soluble vitamins biotin and pantothenic acid, and the metabolically important substrate, lipoate (Prasad et al. 1997; Uchida et al. 2015; Zehnfenning et al. 2015). These nutrients are involved in multiple essential metabolic functions, including intermediary metabolism, protein synthesis, bone density and brain and immune function (Bonjour 1980; Uchida et al. 2015; Moiseenok et al. 2000; Palaniyappan and Alphonese 2011). Thus, it is not surprising that a decrease in cellular availability (i. e., cellular deficiency) of these nutrients leads to disturbance in normal cellular metabolism/function and ultimately to disease manifestations. Indeed, deficiency of each of these nutrients at the whole organism level leads to a variety of abnormalities. For example, systemic biotin deficiency leads to developmental delays (Spilioti et al. 2013), impairment in bone development (Ghosal et al. 2013; Baez-Saldana et al. 2009), immunological changes (Kuroishi 2015) and potential encephalopathy (Sweetman and Nyhan 1986; Ozand et al. 1998; Wolf 2001); systemic pantothenic acid deficiency leads to deficiency of CoA production (Martinez et al. 2014) and neuro-degeneration and immune dysfunction (Moiseenok et al. 2000; Follis and Wintrobe 1945; Kuo et al. 2007); systemic lipoate deficiency leads to immune modulation and slowed cell growth and differentiation (Palaniyappan and Alphonese 2011; Bilska and Wlodek 2005; Li et al. 2014; Cui et al. 2009) and loss of bone density (Roberts and Moreau 2015). The affected child in this study demonstrated this range of clinical symptoms including poor growth and weight gain, developmental delay, microcephaly, cerebral palsy, variable immunodeficiency, and osteopenia, that corresponded to the clinical findings shown in individual vitamin deficiencies described above.
In our characterization of the SLC5A6 mutations found in this patient, the first one (R94X) appeared to lead to an early termination in the hSMVT protein, while the second (R123L) involved a residue predicted to be located extra-cellularly between TMD3 and TMD4 of the SMVT polypeptide. We tested the functionality of both of these two hSMVT clinical mutations experimentally by introducing them into the ORF of hSMVT, followed by expression of the constructs in appropriate human-derived cells [the intestinal epithelial HuTu-80 cells, used by us previously to study functionality of the hSMVT system (Subramanian et al. 2009) and brain U87 cells (Ipas et al. 2015)], then assaying for 3H-biotin uptake. Biotin uptake was severely impaired in both hSMVT mutants compared to uptake by cells expressing the wild-type hSMVT. This result was due to severe reduction in the level of expression of the R94X mutant, and to retention of the R123L mutant protein in the endoplasmic reticulum of cells. Since hSMVT is involved in the delivery of not only biotin but also lipoate (Uchida et al. 2015), and likely pantothenic acid, impairment in the transport function of this carrier is expected to affect the transport of all three nutrients.
This is not the first identification of mutations in a membrane transporter associated with changes in brain function and development. A defect in the glucose transporter Glut1 leads to microcephaly and seizures (Seidner et al. 1998), a defect in the citrate transporter leads to an epileptic encephalopathy in the first days of life (Thevenon et al. 2014), a defect in the thiamine transporter leads to encephalopathy and developmental delays (Kono et al. 2009), a defect in the dopamine-serotonin transporter leads to neonatal hypotonia and bradykinesis (Jacobsen et al. 2016), and a defect in the manganese and zinc transporter affects brain growth and function, as well as bone mineralization (Boycott et al. 2015). That the affected child in our study was found to have normal blood levels of biotin and pantothenic acid is not unexpected since the child was fed via a Broviac appliance. With a functionally defective hSMVT, this does not reflect the cellular level of these essential micronutrients in the different tissues.
Our patient demonstrated fractures early in institution of biotin, pantothenic acid and lipoate therapy, and we postulate that the fractures were the result of his increased activity and movement, on a background of underlying osteopenia. It has been demonstrated in animal models that biotin deficiency leads to osteopenia (Ghosal et al. 2013) and abnormal growth plate chondrocytes (Baez-Saldana et al. 2009). Lipoate deficiency is associated with low bone density as well (Roberts and Moreau 2015). Our patient had osteopenia at the time of diagnosis of his first fractures, and we suggest this was secondary to the role played by the deficiency in biotin and lipoic acid leading to poor bone mineralization.
The child's positive response to high pharmacological doses of these micronutrients, clinically, and as shown in Figure 3, further support that impairment in hSMVT function is the cause of the observed clinical presentation. Uptake of these nutrients by different cells at high pharmacological concentrations occurs via simple diffusion, and may at least partially ameliorate the need for a functional hSMVT system. We do not know at this stage if this is an underappreciated disorder (due, for example, to difficulty in identifying the mutations) that is associated with failure to thrive, developmental delay, nystagmus, immune deficiency, and late appearing osteopenia in different degrees, or that it is an uncommon disorder. Low blood biotin (not found in our patient because of the Broviac/G-tube nutrition) may provide a diagnostic clue for future cases. A practical aspect of identifying hSMVT mutations in our patient (and future others), is that affected patients may routinely be treated with biotin, pantothenic acid, and lipoate supplementation. However, altered brain structure that occurs before birth may be less amenable to therapy unless treatment can begin early in the pregnancy.
Acknowledgements
We thank the patient's family for their participation in this study. This study was supported by the Dept. of VA and NIH grants DK58057 and DK56057 (HMS), DK107474 (VSS) and the Gordon Foundation (PJB).
References
- Baez-Saldana A, Gutierrez-Ospina G, Chimal-Monroy J, Fernandez-Mejia C, Saavedra R. Biotin deficiency in mice is associated with decreased serum availability of insulin-like growth factor-1. Eur J Nutr. 2009;48:137–144. doi: 10.1007/s00394-009-0773-8. [DOI] [PubMed] [Google Scholar]
- Balamurugan K, Ortiz A, Said HM. Biotin uptake by human intestinal and liver epithelial cells: role of the SMVT system. Am J Physiol. 2003;285:G73–G77. doi: 10.1152/ajpgi.00059.2003. [DOI] [PubMed] [Google Scholar]
- Banares FF, Lacruz AA, Gine JJ, Esteve M, Gassull MA. Vitamin statues in patients with inflammatory bowel disease. Am J Gastroenterol. 1989;84:744–748. [PubMed] [Google Scholar]
- Bilska A, Wlodek L. Lipoic acid - the drug of the future? Pharmacol Rep. 2005;57:570–577. [PubMed] [Google Scholar]
- Bonjour JP. Vitamins and alcoholism. Int J Vitam Nutr Res. 1980;50:321–338. [PubMed] [Google Scholar]
- Bonjour JP. Biotin. In: Machlin LJ, editor. Handbook of vitamins; nutritional biochemical and clinical aspects. Marcel Dekker Inc.; New York: 1984. pp. 403–435. [Google Scholar]
- Boycott KM, Beaulieu CL, Kernohan KD, Gebril OH, Mhanni A, Chudley AE, Redl D, Qin W, Hampson S, Küry S, Tetreault M, Puffenberger EG, Scott JN, Bezieau S, Reis A, Uebe S, Schumacher J, hegele RA, McLeod DR, Galvez-Peralta M, Majewski J, Ramaekers VT, Care4Rare Canada Consortium. Nebert DW, Innes AM, Parboosingh JS, Abou Jamra R. Autosomal-recessive intellectual disability with cerebellar atrophy syndrome caused by mutation of the manganese and zinc transporter gene SLC39A8. Am J Hum Genet. 2015;97:886–893. doi: 10.1016/j.ajhg.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Le G, Yang R, Shi Y. Lipoic acid attenuates high fat diet-induced chronic oxidative stress and immunosuppression in mice jejunum: a microarray analysis. Cell Immunol. 2009;260:44–50. doi: 10.1016/j.cellimm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Follis RH, Wintrobe MM. A comparison of the effects of pyridoxine and pantothenic acid deficiencies on the nervous tissues of swine. J Exp Med. 1945;81:539–552. doi: 10.1084/jem.81.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal A, Lambrecht N, Subramanya SB, Kapadia R, Said HM. Conditional knockout of the Slc5a6 gene in mouse intestine impairs biotin absorption. Am J Physiol Gastrointest Liver Physiol. 2013;304:G64–G71. doi: 10.1152/ajpgi.00379.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipas H, Guttin A, Issartel JP. Exosomal microRNAs in tumoral U87 MG versus normal astrocyte cells. Microrna. 2015;4:131–145. doi: 10.2174/2211536604666150820115707. [DOI] [PubMed] [Google Scholar]
- Jacobsen JC, Wilson C, Cunningham V, Glamuzina E, Prosser DO, Love DR, Burgess T, Taylor J, Swan B, Hill R, Robertson SP, Snell RG, Lehnert K. Brain dopamine-serotonin vesicular transport disease presenting as a severe infantile hypotonic parkinsonian disorder. J Inherit Metab Dis. 2016;39:305–308. doi: 10.1007/s10545-015-9897-6. [DOI] [PubMed] [Google Scholar]
- Kono S, Miyajima H, Yoshida K, Togawa A, Shirakawa K, Suzuki H. Mutations in a thiamine-transporter gene and Wernicke's-like encephalopathy. N Engl J Med. 2009;360:1792–1794. doi: 10.1056/NEJMc0809100. [DOI] [PubMed] [Google Scholar]
- Krause KH, Bonjour J, Berlit P, Kochen W. Biotin status of epileptics. Ann New York Acad Sci. 1985;447:297–313. doi: 10.1111/j.1749-6632.1985.tb18447.x. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Hayflick SJ, Gitschier J. Deprivation of pantothenic acid elicits a movement disorder and azoospermia in a mouse model of pantothenate kinase-associated neurodegeneration. J Inherit Metab Dis. 2007;30:310–317. doi: 10.1007/s10545-007-0560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroishi T. Regulation of immunological and inflammatory functions by biotin. Can J Physiol Pharmacol. 2015;93:1091–1096. doi: 10.1139/cjpp-2014-0460. [DOI] [PubMed] [Google Scholar]
- Li Y, Ma QG, Zhao LH, Wei H, Duan G X, Zhang JY, Ji C. Effects of lipoic acid on immune function, the antioxidant defense system, and inflammation-related genes expression of broiler chickens fed aflatoxin contaminated diets. Int J Mol Sci. 2014;15:5649–5662. doi: 10.3390/ijms15045649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez DL, Tsuchiya Y, Gout I. Coenzyme A biosynthetic machinery in mammalian cells. Biochem Soc Trans. 2014;42:1112–1117. doi: 10.1042/BST20140124. [DOI] [PubMed] [Google Scholar]
- Mock DM, Stadler DD, Stratton SL, Mock N I. Biotin status assessed longitudinally in pregnant women. J Nutr. 1997;127:710–716. doi: 10.1093/jn/127.5.710. [DOI] [PubMed] [Google Scholar]
- Moiseenok AG, Komar VI, Khomich TI, Kanunnikova NP, Slyshenkov VS. Pantothenic acid in maintaining thiol and immune homeostasis. BioFactores. 2000;11:53–55. doi: 10.1002/biof.5520110115. [DOI] [PubMed] [Google Scholar]
- Ozand PT, Gascon GG, Al Essa M, Joshi S, Al Jishi E, Bakheet S, Al Watban J, Al-Kawi MZ, Dabbagh O. Biotin-responsive basal ganglia disease: a novel entity. Brain. 1998;121:1267–1279. doi: 10.1093/brain/121.7.1267. [DOI] [PubMed] [Google Scholar]
- Palaniyappan A, Alphonse R. Immunomodulatory effect of DL-α-lipoic acid in aged rats. Exp Gerontol. 2011;46:709–715. doi: 10.1016/j.exger.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Prasad PD, Wang H, Kekuda R, Fujita T, Fei Y, Devoe LD, Leibach FH, Ganapathy V. Cloning and functional expression of a cDNA encoding a mammalian sodium-dependent vitamin transporter mediating the uptake of pantothenate, biotin, and lipoate. J Biol Chem. 1997;273:7501–7506. doi: 10.1074/jbc.273.13.7501. [DOI] [PubMed] [Google Scholar]
- Quick M, Shi L. The sodium/multivitamin transporter: a multipotent system with therapeutic implications. Vitam Horm. 2015;98:63–100. doi: 10.1016/bs.vh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retterer K, Juusola J, Cho MT, Vitazka P, Millan F, Gibellini F, Vertino-Bell A, Smaoui N, Neidich J, Monaghan KG, McKnight D, Bai R, Suchy S, Friedman B, Tahiliani J, Pineda-Alvarez D, Richard G, Brandt T, Haverfield E, Chung WK, Bale S. Clinical application of whole-exome sequencing across clinical indications. Genet Med. 2016:696–704. doi: 10.1038/gim.2015.148. [DOI] [PubMed] [Google Scholar]
- Roberts JL, Moreau R. Emerging role of alpha-lipoic acid in the prevention and treatment of bone loss. Nutr Rev. 2015;73:116–125. doi: 10.1093/nutrit/nuu005. [DOI] [PubMed] [Google Scholar]
- Seidner G, Alvarez MG, Yeh JI, O'Driscoll KR, Klepper J, Stump TS, Wang D, Spinner NB, Birnbaum MJ, De Vivo DC. GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat Genet. 1998;18:188–191. doi: 10.1038/ng0298-188. [DOI] [PubMed] [Google Scholar]
- Spilioti M, Evangeliou AE, Tramma D, Theodoridou Z, Metaxas S, Michailidi S, Bonti E, Frysira H, Haidopoulou A, Asprangathou D, Tsalkidis AJ, Kardaras P, Wevers RA, Jakobs C, Gibson KM. Evidence for treatable inborn errors of metabolism in a cohort of 187 Greek patients with autism spectrum disorder (ASD). Front Hum Neurosci. 2013;7:858–70. doi: 10.3389/fnhum.2013.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian VS, Marchant JS, Boulware MJ, Ma TY, Said HM. Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells. Am J Physiol Cell Physiol. 2009;296:C663–C671. doi: 10.1152/ajpcell.00396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman L, Nyhan WL. Inheritable biotin-treatable disorders and associated phenomena. Ann Rev Nutr. 1986;6:314–343. doi: 10.1146/annurev.nu.06.070186.001533. [DOI] [PubMed] [Google Scholar]
- Thevenon J, Milh M, Feillet F, St-Onge J, Duffourd Y, Jugé C, Roubertie A, Héron D, Mignot C, Raffo E, Isidor B, Wahlen S, Sanlaville D, Villeneuve N, Darmency-Stamboul V, Toutain A, Lefenvre M, Chouchane M, Huet F, Lafon A, de Saint Martin A, lesca G, El Chehadeh S, Thauvin-Robinet C, Masurel-Paulet A, Odent S, Villard L, Phillippe C, Faivre L, Riviere JB. Mutations in SLC13A5 cause autosomal-recessive epileptic encephalopathy with seizure onset in the first days of life. Am J Hum Genet. 2014;95:113–120. doi: 10.1016/j.ajhg.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Ito K, Ohtsuki S, Kubo Y, Suzuki T, Terasaki T. Major involvement of Na(+)-dependent multivitamin transporter (SLC5A6/SMVT) in uptake of biotin and pantothenic acid by human brain capillary endothelial cells. J Neurochem. 2015;134:97–112. doi: 10.1111/jnc.13092. [DOI] [PubMed] [Google Scholar]
- Wang H, Huang W, Fei YJ, Xia H, Yang-Feng T, Leibach FH, Devoe LD, Ganapathy V, Prasad PD. Human placental Na+-dependent multivitamin transporter: Cloning, Functional expression, Gene structure and chromosomal localization. J Biol Chem. 1999;274:14875–14883. doi: 10.1074/jbc.274.21.14875. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nagai Y, Taniguchi A, Ebara S, Kimura S, Fukui T. Effect of biotin deficiency on embryonic development in mice. Nutrition. 2009;25:78–84. doi: 10.1016/j.nut.2008.06.031. [DOI] [PubMed] [Google Scholar]
- Wolf B. In: The metabolic and molecular basis of inherited diseases. C. R. Scriver CR, Beaudet A, editors. McGraw-Hill; New York, NY: 2001. pp. 3935–3962. [Google Scholar]
- Wolf B. Biotinidase deficiency. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. Gene Reviews. University of Washington; Seattle (WA): 2013. [Internet (no page number] [PubMed] [Google Scholar]
- Zehnfenning B, Wiriyasermkul P, Carlson DA, Quick M. Interaction of α-Lipoic acid with the human Na+/multivitamin transporter (hSMVT). J Biol Chem. 2015;290:16372–16382. doi: 10.1074/jbc.M114.622555. [DOI] [PMC free article] [PubMed] [Google Scholar]