Abstract
Background and Purpose
ATP-binding cassette transporter A1 (ABCA1) is a major reverse cholesterol-transporter and plays critical role in the formation of brain high-density-lipoprotein (HDL) cholesterol. Apolipoprotein-E (ApoE) is the most abundant apolipoprotein and transports cholesterol into cells in brain. ABCA1 and ApoE are up-regulated by liver-X-receptors. Activation of liver-X-receptors has neurorestorative benefit for stroke. We tested the hypothesis that ABCA1/ApoE/HDL pathway mediates GW3965, a synthetic dual liver-X-receptor agonist, induced neurorestoration after stroke.
Methods
Middle-aged male specific brain-ABCA1-deficient (ABCA1−B/−B) and floxed-control (ABCA1fl/fl) mice were subjected to distal middle-cerebral-artery occlusion (dMCAo) and gavaged with saline or GW3965 (10mg/kg), or intracerebral-infusion of artificial cerebrospinal-fluid or hHDL3 in ABCA1−B/−B–stroke mice, starting 24h after dMCAo and daily until sacrifice 14 days after dMCAo.
Results
No differences in the blood level of total-cholesterol and triglyceride and lesion-volume were found among the groups. Compared with ABCA1fl/fl-ischemic mice, ABCA1−B/−B-ischemic mice exhibited impairment functional-outcome and decreased ABCA1/ApoE expression, and decreased gray/white-matter densities in the ischemic-boundary-zone 14 days after dMCAo. GW3965-treatment of ABCA1fl/fl-ischemic mice led to increased brain ABCA1/ApoE expression, concomitantly to increased blood-HDL, gray/white-matter densities and oligodendrocyte-progenitor-cell numbers in the ischemic-boundary-zone as well as improved functional-outcome 14 days after dMCAo. GW3965-treatment had negligible beneficial effects in ABCA1−B/−B-ischemic mice. However, intracerebral-infusion of hHDL3 significantly attenuated ABCA1−B/−B–induced deficits. In vitro, GW3965-treatment (5μM) increased ABCA1/synaptophysin level and neurite/axonal-outgrowth in primary cortical neurons derived from ABCA1fl/fl-embryos, but not in neurons derived from ABCA1−B/−B-embryos. HDL-treatment (80μg/mL) attenuated the reduction of neurite/axonal-outgrowth in neurons derived from ABCA1−B/−B-embryos.
Conclusions
ABCA1/ApoE/HDL pathway, at least partially, contributes to GW3965-induced neurorestoration after stroke.
Keywords: ATP-binding cassette transporter A1, high density lipoprotein, apolipoprotein E, liver X receptors, white matter, stroke
Introduction
Stroke is one of the most-frequent causes of death and disability worldwide. Although the pathogenesis and clinical significance of stroke recovery are clearly related to the functional cerebral lesions both in gray matter and white-matter (WM)1, the molecular mechanisms of gray and WM remodeling after stroke are not fully understood. New strategies to promote neurorestoration and to improve long-term neurological deficits after stroke are necessary beyond the hyper-acute phase of ischemia.
Cholesterol plays major structural and functional roles in both the gray matter and WM. The ATP-binding cassette transporter member A1 (ABCA1), a major cholesterol-transporter, regulates efflux of intracellular cholesterol and phospholipids onto lipid-poor apolipoprotein E (ApoE) and plays a critical role in mediating high-density-lipoprotein (HDL) cholesterol and ApoE production in the brain2, 3. ApoE is the most abundant apolipoprotein in the brain. It solubilizes phospholipid and transports cholesterol into cells, while stabilizing HDL-particles, and enables these proteins to be partners with ABCA13–6. Both ABCA1 and ApoE expression are up-regulated by nucleic-transcription-factor liver-X-receptors (LXRs)6–8. Treatment of stroke with GW3965, a synthetic dual LXR (including LXRalpha and LXRbeta) agonist, promotes neuroprotection and reduces brain inflammation9, increases vascularization and WM-remodeling in the ischemic brain, and elevates blood-HDL level as well as improves neurological functional-outcome in experimental stroke10. However, the molecular mechanisms of GW3965-induced neurorestorative effects after stroke are not fully elucidated. In this study, using specific conditional brain-ABCA1 knockout mice, we tested whether the ABCA1/ApoE/HDL signaling pathway mediates GW3965-treatment induced neurorestoration after stroke.
Materials and Methods
For all in vivo studies, the use of animals and procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Health System. All animal groups, treatment and their identity were blinded to the surgeon and the investigators who performed behavior tests, lesion volume and biochemical measurements, and immunostaining analysis.
Animals and stroke model
Specific brain-ABCA1 knockout (ABCA1−B/−B) and ABCA1-floxed control (ABCA1fl/fl) mice were used. The parent pairs of animals were generously provided by Dr. Michael Hayden (University of British Columbia, Canada). Since female with wide range of estrogen’s effects, in this study, we subjected male mice (middle-aged, 13–14 months) to permanent right distal middle-cerebral-artery occlusion (dMCAo) induced by transcranial ligation with a 10–0 nylon filament. The number of animals was calculated a priori by power calculation. Because dMCAo leads to a consistent lesion volume and very low mortality, 9 animals per group were considered to reach 80% power at a significance level of <0.05 assuming 20% difference in both of mean and SD at the 95% confidence level and a two-sided test.
Experimental groups
To investigate whether ABCA1/ApoE mediates GW3965-induced neurorestoration after stroke, ABCA1fl/fl and ABCA1−B/−B stroke mice were randomly divided into 4 groups by a non-team member using the method of drawing different colored balls. Mice were gavaged starting 24h after dMCAo with saline as vehicle control or GW3965 10mg/kg (Sigma) daily for 14 days based on previous dose-dependent study10. After 14 days of treatment, animals were randomly separated into two sets: one set of animals (total 24 mice, n=6/group) were employed for WB and RT-PCR assay; the other set of animals (total 36 mice, n=9/group) were used for behavioral testing, blood-biochemistry, and lesion-volume and immunostaining measurements.
To investigate the effect of HDL on GW3965-induced neurorestoration, ABCA1−B/−B stroke mice were randomly assigned to two groups (total 18 mice, n=9/group) and intraventricularly-infused with artificial cerebrospinal-fluid (CSF, Tocris Bioscience) 100μl as vehicle control, or human-plasma HDL3 (hHDL3, Cell Biolabs Inc.) 25μg in 100μl artificial-CSF by transplanting a micro-osmotic pump (D1002, Alzet) into the right lateral-ventricle initiated at 24h after dMCAo for 14 days. All mice were sacrificed 14 days after dMCAo.
Functional test
The adhesive-removal test, the most sensitive functional test for dMCAO11, was performed prior to dMCAo and at 1, 3, 7 and 14 days after dMCAo.
HDL, total cholesterol and triglyceride measurement
Blood was collected from the tail vein 14 days after dMCAo and the blood level of HDL, total cholesterol and triglyceride were measured using a CardioChek P•A analyzer and test strips (Polymer 285 Technology System).
Lesion-volume, histochemical and immunohisto-staining assessment, RT-PCR/WB assay and neurite/axonal-outgrowth measurement (please see Supplemental Methods)
Data and statistical analysis
Data are presented as mean ± Standard Error (SE). Two-way ANOVA followed Tukey post-hoc test were performed for analysis: 1) gene/protein expression, lesion volume/blood biochemistry/functional outcome measurement and in vitro study for comparison of gene deficient (ABCA1fl/fl vs ABCA1−B/−B) and treatment effect (with vs without GW3965 or HDL, respectively); 2) immunostaining measurement for gene-deficient (ABCA1fl/fl vs ABCA1−B/−B) and ischemic-effect (contralateral vs ipsilateral). Independent t-test was made between ABCA1−B/−B–stroke mice intracerebral-infusion of artificial-CSF and ABCA1−B/−B–stroke mice intracerebral-infusion of hHDL3 groups. A value of p<0.05 was taken as significant.
Results
GW3965-treatment increases brain ABCA1/ApoE expression in ABCA1fl/fl-stroke mice, but not in ABCA1−B/−B–stroke mice
ABCA1/ApoE protein and mRNA level significantly decreased measured by WB and RT-PCR assays in both the contralateral and the ipsilateral-ischemic brain tissues isolated from ABCA1−B/−B–saline control stroke mice compared with tissues isolated from ABCA1fl/fl-saline control stroke mice, respectively; GW3965-treatment of ABCA1fl/fl-stroke mice significantly increased ABCA1/ApoE levels in both the contralateral and the ipsilateral-ischemic brain tissue compared with ABCA1fl/fl-saline control stroke mice, respectively. However, GW3965 had a negligible effect on ABCA1 expression, but increases ApoE expression in ABCA1−B/−B-stroke mice (Figure 1B–D, p<0.05, n=6/group).
Figure 1.
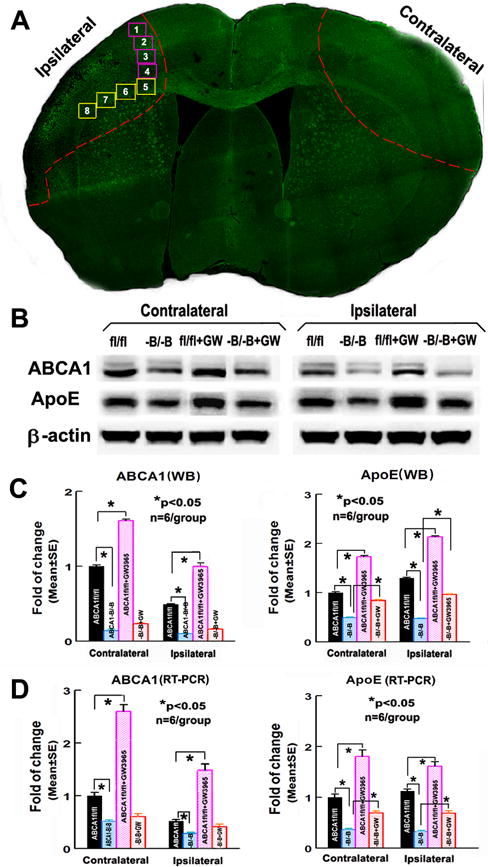
ABCA1−B/−B decreases brain ABCA1/ApoE level; GW3965-treatment increases ABCA1/ApoE level in the ABCA1fl/fl-brain, but not in ABCA1−B/−B-brain 14 days after dMCAo. A: Confocal-micrograph photo schematically shows the areas where the images were taken for Syn (square 1–4) or LFB/BS/SMI31/PDGFRα (square 5–8), and ipsilateral-ischemic tissue and contralateral brain tissue (outlined areas); B: Western blot (WB) image; C: quantitative data of WB; D: RT-PCR assay. *p<0.05, n=6/group.
GW3965-treatment increases blood-HDL level and improves functional outcome in ABCA1fl/fl-stroke mice, but not in ABCA1−B/−B–stroke mice; Intracerebral-infusion of hHDL3 improves functional outcome in ABCA1−B/−B-stroke mice
There were no significant differences in the indirect lesion volume (Supplemental Figure IA), and the blood level of total cholesterol and triglyceride among ABCA1fl/fl and ABCA1−B/−B stroke mice treated with saline or GW3965. However, GW3965-treatment of ABCA1fl/fl-stroke mice, but not ABCA1−B/−B-stroke mice, significantly increased blood HDL level compared with ABCA1fl/fl-saline control stroke mice 14 days after dMCAo (Supplemental Figure IB, p<0.05, n=9/group). Compared with ABCA1fl/fl-saline control stroke mice, ABCA1−B/−B-saline control stroke mice exhibited more severe neurological deficits 3, 7 and 14 days after dMCAo. Treatment of stroke with GW3965 significantly improved functional outcome 7 and 14 days after dMCAo in ABCA1fl/fl-stroke mice, but not in ABCA1−B/−B-stroke mice (Supplemental Figure IC, p<0.05, n=9/group).
Compared with intraventricular-infusion of artificial-CSF in ABCA1−B/−B-stroke mice, no significant differences in the lesion volume (Supplemental Figure IIA) and blood levels of HDL/triglyceride/total cholesterol (Supplemental Figure IIB) were found in ABCA1−B/−B-stroke mice intraventricularly-infused with hHDL3. However, these mice exhibited significantly improved functional outcome 14 days after dMCAo (Supplemental Figure IIC, p<0.05, n=9/group).
GW3965-treatment increases Syn/MBP expression in the ischemic-brain in ABCA1fl/fl-stroke mice, but not in ABCA1−B/−B-stroke mice; Intracerebral-infusion of hHDL3 increases Syn/MBP expression in the ischemic-brain in ABCA1−B/−B-stroke mice
ABCA1−B/−B-saline control stroke mice exhibit significantly decreased Syn-protein level measured by both immunostaining (Figure 2A) and WB-assay (Figure 2B), and MBP-protein/mRNA (Figure 2B) level in both the contralateral and ipsilateral-ischemic brain compared with ABCA1fl/fl-saline control stroke mice, respectively (p<0.05, n=9/group). GW3965-treatment of ABCA1fl/fl-stroke mice, but not ABCA1−B/−B–stroke mice, significantly increased Syn protein and MBP protein/mRNA level in both the contralateral and ipsilateral ischemic brain compared to ABCA1fl/fl–saline control stroke mice (Figure 2A, 2B, p<0.05, n=9/group).
Figure 2.
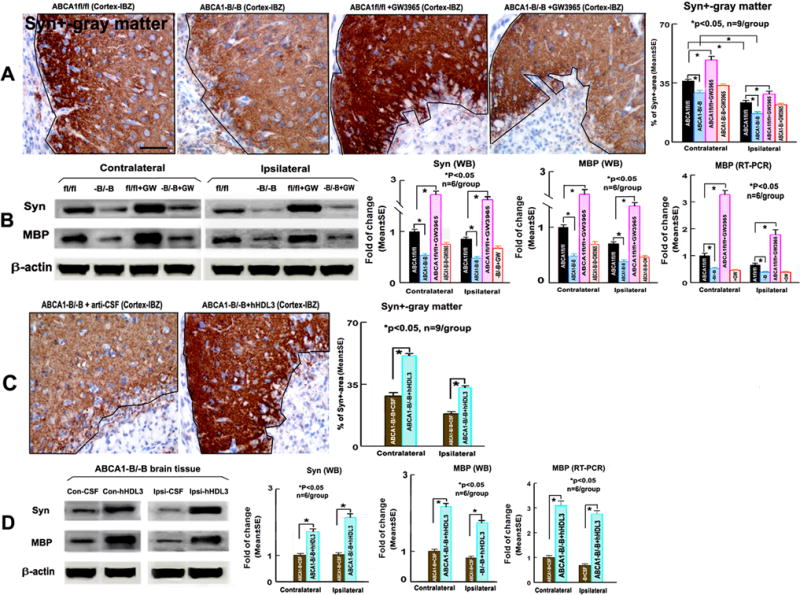
ABCA1−B/−B–stroke mice exhibit decreased Syn/MBP level. GW3965-treatment of ABCA1fl/fl-stroke mice, but not ABCA1−B/−B-stroke mice, increases Syn/MBP expression in both the contralateral-brain and the ischemic-brain. Intraventricular-infusion of hHDL3 increased Syn/MBP level in the contralateral and the ischemic-brain in ABCA1−B/−B-stroke mice 14 days after stroke. A, B: Syn-immunostaining and quantitative data (A, *p<0.05, n=9/group), and Syn/MBP WB/RT-PCR assay and quantitative data (B, *p<0.05, n=6/group) in ABCA1fl/fl and ABCA1−B/−B stroke mice treated with saline or GW3965; C, D: Syn-immunostaining and quantitative data (C, *p<0.05, n=9/group), and Syn/MBP WB/RT-PCR assay and quantitative data (D, *p<0.05, n=6/group) in ABCA1−B/−B–stroke mice with intracerebral-infusion of artificial-CSF or hHDL3. Scare bar = 40μm.
Intraventricular-infusion of hHDL3 in ABCA1−B/−B–stroke mice significantly increased Syn protein level measured by immunostaining (Figure 2C, p<0.05, n=9/group) and Syn/MBP protein/mRNA level (Figure 2D, p<0.05, n=6/group) compared with intraventricular-infusion of artificial-CSF in ABCA1−B/−B–stroke mice.
GW3965-treatment of stroke increases WM-densities and OPC-numbers in the IBZ of ABCA1fl/fl-stroke mice, but not in ABCA1−B/−B-stroke mice; Intracerebral-infusion of hHDL3 increases WM-densities and OPC-numbers in the IBZ of ABCA1−B/−B-stroke mice
Compared with contralateral-CC, the densities of LBF+-myelin, BS+-axons and SMI31+-phosphorylated neurofilament in the IBZ of ipsilateral-CC significantly decreased both in ABCA1fl/fl-saline control stroke mice and ABCA1−B/−B-saline control stroke mice, respectively. The number of PDGFRα+-OPCs in the IBZ of ipsilateral-CC significantly increased in ABCA1fl/fl-saline control stroke mice, but not in ABCA1−B/−B-saline control stroke mice. The densities of LFB+/BS+/SMI31+-WM and the numbers of PDGFRα+-OPCs significantly decreased in both the contralateral-CC and the IBZ of the ipsilateral-CC in ABCA1−B/−B-saline control stroke mice compared with ABCA1fl/fl-saline control stroke mice. However, GW3965-treatment of ABCA1fl/fl-stroke mice, but not ABCA1−B/−B-stroke mice, exhibited increased densities of LFB+/SMI31+ in the contralateral-CC, and increased the densities of LFB+/BS+/SMI31+ and the number of PDGFRα+-OPC in the IBZ of the ipsilateral-CC compared with ABCA1fl/fl-stroke saline-control mice (Figure 3A, p<0.05, n=9/group).
Figure 3.
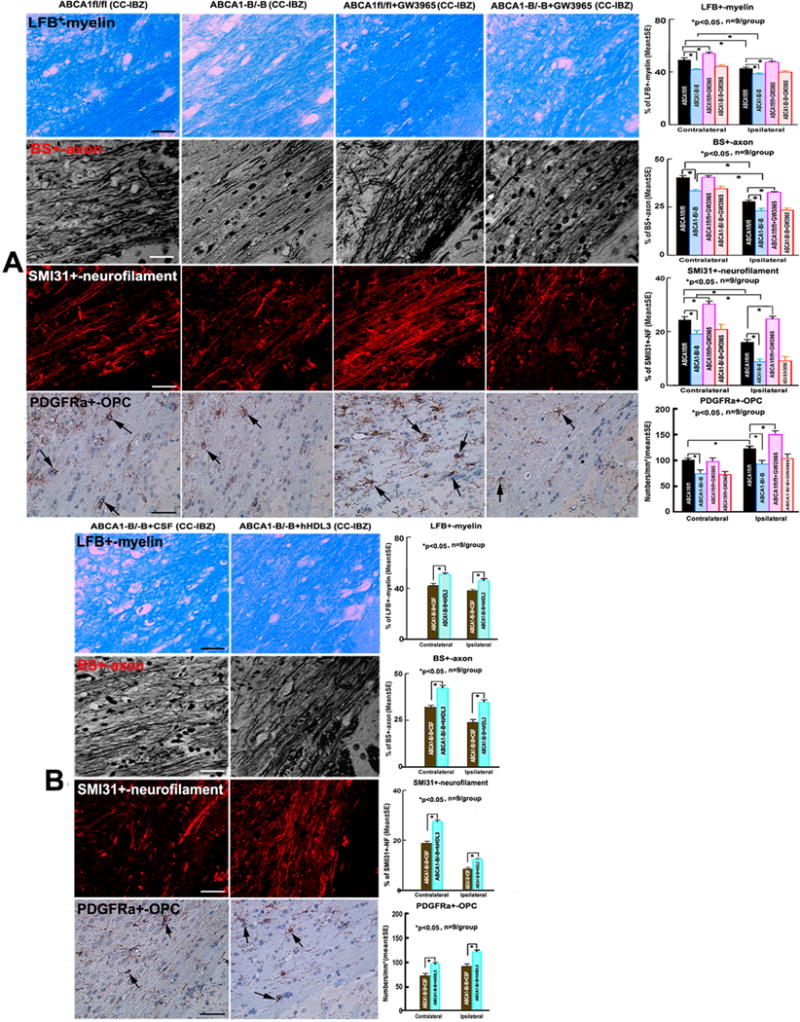
ABCA1−B/−B decreases WM-density/OPC-numbers, GW3965-treatment of ABCA1fl/fl-mice, but not ABCA1−B/−B–mice, increases WM-density/OPC-numbers in the IBZ of ipsilateral-CC; Intraventricular-infusion of hHDL3 significantly increased WM-density/OPC-numbers in both of contralaterlal-CC and IBZ of ipsilateral-CC 14 days after stroke. A: Immunostainings of LFB+-myelin/BS+-axon/SMI31+-phosphorylated-neurofilament/PDGFRα+-OPCs and quantitative data in ABCA1fl/fl and ABCA1−B/−B stroke mice treated with saline or GW3965, respectively; B: Immunostainings of LFB+-myelin/BS+-axon/SMI31+-phosphorylated-neurofilament/PDGFRα+-OPCs and quantitative data in ABCA1−B/−B-stroke mice intraventricularly-infused with artificial-CSF or hHDL3, respectively. Scale bar = 20 μm in LFB+-myelin and BS+-axon images, 40μm in SMI31+-neurofilament and PDGFRa+-OPC images. *p<0.05, n=9/group.
Compared with Intraventricular-infusion of artificial-CSF in ABCA1−B/−B-stroke mice, intraventricular-infusion of hHDL3 in ABCA1−B/−B-stroke mice significantly increased the densities of LFB+/BS+/SMI31+ and the number of PDGFRα+-OPC in both the contralateral and the ipsilateral-CC (Figure 3B, p<0.05, n=9/group).
GW3965-treatment increases ABCA1/Syn level and neurite/axonal outgrowth in ABCA1fl/fl-PCNs, but not in ABCA1−B/−B-PCNs; HDL attenuates the reduction of neurite/axonal outgrowth in ABCA1−B/−B-PCNs
The WB and RT-PCR assay and quantitative data show that both the Syn protein and ABCA1 protein/mRNA levels were significantly decreased in ABCA1−B/−B-PCNs compared with ABCA1fl/fl-PCNs after OGD. GW3965-treatment significantly increased ABCA1 protein/mRNA and Syn protein levels in ABCA1fl/fl-PCNs, but not in ABCA1−B/−B-PCNs, after OGD (Figure 4A, p<0.05, n=6/group).
Figure 4.
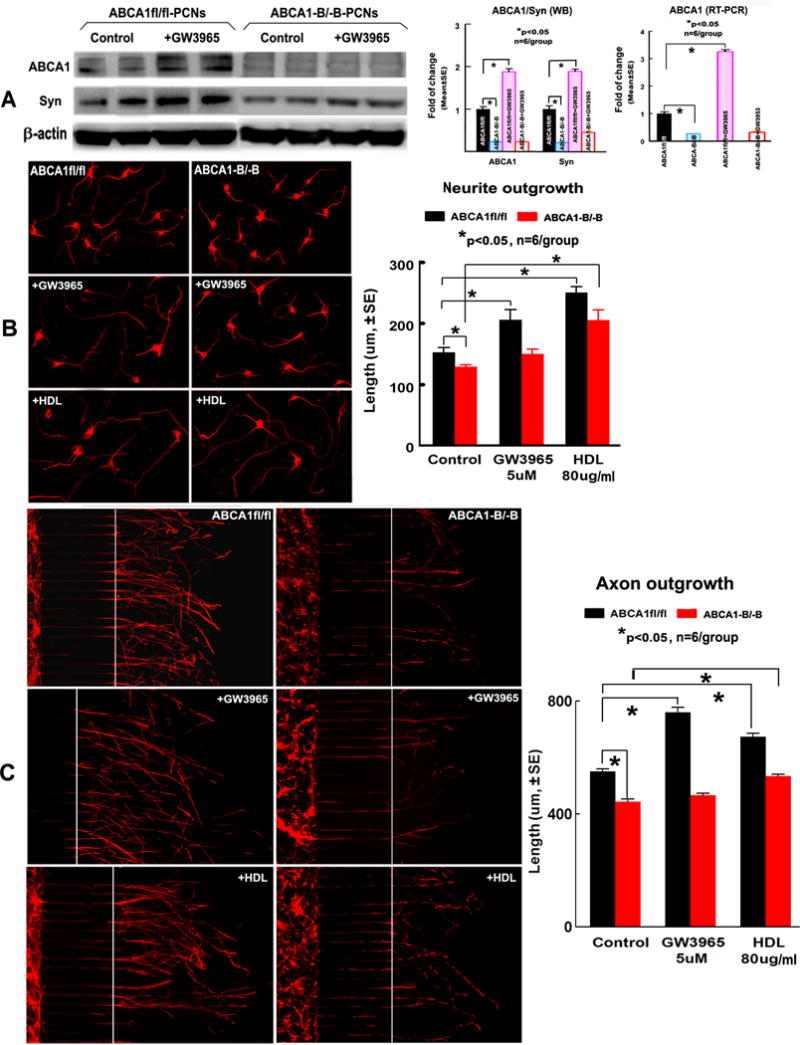
ABCA1−B/−B decreased ABCA1/Syn level and neurite/axonal outgrowth in PCNs. GW3965-treatment increased ABCA1/Syn levels and neurite/axonal outgrowth in ABCA1fl/fl-PCNs, but not in ABCA1−B/−B–PCNs after OGD; HDL attenuated ABCA1−B/−B–induced neurite/axonal outgrowth with or without hypoxic condition. A: WB image and quantitative data of WB and RT-PCR; B: Neurite-outgrowth after OGD and quantitative data; C: Axonal-outgrowth and quantitative data. *p<0.05, n=6/group.
The neurite-outgrowth measurement revealed that ABCA1−B/−B-PCNs had significantly decreased neurite-outgrowth after OGD compared with ABCA1fl/fl-PCNs; GW3965-treatment significantly increased neurite-outgrowth in ABCA1fl/fl-PCNs, but not in ABCA1−B/−B-PCNs, after OGD. HDL-treatment not only increased neurite-outgrowth in ABCA1fl/fl-PCNs, but also attenuated the reduction of neurite-outgrowth in ABCA1−B/−B-PCNs after OGD (Figure 4B, p<0.05, n=6/group).
ABCA1−B/−B-PCNs exhibited significantly decreased axonal-outgrowth compared with ABCA1fl/fl-PCNs. GW3965-treatment increased the axonal-outgrowth in ABCA1fl/fl-PCNs, but did not do so in ABCA1−B/−B-PCNs. However, HDL-treatment significantly increased axonal-outgrowth in both ABCA1fl/fl-PCNs and ABCA1−B/−B-PCNs (Figure 4C, p<0.05, n=6/group).
Discussion
WM is composed of bundles of myelinated axons. WM-remodeling (including axonal-regeneration and remyelination) in the IBZ of cerebral infarcts is essential for long-term stroke recovery, and the process of remyelination is mediated by abundant OPCs located throughout the adult brain12, 13. Cholesterol is a major component of myelin, and axonal regeneration is in-part dependent on local cholesterol utilization in regenerating neurons14, 15. Cholesterol is also an important component of neuronal membranes, participates in neuronal survival16 and neuronal function, such as membrane trafficking, signal transduction, and neurotransmitter release15, 17, 18. Synaptic protein is formed from neuronal synapses, and cholesterol is essential lipid substrates for massive synaptogenesis in neurons18, 19. Synaptogenesis parallels functional recovery after cortical injury, including stroke20.
HDL-cholesterol plays an important role in WM-remodeling and neurological functional recovery after brain injury. Almost all of HDL-cholesterol in the central nervous system is derived from in situ biosynthesis mainly by ABCA121, 22. Mutations in the human ABCA1 gene cause HDL deficiency syndrome, i.e., Tangier disease, characterized by little or virtual absence of plasma HDL and prominent cholesterol deposition in tissues, cells and prevalent atherosclerosis23, 24. In contrast, ABCA1 transgenic mice have a significant increase in cholesterol efflux in different tissues and marked elevation in HDL-cholesterol levels8. Deficiency of ABCA1 also decreases ApoE level and impairs ApoE metabolism in brain3, 5, 25–27. Both ABCA1 and ApoE play a vital role in cholesterol homeostasis, neuronal repair and synaptic plasticity28, 29. ABCA1−B/−B mice are generated by crossing loxP-flanked (floxed) ABCA1 mice with transgenic mice expressing Cre recombinant under the control of the neuronal and glial specific nestin promoter25. This leads to an absence of ABCA1 in all nestin-lineage cells (neural stem cells) including neurons and glia. These mice exhibit selective loss of brain-ABCA1 and very low level of HDL and ApoE in both brain tissue and CSF, and reduced synapse and synaptic vesicle numbers in the brain25–27. Our previous study showed that there is no significant difference in both the WM-density and the number of oligodendrocytes or OPCs in the brain of young (2–3 months) non-stroke ABCA1−B/−B mice; however, ABCA1−B/−B-stroke mice exhibited more severe WM-damage in the ischemic brain and worse functional outcome compared with ABCA1fl/fl–stroke mice 7 days after stroke30. In the present study, we found the middle-aged ABCA1−B/−B-stroke mice exhibited decreased brain ABCA1/ApoE level, decreased WM-remodeling and Syn-protein in both the contralateral and the ipsilateral-ischemic brain, as well as decreased functional outcome compared with ABCA1fl/fl–stroke mice 14 days after dMCAo. Using PCN cultures, both ABCA1/Syn level and the neurite/axonal-outgrowth significantly decreased in the ABCA1−B/−B-PCNs with or without OGD condition. These data indicate that ABCA1/ApoE play an important role in both the gray/WM-remodeling after stroke.
Increase of HDL functionality may have important implications for treatment and prevention of cerebral WM-damage after stroke31. The regulation of ABCA1/ApoE expression by LXRs is physiologically relevant in vivo because both ABCA1/ApoE and LXRs are expressed in neurons and glia in the brain28, 32–34. Activation of LXRs, which heterodimerizes with retinoid X receptors (RXRs), induces the transcription of both ABCA1 and ApoE in vivo and in vitro35, 36. In addition, upregulation of ApoE via the LXR activation plays an important role in stroke recovery37. GW3965 crosses the blood-brain barrier and stimulates expression of many target genes, including ABCA1/ApoE6. GW3965 treatment raises HDL-level in plasma, liver and macrophages10, 32, 38, 39, and increases expression of ABCA1/ApoE in the hippocampus and cerebral cortex and rescues hippocampus long-term synaptic plasticity in an Alzheimer’s disease mouse model29. In the present study, we found that GW3965-treatment of ABCA1fl/fl-stroke mice significantly increased the levels of brain-ABCA1/ApoE and blood-HDL, increased gray/WM-remodeling as well as improved functional-outcome. In vitro, GW3965-treatment significantly increased ABCA1/Syn level and increased neurite/axonal-outgrowth in ABCA1fl/fl-PCNs with or without hypoxic condition. Although GW3965-treatment elevated brain-ApoE level in ABCA1−B/−B-stroke mice, however, ABCA1-deficiency abolishes GW3965-treatment induced benefits in vivo and in vitro. In contrast, HDL-supplemental treatment attenuated ABCA1−B/−B-induced deficits both in vivo and in vitro. These data indicate that substrate (HDL) availability is a predominant factor for neurorestoration through ABCA1/ApoE cholesterol transporters after stroke.
Limitations: 1. the risk of hepatic steatosis upon pharmaceutical targeting of LXR may be a particularly serious consequence in humans40. T0901317, an LXRalpha agonist, which induces liver steatosis through enhanced Srebp1-induced fatty acid syntheses and lipoprotein transcription41, 42. However, our present study shown that GW3965-treatment did not change blood triglyceride and total-cholesterol. Consistent with our study, GW3965 raises HDL without inducing hepatic steatosis and hypertriglyceridemia32, 38, 39, and do not affect hepatic insulin sensitivity43 in rodents. Therefore, we should carefully to choose LXRs for clinical use. 2. In the present study, we are unable to provide evidence that which cell types, neurons, astrocytes or oligodendrocytes, paly a primary role in mediating ABCA1-deficient induced WM-impairment or GW3965-treatment induced WM-remodeling, which is warranted to further studies.
Conclusions
We demonstrated, to our knowledge for the first time, GW3965-treatment increases ABCA1/ApoE/HDL level, promotes gray/WM-remodeling and improves neurological functional outcome in ABCA1fl/fl–stroke mice 14 days after dMCAo. These beneficial responses were absent in ABCA1−B/−B–stroke mice; however, intracerebral-infusion of hHDL3 attenuated ABCA1-deficient induced the deficits. Our data indicate that ABCA1/ApoE/HDL signaling pathway, at least partially, contribute to GW3965-induced neurorestoration after stroke.
Supplementary Material
Acknowledgments
The authors wish to thank Cynthia Roberts, Qinge Lu and Sutapa Santra for technical assistance.
Sources of funding
This work was supported by National Institute of Neurological Disorders and Stroke R01NS092917 (Xu Cui), R01NS083078 and R01NS099030 (Jieli Chen), R01NS079612 (Zhenggang Zhang), and R01NS088656 (Michael Chopp), and American Heart Association grant 12SDG9300009 (Xu Cui).
Footnotes
Disclosure
None.
References
- 1.Sierra C. Essential hypertension, cerebral white matter pathology and ischemic stroke. Current medicinal chemistry. 2014;21:2156–2164. doi: 10.2174/0929867321666131227155140. [DOI] [PubMed] [Google Scholar]
- 2.Danik M, Champagne D, Petit-Turcotte C, Beffert U, Poirier J. Brain lipoprotein metabolism and its relation to neurodegenerative disease. Critical reviews in neurobiology. 1999;13:357–407. doi: 10.1615/critrevneurobiol.v13.i4.20. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch-Reinshagen V, Zhou S, Burgess BL, Bernier L, McIsaac SA, Chan JY, et al. Deficiency of abca1 impairs apolipoprotein e metabolism in brain. J Biol Chem. 2004;279:41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- 4.Lund-Katz S, Phillips MC. High density lipoprotein structure-function and role in reverse cholesterol transport. Sub-cellular biochemistry. 2010;51:183–227. doi: 10.1007/978-90-481-8622-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, et al. Abca1 is required for normal central nervous system apoe levels and for lipidation of astrocyte-secreted apoe. J Biol Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- 6.Donkin JJ, Stukas S, Hirsch-Reinshagen V, Namjoshi D, Wilkinson A, May S, et al. Atp-binding cassette transporter a1 mediates the beneficial effects of the liver x receptor agonist gw3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. J Biol Chem. 2010;285:34144–34154. doi: 10.1074/jbc.M110.108100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hozoji-Inada M, Munehira Y, Nagao K, Kioka N, Ueda K. Liver x receptor beta (lxrbeta) interacts directly with atp-binding cassette a1 (abca1) to promote high density lipoprotein formation during acute cholesterol accumulation. J Biol Chem. 2011;286:20117–20124. doi: 10.1074/jbc.M111.235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singaraja RR, Bocher V, James ER, Clee SM, Zhang LH, Leavitt BR, et al. Human abca1 bac transgenic mice show increased high density lipoprotein cholesterol and apoai-dependent efflux stimulated by an internal promoter containing liver x receptor response elements in intron 1. J Biol Chem. 2001;276:33969–33979. doi: 10.1074/jbc.M102503200. [DOI] [PubMed] [Google Scholar]
- 9.Morales JR, Ballesteros I, Deniz JM, Hurtado O, Vivancos J, Nombela F, et al. Activation of liver x receptors promotes neuroprotection and reduces brain inflammation in experimental stroke. Circulation. 2008;118:1450–1459. doi: 10.1161/CIRCULATIONAHA.108.782300. [DOI] [PubMed] [Google Scholar]
- 10.Cui X, Chopp M, Zacharek A, Cui Y, Roberts C, Chen J. The neurorestorative benefit of gw3965 treatment of stroke in mice. Stroke. 2013;44:153–161. doi: 10.1161/STROKEAHA.112.677682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freret T, Bouet V, Leconte C, Roussel S, Chazalviel L, Divoux D, et al. Behavioral deficits after distal focal cerebral ischemia in mice: Usefulness of adhesive removal test. Behavioral neuroscience. 2009;123:224–230. doi: 10.1037/a0014157. [DOI] [PubMed] [Google Scholar]
- 12.Alix JJ, Domingues AM. White matter synapses: Form, function, and dysfunction. Neurology. 2011;76:397–404. doi: 10.1212/WNL.0b013e3182088273. [DOI] [PubMed] [Google Scholar]
- 13.Bakiri Y, Karadottir R, Cossell L, Attwell D. Morphological and electrical properties of oligodendrocytes in the white matter of the corpus callosum and cerebellum. The Journal of physiology. 2011;589:559–573. doi: 10.1113/jphysiol.2010.201376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodrum JF, Brown JC, Fowler KA, Bouldin TW. Axonal regeneration, but not myelination, is partially dependent on local cholesterol reutilization in regenerating nerve. J Neuropathol Exp Neurol. 2000;59:1002–1010. doi: 10.1093/jnen/59.11.1002. [DOI] [PubMed] [Google Scholar]
- 15.Pfrieger FW. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci. 2003;60:1158–1171. doi: 10.1007/s00018-003-3018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michikawa M, Yanagisawa K. Inhibition of cholesterol production but not of nonsterol isoprenoid products induces neuronal cell death. J Neurochem. 1999;72:2278–2285. doi: 10.1046/j.1471-4159.1999.0722278.x. [DOI] [PubMed] [Google Scholar]
- 17.Pfrieger FW. Role of cholesterol in synapse formation and function. Biochim Biophys Acta. 2003;1610:271–280. doi: 10.1016/s0005-2736(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 18.Koudinov AR, Koudinova NV. Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J. 2001;15:1858–1860. doi: 10.1096/fj.00-0815fje. [DOI] [PubMed] [Google Scholar]
- 19.Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, et al. Cns synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 20.Kolb B. Synaptic plasticity and the organization of behaviour after early and late brain injury. Can J Exp Psychol. 1999;53:62–76. doi: 10.1037/h0087300. [DOI] [PubMed] [Google Scholar]
- 21.DeMattos RB, Brendza RP, Heuser JE, Kierson M, Cirrito JR, Fryer J, et al. Purification and characterization of astrocyte-secreted apolipoprotein e and j-containing lipoproteins from wild-type and human apoe transgenic mice. Neurochem Int. 2001;39:415–425. doi: 10.1016/s0197-0186(01)00049-3. [DOI] [PubMed] [Google Scholar]
- 22.Ito J, Zhang LY, Asai M, Yokoyama S. Differential generation of high-density lipoprotein by endogenous and exogenous apolipoproteins in cultured fetal rat astrocytes. J Neurochem. 1999;72:2362–2369. doi: 10.1046/j.1471-4159.1999.0722362.x. [DOI] [PubMed] [Google Scholar]
- 23.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, et al. Mutations in abc1 in tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 24.van Dam MJ, de Groot E, Clee SM, Hovingh GK, Roelants R, Brooks-Wilson A, et al. Association between increased arterial-wall thickness and impairment in abca1-driven cholesterol efflux: An observational study. Lancet. 2002;359:37–42. doi: 10.1016/S0140-6736(02)07277-X. [DOI] [PubMed] [Google Scholar]
- 25.Karasinska JM, Rinninger F, Lutjohann D, Ruddle P, Franciosi S, Kruit JK, et al. Specific loss of brain abca1 increases brain cholesterol uptake and influences neuronal structure and function. J Neurosci. 2009;29:3579–3589. doi: 10.1523/JNEUROSCI.4741-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karasinska JM, de Haan W, Franciosi S, Ruddle P, Fan J, Kruit JK, et al. Abca1 influences neuroinflammation and neuronal death. Neurobiology of disease. 2013;54:445–455. doi: 10.1016/j.nbd.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Jan A, Karasinska JM, Kang MH, de Haan W, Ruddle P, Kaur A, et al. Direct intracerebral delivery of a mir-33 antisense oligonucleotide into mouse brain increases brain abca1 expression. [corrected] Neuroscience letters. 2015;598:66–72. doi: 10.1016/j.neulet.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Ballerini P, Ciccarelli R, Di Iorio P, Buccella S, D’Alimonte I, Giuliani P, et al. Guanosine effect on cholesterol efflux and apolipoprotein e expression in astrocytes. Purinergic signalling. 2006;2:637–649. doi: 10.1007/s11302-006-9011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandoval-Hernandez AG, Buitrago L, Moreno H, Cardona-Gomez GP, Arboleda G. Role of liver x receptor in ad pathophysiology. PLoS ONE. 2015;10:e0145467. doi: 10.1371/journal.pone.0145467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui X, Chopp M, Zacharek A, Karasinska JM, Cui Y, Ning R, et al. Deficiency of brain atp-binding cassette transporter a-1 exacerbates blood-brain barrier and white matter damage after stroke. Stroke. 2015;46:827–834. doi: 10.1161/STROKEAHA.114.007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crisby M, Bronge L, Wahlund LO. Low levels of high density lipoprotein increase the severity of cerebral white matter changes: Implications for prevention and treatment of cerebrovascular diseases. Curr Alzheimer Res. 2010;7:534–539. doi: 10.2174/156720510792231694. [DOI] [PubMed] [Google Scholar]
- 32.Miao B, Zondlo S, Gibbs S, Cromley D, Hosagrahara VP, Kirchgessner TG, et al. Raising hdl cholesterol without inducing hepatic steatosis and hypertriglyceridemia by a selective lxr modulator. J Lipid Res. 2004;45:1410–1417. doi: 10.1194/jlr.M300450-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Brunham LR, Singaraja RR, Duong M, Timmins JM, Fievet C, Bissada N, et al. Tissue-specific roles of abca1 influence susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:548–554. doi: 10.1161/ATVBAHA.108.182303. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi H, Campenot RB, Vance DE, Vance JE. Glial lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J Biol Chem. 2004;279:14009–14015. doi: 10.1074/jbc.M313828200. [DOI] [PubMed] [Google Scholar]
- 35.Fukumoto H, Deng A, Irizarry MC, Fitzgerald ML, Rebeck GW. Induction of the cholesterol transporter abca1 in central nervous system cells by liver x receptor agonists increases secreted abeta levels. J Biol Chem. 2002;277:48508–48513. doi: 10.1074/jbc.M209085200. [DOI] [PubMed] [Google Scholar]
- 36.Whitney KD, Watson MA, Collins JL, Benson WG, Stone TM, Numerick MJ, et al. Regulation of cholesterol homeostasis by the liver x receptors in the central nervous system. Molecular endocrinology. 2002;16:1378–1385. doi: 10.1210/mend.16.6.0835. [DOI] [PubMed] [Google Scholar]
- 37.Kim E, Woo MS, Qin L, Ma T, Beltran CD, Bao Y, et al. Daidzein augments cholesterol homeostasis via apoe to promote functional recovery in chronic stroke. J Neurosci. 2015;35:15113–15126. doi: 10.1523/JNEUROSCI.2890-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunham LR, Kruit JK, Pape TD, Parks JS, Kuipers F, Hayden MR. Tissue-specific induction of intestinal abca1 expression with a liver x receptor agonist raises plasma hdl cholesterol levels. Circulation research. 2006;99:672–674. doi: 10.1161/01.RES.0000244014.19589.8e. [DOI] [PubMed] [Google Scholar]
- 39.Zhang XQ, Even-Or O, Xu X, van Rosmalen M, Lim L, Gadde S, et al. Nanoparticles containing a liver x receptor agonist inhibit inflammation and atherosclerosis. Adv Healthc Mater. 2015;4:228–236. doi: 10.1002/adhm.201400337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotokorpi P, Ellis E, Parini P, Nilsson LM, Strom S, Steffensen KR, et al. Physiological differences between human and rat primary hepatocytes in response to liver x receptor activation by 3-[3-[n-(2-chloro-3-trifluoromethylbenzyl)-(2,2-diphenylethyl)amino]propyloxy]phe nylacetic acid hydrochloride (gw3965) Mol Pharmacol. 2007;72:947–955. doi: 10.1124/mol.107.037358. [DOI] [PubMed] [Google Scholar]
- 41.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, et al. Role of lxrs in control of lipogenesis. Genes & development. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, et al. Stimulation of lipogenesis by pharmacological activation of the liver x receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 43.Grefhorst A, van Dijk TH, Hammer A, van der Sluijs FH, Havinga R, Havekes LM, et al. Differential effects of pharmacological liver x receptor activation on hepatic and peripheral insulin sensitivity in lean and ob/ob mice. American journal of physiology. Endocrinology and metabolism. 2005;289:E829–838. doi: 10.1152/ajpendo.00165.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


