Abstract
Interleukin-10 (IL-10) is essential to maintain intestinal homeostasis. CD4+ T regulatory type 1 (TR1) cells produce large amounts of this cytokine and being therefore currently examined in clinical trials as T-cell therapy in patients with inflammatory bowel disease (IBD). However, factors and molecular signals sustaining TR1 cell regulatory activity still need to be identified in order to optimize the efficiency and to ensure the safety of these trials. We investigated the role of IL-10 signaling in mature TR1 cells in vivo.
Double IL-10eGFP Foxp3mRFP reporter mice and transgenic mice with impairment in IL-10 receptor signaling were used to test the activity of TR1 cells in a murine IBD model, a model that resembles the trials performed in humans. The molecular signaling was elucidated in vitro. Finally, we used human TR1 cells, currently employed for cell therapy, to confirm our results.
We found that murine TR1 cells expressed functional IL-10 receptor α. TR1 cells with impaired IL-10 receptor signaling lost their regulatory activity in vivo. TR1 cells required IL-10 receptor signaling in order to activate p38 MAP kinase, thereby sustaining IL-10 production, which ultimately mediated their suppressive activity. Finally, we confirmed these data using human TR1 cells.
In conclusion TR1 cell regulatory activity is dependent on IL-10 receptor signaling. These data suggest that in order to optimize TR1 cell-based therapy, IL-10 receptor expression has to be taken into consideration.
Keywords: IBD, T cell therapy, IL-10, immune regulation
Introduction
Interleukin-10 (IL-10) plays an essential role in controlling inflammation. IL-10-deficient mice spontaneously develop inflammatory disease and, patients with mutations in either IL-10 or IL-10R suffer from severe early onset colitis (1, 2). Thus IL-10 plays a fundamental role in maintaining intestinal immune homeostasis (3, 4). Resetting the physiological concentration of IL-10 in the intestine is considered the ‘Holy Grail’ for all therapies, which aim to cure inflammatory mediated diseases, especially in the intestine.
CD4+ T regulatory type 1 cells (TR1) secrete high levels of IL-10 and are known to play a major role in maintaining immune tolerance through their strong immune-regulatory activity (5–10). Due to the strong immune-regulatory potential of TR1 cells, which has been proved in several pre-clinical mouse models (6–10), they are a major target of new approaches in the field of T cell-based therapy (11–13).
TR1 cells are characterized by co-expression of CD49b and LAG-3, high secretion of IL-10 and the lack of Foxp3 expression (14, 15). The expression of Granzyme B and TGF-β1 also contributes to the suppressive capacity of TR1 cells and the expression of CTLA-4 allows a cell-cell contact dependent suppression of T cells by TR1 cells (16, 17). Chronic stimulation of CD4+ T-helper cells with IL-10 is sufficient to induce in vitro functional mouse and human TR1 cells. However IL-10 is dispensable to induce mouse TR1 cells in vivo (18). Indeed, interleukin-27 (IL-27) was found to promote the differentiation of TR1 cells in vitro and in vivo. Consistent with this observation, mice deficient in IL-27R (WSX1−/−) have a strong reduction of TR1 cells upon infection (19). IL-27 induces the differentiation of mouse TR1 like cells by activating STAT1 and STAT3, which promotes LAG-3 expression and IL-10 production through the transcriptional factors Egr-2 and Blimp1 (encoded by Prdm1) (20). Other factors, such as Ahr, c-Maf, Nfil3 and ROR-α play a key role in the differentiation of TR1 cells (20–22). Although several transcriptional factors have been shown to drive the differentiation of TR1 cells, whether this requires one “master regulator” factor or a finely tuned network comprising several factors, still remains unknown.
On the basis of global transcriptomic analysis, it has been shown that TR1 cells are distinct, not unlike how the other T-helper cell subsets are from each other (23, 24). Recently, their peculiar metabolic activity and their specific activity during the night/day cycle have further highlighted the distinction of TR1 cells from TH17 and Foxp3+ Treg cells (25). As a consequence of all these differences, the protective and anti-inflammatory function of TR1 cells is what ultimately distinguishes them from other subsets such as TH1, TH2 and TH17 cells (17, 26, 27).
Several pre-clinical studies and mouse models of immune mediated disease have sustained the translation of TR1 cell-based therapy into clinic (8, 9, 28, 29). Human TR1 cells are being tested in clinical trials to treat autoimmune diseases, such as inflammatory bowel disease (IBD) or to limit donor vs. host-reactivity (GvHD) after hematopoietic stem cell transplantation (HSCT) (11, 13). Treatment of Crohn’s patients with antigen-specific TR1 cells showed good tolerability and high potential. Also the proof-of-concept trial with HSCT patients showed a positive outcome for patients who were treated with IL-10-anergized T cells containing TR1 cells (11, 13, 30). A new clinical trial for kidney transplanted patients is planned (31).
Although TR1 cell-based therapies are ongoing, it is unknown which molecular signal maintains TR1 cell regulatory activity: a basic biological aspect which is fundamental to design successful therapies. IL-10, the signature cytokine produced by TR1 cells, has been shown to regulate Foxp3+ Treg cells (Tregs) by maintaining their regulatory function (32). However, it is still unclear whether TR1 cells also express IL-10R and whether IL-10 signaling is responsible for maintaining the functional stability of these cells.
The best-studied IL-10 signaling pathway is the activation of JAK1 and STAT3(33). PI3 kinases as well as p38 MAP kinase pathways can also act downstream of IL-10 by binding to the activated IL-10 receptor complex (34, 35). Whether mature TR1 cells respond to IL-10, and if so, through which signaling pathway, is currently unknown.
The aim of our study was to analyze the role of IL-10 signaling for the anti-inflammatory activity of mature mouse and human TR1 cells. Our data show that IL-10 plays a key role in sustaining the function of murine and human TR1 cells. Mouse TR1 cells with impaired IL-10 signaling are unstable and lose their regulatory activity in vivo in a T cell transfer mediated IBD model (13). Finally, in vitro induced human TR1 cells, which can potentially be employed in a cell therapy approach, do also require IL-10 receptor signaling to maintain IL-10 production.
Materials and Methods
Mice
C57BL/6, C57BL/6 Rag1−/−, and C57BL/6 Rag1−/− CD45.1+ were obtained from the Jackson Laboratory. CD4-DNIL-10R transgenic mice, Foxp3RFP, IL-17AeGFP, and IL-10eGFP reporter mice are described elsewhere (26, 36–39). Age and sex matched littermates between 8–16 weeks of age were used.
Flow cytometry
Anti-CD4, anti-CD62L, anti-CD44, anti-CD45.1, anti-CD45.2, anti-CD45RB, anti-TCR-β, anti-IL-10Rα (clone: 1B1.3a, PE) and isotype control (rat IgG1,K, PE) were purchased from BioLegend. Anti-STAT3 (pY705) and anti-pp38 MAPK were purchased from BD Biosciences. To identify dead cells, 7-AAD (Biolegend) staining was performed.
Anti-human anti-CD4, anti-CD45RA and anti-CD49b (clone: P1E6-C5) were purchased from BioLegend. Anti-LAG-3 was purchased from eBioscience (clone: 3DS223H). The staining for LAG-3 and CD49b was performed at 37°C for 30 min.
For intracellular pSTAT3 and pp38 MAPK staining, cells were fixed with PhosFlow Lyse/Fix Buffer (BD Bioscience) for 10 min at 37°C and permeabilized with Perm Buffer III (BD Bioscience) for 30 min on ice. The cells were stained for pSTAT3 or pp38 MAPK and extracellular markers for 1 hour at room temperature before they were acquired on a LSRII flow cytometer (BD Bioscience).
In vitro TR1 cells and TH17 cells differentiation
CD4+ T cells were enriched from splenocytes of IL-10eGFP Foxp3RFP double reporter mice with CD4-microbeads using MACS (Miltenyi Biotec). For naïve T cell enrichment, CD44+ and CD25+ T cells were depleted using biotinylated antibodies and Streptavidin beads (Miltenyi Biotec). TR1 cell differentiation: naive T cells were cultured for 5 days at a density of 106 cells/ml with plate-bound anti-CD3 (2 µg/ml) and soluble anti-CD28 (2 µg/ml) in medium (Click’s medium supplemented with 10% FCS, l-glutamine, penicillin, streptomycin and β-Mercaptoethanol) under TR1-inducing conditions (0.5 ng/ml TGF-β1, 30 ng/ml IL-27). IL-10 (eGFP) and Foxp3 (mRFP) expression were determined by flow cytometry. TH17 cell differentiation: naïve T cells were cultured for 5 days at a density of 106 cells/ml with soluble anti-CD3 (3 µg/ml) and soluble anti-CD28 (1 µg/ml) in the presence of irradiated APCs (ratio 1:4) in medium (Click’s medium supplemented with 10% FCS, l-glutamine, penicillin, streptomycin and β-Mercaptoethanol) under TH17 polarizing conditions (0.5 ng/ml TGF-β1, 10 ng/ml IL-6, 20 ng/ml IL-23, 10 ng/ml IL-1β). IL-17A (eGFP) expression was determined by flow cytometry.
In vitro suppression assay
Responder T cells were isolated from C57Bl/6 mice and labelled with 5 µM violet dye. The cells were activated in the presence of irradiated APCs and 1.5 µg/ml anti-CD3 antibody and cultured either alone or in the presence of IL-10RαWT or IL-10RαImpaired TR1 cells at a 1:2 (TR1:Responder) ratio. After 72 hours the proliferation of the responder T cells was measured via flow cytometry.
In vitro kinase inhibition
SB203580, PD98059, JNK inhibitor II or STAT3 inhibitor VI in DMSO were added to the culture medium in the indicated concentrations every 24 hours (Calbiochem, Darmstadt, Germany). DMSO was added to control cultures at equivalent concentrations.
In vitro IL-10 receptor blocking
In vitro differentiated wild type TR1 cells were re-stimulated (CD3/CD28 antibodies) in the presence of 50 µg/ml IL-10R antibody (clone: 1B1) or isotype control antibody (rat IgG1,K).
In vivo T cell stimulation and Intestinal lymphocyte isolation
Mice were injected with anti-CD3 (clone 2C11, 15µg,) i.p. two times every other day, and sacrificed 4 hours or 48 hours after the second injection. As controls, mice were injected with isotype-matched antibody or PBS. We collected intraepithelial lymphocytes (IEL) after the dissection of the peyer’s patches by incubating the small intestine in the presence of 5mM EDTA at 37°C for 30 min. Lamina propria lymphocytes (LPL) were collected by digesting gut tissue with collagenase IV (100 U, Sigma) at 37°C for 45 min. The cells were further separated with a Percoll gradient (GE Healthcare).
Adoptive Transfer models
Splenocytes were collected from 8 to 12 week old IL-17AeGFP reporter mice (CD45.1/2) and CD4+ T cells were enriched by MACS system (Miltenyi Biotec). The CD4+ T cells were further sorted to collect CD45RBhi Foxp3RFP− cells using FACS Aria II. 1.5 × 105 CD45RBhi cells were i.p. injected either alone or together with in vitro differentiated 1.5 × 105 wild type or IL-10RImpaired TR1 cells into Rag1−/− mice. For generating highly pathogenic TH17 cells 4 × 105 CD45RBhi cells were injected i.p. into Rag1−/− mice (CD45.1). The mice were weighed once a week to monitor IBD development. After establishment of colitis as determined by endoscopy, mice were sacrificed and lymphocytes were isolated from the colon and mesenteric lymph nodes. The cells were further FACS-sorted to purify IL-17AeGFP+ T cells (eTH17 cells). (e)TH17 cells (3 × 104) were transferred, and isolated WT or Tg TR1 cells (3 × 104) from the small intestine of anti-CD3 treated IL-10eGFP reporter mice were transferred either alone or with (e)TH17 cells into CD45.1 Rag1−/− mice. Colitis development was monitored using endoscopy.
Endoscopic procedure
We performed colonoscopy in a blinded fashion for colitis scoring using the Coloview system (Karl Storz, Germany) (40). Colitis scoring was based on the granularity of the mucosal surface, stool consistency, vascular pattern, translucency of the colon and number of fibrin visible (0–3 points for each).
Histopathology procedure
Colons were fixed in Bouin's fixative solution or 4% PFA in PBS and embedded in paraffin. HE stained sections were evaluated by a semi quantitative criterion-based method (score 0–5) as described before (41).
Cytokine assay
We stimulated 4 × 104 mouse T cells/ml for 60 hours with plate-bound CD3 antibodies (10 µg/ml) and soluble CD28 antibodies (10 µg/ml) in medium (Click’s medium supplemented with 10% FCS, l-glutamine, penicillin, streptomycin and β-Mercaptoethanol). Cytokines were quantified by Cytometric Bead Array (mouse TH1/TH2/TH17 Cytokine Kit, BD Bioscience) following the manufacturer’s instructions.
We stimulated 3 × 104 human T cells/200 µl for 96 hours with CD3/CD28 beads (Dynabeads Human T-Activator CD3/CD28 for T Cell Expansion and Activation, Life technologies) in a 1:1 ratio in medium (Click’s medium supplemented with 10% FCS, l-glutamine, penicillin, streptomycin and β-Mercaptoethanol). As indicated, 50 µg/ml human IL-10 Rα Antibody (R&D Systems) were added to the cell culture. Cytokines were quantified by Legendplex Assay (Human T helper Cytokine Panel, BioLegend).
Relative gene expression analysis
RNA from cells was isolated with TRIzol LS reagent (Life Technologies) in accordance with the manufacturer’s protocol. RNA was subjected to reverse transcription with Superscript II (Invitrogen) with oligo (dT) primer in accordance with the manufacturer’s protocol. cDNA was semi-quantified using commercially available primer/probe sets (Applied Biosystems) and analyzed with the ΔCt (change in cycle threshold) method. All results were normalized to Hprt quantified in parallel amplification reactions during each PCR quantification.
Western Blot
To analyze STAT3 activation, total cell lysates of indicated cell populations were separated in a 10% Sodium Dodecyl Sulphate Polyacrylamide Gel electrophoresis (SDS-PAGE) assay, transferred to PVDF membranes (Merck Millipore), probed with anti-phospho-Stat3 (Tyr705) or STAT3 antibody (Cell signaling), followed by incubation with the appropriate HRP-conjugated secondary antibodies (Dako) and were visualized with the enhanced chemiluminescence substrate (Merck Millipore).
Immunofluorescence
In brief, cells were fixed for 10 min in 4% PFA at RT. Cells were washed with PBS and incubated in PBS-Triton 0.3% for 5 min. After washing they were incubated for 60 min in blocking buffer. Samples were stained overnight with biotinylated IL-10Rα antibody (primary rat anti-mouse antibody, 1:100, clone: 1B1.3a, BioLegend) at 4 °C. After washing secondary antibody (Alexa Fluor 568 goat anti-rat IgG, Invitrogen) staining was performed (1 hour, RT) followed by 5 min staining with Hoechst 33258 (1:5000). For isotype control, the primary antibody was omitted.
Isolation of circulating human TR1 cells
PBMC were isolated from buffy coats of healthy donors using the Biocoll Separating Solution (Biochrome AG). CD4+ T cells were enriched with the Human CD4+ T Cell Enrichment Kit in accordance with the manufacture’s protocol (Stemcell Technologies). The CD4+ T cells were further sorted to collect CD4+ CD45RAlow LAG-3+ CD49b+ TR1 cells using FACS Aria II.
Generation of human TR1 cells in vitro
TR1 cells were generated in vitro as previously described (42). Briefly, naïve CD4+ cells were cultured for 10 days with tolerogenic DC-10 in an allogeneic setting, in the presence of exogenous rhIL-10 to produce a TR1-enriched product (TR1 cells) or with mDC in the absence of exogenous rhIL-10 as control (non-TR1 cells).
ELISPOT
Dual IFNγ/IL-10 ELISPOT (Diaclone, Besancon, France) was performed according to manufacturer’s instructions, with slight modifications. Briefly, the filter-bottom plate was wetted then coated with both anti-IFNγ and anti-IL-10 capture. TR1 and non-TR1 cells were plated after thawing and overnight resting in serum-free X-VIVO15 (tissue-culture grade, Lonza). Stimulation was performed with aCD3 (2µg/ml)/TPA (20ng/ml) or with allogeneic mDC in the presence of rat anti-human IL-10R blocking antibody (50µg/ml) or its isotype control (BD Biosciences). After a 48-hour incubation, detection antibodies were added then visualization was performed with AEC and Vector Blue (Vector Labs). The plate was acquired on an A.EL.VIS 4-Plate ELISPOT Reader (A.EL.VIS GmbH). Analysis was performed using ImageJ (version 1.48) to quantify IFNγ-producing cells (red spots) and IL-10-producing cells (blue spots).
Microarray analysis
Gene expression analysis
Affymetrix Mouse Gene 1.0ST Array data were analyzed using R and the Bioconductor package "affy". Samples were normalized by RMA with standard parameters. To define genes functionally related to mouse TR1 signature genes, genes interacting with their human orthologs with an interaction probability of at least 80% were extracted from the Immunet database (43) downloaded on May 11, 2016. Mouse orthologs were then found for these functionally related genes. Data are available at NCBI GEO (https://www.ncbi.nlm.nih.gov/geo/ Accession number: GSE89080).
Gene Ontology analysis
Gene Ontology (GO) term analysis was performed using GOrilla (44). GO terms enriched for TR1 signature genes (p-Value threshold 0.001) were exported. Any genes >2-fold differentially expressed also mapping to these terms were retrieved using Biomart (http:\\www.biomart.org). Enrichment of these genes in the GO term category was calculated as described in (44). Log2-values of enrichment were calculated to better visualize the spread of the data.
Statistics
The Mann–Whitney U test, paired t test or one-way ANOVA (post-test Tukey) were used to calculate statistical significance. A p-value < 0.05 was considered significant. Statistical calculations were performed using Prism program 5.0 (GraphPad Software, Inc.)
Study approval
All experiments involving animals were carried out in accordance with the Institutional Animal Care and Use Committee of Yale University or in accordance with the Institutional Review Board ‘Behörde für Soziales, Familie, Gesundheit und Verbraucherschutz’ (Hamburg, Germany).
Results
Intestinal TR1 cells express IL-10Rα and can respond to IL-10
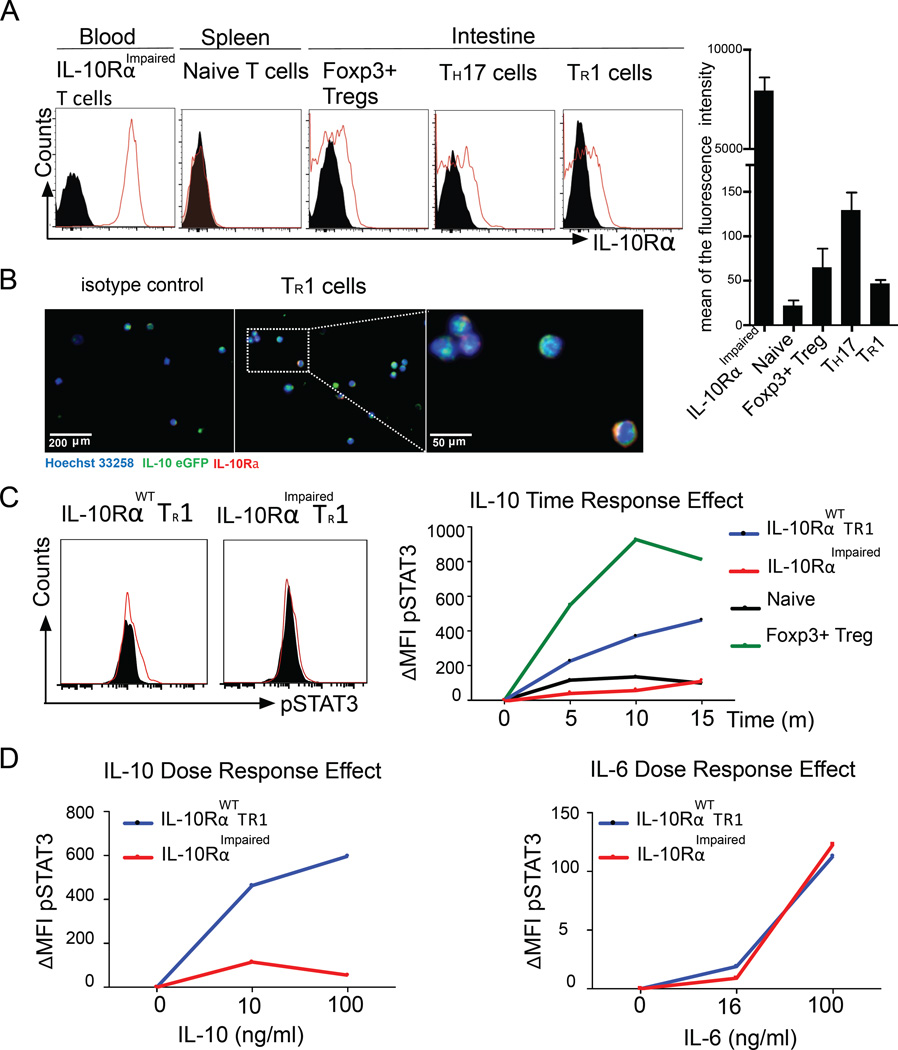
We used Foxp3RFP IL-10eGFP double reporter mice and a model of anti-CD3-specific antibody mediated transient intestinal inflammation to test whether a pure population of intestinal TR1 cells can respond to IL-10 (36, 37). This murine model has already been largely validated by others and by us as being capable of inducing CD4+ Foxp3RFP− IL-10eGFP+ CD49b+ LAG-3+ bona fide TR1 cells in the small intestine (26). First, we found that ex vivo isolated TR1 cells express IL-10Rα at a comparable level to intestinal Foxp3+ pTreg cells and levels about 6 times higher than splenic naïve T cells (Fig. 1A and 1B).
Fig. 1. In vivo differentiated TR1 cells can respond to IL-10.
(A) IL-10Rα expression and MFI of indicated cell populations. Black area represents the isotype control. Four independent experiments were performed. (B) Immunofluorescence staining (two independent experiments) of IL-10Rα on wild type TR1 cells. (C–D) ΔMFI (compared to unstimulated cells) of pSTAT3 levels. (C) Naïve T cells, Foxp3+ T cells and IL-10RαWT or IL-10RαImpaired TR1 cells were stimulated with IL-10 (100 ng/ml) for indicated time points. Black area represents the unstimulated control. (D) IL-10RαWT or IL-10RαImpaired TR1 cells were stimulated for 20 min with the indicated concentrations of IL-10 or IL-6. Data are representative of three independent experiments.
We then wanted to test whether IL-10Rα was functional on TR1 cells. We therefore checked the level of phosphorylated STAT3 (45), the main downstream molecule of the IL-10R signaling, in intestinal TR1 cells upon in vitro stimulation with IL-10. (45).
By testing STAT3 phosphorylation at different times and in response to different doses of IL-10 we found that intestinal TR1 cells respond to IL-10 (Fig.1C and 1D). As controls, splenic naïve T cells, in which IL-10R was very low, did not show an elevated level of pSTAT3 after stimulation with IL-10 (Fig. 1C), whereas Foxp3+ Treg cells responded to IL-10 with a distinct increase of pSTAT3 level (Fig. 1C). To further strengthen our data, we used CD4-DN-IL10R transgenic (IL-10RImpaired) TR1 cells, which overexpress a dominant-negative IL10Rα chain and therefore have largely impaired IL-10 signaling (37). We detected that these IL-10RImpaired TR1 cells only slightly up-regulated pSTAT3 upon IL-10 stimulation (Fig.1C and 1D). STAT3 can also be phosphorylated by pro-inflammatory cytokine IL-6. As a control, to confirm that STAT3 activation in CD4-DN-IL10R transgenic TR1 cells is not impaired per se, we stimulated wild type (WT) and IL-10RImpaired TR1 cells with IL-6. In contrast to what we observed with IL-10 stimulation, the activation of pSTAT3 by IL-6 was normal in IL-10RImpaired TR1 cells (Fig. 1D).
Finally, we wanted to test whether the capacity of TR1 cells to respond to IL-10 is restricted to the mouse model used or if it is a more general feature of TR1 cells. We therefore used the standard protocol (i.e. IL-27+TGF-β1) (21, 46) to induce bona fide TR1 cells in vitro. Initially, we confirmed IL-10Rα expression on in vitro differentiated wild type and IL-10RImpaired TR1 cells using Immunofluorescence (Fig. S1A). Next, we FACS-sorted a pure population of differentiated TR1 cells and stimulated them at different times and with different doses of IL-10 and measured STAT3 phosphorylation via FACS and western blot analysis. As for the in vivo induced intestinal TR1 cells, we observed that also mature TR1 cells induced in vitro respond to IL-10 by activating STAT3 (Fig. S1B–S1E).
Altogether these data show that in vivo and in vitro differentiated murine TR1 cells express functional IL-10Rα and are, in principle, able to respond to IL-10 stimulation.
No phenotypically and early functional defect among wild type and DN-IL10R transgenic TR1 cells
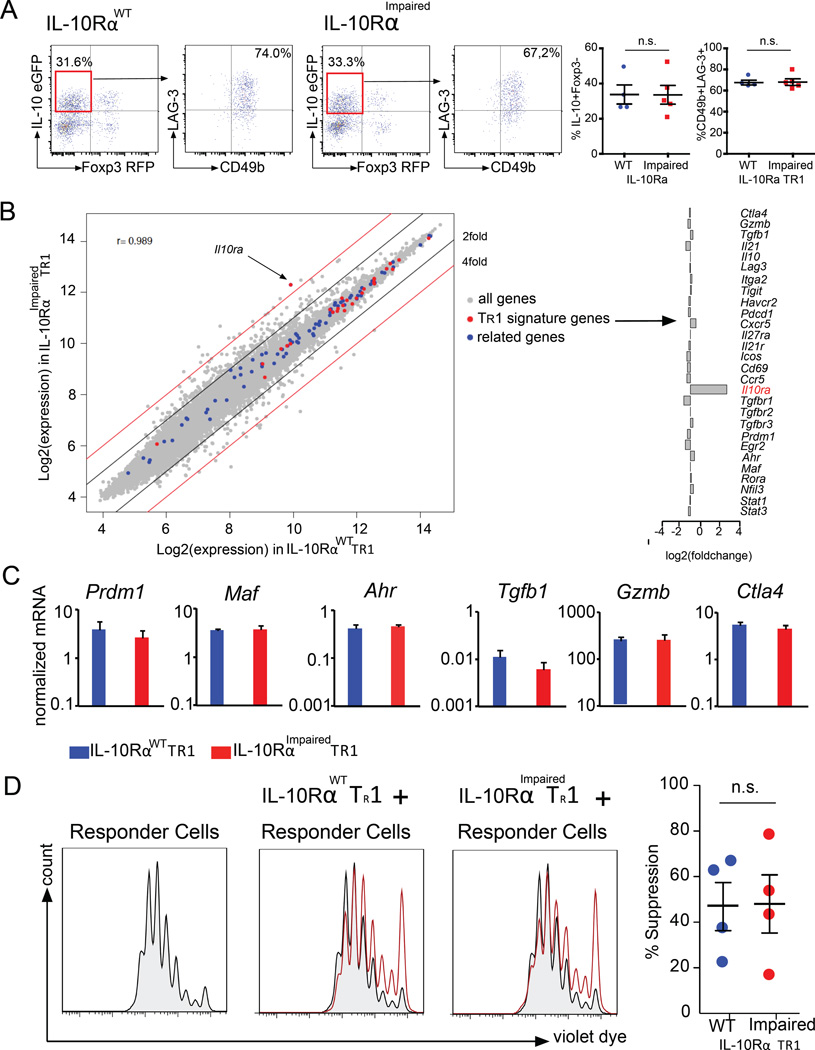
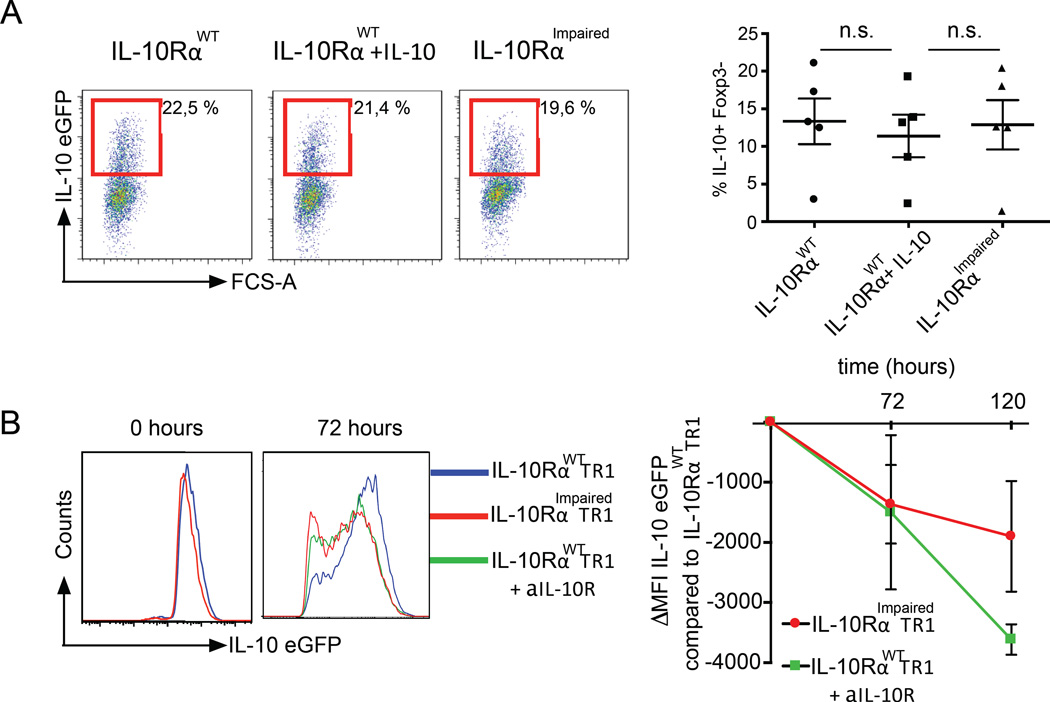
The above results show that TR1 cells express functional IL-10R. However, what exactly the role of this receptor is in TR1 cell biology remained elusive. To investigate this, wild type (WT) and CD4-DN-IL10R transgenic (IL-10RImpaired) mice were treated with anti-CD3 mAb. Shortly after the second injection, the induction of intestinal TR1 cells was evaluated without any in vitro manipulation due to the use of the Foxp3RFP IL-10eGFP double reporters. Surprisingly, we did not observe any changes in the frequency and number of intestinal TR1 cells between WT and CD4-DN-IL10R transgenic mice upon treatment (Fig. 2A).
Fig. 2. IL-10 signaling in T cells is not essential for the differentiation of TR1 cells.
IL-10RαWT or IL-10RαImpaired Foxp3RFP IL-10eGFP double reporter mice were treated with anti-CD3. (A) Frequency of TR1 cells in the small intestine and CD49b and LAG-3 expression by TR1 cells are shown (IL-10RαWT n=4; IL-10RαImpaired n=5) (B) Gene expression analysis, comparing IL-10RαWT TR1 cells and IL-10RαImpaired TR1 cells. Fold change expression of TR1 cell signature genes of IL-10RαImpaired TR1 cells compared to IL-10RαWT TR1 cells. (C) Maf, Ahr, Prdm1, Tgfb1, Ctla4 and Gzmb mRNA expression normalized to Hprt (data pooled of three independent experiments). (D) TR1-mediated in vitro suppression. Data are representative of five independent experiments.
We further analyzed the surface markers, which typically characterize TR1 cells. The FACS analysis on WT and IL-10RImpaired TR1 cells showed similar levels of CD49b and LAG-3 expression (Fig. 2A). To further broaden our data, we performed a microarray analysis of IL-10RWT TR1 and IL-10RImpaired TR1 cells. The gene expression profiles between IL-10RWT TR1 and IL-10RImpaired TR1 cells were largely similar (Pearson correlation coefficient r=0.989). For all transcriptional factors, extracellular receptor and cytokines known to be crucial for TR1 cell differentiation and function (TR1 signature genes) such as Prdm1, Maf and Ahr, as well as Gzmb, Tgfb1 and Ctla4 mRNA expression was unaffected in WT TR1 cells compared to IL-10RImpaired TR1 cells (Fig. 2B). The similar expression of some of these genes was also confirmed by conventional mRNA analysis (Fig. 2C). We further expanded our analysis to those genes that were found to be functionally related to the TR1 signature genes (related genes) based on the ImmuNet Data Base (43): none of those genes were more than two fold differentially expressed among the two genotypes. Although most genes were similarly expressed, we found 300 genes higher than two fold differentially expressed among IL-10RImpaired TR1 cells compared to wild type TR1 cells (NCBI GEO https://www.ncbi.nlm.nih.gov/geo/ Accession number: GSE89080). We therefore asked whether any of these genes are functionally related to the genes known to be involved in TR1 cell differentiation. A Gene Ontology (GO) analysis revealed 379 GO terms enriched for TR1 signature genes. Within these GO categories, enrichment of TR1 signature genes was consistently higher than enrichment of the genes more than two fold differentially expressed between IL-10RImpaired TR1 cells and wild type TR1 cells (Fig. S2). Based on the current knowledge about TR1 cells, we could not reveal any significant defect of IL-10RImpaired TR1 cells. However we cannot exclude that among the differentially expressed genes are some genes, which could affect TR1 cells. Thus we tested the functional activity of WT and IL-10RImpaired TR1 cells isolated shortly after the in vivo induction using anti-CD3 antibody treatment to further exclude a defect of IL-10RImpaired TR1 cells. The in vitro suppressive assay showed that both WT and IL-10RImpaired TR1 cells have similar suppressive capacities (Fig. 2D). These data indicate that shortly after TR1 cell induction, IL-10RImpaired TR1 cells do not show any obvious defect compared to WT TR1 cells, suggesting that IL-10 signaling is not essential for TR1 cell differentiation.
IL-10 signaling in TR1 cells maintains their suppressive function in vivo in a murine IBD model
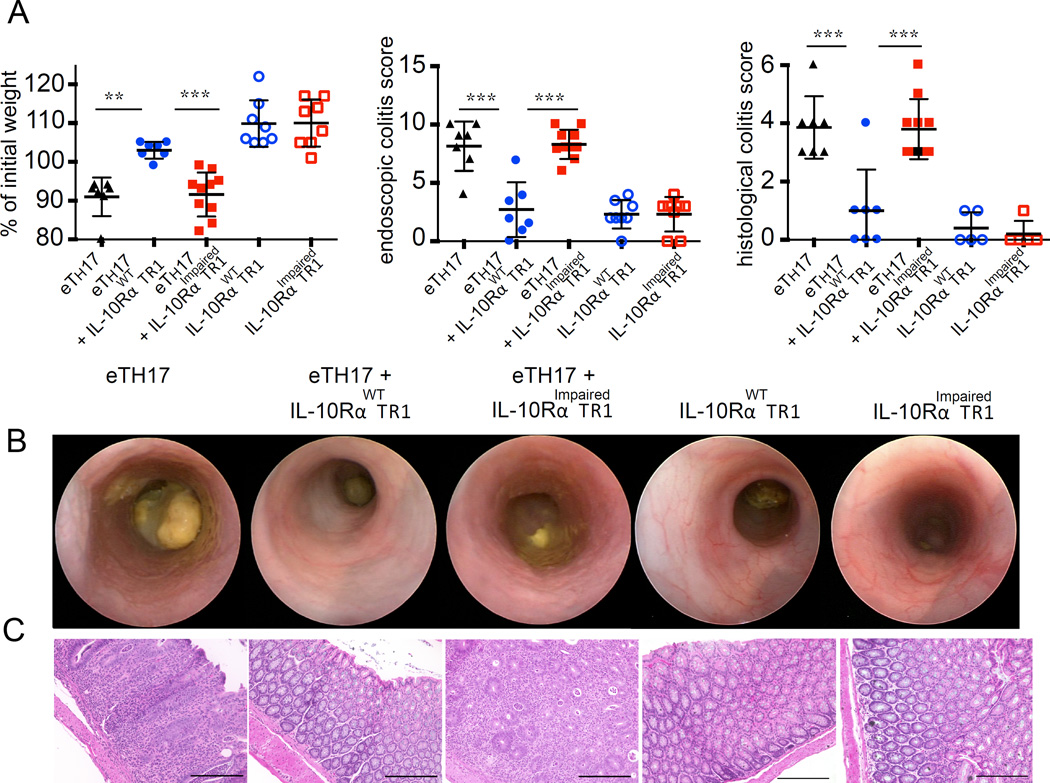
Although IL-10R signaling is dispensable for acquiring a TR1 like phenotype and in vitro suppressive function, we wondered whether IL-10R signaling influences the long-term functional stability of TR1 cells, as is the case with Foxp3+ Treg cells (32, 47). We tested the functionality of IL-10RImpaired TR1 cells and wild type TR1 in the CD45RBhi T cells transfer colitis model. Transfer of CD4+Foxp3−CD45RBhi cells caused severe disease that could be prevented by the co-transfer of in vitro differentiated wild type TR1 cells. IL-10RImpaired TR1 cells showed a significantly reduced regulatory activity compared to wild type TR1 cells. Interestingly, we observed that IL-10RImpaired TR1 cells still have the ability to reduce the colitis caused by the transfer of CD4+Foxp3−CD45RBhi T cells into Rag1−/− by trend (Fig. S3). Nevertheless, in patients suffering from Crohn’s disease a possible TR1-based cell therapy is required to not only suppress the development of naïve T cells into pathogenic effector T cells, but to control already differentiated cells. Thus, we wanted to test IL-10RImpaired TR1 cells and wild type TR1 in the more challenging model of TH17 cell induced colitis (26). CD4+ IL-17AeGFP+ effector (e)TH17 cells were generated using the CD45RBhi transfer colitis model and isolated from the intestine and mesenteric lymph nodes of diseased mice. The (e)TH17 cells were then co-transferred into Rag1−/− recipients together with wild type TR1 cells or IL-10RImpaired TR1 cells. Transfer of (e)TH17 cells caused severe disease, characterized by histological and endoscopic findings of colitis and weight loss. Transfer of wild type TR1 cells prevented the development of colitis mediated by (e)TH17 cells. In contrast, IL-10RImpaired TR1 cells failed to block colitis mediated by (e)TH17 cells (Fig. 3A). These recipient mice showed similar weight loss, endoscopic and histological colitis score to the experimental group of mice that received (e)TH17 cells alone (Fig. 3B and 3C). Adoptive transfer of IL-10RImpaired TR1 cells alone did not cause colitis, nor did the transfer of wild type TR1 cells alone. The recipient mice showed no signs of weight loss (Fig. 3A) or colitis as evaluated by endoscopic and histological colitis score (Fig. 3B and 3C).
Fig. 3. IL-10 signaling in TR1 cells is essential for their in vivo function.
In vivo induced IL-10RαWT or IL-10RαImpaired TR1 were injected alone or together with in vivo differentiated effector (e)TH17 cells. (A) Mass loss, endoscopic and histological colitis score 5 weeks upon transfer (eTH17 n=7; eTH17+ IL-10RαWT TR1 n=7; eTH17+ IL-10RαImpaired TR1 n=10; IL-10RαWT TR1 n=8; IL-10RαImpaired TR1 n=8; lines indicate mean ± SEM). Representative endoscopic pictures (B) and histology (scale bars, 200 µm) (C) are shown. Results are cumulative of two independent experiments. One-way ANOVA (post-test Tukey) was used to calculate significance (** p < 0.01; *** p<0.001).
In summary, wild type, but not TR1 cells with impaired IL-10 signaling, could suppress colitis development. However, 10RImpaired TR1 did not aggravate disease caused by (e)TH17. Furthermore, transfer of TR1 cells with impaired IL-10 signaling alone did not cause disease, backing up the argument for the safety of TR1 cells in human trials.
IL-10 receptor signaling in TR1 cells maintains IL-10 production
Although IL-10 receptor is dispensable for the development of TR1 cells, this receptor becomes fundamental to maintain the functional activity of TR1 cells in vivo.
However, the reason why TR1 cells require IL-10 receptor signaling to maintain their functional activity remained unresolved. Knowing that other CD4+ T cells, such as TH17, TH2 and Foxp3+ Treg cells are required to respond to their own cytokines to maintain their functional activity, we wondered if TR1 cells are also required to respond to IL-10 to maintain the production of IL-10, which mediates their suppressive activity.
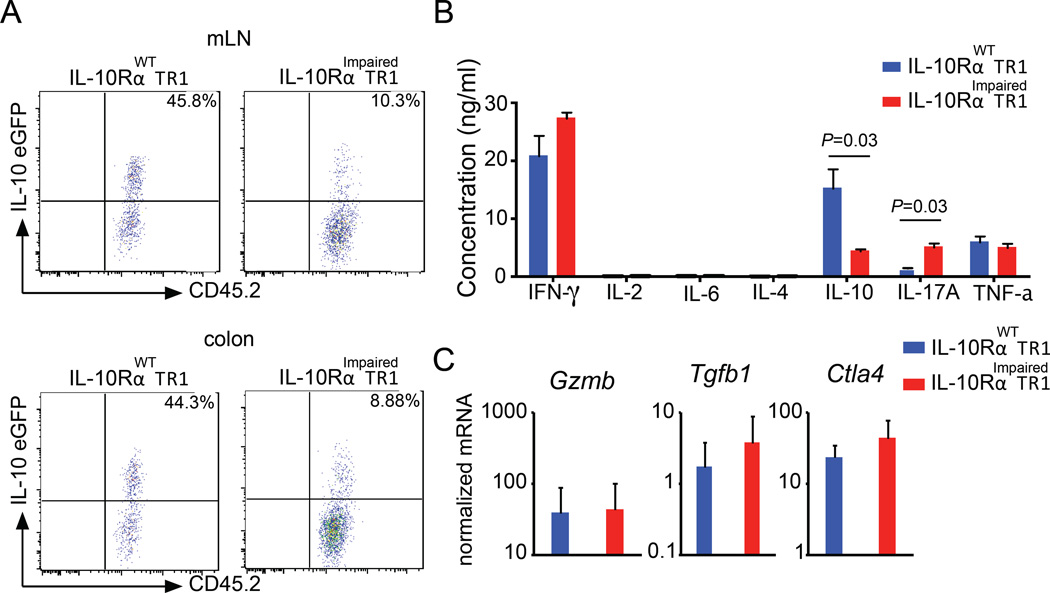
To test this, we adoptively transferred in vivo generated wild type and IL-10RImpaired TR1 cells, which had been isolated from the small intestine of Foxp3RFP IL-10eGFP double reporter mice, into congenic Rag1−/− mice. Five weeks later, transferred cells were isolated from different organs and IL-10eGFP expression was analyzed. Approximately 45% of the previously transferred wild type TR1 cells isolated from the colon or mesenteric lymph nodes still expressed IL-10. However, only 10% of the previously transferred CD4-DN-IL10R transgenic TR1 cells still demonstrated IL-10 production (Fig. 4A). To balance out the bias resulting from the transfer of TR1 cells into immune deficient mice, we continued testing this aspect. However, this time we used an in vitro reductionist approach to investigate whether intestinal TR1 cells require IL-10 receptor signaling to sustain the expression of IL-10 upon in vitro reactivation. In line with the in vivo experiments, in vitro re-stimulated IL-10RImpaired TR1 cells produce significantly lower amounts of IL-10 as compared to wild type TR1 cells. As expected based on our previous results which show that IL-10 suppresses IL-17A but not IFN-γ production by T cells in vivo (26), we observed in vitro that the production of IFN-γ was similar, while IL-10Rimpaired T cells produced more IL-17A (Fig. 4B). Notably, the mRNA level of Tgfb1, Ctla4 and Gzmb, which are involved in the suppressive function of TR1 cells, were not altered in IL-10RImpaired TR1 cells compared to WT TR1 cells (Fig. 4C). Altogether, these data suggest that IL-10 is fundamental to sustain IL-10 production in TR1 cells over time.
Fig. 4. IL-10 signaling in TR1 cells sustains IL-10 expression.
(A) In vivo induced IL-10RαWT or IL-10RαImpaired TR1 cells were injected into Rag1−/− mice. Cells were isolated 5 weeks after transfer. Representative dot plots of IL-10eGFP expression of 4 pooled mice per group are shown. Data are representative of three independent experiments. (B) Cytokine production of TR1 cells was quantified using Cytometric Bead array. Mean ± SEM from three independent experiments are shown. Mann-Whitney U test was used to calculate significance. (C) Tgfb1, Ctla4 and Gzmb mRNA expression normalized to Hprt. Data are cumulative of three independent experiments.
When cells are not synchronized in vivo, it is difficult to clearly dissect early IL-10 receptor related biological effects occurring during the cell differentiation phase from later effects happening when cells have already acquired their phenotype. We had to find a solution when faced with this technical limitation, so once more we took advantage of a better controllable in vitro experimental setting. We therefore differentiated TR1 cells starting with naïve wild type and IL-10RImpaired T cells in the presence of IL-27 and TGF-β1. In line with the in vivo experiment, we did not observe any difference after the first 5 days of TR1 differentiation, even when IL-10 was added to the culture (Fig. 5A). Then, we FACS-sorted the TR1 cells and monitored IL-10 expression over time after in vitro poly-clonal TCR stimulation. To exclude that the observed defect in IL-10 production by IL-10RImpaired TR1 cells was caused by side effects due to the over expression of a dominant negative IL-10Rα, we included an additional control. We re-stimulated wild type TR1 cells either in the presence of a blocking IL-10R antibody or isotype control. Thus, we were able to assess the influence of IL-10 signaling on the IL-10 production of mature TR1 cells starting from the same pool of cells. The resulting data confirmed our in vivo findings that IL-10RImpaired TR1 cells showed a faster decrease of IL-10 expression over time compared to wild type TR1 cells (Fig. 5B). Likewise, TR1 cells re-stimulated in the presence of blocking IL-10R antibody demonstrated a strong decrease of IL-10 expression over time compared to TR1 cells in the presence of an isotype antibody (Fig. 5B).
Fig. 5. IL-10 signaling is dispensable for the in vitro differentiation of TR1 cells with IL-27 and IL-10 sustains IL-10 production in in vitro differentiated TR1 cells.
(A) In vitro differentiation of IL-10RαWT and IL-10RαImpaired TR1 cells. Five independent experiments were performed. (B) IL-10eGFP ΔMFI of re-stimulated IL-10RαImpaired TR1 cells and IL-10RαWT TR1 cells + αIL-10R antibody compared to IL-10RαWT TR1 cells are shown. Results are cumulative of three independent experiments.
IL-10 maintains IL-10 production in TR1 cells via activation of p38 MAP kinase
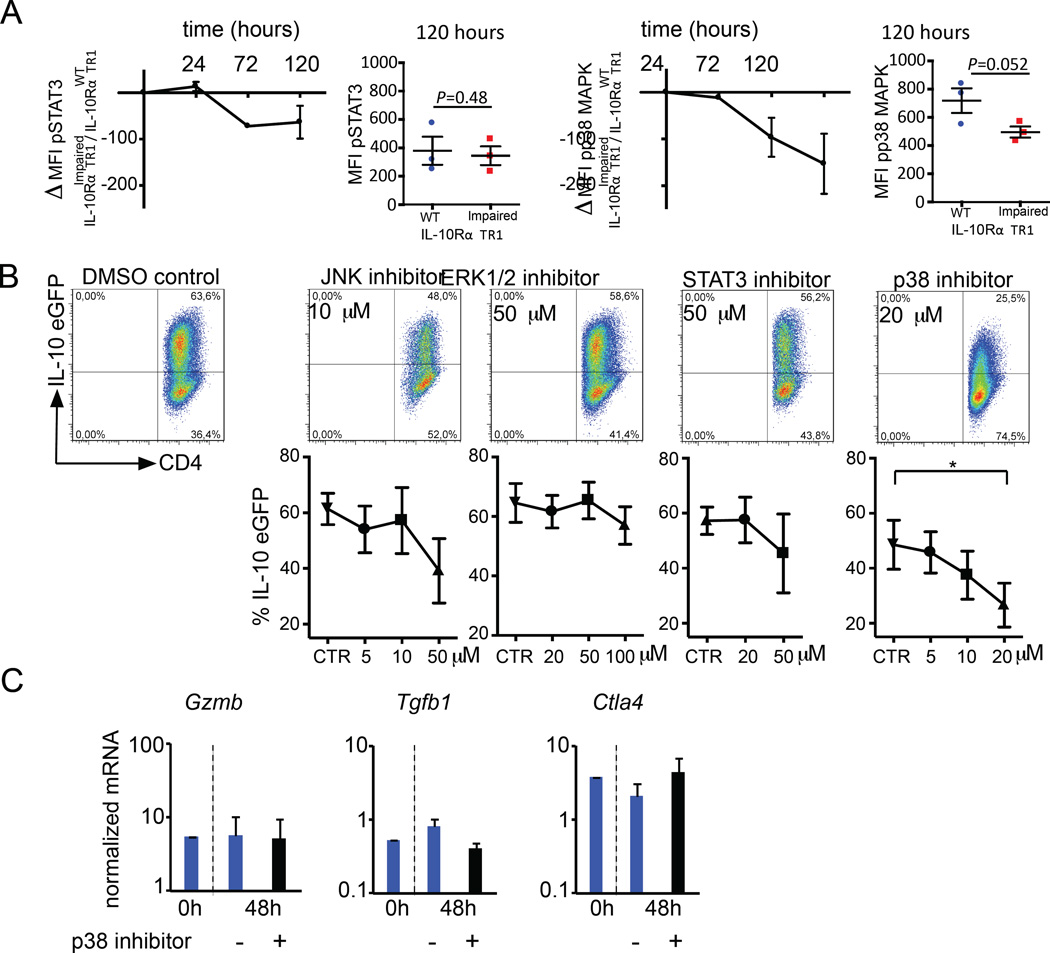
It has previously been shown that IL-10 production by Foxp3+ Treg cells is controlled in a STAT3 dependent manner following IL-10R signaling. Furthermore, induction of IL-10 during in vitro differentiation of TR1 cells with IL-27 also depends on STAT3 activation (48, 49). However, p38 MAP kinase signaling has also been linked to IL-10 production in cell types other than T cells, such as human monocytes and macrophages, and it has also been linked to the regulatory function of iTreg cells (50–53). To address the role of STAT3 and p38 MAP kinase in maintaining IL-10 production in TR1 cells, we compared the phosphorylation status of STAT3 and p38 MAP kinase in a pure population of in vitro induced WT and IL-10RImpaired TR1 cells over time upon re-stimulation.
We could not observe a reduction of pSTAT3 in IL-10RImpaired TR1 cells compared to WT TR1 cells over time (Fig. 6A). In contrast, phosphorylation of p38 MAP kinase was reduced by trend in IL-10RImpaired TR1 cells compared to WT TR1 cells (Fig. 6A). These results suggested a possible correlation between IL-10 production and the activation level of p38 MAP kinase in TR1 cells. To further test the functional role of STAT3 and p38 MAP kinase signaling in TR1 cells, kinase inhibitors were used. As a control, we also tested inhibitors of other major MAPK pathways, ERK1/2 and JNK, since these kinases have also been linked to IL-10 expression in other immune cell types, such as TH1 and TH2 cells or human monocytes and macrophages (54–56). Wild type TR1 cells were differentiated in vitro from CD4+ T cells, isolated from Foxp3RFP IL10eGFP double reporter mice, and re-activated for 48 hours. To test the role of p38 MAP kinase signaling in the stability of TR1 cells, a specific p38 inhibitor (SB203580), which has previously been shown by others and us to selectively block p38 MAP kinase signaling in T cells (57, 58), was added during the re-activation (Fig. 6B). The frequency of IL-10eGFP+ cells was tested. Both STAT3 and JNK inhibitors slightly reduced the frequency of IL-10eGFP+. However, high concentrations of STAT3 or JNK inhibitor also decrease the viability of TR1 cells (data not shown). Inhibition of ERK1/2 activation did not have any effect on the IL-10 production of TR1 cells. Strikingly, inhibition of p38 MAP kinase resulted in a significant and strong reduction in the frequency of IL-10eGFP+ in a dose dependent manner (Fig. 6B). p38 MAP kinase inhibitor neither compromised viability of the cells nor resulted in overgrowth of IL-10eGFP− cells in culture (Fig. S4A). Moreover, in line with our previous in vivo data, in vitro differentiated wild type TR1 cells, re-activated in the presence of p38 MAP kinase inhibitor, did not show significant changes in the expression of Gzmb, Tgfb1 and Ctla4 genes (Fig. 6C).
Fig. 6. IL-10 signaling sustains IL-10 production in TR1 cells via activation of p38 MAPK.
(A) pSTAT3 and pp38 ΔMFI of re-stimulated IL-10RαImpaired TR1 cells compared to IL-10RαWT TR1 cells are shown. Results are cumulative of three independent experiments. Paired t test was used to test significance. (B) Frequency of IL-10eGFP of re-stimulated TR1 cells in the presence of indicated inhibitors are shown (mean ± SEM of three independent experiments). One-way ANOVA (post-test Tukey) was used to calculate significance (* P<0,05). (C) Tgfb1, Ctla4 and Gzmb mRNA expression normalized to Hprt. Results are cumulative of three independent experiments.
STAT3 has been previously reported to be important during the differentiation of TR1 cells in the presence of IL-27 (59, 60). We therefore tested the effect of STAT3 on the differentiation of TR1 cells from naïve T cells. As expected, the addition of STAT3 inhibitor blocked TR1 cell differentiation demonstrating the activity of this compound (Fig S4B). We also tested p38 MAP kinase inhibitors and we observed that this compound also blocks the differentiation of TR1 cells almost completely, whereas inhibition of ERK1/2 or JNK did not affect the differentiation of TR1 cells in vitro. (Figure S4B).
In summary, our findings suggest that IL-27 initiates the differentiation of naïve CD4+ T cells into TR1 cells through STAT3 and p38 MAP kinase activation. In addition, differentiated TR1 cells, which express functional IL-10R, are required to respond to IL-10 in order to maintain p38 MAP kinase activation and in turn, sustain IL-10 production.
IL-10 maintains IL-10 production in human TR1 cells
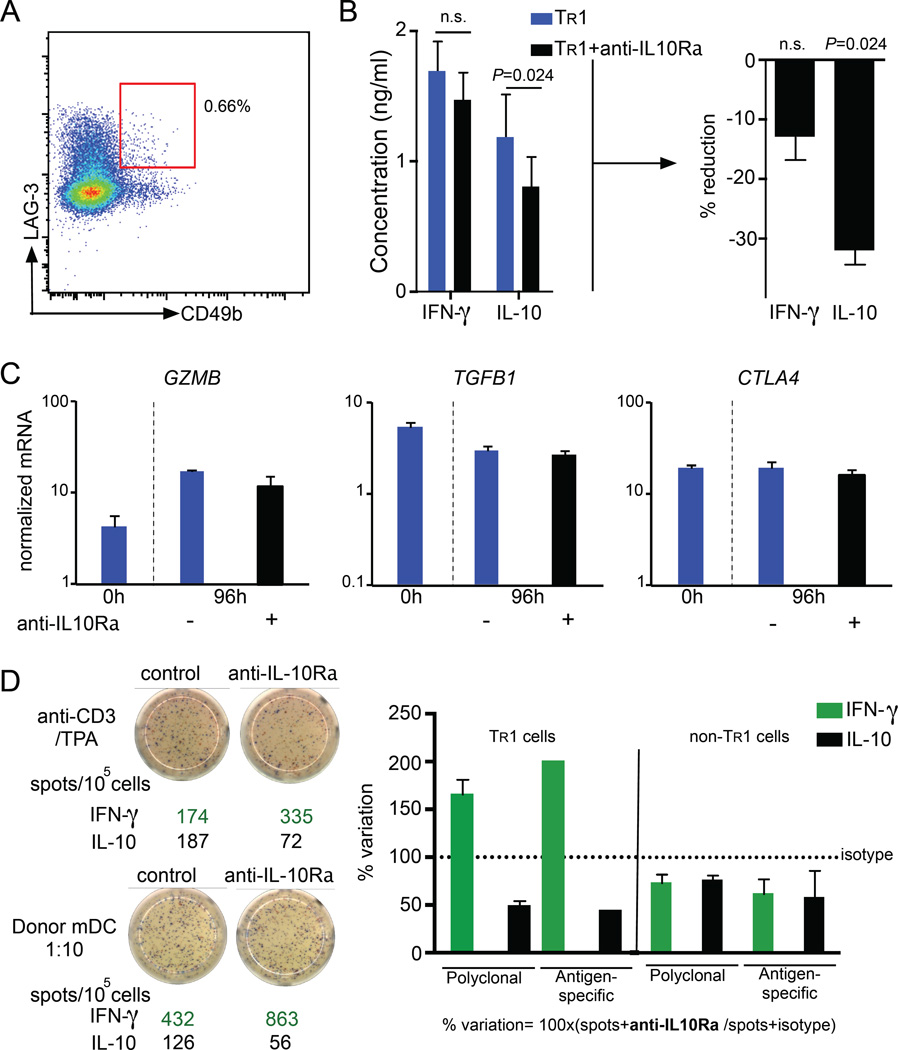
Human TR1 cells are of great interest for regulatory T cell-based immunotherapy, because of their strong potential to induce immune homeostasis (14, 61, 62). Thus, we aimed to investigate the role of IL-10 signaling in mature human TR1 cells. Using CD49b and LAG-3 as markers, we isolated circulating human TR1 cells from PBMCs of healthy donors (Fig. 7A). We re-stimulated the isolated TR1 cells in vitro together with IL-10Rα blocking antibody (or isotype) and measured cytokine release. In line with the data obtained with mouse TR1 cells, we did not observe differences in the production of IFN-γ, IL-4 or IL-6 between TR1 cells upon blocking IL10R compared to control. IL-10 production of human TR1 cells was however decreased by more than 30% in the presence of IL-10Rα blocking antibody, highlighting the importance of IL-10 signaling to maintain the production of IL-10 (Fig. 7B and data not shown). In line with our murine data, we saw no significant difference in the mRNA expression of GZMB, TGFB1 and CTLA4 between re-stimulated TR1 cells in the presence or absence of IL-10Rα blocking antibody (Fig. 7C).
Fig. 7. IL-10 signaling sustains IL-10 production in human TR1 cell.
(A–C) Circulating human TR1 cells (CD4+CD45RAlowCD49b+LAG-3+) were FACS-sorted from PBMCs of healthy donors (n=5) (A). TR1 cells were re-stimulated with anti-CD3 and anti-CD28 for 96 hours with either 50 µg/ml human IL-10Rα or isotype control antibody and the indicted cytokines were quantified. A paired t tested was used to calculate significance. (B). TGFB1, CTLA4 and GZMB mRNA expression normalized to HPRT (C). (D) In vitro differentiated TR1 and non-TR1 cells were re-stimulated with anti-CD3/TPA or allogeneic mDC for 48 hours with 50 µg/ml human IL-10Rα blocking antibody or isotype control. A dual IFNγ/IL-10 ELISPOT was performed. Data are cumulative of two independent experiments.
Next, we used IL-10-anergized T cells containing TR1 cells that were generated in vitro with tolerogenic dendritic cells (DC-10) (42). This is the most efficient protocol to induce human TR1 cells which are going to be used in the upcoming clinical trials (63). We observed that differentiated TR1 cells re-activated in the presence of IL-10Rα blocking antibody showed reduced numbers of IL-10 producer cells compared to isotype treated cells. Of note, the effect we observed was independent of the activation used: both polyclonal and allogeneic mDC stimulation in the presence of IL-10Rα blocking antibody led to a reduction of IL-10-producing cells (Fig. 7D), confirming our results obtained with circulating human TR1 cells regarding the IL-10 production. Interestingly, blockade of IL-10 signaling led to increased IFN-γ production in the pool of IL-10-anergized T cells. This difference might be due to the presence of non-TR1 cells in the IL-10-anergized T cell pool, which might be affected differently by the blocking of IL-10 signaling. CD4+ T cells generated in vitro in the absence of IL-10 (non-TR1 cells) were also tested and they did not show an increased IFN-γ production and only a slight reduction in IL-10 production upon blockage of IL-10 signaling (Fig. 7D).
In summary, IL-10 signaling is also essential to maintain IL-10 production by freshly isolated and in vitro induced human TR1 cells.
Discussion
Chronic TCR stimulation in the presence of IL-10 has been shown to be sufficient to induce highly regulatory mouse and human TR1 cells (29, 64). However, findings by Maynard et al. (2007) demonstrated that in a totally IL-10 deficient mouse, TR1 cells were still present in the intestine (18). Our study allowed us to test the direct effect of IL-10 on intestinal TR1 cells and therefore exclude possible extrinsic effects and side effects related to the use of a totally IL-10 deficient mouse model.
We found that IL-10 signaling is not essential to induce TR1 cells in vivo. Of note, the over expression of a dominant negative IL-10Rα suppressed IL-10 mediated STAT3 activation. Our data suggest that the differentiation of TR1 cells is depended on STAT3 activation, but independent of IL-10 signaling. However, the activation of STAT3 by other factors, such as IL-27 and IL-6 is not affected in transgenic mice with impaired IL-10 signaling, which explains this finding. Furthermore, it is widely accepted that IL-27 is sufficient to induce TR1 cells in vivo (46, 48, 59, 60). Our data further support this as they show that TR1 cells with a strongly impaired IL-10 signaling do not show any obvious defects shortly after induction. However, we showed that mature TR1 cells can respond to IL-10. More importantly IL-10 signaling is crucial for their function: By responding to IL-10, TR1 cells maintain the production of IL-10. This allows them to preserve their regulatory activity. When mouse intestinal TR1 cells do not respond to IL-10, they lose IL-10 production and therefore their suppressive function. Accordingly, IL-10 signaling in TR1 cells was crucial to maintain their high level of potential to prevent CD4+Foxp3−CD45RBhi T cell and TH17 cell mediated colitis. Interestingly, we observed that IL-10RImpaired TR1 cells still have the ability to reduce the colitis caused by CD45RBhi T cells by trend. In contrast IL-10RImpaired TR1 cells were not able to improve colitis induced by the transfer of TH17 cells. This difference could be due to the different disease severity in the two colitis models or the different T helper cell composition, since the CD45RBhi colitis model is dominated by TH1 and TH17 cells. Thus, it would be possible that IL-10 signaling in TR1 cells is more important to control TH17 cells than TH1 cells like it has been proposed for Foxp3 Treg (32). Taken together these studies with our current findings suggests that Foxp3+ Tregs and TR1 cells need to respond to IL-10 to sustain IL-10 production, which is then essential to directly control TH17 cells. Nevertheless, we cannot exclude that other TR1 associated suppressive mechanisms, such as the secretion of Granzyme B or TGF-β, are more important to suppress other cell types.
TR1 cells can exert their regulatory function through several mechanisms (16, 17, 65). Among these, we found that TR1 cells that do not respond to IL-10 lose their IL-10 production and their capacity to suppress TH17 mediated colitis, but still express normal levels of, Granzyme B and CTLA-4. All of these molecules have been considered as being involved in alternative regulatory mechanisms of TR1 cells (29, 66, 67). Nevertheless IL-10 seems to be essential to control TH17 cells (26, 32). The possibility remains that in the absence of IL-10 secretion, other regulatory mechanisms of TR1 cells would be directed to other pro-inflammatory cells. For example, Granzyme B secreted by TR1 cells could kill antigen presenting cells (16).
Several signaling molecules have been identified to induce and maintain IL-10 expression in a variety of cells, for example STAT3, p38 MAP kinase and ERK1 and ERK2 (ERK1/2) (50–53, 68). Furthermore, it has been previously shown that IL-10 signaling sustains the production of IL-10 via activation of STAT3 in Foxp3+ Treg cells. TR1 cells also depend on STAT3 signaling for differentiation (32, 48), but surprisingly we found that it is dispensable for the maintenance of IL-10 secretion in mature TR1 cells, whereas p38 MAP kinase plays a crucial role in this process. However, since addition of JNK and STAT3 inhibitors affected cell viability at high concentrations, we cannot completely exclude that JNK or STAT3 signaling in addition to p38 MAP kinase signaling might affect IL-10 production. But STAT3 requires Foxp3 and histone acetyl transterase-1 to epigenetically modify the Il10 promoter region to allow for gene regulation via STAT3 in Foxp3+ Treg cells (69). The absence of Foxp3 in mature TR1 cells could mitigate the role of STAT3 in this process and explain why p38 MAP kinase, which does not require Foxp3 mediated stabilization, sustains IL-10 expression in TR1 cells.
Considering the literature, we propose IL-27/Erg-2/Blimp1 in conjunction with STAT3, Ahr and c-Maf as the driving force for TR1 cell differentiation. However, IL-10 signaling, through p38 MAP kinase, is required for the stabilization and function of intestinal TR1 cells. Whether and how IL-10 and IL-21 (another cytokine which has been proposed to sustain the TR1 cell phenotype) synergistically sustain IL-10 production, remains to be further investigated (21, 46, 59).
Human TR1 cells display a high level of potential to maintain and re-establish immune homeostasis. They therefore receive major focus in current immunological and clinical research into the design of TR1 cell-based therapies to treat human diseases such as IBD (61, 62). In such trials the success of a TR1-based therapy is strongly linked to their high IL-10 production (67). The functional stability of TR1 cells could be critical for the efficiency and safety of these cells as therapeutics.
We extended our key findings to human biology. We show that IL-10 is also essential for human TR1 cells to maintain their IL-10 production. The capacity of TR1 cells to sustain themselves by IL-10 is therefore key to determine the long-term success of the therapy. Currently, it has been proposed to select in vitro induced TR1 cells based on the expression of CD49b and LAG-3 (67). Further enriching TR1 cells by high expression of IL-10 receptor could optimize the efficiency and ensure the safety of TR1 cell-based trials. Furthermore, measuring the expression of IL-10 receptor could serve as a marker to determine the efficacy of a TR1 cell therapy.
Collectively, we show that TR1 cells require the presence of their immunoregulatory cytokine IL-10 to maintain the cell function. Accordingly, TR1 cell stability is dependent on IL-10 signaling in TR1 cells themselves. Overall, our data indicate the crucial role of IL-10 signaling in TR1 cells and suggest that TR1-based T cell therapy is safe and efficient for the treatment of IBD.
Supplementary Material
Acknowledgments
We thank Cathleen Haueis and Sandra Wende for excellent technical assistance. Furthermore, we thank the FACS Sorting Core Unit of the Universitätsklinikum Hamburg-Eppendorf for the excellent support.
S.H. is supported by the Hofschneider Stiftung für Experimentelle Biomedizin and Ernst Jung-Stiftung. This work was supported in part by Howard Hughes Medical Institute (to R.A.F.), the Deutsche Forschungsgemeinschaft (DFG HU 1714/3-1; to S.H.; SFB841 to J.H. and S.H.; SFB1192 to SH and CK), and 7th Framework Programme of the EU (Marie Curie Actions Initial Training Network -FP7-PEOPLE-2011-ITN), under the Marie Skłodowska-Curie grant agreement No. 289903 (to B.M.).
Footnotes
The authors have declared that there is no conflict of interest.
Contribution:
LB: study concept and design, acquisition of data, drafting of the manuscript; NG: study concept and design, drafting of the manuscript; BS: performed the microarray analysis; AG, JK, PP: acquisition of data; BM,MB: interpretation of data: MG: material support; YW: material support; EE: interpretation of data; FH: revised the manuscript; JH: critical revision of the manuscript for important intellect00ual content; RF, SH: study supervision
References
- 1.Glocker EO, Frede N, Perro M, Sebire N, Elawad M, Shah N, Grimbacher B. Infant colitis--it's in the genes. Lancet. 2010;376:1272. doi: 10.1016/S0140-6736(10)61008-2. [DOI] [PubMed] [Google Scholar]
- 2.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England journal of medicine. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 4.Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, Sordat B, Gibbs VC, Aguet M. The orphan receptor CRF2–4 is an essential subunit of the interleukin 10 receptor. The Journal of experimental medicine. 1998;187:571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pot C, Apetoh L, Kuchroo VK. Type 1 regulatory T cells (Tr1) in autoimmunity. Seminars in immunology. 2011;23:202–208. doi: 10.1016/j.smim.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. Journal of immunology. 2002;169:6112–6119. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- 7.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O'Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. The Journal of experimental medicine. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gagliani N, Jofra T, Stabilini A, Valle A, Atkinson M, Roncarolo MG, Battaglia M. Antigen-specific dependence of Tr1-cell therapy in preclinical models of islet transplant. Diabetes. 2010;59:433–439. doi: 10.2337/db09-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gagliani N, Gregori S, Jofra T, Valle A, Stabilini A, Rothstein DM, Atkinson M, Roncarolo MG, Battaglia M. Rapamycin combined with anti-CD45RB mAb and IL-10 or with G-CSF induces tolerance in a stringent mouse model of islet transplantation. PloS one. 2011;6:e28434. doi: 10.1371/journal.pone.0028434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang J, Garabatos N, Izquierdo C, Agrawal S, Keough MB, Yong VW, James E, Moore A, Yang Y, Stratmann T, Serra P, Santamaria P. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:434–440. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- 11.Bacchetta R, Lucarelli B, Sartirana C, Gregori S, Lupo Stanghellini MT, Miqueu P, Tomiuk S, Hernandez-Fuentes M, Gianolini ME, Greco R, Bernardi M, Zappone E, Rossini S, Janssen U, Ambrosi A, Salomoni M, Peccatori J, Ciceri F, Roncarolo MG. Immunological Outcome in Haploidentical-HSC Transplanted Patients Treated with IL-10-Anergized Donor T Cells. Frontiers in immunology. 2014;5:16. doi: 10.3389/fimmu.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hippen KL, Riley JL, June CH, Blazar BR. Clinical perspectives for regulatory T cells in transplantation tolerance. Seminars in immunology. 2011;23:462–468. doi: 10.1016/j.smim.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desreumaux P, Foussat A, Allez M, Beaugerie L, Hebuterne X, Bouhnik Y, Nachury M, Brun V, Bastian H, Belmonte N, Ticchioni M, Duchange A, Morel-Mandrino P, Neveu V, Clerget-Chossat N, Forte M, Colombel JF. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn's disease. Gastroenterology. 2012;143:1207–1217. e1201–e1202. doi: 10.1053/j.gastro.2012.07.116. [DOI] [PubMed] [Google Scholar]
- 14.Roncarolo MG, Gregori S, Lucarelli B, Ciceri F, Bacchetta R. Clinical tolerance in allogeneic hematopoietic stem cell transplantation. Immunological reviews. 2011;241:145–163. doi: 10.1111/j.1600-065X.2011.01010.x. [DOI] [PubMed] [Google Scholar]
- 15.Okamura T, Fujio K, Shibuya M, Sumitomo S, Shoda H, Sakaguchi S, Yamamoto K. CD4+CD25−LAG3+ regulatory T cells controlled by the transcription factor Egr-2. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13974–13979. doi: 10.1073/pnas.0906872106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnani CF, Alberigo G, Bacchetta R, Serafini G, Andreani M, Roncarolo MG, Gregori S. Killing of myeloid APCs via HLA class I, CD2 and CD226 defines a novel mechanism of suppression by human Tr1 cells. European journal of immunology. 2011;41:1652–1662. doi: 10.1002/eji.201041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunological reviews. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 18.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nature immunology. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 19.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. Journal of immunology. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki Y, Fujio K, Okamura T, Yanai A, Sumitomo S, Shoda H, Tamura T, Yoshida H, Charnay P, Yamamoto K. Egr-2 transcription factor is required for Blimp-1-mediated IL-10 production in IL-27-stimulated CD4+ T cells. European journal of immunology. 2013;43:1063–1073. doi: 10.1002/eji.201242942. [DOI] [PubMed] [Google Scholar]
- 21.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nature immunology. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu C, Sakuishi K, Xiao S, Sun Z, Zaghouani S, Gu G, Wang C, Tan DJ, Wu C, Rangachari M, Pertel T, Jin HT, Ahmed R, Anderson AC, Kuchroo VK. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nature communications. 2015;6:6072. doi: 10.1038/ncomms7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinemann C, Heink S, Petermann F, Vasanthakumar A, Rothhammer V, Doorduijn E, Mitsdoerffer M, Sie C, Prazeres da Costa O, Buch T, Hemmer B, Oukka M, Kallies A, Korn T. IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1. Nature communications. 2014;5:3770. doi: 10.1038/ncomms4770. [DOI] [PubMed] [Google Scholar]
- 24.Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limon P, Paiva RS, Ching T, Weaver C, Zi X, Pan X, Fan R, Garmire LX, Cotton MJ, Drier Y, Bernstein B, Geginat J, Stockinger B, Esplugues E, Huber S, Flavell RA. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, Siddiqui S, Basso AS, Otterbein LE, Pardoll DM, Pan F, Priel A, Clish CB, Robson SC, Quintana FJ. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nature medicine. 2015;21:638–646. doi: 10.1038/nm.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber S, Gagliani N, Esplugues E, O'Connor W, Jr, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, Roncarolo MG, Battaglia M, Flavell RA. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(−) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gagliani N, Jofra T, Valle A, Stabilini A, Morsiani C, Gregori S, Deng S, Rothstein DM, Atkinson M, Kamanaka M, Flavell RA, Roncarolo MG, Battaglia M. Transplant tolerance to pancreatic islets is initiated in the graft and sustained in the spleen. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:1963–1975. doi: 10.1111/ajt.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nature immunology. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 29.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 30.Himmel ME, Yao Y, Orban PC, Steiner TS, Levings MK. Regulatory T-cell therapy for inflammatory bowel disease: more questions than answers. Immunology. 2012;136:115–122. doi: 10.1111/j.1365-2567.2012.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trzonkowski P, Bacchetta R, Battaglia M, Berglund D, Bohnenkamp HR, ten Brinke A, Bushell A, Cools N, Geissler EK, Gregori S, Marieke van Ham S, Hilkens C, Hutchinson JA, Lombardi G, Madrigal JA, Marek-Trzonkowska N, Martinez-Caceres EM, Roncarolo MG, Sanchez-Ramon S, Saudemont A, Sawitzki B. Hurdles in therapy with regulatory T cells. Science translational medicine. 2015;7 doi: 10.1126/scitranslmed.aaa7721. 304ps318. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W, Rudensky AY. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber-Nordt RM, Riley JK, Greenlund AC, Moore KW, Darnell JE, Schreiber RD. Stat3 recruitment by two distinct ligand-induced, tyrosine-phosphorylated docking sites in the interleukin-10 receptor intracellular domain. The Journal of biological chemistry. 1996;271:27954–27961. doi: 10.1074/jbc.271.44.27954. [DOI] [PubMed] [Google Scholar]
- 34.Gunzl P, Bauer K, Hainzl E, Matt U, Dillinger B, Mahr B, Knapp S, Binder BR, Schabbauer G. Anti-inflammatory properties of the PI3K pathway are mediated by IL-10/DUSP regulation. Journal of leukocyte biology. 2010;88:1259–1269. doi: 10.1189/jlb.0110001. [DOI] [PubMed] [Google Scholar]
- 35.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annual review of biochemistry. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 36.Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, Harhaj E, Flavell RA. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Kamanaka M, Huber S, Zenewicz LA, Gagliani N, Rathinam C, O'Connor W, Jr, Wan YY, Nakae S, Iwakura Y, Hao L, Flavell RA. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. The Journal of experimental medicine. 2011;208:1027–1040. doi: 10.1084/jem.20102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O'Connor W, Jr, Rongvaux A, Van Rooijen N, Haberman AM, Iwakura Y, Kuchroo VK, Kolls JK, Bluestone JA, Herold KC, Flavell RA. Control of TH17 cells occurs in the small intestine. Nature. 2011;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becker C, Fantini MC, Neurath MF. High resolution colonoscopy in live mice. Nature protocols. 2006;1:2900–2904. doi: 10.1038/nprot.2006.446. [DOI] [PubMed] [Google Scholar]
- 41.O'Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nature immunology. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, Hauben E, Roncarolo MG. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116:935–944. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 43.Gorenshteyn D, Zaslavsky E, Fribourg M, Park CY, Wong AK, Tadych A, Hartmann BM, Albrecht RA, Garcia-Sastre A, Kleinstein SH, Troyanskaya OG, Sealfon SC. Interactive Big Data Resource to Elucidate Human Immune Pathways and Diseases. Immunity. 2015;43:605–614. doi: 10.1016/j.immuni.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC bioinformatics. 2009;10:48. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–487. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. Journal of immunology. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nature immunology. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature immunology. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adler HS, Kubsch S, Graulich E, Ludwig S, Knop J, Steinbrink K. Activation of MAP kinase p38 is critical for the cell-cycle-controlled suppressor function of regulatory T cells. Blood. 2007;109:4351–4359. doi: 10.1182/blood-2006-09-047563. [DOI] [PubMed] [Google Scholar]
- 51.Dobreva ZG, Miteva LD, Stanilova SA. The inhibition of JNK and p38 MAPKs downregulates IL-10 and differentially affects c-Jun gene expression in human monocytes. Immunopharmacology and immunotoxicology. 2009;31:195–201. doi: 10.1080/08923970802626276. [DOI] [PubMed] [Google Scholar]
- 52.Horie K, Ohashi M, Satoh Y, Sairenji T. The role of p38 mitogen-activated protein kinase in regulating interleukin-10 gene expression in Burkitt's lymphoma cell lines. Microbiology and immunology. 2007;51:149–161. doi: 10.1111/j.1348-0421.2007.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 53.Ma W, Lim W, Gee K, Aucoin S, Nandan D, Kozlowski M, Diaz-Mitoma F, Kumar A. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. The Journal of biological chemistry. 2001;276:13664–13674. doi: 10.1074/jbc.M011157200. [DOI] [PubMed] [Google Scholar]
- 54.Chang HD, Helbig C, Tykocinski L, Kreher S, Koeck J, Niesner U, Radbruch A. Expression of IL-10 in Th memory lymphocytes is conditional on IL-12 or IL-4, unless the IL-10 gene is imprinted by GATA-3. European journal of immunology. 2007;37:807–817. doi: 10.1002/eji.200636385. [DOI] [PubMed] [Google Scholar]
- 55.Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O'Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31:209–219. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu J, Yang Y, Qiu G, Lal G, Wu Z, Levy DE, Ochando JC, Bromberg JS, Ding Y. c-Maf regulates IL-10 expression during Th17 polarization. Journal of immunology. 2009;182:6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huber S, Schrader J, Fritz G, Presser K, Schmitt S, Waisman A, Luth S, Blessing M, Herkel J, Schramm C. P38 MAP kinase signaling is required for the conversion of CD4+CD25− T cells into iTreg. PloS one. 2008;3:e3302. doi: 10.1371/journal.pone.0003302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar S, Jiang MS, Adams JL, Lee JC. Pyridinylimidazole compound SB 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochemical and biophysical research communications. 1999;263:825–831. doi: 10.1006/bbrc.1999.1454. [DOI] [PubMed] [Google Scholar]
- 59.Pot C, Apetoh L, Awasthi A, Kuchroo VK. Induction of regulatory Tr1 cells and inhibition of T(H)17 cells by IL-27. Seminars in immunology. 2011;23:438–445. doi: 10.1016/j.smim.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H, Meng R, Li Z, Yang B, Liu Y, Huang F, Zhang J, Chen H, Wu C. IL-27 induces the differentiation of Tr1-like cells from human naive CD4+ T cells via the phosphorylation of STAT1 and STAT3. Immunology letters. 2011;136:21–28. doi: 10.1016/j.imlet.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Allan SE, Broady R, Gregori S, Himmel ME, Locke N, Roncarolo MG, Bacchetta R, Levings MK. CD4+ T-regulatory cells: toward therapy for human diseases. Immunological reviews. 2008;223:391–421. doi: 10.1111/j.1600-065X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 62.Bacchetta R, Gregori S, Serafini G, Sartirana C, Schulz U, Zino E, Tomiuk S, Jansen U, Ponzoni M, Paties CT, Fleischhauer K, Roncarolo MG. Molecular and functional characterization of allogantigen-specific anergic T cells suitable for cell therapy. Haematologica. 2010;95:2134–2143. doi: 10.3324/haematol.2010.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gregori S, Passerini L, Roncarolo MG. Clinical Outlook for Type-1 and FOXP3(+) T Regulatory Cell-Based Therapy. Frontiers in immunology. 2015;6:593. doi: 10.3389/fimmu.2015.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E, Roncarolo MG. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006;55:40–49. [PubMed] [Google Scholar]
- 65.Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. Journal of immunology. 2001;166:5530–5539. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 66.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, Kegel C, Disch R, Schmidt-Weber CB, Blaser K, Akdis CA. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. The Journal of experimental medicine. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roncarolo MG, Gregori S, Bacchetta R, Battaglia M. Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Current topics in microbiology and immunology. 2014;380:39–68. doi: 10.1007/978-3-662-43492-5_3. [DOI] [PubMed] [Google Scholar]
- 68.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nature medicine. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 69.Hossain DM, Panda AK, Manna A, Mohanty S, Bhattacharjee P, Bhattacharyya S, Saha T, Chakraborty S, Kar RK, Das T, Chatterjee S, Sa G. FoxP3 acts as a cotranscription factor with STAT3 in tumor-induced regulatory T cells. Immunity. 2013;39:1057–1069. doi: 10.1016/j.immuni.2013.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.