Abstract
Interleukin-15 (IL-15) is essential for development and differentiation of natural killer (NK) and memory (m)CD8+ T cells. Our lab previously showed that NK and CD8+ T lymphocytes facilitate the pathobiology of septic shock. However, factors that regulate NK and CD8+ T lymphocyte functions during sepsis are not well characterized. We hypothesized that IL-15 promotes the pathogenesis of sepsis by maintaining NK and mCD8+ T cell integrity. To test our hypothesis, the pathogenesis of sepsis was assessed in IL-15-deficient (IL-15 KO) mice. IL-15 KO mice showed improved survival, attenuated hypothermia, and less pro-inflammatory cytokine production during septic shock caused by cecal ligation and puncture (CLP) or endotoxin-induced shock. Treatment with IL-15 superagonist (IL-15 SA, IL-15/IL-15Rα complex) regenerated NK and mCD8+ T cells and re-established mortality of IL-15 KO mice during septic shock. Preventing NK cell regeneration attenuated the restoration of mortality caused by IL-15 SA. If given immediately prior to septic challenge, IL-15 neutralizing IgG M96 failed to protect against septic shock. However, M96 caused NK cell depletion if given 4 days prior to septic challenge and conferred protection. IL-15 SA treatment amplified endotoxin shock, which was prevented by NK cell or IFNγ depletion. IL-15 SA treatment also exacerbated septic shock caused by CLP when given after the onset of sepsis. In conclusion, endogenous IL-15 doesn’t directly augment the pathogenesis of sepsis but enables the development of septic shock by maintaining NK cell numbers and integrity. Exogenous IL-15 exacerbates the severity of sepsis by activating NK cells and facilitating IFNγ production.
Keywords: IL-15, sepsis, NK cells, IFNγ, NK cell integrity and effector function
Introduction
Interleukin (IL)-15 is a cytokine that is essential for maintaining the homeostasis and effector functions of natural killer (NK) and memory CD8+ (mCD8+) T lymphocytes. IL-15 prompts the generation of mature NK cells in the bone marrow (1); it potently expands and activates peripheral NK cells to perform cytotoxic functions and facilitate cytokine secretion during viral and bacterial infections (2, 3); IL-15 also plays a pivotal role in the generation, cytotoxicity and survival of CD8+ T lymphocytes, especially the mCD8+ subset (4, 5); and is essential for survival of natural killer T (NKT) and intestinal intraepithelial lymphocytes (IELs) (6, 7). Germline deletion of IL-15 in mice causes deficiency in NK, mCD8+ T, NKT cells and intraepithelial lymphocytes (8). IL-15 is constitutively expressed by multiple types of cells including monocytes, macrophages, dendritic cells (DCs), fibroblasts and epithelial cells (9, 10). Its expression is induced by cytokines such as type I (IFNα/β) and type II (IFNγ) interferons (IFN) as well as microbial products such as lipopolysaccharide, poly I:C, and viruses (11, 12). IL-15 is primarily presented in association with the unique high-affinity IL-15 receptor alpha (IL-15Rα) subunit that is expressed on the surface of IL-15 producing cells and delivers signals to target cells that express the IL-2R β and γ receptor subunits, a process called trans-presentation. The IL-15/IL-15Rα complex can also be released in soluble form after cleavage of the transmembrane domain of the receptor α (13–15). The IL-15/IL-15Rα complex can also be generated in solution by mixing the individual components. The generated IL-15/IL-15Rα complex. possesses longer half-life and greater biological activity than free IL-15 and is thus termed IL-15 superagonist (IL-15 SA) (16).
NK and CD8+ T lymphocytes have been shown to facilitate physiological dysfunction and systemic inflammation during sepsis (17–19). However, little is known about the factors that regulate the functions of NK and CD8+ T lymphocytes in the context of sepsis. IL-15 appears to be an essential pro-inflammatory mediator during sepsis, as IL-15 KO mice show resistance to sepsis (20). In clinical studies, elevated blood IL-15 concentrations are associated with the development of organ injury and mortality in high risk gastrointestinal surgery patients and patients with severe sepsis, respectively (21, 22). However, the underlying mechanisms by which IL-15 facilitates the pathogenesis of sepsis have not been well characterized. In addition, IL-15 KO mice are not only deficient in IL-15, but have markedly decreased numbers of NK and CD8+ T cells, which are implicated in the pathogenesis of sepsis (17–19). Therefore, it is unclear if lack of IL-15 alone or lack of IL-15-dependent NK and mCD8+ T cells in IL-15 KO mice confers protection against septic shock. In addition, it remains unclear whether IL-15 treatment exacerbates the pathogenesis of sepsis by activating NK and mCD8+ T cells.
In this paper, the role of endogenous and exogenous IL-15 in the pathogenesis of sepsis and its regulatory effect on NK and mCD8+ T cell viability and activity during sepsis was investigated. The studies were designed to address the hypotheses that IL-15 KO mice are resistant to septic shock due to an intrinsic deficiency of NK and mCD8+ T cells and treatment of wild type mice with IL-15 will facilitate the pathogenesis of septic shock by augmenting NK and mCD8+ T cell activation. The response of IL-15-deficient mice to sepsis caused by CLP or lipopolysaccharide (LPS) challenge was fully examined. Specific endpoints included survival, organ injury, systemic cytokine production and bacterial clearance. Whether regeneration of NK and mCD8+ T cells by treatment with IL-15 SA would alter the response of IL-15-deficient mice to sepsis was also assessed. Additional experiments examined whether sustained administration of IL-15 SA to wild type mice or acute administration after the onset of sepsis altered sepsis-associated pathobiology. Studies employing NK or mCD8+ T cell depletion, and IFNγ-deficient mice were performed to provide mechanistic insights.
Materials and Methods
Mice
Female and male, 8- to 12-week-old C57BL/6Tac were purchased from Taconic Farms (Hudson, NY). Breeding pairs of homozygous IL-15 null mice (C57BL/6NTac-IL15tm1Imx N5, IL-15 KO) were purchased from Taconic and genotype of offspring was verified by PCR analysis performed by Transnetyx (Memphis, TN). Female, 8- to 12-week-old homozygous IFN-gamma null mice (B6.129S7-Ifngtm1Ts/J, IFNγ KO) and wild type C57BL/6J (WT) control mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All studies were approved by IACUC at Vanderbilt University. Eight- to 12-week-old wild type and knockout mice were used in all experiments except in experiments in which IL-15 SA was administrated after the onset of sepsis, (Figure 11), in which 16- to 20-week-old wild type mice were used.
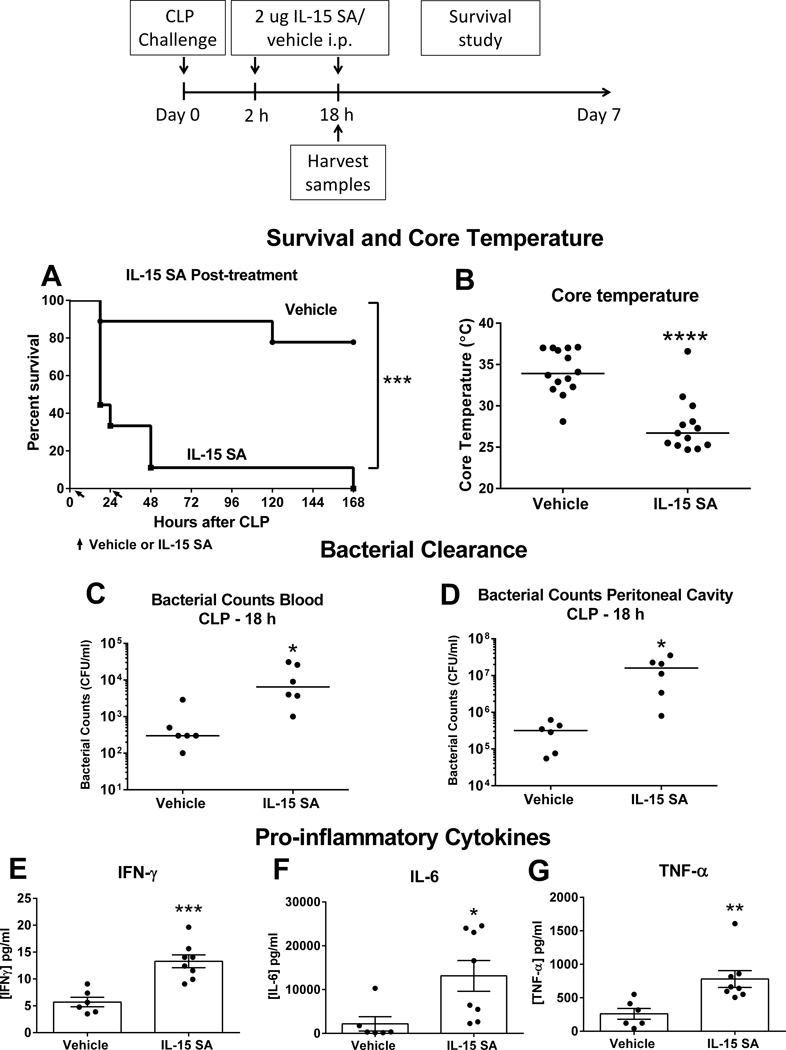
Figure 11. High dose IL-15 SA post-treatment accentuates lethality to septic shock.
Wild type mice (16- to 20-week-old) received vehicle or IL-15 SA (2 µg) at 2 and 18 hours after CLP challenge and survival rate was monitored over 7 days (A). Core temperature, bacterial counts in blood and peritoneal fluid as well as IFNγ, IL-6 and TNF-α concentrations in the plasma were measured at 18 hours after LPS (B–G). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 compared to vehicle wild type control. n= 8–9 mice per group. Data are representative of two separate experiments.
Cell depletion procedure
NK cells were depleted in mice by intraperitoneal injection with anti-asialoGM1 IgG (50 µg/mouse, Cedarlane Laboratories, Canada) at 24 hours prior to initiation of IL-15 SA treatment or LPS challenge. CD8 T cells were depleted by treatment with anti-CD8α IgG (clone 53-6.7, 50 µg/mouse, eBioscience) at 24 hours prior to IL-15 SA treatment or LPS challenge. Isotype-specific or non-specific IgG served as control in all antibody-induced leukocyte depletion experiments.
Cecal ligation and puncture model
The protocol was described previously (23). In brief, mice were anesthetized with 2% isoflurane in oxygen. A 1- to 2-cm midline incision was made through the abdominal wall. The cecum was identified and ligated 1.0 cm from the tip using a 3-0 silk tie. A double puncture of the ligated cecum was performed using a 20-gauge needle. The incision was closed using autoclips. Buprenorphine (0.1 mg/kg) was administered subcutaneously for analgesia 30 minutes before CLP and twice daily thereafter. All mice received fluid resuscitation (Lactated Ringers solution, 1 ml, intraperitoneal) immediately after injury and twice daily thereafter.
Endotoxin shock model
Ultrapure LPS-EB (from E. coli 0111:B4) from InvivoGen (San Diego, CA) was administered at a dose of 100 or 150 µg/mouse via intraperitoneal injection. Measurements of rectal temperature, pro-inflammatory cytokine concentrations and indices of acute organ injury were performed at 6 and 24 hours after LPS challenge.
IL-15 Superagonist (IL-15 SA) Preparation
Recombinant mouse IL-15 was purchased from eBioscience (San Diego, CA, Cat. no. 34-8151-85). Mouse IL-15 Rα subunit Fc chimera (IL-15 Ra) was purchased from R&D Systems (Minneapolis, MN, Cat. no. 551-MR-100). For preparation of IL-15 SA, 20 µg IL-15 and 90 µg IL-15Ra were incubated in 400 µl of sterile phosphate buffered saline (PBS) at 37 °C for 20 minutes to form the IL-15/IL-15 Ra complex. The complex was then diluted with sterile PBS to reach a concentration of 0.625 or 10 µg IL-15 SA/ml, then aliquoted and frozen. The IL-15 SA doses reported in the study are based on the amount of IL-15 present in the IL-15 SA complex.
IL-15 SA treatment
To regenerate NK cells, IL-15 KO mice received intraperitoneal injections of 0.125 µg IL-15 SA (low-dose) in 0.2 ml PBS for 4 days (day 0–3). IL-15 KO mice that were treated with vehicle served as control. On day 4, spleens and livers were harvested for measurement of leukocyte numbers and activation. In additional experiments, after the same treatment regimen of IL-15 SA, IL-15 KO mice were subjected to CLP or LPS (150 µg) on day 4. In survival studies, IL-15 SA (0.125 µg) injection was continued daily to maintain the regenerated NK cell population. In additional experiments, wild type mice received intraperitoneal injections with a high dose (2 µg) of IL-15 SA at 30 minutes prior to or 2 hours after challenge with 100 µg of LPS. A second dose of IL-15 SA was performed at 18 hours after LPS challenge.
M96 treatment
IL-15 neutralizing antibody M96 was generously provided by Amgen (Thousand oaks, CA) (24). Wild type mice received intraperitoneal injection with 20 µg M96 either 2 hours or 4 days prior to CLP or LPS challenge. A survival study was followed for 7 days after the initiation of septic shock. When M96 was given 4 days prior to CLP or LPS, a second administration of M96 at the same dose was given at the time of septic insults to maintain the effect of M96 on depletion of NK cells.
ALT, AST, BUN and creatinine measurement
Plasma was obtained from heparinized whole blood after centrifugation (2000g×10 minutes). Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) concentrations were measured as indices of acute liver injury, and blood urea nitrogen (BUN) and creatinine concentrations as indices of renal injury. They were measured in the Translational Pathology Core Laboratory at Vanderbilt University using an ACE Alera Chemistry Analyzer (Alfa Wassermann, Inc. West Caldwell, NJ).
Cytokine measurement
Concentrations of IL-6, IFN-γ, IL-12p70, IL-18, TNF-α, IL-1β and IL-10 in plasma were measured using a Bio-Plex Multiplex Assay with the Magpix Multiplex Reader (Bio-Rad, Hercules, CA). Results were analyzed with Bio-Plex Manager Software 6.1. Concentration of soluble IL-15/IL-15Rα complex in plasma was measured using Mouse IL-15/IL-15R Complex ELISA Ready-Set-Go (eBioscience, San Diego, CA).
Microbiology
Bacterial counts were performed on aseptically harvested blood and peritoneal fluid. Blood was harvested via carotid laceration. Peritoneal lavage fluid was obtained by injection and aspiration of 2 ml sterile PBS into the peritoneal cavity. Samples were serially diluted in sterile PBS and cultured on tryptic soy agar plates. Plates were incubated at 37°C for 24 hours and bacterial colonies were counted.
Flow cytometry
Splenocytes and hepatic leukocytes were isolated as previously described. Briefly, the spleen was smashed in PBS with the plunger from a 10-ml syringe and the homogenate was passed through a 70-µm cell strainer. Erythrocytes were lysed with Red Blood Cell Lysis Buffer (Sigma Life Sciences, St Louis, MO). The cells were counted using TC20™ Automated Cell Counter (Bio-Rad, Hercules, CA), centrifuged (300g × 5 minutes) and the cell pellet was resuspended in PBS. Livers were harvested after perfusion, which was achieved by cutting of the hepatic portal vein, insertion of a 25 G needle into the left ventricle of the heart and perfusion with 10 ml PBS. Liver homogenate was passed through a 70-µm cell strainer and then was washed, resuspended with 10 ml of 37.5% Percoll Plus (GE Healthcare Life Sciences) and centrifuged (680g×12 minutes at room temperature), The supernatant containing hepatocytes was discarded, erythrocytes were lysed, and the resulting mononuclear cells were counted using TC20™ Automated Cell Counter.
For surface marker staining, cells were suspended in PBS (1 × 107 cells/ml) and incubated with anti-mouse CD16/32 (eBioscience, 1 µl/ml) for 5 minutes to block nonspecific Fc receptor–mediated antibody binding. One million cells were then transferred into polystyrene tubes. Fluorochrome-conjugated antibodies or isotype controls (0.5 µg /tube) were added, incubated (4°C) for 30 min, and washed with 2 ml of cold PBS. After centrifugation (300g × 5 minutes), cell pellets were fixed with 250 µl of 1% paraformaldehyde. Antibodies used for surface marker labeling included CD3-Alex Fluor 488, NK1.1-PE-Cy7, CD8-FITC, CD4-FITC, CD44-PE-Cy5, CD19-PE, CD69-PE, CD11b-PE and CD27-APC (eBioScience, San Diego, BD Biosciences, San Diego). Appropriate isotype-specific antibodies were used as controls.
For intracellular staining, 1 × 106 splenocytes were incubated with 4 ul PMA and ionomycin (cell stimulation cocktail, eBioScience, San Diego) in 1 ml of RPMI-1640 media with 10% FBS at 37°C for 5 hours. After one hour, the protein transport inhibitors brefeldin A and monensin (2 ul/ml, eBioScience, San Diego) were added into cell cultures for the remaining 4 hours. After incubation, cell suspensions were labeled with fluorochrome-conjugated antibodies to surface markers as described above. Cells were then fixed and permeabilized with Cytofix/Cytoperm Plus (BD Biosciences, 250 µl /tube) for 20 minutes at 4°C. After washing with BD Perm/Wash solution, anti-IFNγ-PE (clone XMG1.2, eBioscience) was used to detect intracellular IFNγ production. Fluorochrome-conjugated isotype-specific IgG served as controls. All samples were analyzed using an Accuri C6 flow cytometer (BD Biosciences, San Diego, CA). Data were analyzed using Accuri C6 software.
Statistics
All values are presented as the mean ± SEM, except for body temperature, ALT and AST, BUN and creatinine for which median values are designated. A Student’s t test was used to examine the difference between two experimental groups. Data from multiple group experiments were analyzed using one-way ANOVA followed by a post hoc Tukey’s test to compare groups. Survival data were analyzed using the Mantel-Cox log-rank test. A value of p < 0.05 was considered statistically significant for all experiments.
Results
IL-15 KO mice are resistant to CLP-induced septic shock
IL-15 null mice have been reported to be deficient of NK, NKT and mCD8+ T cells (25). Examination of our colony showed that NK and mCD8+ T cells were significantly decreased in the spleens and livers of IL-15 KO mice whereas NKT cell numbers were not significantly different in either tissue as compared to wild type controls (Supplemental Figure 1). CD4+ T, naïve CD8+ T and B cell numbers were not significantly different when comparing wild type and IL-15 KO mice (data not shown).
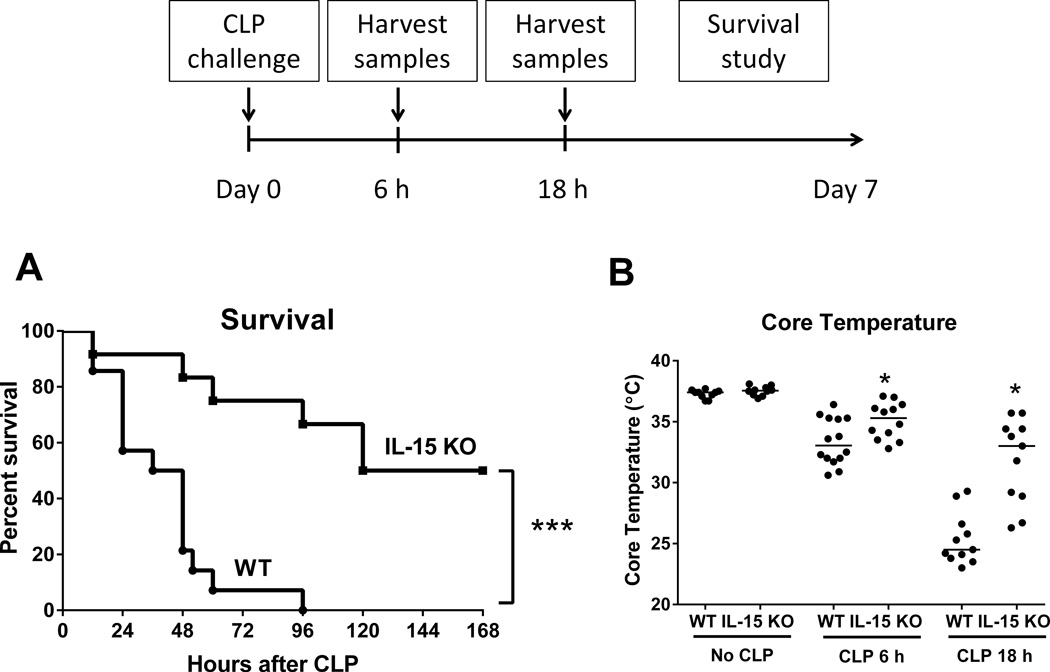
A survival study was performed to assess mortality in IL-15 KO and wild type mice during sepsis induced by cecal ligation and puncture (CLP). A significant survival advantage was observed in IL-15 KO mice, in which 50% long-term survival and a 120 hour median survival time were observed as compared to 0% survival and 36 hour median survival in wild type mice (Figure 1A). Both wild type and IL-15 KO mice developed sepsis-induced hypothermia (Figure 1B). However, core body temperature was significantly higher in IL-15 KO mice at 6 and 18 hours after CLP as compared to wild type controls (Figure 1B).
Figure 1. IL-15 KO are resistant to CLP-induced septic shock.
Wild type and IL-15 KO mice were subjected to CLP and were monitored for 7-day survival (A). Body temperature (B) was measured at 6 and 18 hours after CLP. The median value is designated in Figure 1B. * p < 0.05, *** p < 0.001, compared to wild type (WT) mice. n=11–14 mice per group. Data are representative of two to three separate experiments.
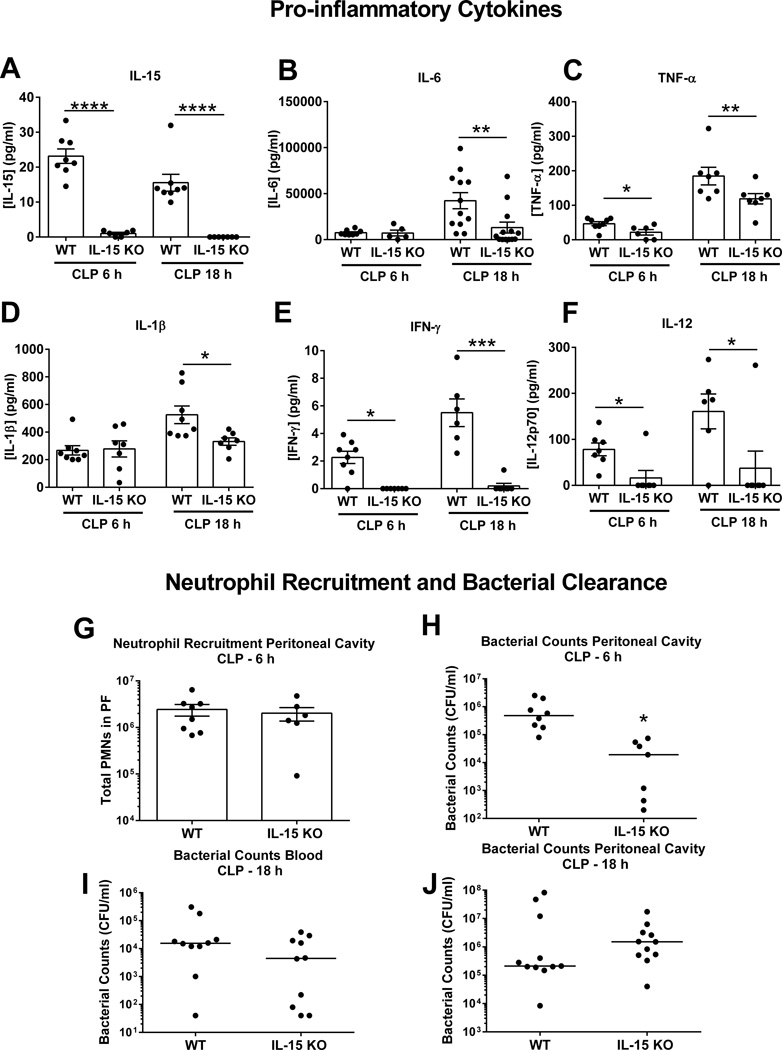
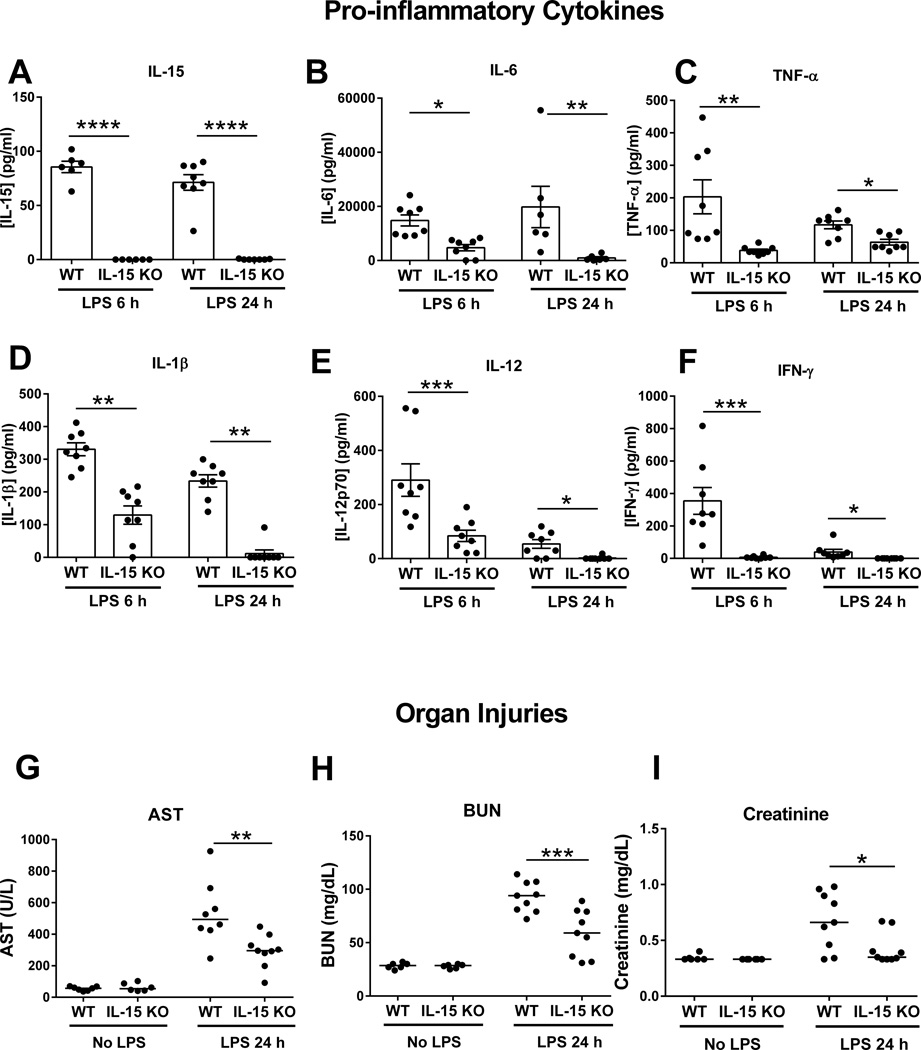
Further studies were undertaken to assess the effect of IL-15 deficiency on pro-inflammatory cytokine production and bacterial clearance after CLP (Figure 2). At 6 and 18 hours after CLP, IL-15 was detected in the plasma of wild type mice but not in IL-15 KO mice (Figure 2A). Concentrations of several pro-inflammatory cytokines, including IL-6, TNFα, IL-1β, IFNγ and IL-12, were significantly lower in the plasma of IL-15 KO mice compared to wild type controls (Figure 2B–F). Meanwhile, neutrophil recruitment into the peritoneal cavity at 6 hours after CLP was not different between IL-15 KO and wild type mice (Figure 2G). The numbers of bacteria were lower in peritoneal lavage fluid of IL-15 KO mice than wild type control mice at 6 hours after CLP (Figure 2H) but showed no significant difference between groups in blood or peritoneal cavity at 18 hours after CLP (Figure 2I, J).
Figure 2. IL-15 KO mice exhibit attenuated proinflammatory cytokine production during CLP-induced septic shock.
Blood was harvested at 6 and 18 hours after CLP challenge for measurement of pro-inflammatory cytokines (A–F). Neutrophil numbers in peritoneal cavity (G) and bacterial colony forming units in blood and peritoneal fluid (H–J) were also measured at designated time points in wild type and IL-15 KO mice. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to wild type mice at designated time points. n=6–8 mice per group. Data are representative of two separate experiments.
IL-15 KO mice are resistant to endotoxin-induced shock
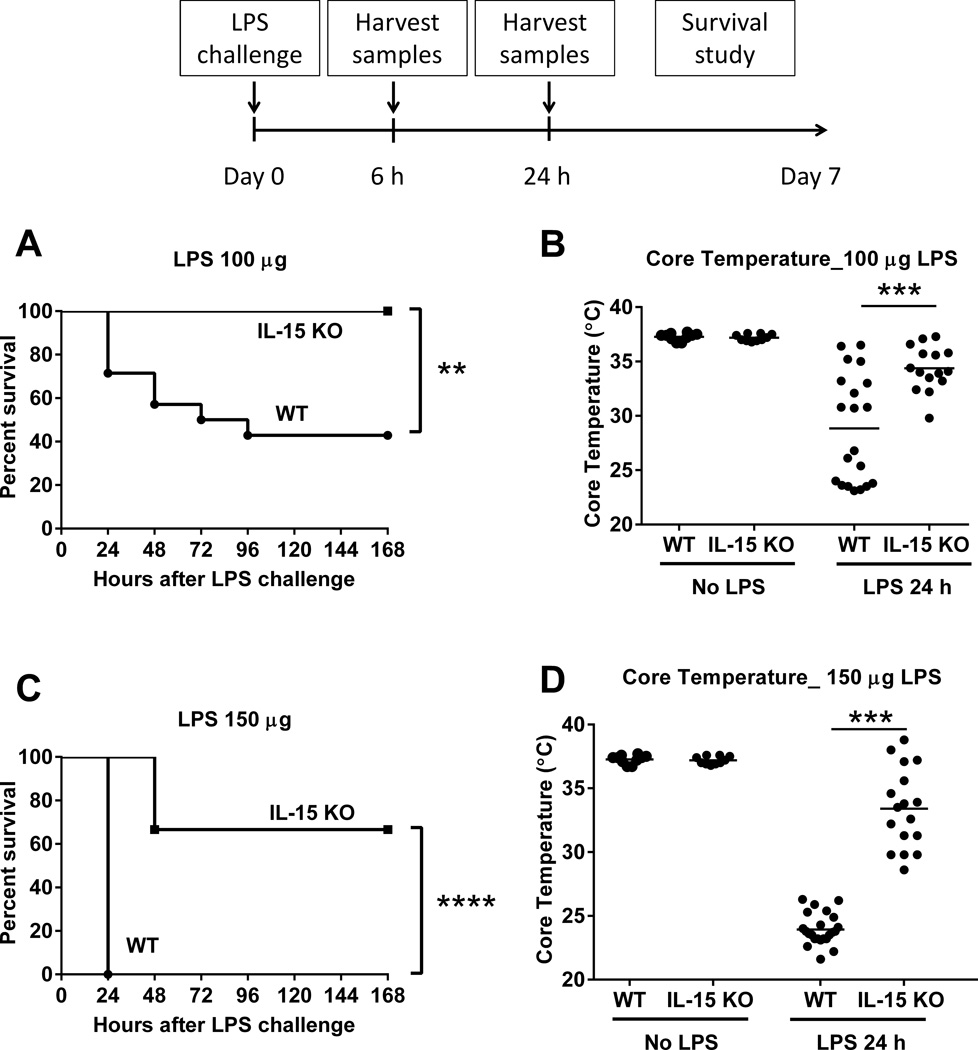
In a dose finding study, IL-15 KO and wild type mice were injected with LPS at doses of 100 or 150 ug and monitored for sepsis-induced mortality over 7 days. Wild type mice showed dose-dependent mortality with 43% survival after 100 µg LPS and 0% after 150 µg LPS, while IL-15 KO mice showed improved survival at both doses of LPS (100% survival after 100 µg LPS and 67% after 150 µg LPS) (Figure 3A, C). IL-15 KO mice developed significantly less hypothermia than wild type controls at 24 hours after LPS challenge (Figure 3B, D).
Figure 3. IL-15 KO are resistant to LPS-induced septic shock.
In a dose escalation study, IL-15 KO and wild type mice received intraperitoneal injection with 100 or 150 µg LPS and were observed for 7-day survival (A and B). Body temperature was measured at 24 hours after 100 or 150 µg LPS injection (A and B). ** p < 0.01, *** p < 0.001, **** p < 0.0001 when compared to wild type mice; n=8–23 mice per group. Data are representative of two to four separate experiments.
Further studies assessed organ injury and pro-inflammatory cytokine production after 150 µg LPS challenge. IL-15 concentration in the plasma was detected in WT, but not in IL-15 KO mice at 6 and 24 hours after LPS (Figure 4A). Meanwhile, lower concentrations of other pro-inflammatory cytokines in plasma were observed in IL-15 KO mice compared to wild type mice (Figure 4B–F). Also, concentrations of AST, BUN and creatinine were lower in the plasma of IL-15 KO mice compared to wild type controls at 24 hours after LPS challenge, indicative of reduced acute hepatocellular and kidney injury (Figure 4G–I).
Figure 4. IL-15 KO have attenuated proinflammatory cytokine production and organ injury after LPS-induced septic shock.
Blood was harvested at 6 and 24 hours after 150 µg LPS challenge for measurement of proinflammatory cytokines in plasma (A–F). AST (aspartate transaminase), BUN (blood urea nitrogen) and creatinine (G–I) concentrations in plasma were also measured at 24 hours post 150 µg LPS. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 when compared to wild type mice. n=8 mice per group. Data are representative of two separate experiments.
IL-15 SA treatment restores NK and mCD8+ T cell numbers and susceptibility to CLP- and LPS-induced shock in IL-15 KO mice
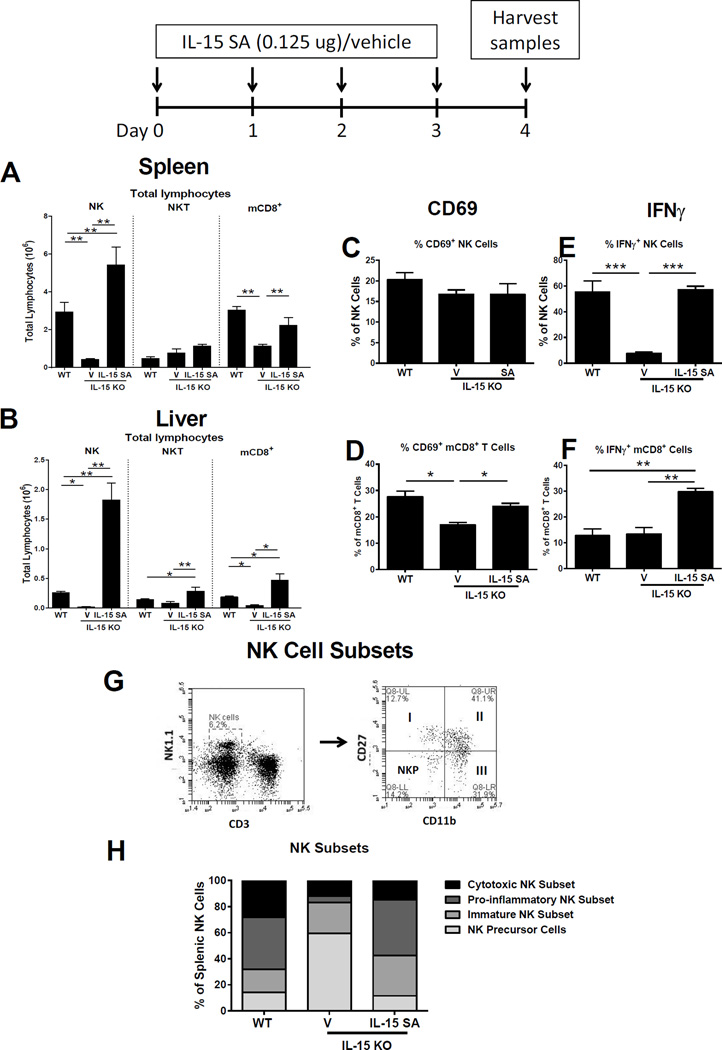
IL-15 KO mice were treated with IL-15 superagonist (0.125 µg/day) for 4 days and lymphocyte numbers in spleen and liver were examined. Total NK cell (CD3− NK1.1+) numbers in spleen and liver were significantly higher in IL-15 SA-treated IL-15 KO mice compared to vehicle-treated IL-15 KO mice and were ~1.9 fold higher in spleen and ~7.0 fold higher in liver compared to wild type mice (Figure 5A, B). After IL-15 SA treatment, splenic mCD8+ T cells (CD8+CD44high) increased to levels equal to those observed in wild type mice and hepatic mCD8+ T cells to ~2.5 fold higher than in wild type mice (Figure 5A, B). NKT (CD3+ NK1.1+) cell numbers were increased in liver, but not in spleen, in IL-15 KO mice treated with IL-15 SA compared to vehicle-treated controls and wild type mice (Figure 5A,B). Naïve CD8+ T (CD8+CD44low), CD4+ T and B cells were not significantly different when comparing IL-15 KO mice treated with IL-15 SA or vehicle (data not shown).
Figure 5. Low dose IL-15 SA treatment restores NK and memory CD8+ T cells in IL-15 KO mice.
IL-15 KO mice were treated with 0.125 µg IL-15 SA for 4 consecutive days. At 24 hours following the last treatment, splenic (A) and liver (B) NK (CD3−NK1.1+), NKT (CD3+NK1.1+) and memory CD8+ T (CD8+CD44high) lymphocyte numbers were measured using flow cytometry. The activation status (CD69 expression) and IFNγ expression by NK (C and D) and memory CD8+ T cells (E and F) upon IL-15 SA treatment were also analyzed. For IFNγ expression, splenocytes from wild type mice and vehicle- and IL-15 SA-treated IL-15 KO mice were restimulated with PMA/ionomycin for 5 hours to amplify intracellular cytokine signaling (for details see method session). In the representative dot plot, NK cells are divided into four subpopulations based on CD11b and CD27 expression, namely precursor (CD11blowCD27low), immature (I, CD11blowCD27high), mature pro-inflammatory (II, CD11bhighCD27high) and mature cytotoxic (III, CD11bhighCD27low) NK cells (G). The graph in Figure H shows the relative number of splenic NK subsets among intact wild type mice, vehicle- and IL-15 SA-treated IL-15 KO mice. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 compared to designated groups. n=4–12 mice per group. Data are representative of two to four separate experiments.
The impact of IL-15 SA treatment on regenerated NK and mCD8+ T cell activation and function was determined by examining expression of the early surface activation marker CD69 and production of IFNγ. Expression of CD69 on splenic NK cells in IL-15 KO mice treated with IL-15 SA was not different compared to vehicle-treated IL-15 KO mice or wild type mice (Figure 5C). The numbers of mCD8+ T cells expressing CD69 was increased in IL-15 KO mice treated with IL-15 SA as compared to vehicle-treated IL-15 KO controls but not compared to wild type control (Figure 5D). PMA/ionomycon-induced IFNγ production by NK cells was significantly higher in IL-15 KO mice treated with IL-15 SA as compared to those treated with vehicle and was not different than in wild type controls (Figure 5E). PMA/ionomycin-induced IFNγ production by mCD8+ T cells was significantly higher in IL-15 SA-treated mice compared to wild type controls and vehicle-treated IL-15 KO mice (Figure 5F).
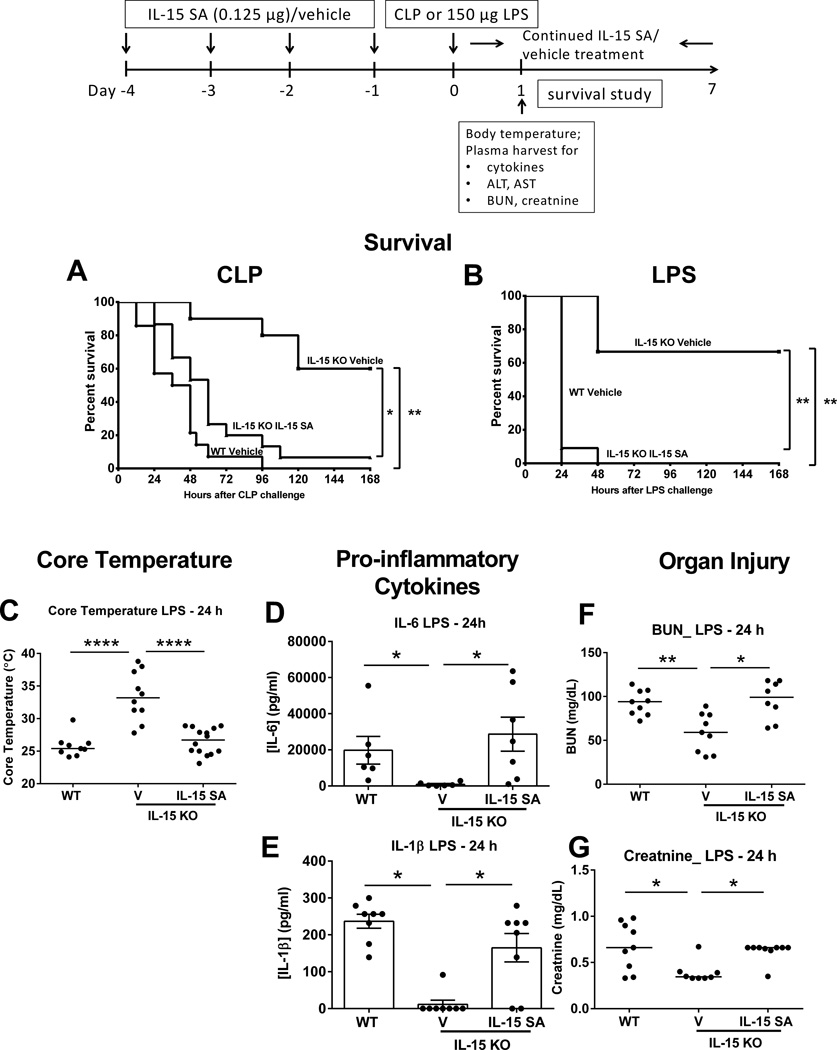
NK cell subsets were characterized based on CD11b and CD27 expression and classified as precursor (CD11blowCD27low), immature (I, CD11blowCD27high), pro-inflammatory (II, CD11bhighCD27high) or cytotoxic (III, CD11bhighCD27low) (Figure 5G) (26). IL-15 SA treatment increased the numbers of immature and pro-inflammatory NK cells in IL-15 KO mice to a level significantly higher or comparable to wild type controls but did not increase the numbers of the cytotoxic subset (Figure 5H). Further studies were undertaken to assess CLP- or LPS-induced mortality of IL-15 KO mice treated with IL-15 SA for 4 days prior to septic challenge. IL-15 SA treatment was continued daily throughout the experimental period to maintain regenerated NK and mCD8+ T cell populations. During septic shock elicited by CLP or 150 µg LPS, IL-15 SA reestablished mortality in IL-15 KO mice to a level comparable to wild type control and significantly higher than IL-15 KO mice treated with vehicle (Figure 6A, B). IL-15 KO mice treated with IL-15 SA also showed a significant decrease in core body temperature after LPS challenge compared to vehicle-treated IL-15 KO mice which was comparable to that observed in wild type mice (Figure 6C). Production of pro-inflammatory cytokines, including IL-6 and IL-1β, was also increased in IL-15 SA-treated IL-15 KO mice at 24 hours after LPS compared to vehicle-treated IL-15 KO mice (Figure 6D, E). IL-15 SA treatment also prompted the development of acute kidney injury in IL-15 KO mice as indicated by elevated plasma BUN and creatinine concentrations after LPS challenge (Figure 6F, G). But IL-15 SA did not exacerbate liver injury as it did not increase concentrations of ALT and AST in the plasma following LPS challenge (data not shown).
Figure 6. Low dose IL-15 SA treatment restores IL15-mediated lethality to septic shock in IL-15 KO mice.
IL-15 SA (0.125 µg) treatment was initiated 4 days prior to septic challenge and continued throughout the experimental period to maintain regenerated NK and mCD8+ T cell populations. Mice were monitored for survival rate over 7 days during CLP- or 150 µg LPS-induced sepsis (A and B). Core temperature was measured at 24 hours after 150 µg LPS challenge (C). Vehicle-treated wild type and IL-15 KO mice served as control. Blood was collected from wild type and IL-15 KO mice at 24 hours after 150 µg LPS challenge for measurement of IL-6 and IL-1β (D and E) as well as ALT, AST, BUN and creatinine (F and G) concentrations in plasma. * p < 0.05, ** p < 0.01, **** p < 0.0001 compared to designated groups. n=7–14 mice per group. Data are representative of two to four separate experiments.
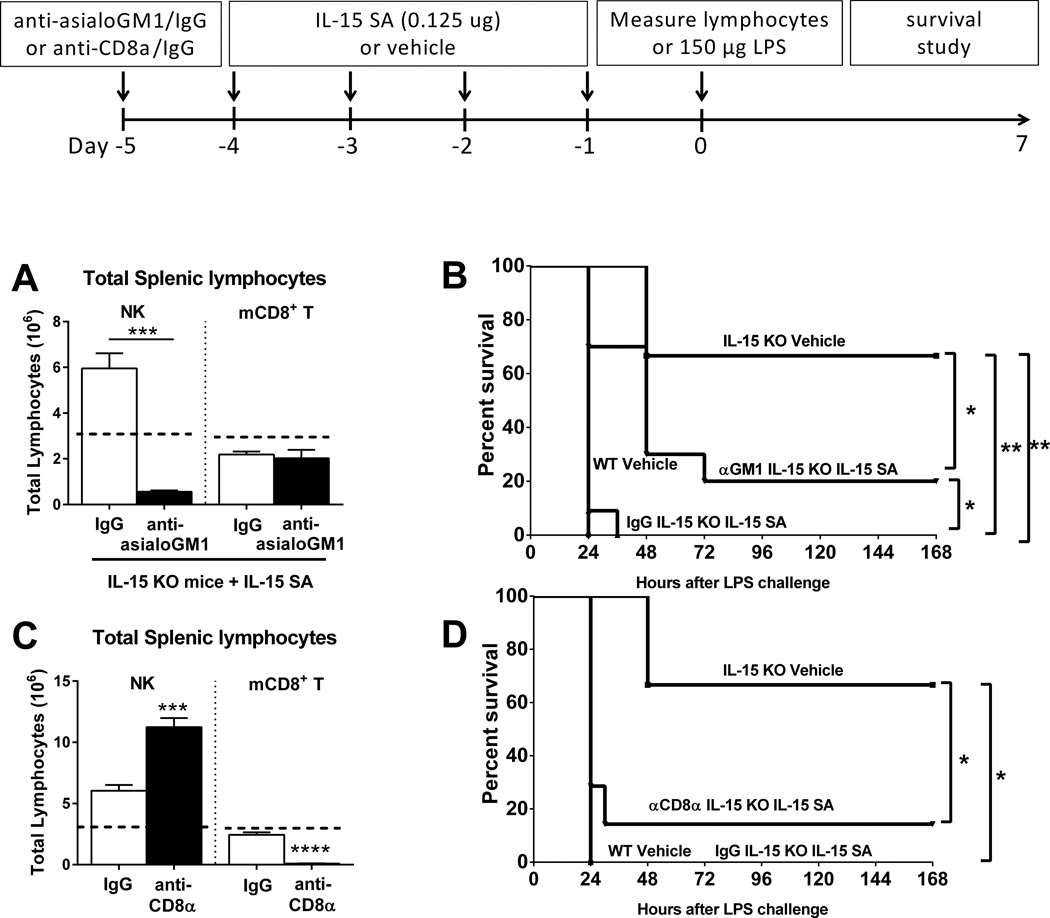
Blockade of IL-15 SA-induced NK cell, but not CD8+ T cell, regeneration protects IL-15 KO mice from septic shock
Treatment with anti-asialoGM1 prior to the initiation of IL-15 SA treatment, prevented NK cell regeneration in IL-15 KO mice (Figure 7A) and also partially, but significantly, reversed endotoxin-induced mortality in IL-15 SA-treated IL-15 KO mice upon 150 µg LPS challenge (Figure 7B). However, preventing CD8+ T cell regeneration by treatment with anti-CD8α (Figure 7C) failed to rescue IL-15 KO mice treated with IL-15 SA during LPS-induced shock (Figure 7D).
Figure 7. Neutralization of NK cells ablates IL-15 SA treatment-induced restoration of lethality to septic shock in IL-15 KO mice.
IL-15 KO mice received anti-asialoGM1 or anti-CD8α at 24 hours prior to the initiation of IL-15 SA (0.125 µg) and NK and memory CD8+ T lymphocyte counts were measured at 24 hours after the lastIL-15 SA treatment. Dotted lines represent the baseline levels of NK and memory CD8+ T cells in wild type controls (A and C). A survival study was also undertaken to assess the effect of anti-asialoGM1 or anti-CD8α on the survival of IL-15 SA-treated IL-15 KO mice upon 150 µg LPS challenge (B and D). Isotype-specific IgG served as control. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 compared to designated groups. n=5–15 mice per group. Data are representative of two to four separate experiments.
Prolonged, rather than acute, neutralization of IL-15 protects against septic shock
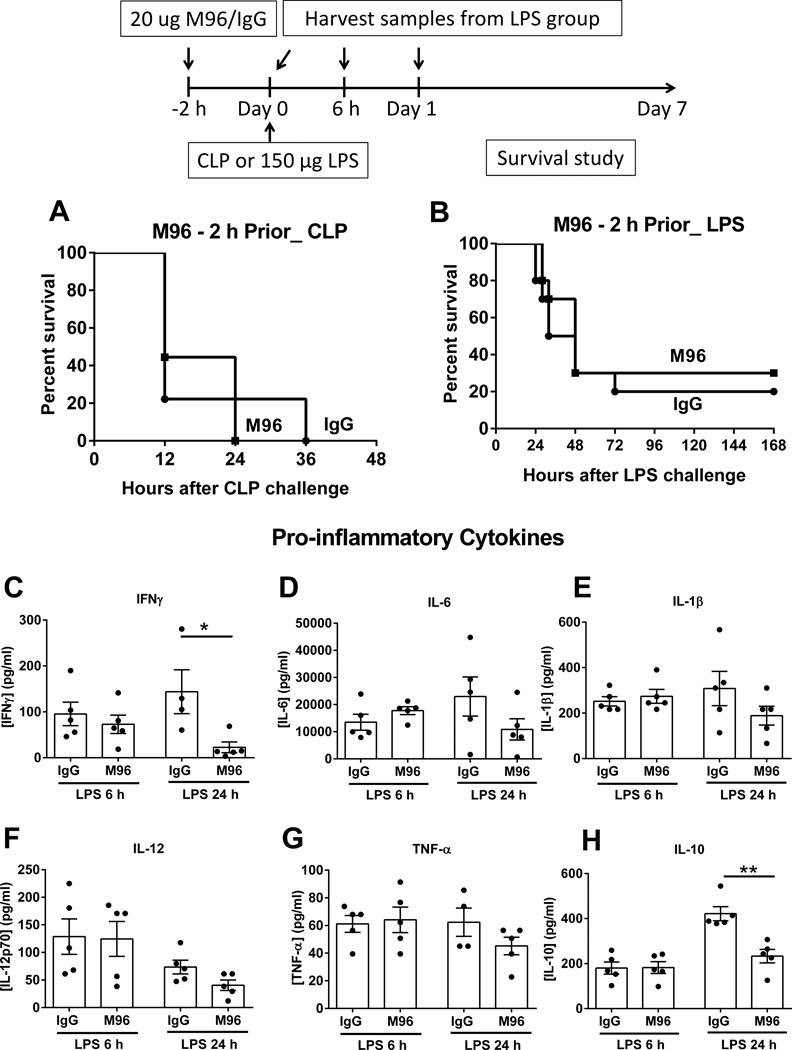
Generally, IL-15 is retained inside the cell until it is chaperoned with the high-affinity receptor α and shuttled to the cell surface for delivery to target cells, a process called trans-presentation. But the IL-15/IL-15Rα complex in a soluble form is also present in plasma after proteolytic cleavage of the transmembrane domain of IL-15Rα from the cell surface. Plasma IL-15/IL-15Rα complex concentration was elevated in wild type mice during shock induced by CLP or LPS and was neutralized by treatment with M96, an IL-15 neutralizing antibody, when given to WT mice 2 hours prior to CLP or LPS (Supplemental Figure 2). However, short-term neutralization of IL-15 by M96 failed to confer survival benefit to wild type mice compared to IgG-treated controls after CLP and 150 µg LPS challenges (Figure 8A, B). Examination of cytokine expression profile shows that the acute neutralization of IL-15 did not alter concentrations of other plasma pro-inflammatory cytokines except IFN-γ, which was lower compared to IgG control at 24 hours after 150 µg LPS challenge (Figure 8C–G). The level of anti-inflammatory cytokine IL-10 was lower in M96-treated wild type mice at 24 hours after LPS (Figure 8H). Under the current treatment regimen, M96 didn’t decrease the number of NK cells until 24 hours after LPS challenge. M96 did not alter the number of mCD8+ T cells or the activation of NK and mCD8+ T cells as indicated by CD69 expression at 6 and 24 hours after LPS challenge as compared to IgG control (Supplemental Figure 3).
Figure 8. Acute neutralization of IL-15 does not mediate resistance to septic shock.
Wild type mice received 20 µg of M96, an IL-15 neutralizing antibody i.p. at 2 hours prior to CLP or 150 µg LPS challenge and a survival study was followed (A and B). Specific IgG serve as control. Concentrations of IFNγ, IL-6, IL-1β, IL-12, TNF-α and IL-10 in the plasma were measured at 6 and 24 hours after 150 µg LPS challenge (C–H). * p < 0.05, ** p < 0.01 compared to IgG control. n=5–10 mice per group Data are representative of two to three separate experiments.
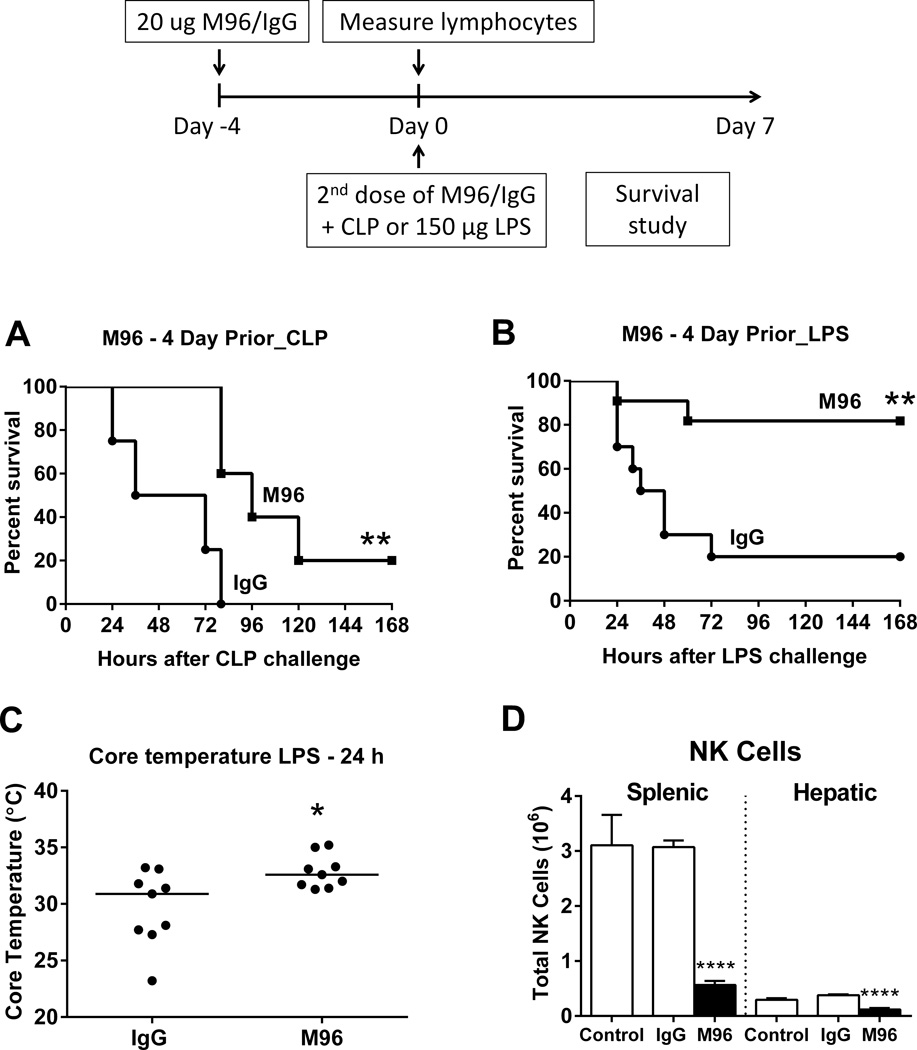
Further studies were undertaken to assess the effects of prolonged M96 pretreatment on CLP- or LPS-induced mortality in wild type mice. M96 treatment was initiated 4 days prior to septic challenge and a second administration was given at the time of CLP or 150 µg LPS. As opposed to acute neutralization of IL-15, long-term IL-15 neutralization partially reversed septic mortality of wild type mice upon CLP challenge and significantly protected wild type mice from shock induced by LPS (Figure 9A, B). Under the current treatment regimen of M96, wild type mice exhibited attenuated hypothermia at 24 hours after LPS challenge (Figure 9C). Analysis of splenic and hepatic lymphocyte populations at 4 days after M96 treatment showed depletion of splenic and hepatic NK cells (Figure 9D). However, mCD8+, NKT, naïve CD8+, CD4+ and B lymphocyte numbers were not altered by pretreatment with M96 (data not shown).
Figure 9. Prolonged neutralization of IL-15 mediates resistance to septic shock.
Wild type mice received 20 µg of IL-15 neutralizing antibody M96 or IgG i.p. at 4 days prior to (day -4) and at the time of CLP or 150 µg LPS challenge (day 0). A survival study was undertaken over 7 days (A and B). n=8–11 mice per group. Core temperature was measured at 24 hours after 150 µg LPS challenge (C). n= 9 mice per group. Splenic and hepatic NK cell number was measured at day 0 without LPS challenge (D). n= 6–9 mice/group. ** p < 0.01, **** p < 0.0001, compared to IgG control. n=8–11 mice per group in survival studies. Data are representative of two to three separate experiments.
High-dose IL-15 SA treatments augment sepsis-induced pathogenesis in WT mice
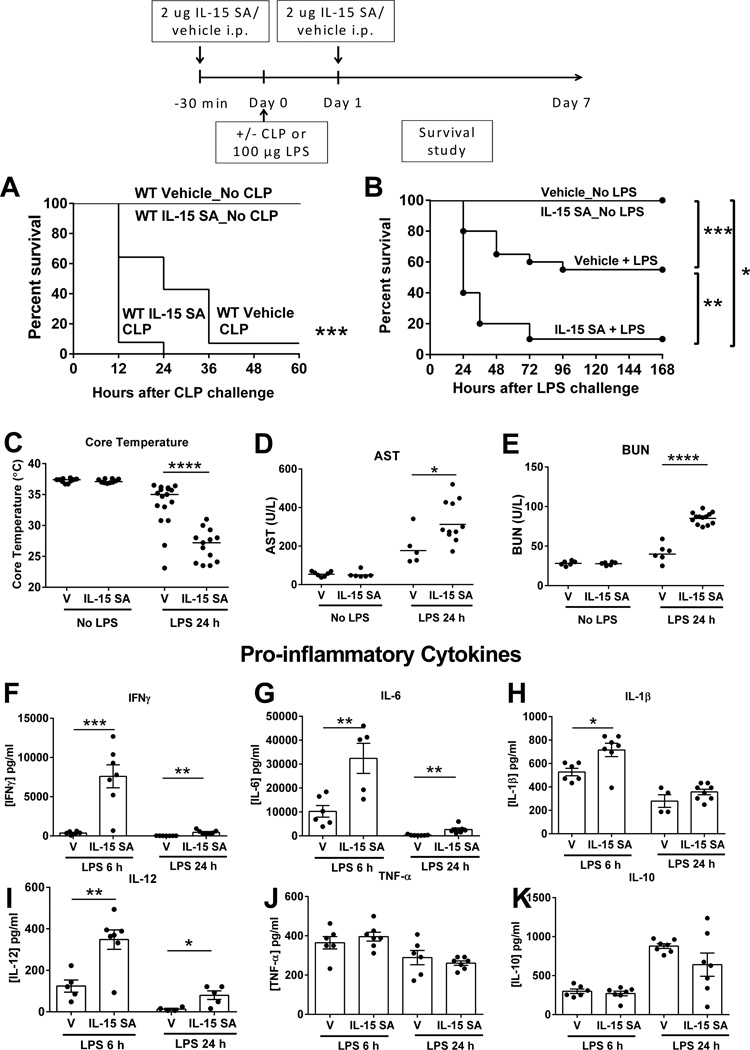
Previous studies from our lab showed that systemic administration of IL-15 SA at 2 µg for 4 consecutive days caused immuno-toxicity characterized by hypothermia, weight loss, liver injury, and mortality in wild type mice (27). Here, studies were performed to examine the effect of exogenous 2 µg IL-15 SA on survival of wild type mice upon CLP or LPS (100 µg) challenge. If given 30 minutes prior to and 24 hours after LPS challenge, IL-15 SA (2 µg) worsened mortality compared to vehicle control, while treatment with IL-15 SA alone did not cause any signs of toxicity or mortality (Figure 10A, B). IL-15 SA treatment also exacerbated LPS-induced hypothermia and increased levels of AST and BUN in the plasma of wild type mice after LPS challenge (Figure 10C–E). Concentrations of IFNγ, IL-6, IL-1β and IL-12 were significantly higher in the plasma of wild type mice treated with IL-15 SA at 6 and/or 24 hours after LPS challenge, while levels of TNF-α and IL-10 were not altered (Figure 10F–K). IL-15 SA facilitated the rapid mobilization of NK cells out of spleens at 1 hour following LPS challenge. IL-15 also promoted the activation of NK and mCD8+ T cells as indicated by the upregulation in CD69 expression as early as 1 hour after LPS challenge (Supplemental Figure 4).
Figure 10. High dose IL-15 SA pre-treatment accentuates lethality to septic shock.
Wild type mice received vehicle or IL-15 SA at 2 µg 30 minutes prior to and 24 hours after CLP or LPS (100 µg) and survival studies were followed (A, B). Core temperature, AST and BUN levels, and IFNγ, IL-6, IL-1β, IL-12, TNF-α and IL-10 concentrations in the plasma were measured at 24 hours after 100 µg LPS (C–K)..* p < 0.05, ** p < 0.01, *** p < 0.001, compared to vehicle wild type control. n=5–20 mice per group. Data are representative of two to three separate experiments.
Additional studies were performed to determine the effect of IL-15 SA on the pathogenesis of sepsis in wild type mice when given after the onset of sepsis. IL-15 SA (2 ug) or vehicle treatment was given to wild type mice (16– to 20-week-old) at 2 and 18 hours after CLP challenge. Post-treatment with IL-15 SA induced 100% mortality as compared to 22% mortality in vehicle-treated mice (Figure 11A). At 18 hours after CLP challenge, IL-15 SA-treated mice exhibited more hypothermia, increased bacterial burden in the blood and peritoneal cavity as compared to vehicle controls (Figure 11B–D). In addition, concentrations of IFNγ, IL-6 and TNF-α were significantly higher in the plasma of wild type mice treated with IL-15 SA after otherwise sublethal CLP challenge (Figure 11E–G).
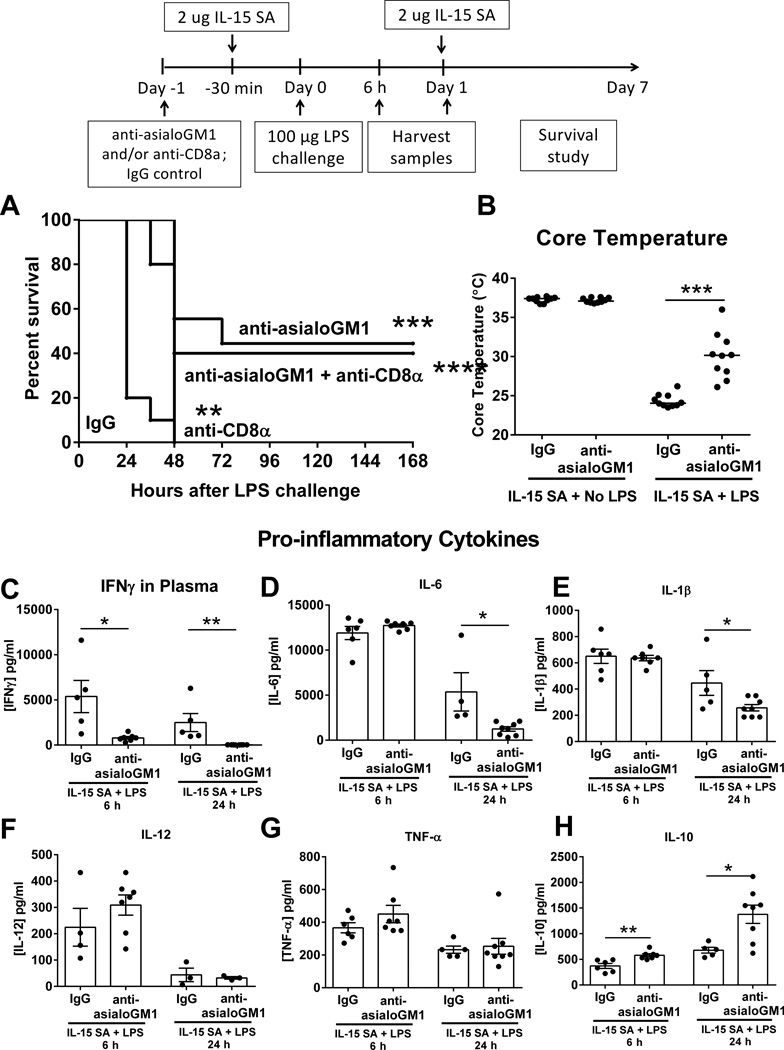
Further studies determined the contributions of NK and CD8+ T cells to IL-15 SA-exacerbated mortqality caused by LPS challenge. Depletion of NK cells alone or in combination with CD8+ T cells attenuated mortality caused by the combined administration of IL-15 SA and 100 µg LPS (Figure 12A). Depletion of CD8+ T cells alone prolonged the time to 100% mortality but did not improve long-term survival. (Figure 12A). Moreover, NK cell depletion attenuated hypothermia and production of some, but not all, pro-inflammatory cytokines in wild type mice treated with IL-15 SA and LPS (Figure 12B–H).
Figure 12. Neutralization of NK cells and CD8+ T cells combined, NK cells, but not CD8 T cells alone, mediates resistance of IL-15 SA treated mice to septic shock.
Wild type mice received anti-asialoGM1 and/or anti-CD8α i.p. at 24 hours prior to IL-15 SA treatment and then were challenged with 100 µg LPS and a second dose of IL-15 SA at 24 hours thereafter. Survival rate was monitored over 7 days (A). Isotype-specific IgG serve as control. Core temperature and IFNγ, IL-6, IL-1β, IL-12, TNF-α and IL-10 concentrations in the plasma were measured at 6 and/or 24 hours after 100 µg LPS (B–H). ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared to IgG wild type mice that were treated with 2 µg IL-15 SA. n=5–10 mice per group. Data are representative of two to three separate experiments.
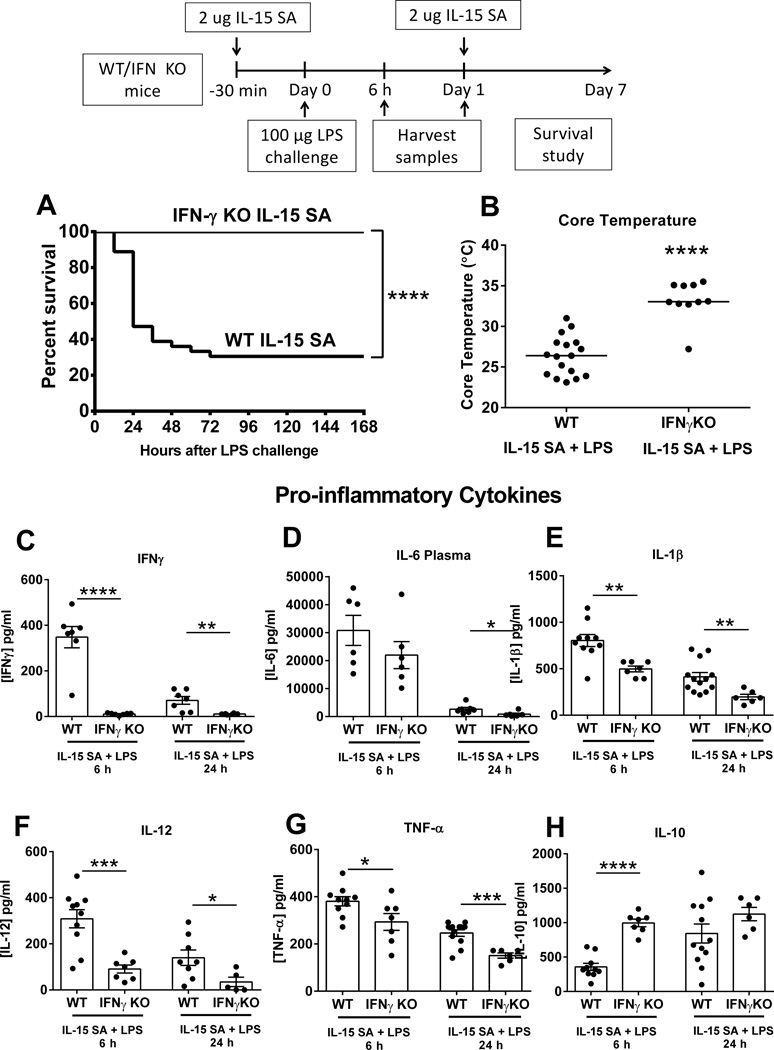
IFN KO mice are resistant to high dose-IL-15 SA-mediated lethality after septic shock
Additional studies were performed to examine the functional importance of IFNγ in the IL-15 SA-mediated exacerbation of septic severity. IFNγ KO mice exhibited improved survival and attenuated hypothermia during shock induced by co-administration of IL-15 SA and LPS (Figure 13A, B). At 6 and/or 24 hours after LPS challenge, IFNγ was not detected in the plasma of IFNγ KO, while IL-6, IL-1β, IL-12 and TNF-α concentrations were significantly lower in the plasma of IFNγ KO mice compared to wild type controls (Figure 13C–H). The anti-inflammatory cytokine IL-10 was significantly elevated after LPS challenge in IFNγ KO mice after IL-15 SA treatment.
Figure 13. IFNγ KO mice are resistant to IL-15 SA-induced accentuation of septic shock.
IFNγ KO mice received intraperitoneal injection with 2 µg of IL-15 SA 30 minutes prior to and 24 hours after LPS (100 µg) challenge. A survival study was performed over 7 days (A). Core temperature and IFNγ, IL-6, IL-1β, IL-12, TNF-α and IL-10 concentrations in the plasma were measured at 6 and/or 24 hours after 100 µg LPS (B–H). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 compared to wild type control treated with IL-15 SA. n=5–10 mice per group. Data are representative of two separate experiments.
Discussion
Although previous studies have shown that IL-15 exhibits toxicity (27) and aggravates chronic inflammatory disorders (32–38), there are few studies examining the role of endogenous and exogenous IL-15 in the pathogenesis of acute inflammation, which is typical of sepsis (40). This study supports the hypothesis that IL-15 plays a role in the pathogenesis of septic shock via maintenance and activation of NK cells and, to a lesser degree, CD8+ T cells. We report that mice genetically deficient in IL-15 show a significant survival benefit over wild type mice during CLP- and LPS-induced septic shock. IL-15 KO mice display markedly decreased NK and mCD8+ T cell numbers and attenuated sepsis-induced hypothermia, hepatocellular and kidney injuries and systemic pro-inflammatory cytokine production compared to wild type mice. Similarly, prolonged neutralization of IL-15 over a 4-day period causes NK cell depletion and provides protection from septic shock. Treatment of IL-15 KO mice with low-dose IL-15 SA regenerates NK cells and reestablishes susceptibility to septic shock, whereas acute neutralization of IL-15 in wild type mice fails to provide protection. In addition, treatment of wild type mice with high-dose IL-15 SA prompts the activation of NK cells and worsens septic outcomes, which is prevented by NK cell depletion or IFNγ neutralization. Thus, we conclude that IL-15 contributes to the pathogenesis of acute septic shock by maintaining and activating NK and, possibly, mCD8+ T cells. These cells facilitate systemic inflammation and organ injury during septic shock through a mechanism that is dependent on the production of IFNγ.
Orinska et al previously showed the genetic deletion of IL-15 results in improved survival and better bacterial clearance during the early phases of septic shock induced by CLP (28). The present study also demonstrates improved survival in IL-15 KO mice and a small, but significant, improvement in bacterial clearance at 6 hours after CLP. However, we didn’t observe differences in bacterial burden in blood or peritoneal cavity among groups at 18 hours after CLP. In another experimental model of septic shock elicited by injection with LPS, IL-15 KO mice also display protection, although no live bacterial infection is present. Thus, our studies suggest that the improved outcomes in IL-15 KO mice are associated with attenuated inflammation as evidenced by decreased systemic cytokine production in both the CLP and LPS models rather than alterations in bacterial clearance mechanisms.
NK cells are large granular innate lymphoid cells that play an essential role in tumor surveillance and elimination of virus-infected cells (29, 30). However, as shown in previous studies from our lab and others, NK cells participate in the propagation of acute inflammation and physiological dysfunctions in experimental models of polymicrobial peritonitis (31), endotoxin shock (32), pneumococcal pneumonia (33), systemic Escherichia coli and Streptococcus pyogenes infection (34, 35), and polytrauma (36). Our lab previously showed that NK cells quickly migrate to sites of infection during intraabdominal sepsis, in a manner regulated by the chemokines CXCL9 and CXCL10 (23, 37–39). During sepsis, activated NK cells increased production of IFN-γ, which is known to potentiate inflammation by activating macrophages, dendritic cells and other innate immune cells (31, 40). In the current study, we show that loss of NK cells is a major factor by which IL-15 KO mice are protected from septic shock. Thus, using a unique model, we provide further evidence that NK cells play an important role in augmenting acute inflammation and contributing to the pathogenesis of organ injury and physiological dysfunction during septic shock.
Our lab also reported the contribution of total CD8+ T cells to the pathogenesis of septic shock as CD8+ T cell-deficient mice show improved survival during intraabdominal sepsis induced by CLP (17, 19, 41). However, there is some controversy about the role of CD8+ T cells in the pathogenesis of septic shock. β2-microglobin KO mice, which lack CD8+ T cells, are more susceptible to LPS-induced endotoxin shock, although these mice have been shown to be protected from CLP (41). The present study further showed antibody-mediated depletion of CD8+ T cells in wild type mice fails to confer protection from LPS-induced septic shock. Preventing CD8+ T cell regeneration fails to attenuate septic mortality in IL-15 SA-treated IL-15 KO mice. Thus, we conclude that lack of CD8+ T cells doesn’t contribute significantly to the resistance of IL-15 KO mice to septic shock. However, the specific contribution of memory CD8+ T cell subset to the pathogenesis of septic shock has not been well characterized, since it is currently not possible to selectively deplete these cells.
NKT cells are increased in IL-15 KO mice after treatment with low-dose IL-15 SA. It is possible that NKT cells also play a pathogenic role during septic shock as NKT-deficient mice are shown to be resistant to CLP-induced septic shock (42). However, currently there are no antibodies available that can selectively deplete NKT cells in IL-15 SA-treated IL-15 KO mice so our exploration of the role of NKT cells in this setting is limited. Nevertheless, we did not note a significant decrease in NKT cell numbers in IL-15 KO mice. Thus, loss of NKT cells does not appear to contribute to the resistance of IL-15 KO mice to septic shock.
IL-15 KO mice lack not only NK cells but also the cytokine itself. It is unclear whether IL-15 alone plays an acute role in the pathobiology of sepsis. Here, we showed that concentration of soluble IL-15/IL-15Rα was elevated in the plasma of septic mice compared to non-septic mice. Recent studies correlate elevated serum IL-15 concentration with the development of organ dysfunction and mortality in patients with septic shock (43). Thus, it is possible that IL-15 alone plays a detrimental role in the pathogenesis of septic shock. The current study shows that acute neutralization of IL-15 at the onset of septic shock failed to confer protection, while long-term neutralization of IL-15 prior to septic shock conferred protection. Further studies showed that acute neutralization of IL-15 neither depletes NK cells nor blocks NK cell activation at the early phase of septic shock. In contrast, long-term neutralization of IL-15 depleted 80.8% of splenic NK cells. Therefore, our studies suggest that endogenous IL-15 does not play an acute role in the pathogenesis of septic shock but contributes to septic mortality by maintaining NK cells which aggravate systemic inflammation.
Lastly, we reported that treatment of wild type mice with higher doses of IL-15 SA (2 µg) exacerbates physiological dysfunctions and mortality during septic shock when initiated either immediately before or shortly after CLP and/or LPS challenge. Activated NK cells primarily mediate the worsened septic lethality caused by co-administration of IL-15 SA. Another recent paper from our laboratory showed that IL-15 SA is effective in expanding NK, NKT and mCD8+ T cells in burned mice but does not improve survival in a model of Pseudomonas burn wound infection (44). In contrast, Inoue et al reported that treatment with exogenous IL-15 after CLP challenge attenuates sepsis-induced apoptosis, reverses associated immune dysfunction and improves survival during sepsis (45). The reasons for differences observed between the studies are not entirely clear but could be due to the use of different mouse strains, dosage of IL-15 SA, delivery routes of IL-15 SA as well as different severity of our models of sepsis. Inbred male and female mice on the C57Bl/6J background were employed in the present study whereas Inoue and colleagues employed male mice in the outbred CD-1 strain of mice. Investigators have noted differences in the response to sepsis among inbred and outbred strains of mice and these differences may partly explain the dissimilarities in our results (46). It is unlikely that gender differences played a role given that many experiments in each study were performed using male mice. A slightly higher dose of IL-15 SA (2 vs 1.5 µg) was used in our study and could have resulted in a more pronounced pro-inflammatory response. Finally, the severity of models in our post-treatment experiments were different. Our study employed a low lethality model (22% mortality in vehicle treated mice) whereas a more severe model was employed in their study (75–90% mortality). Regardless of these differences, our current study indicates that IL-15 should be used with caution as an immunomodulator in subjects with acute sepsis due to its potential to augment systemic inflammatory responses. Nevertheless, it is important to consider proper dosing of IL-15 SA since lower doses than used in either of our studies could provide therapeutic benefit and minimal toxicity.
In conclusion, the present study demonstrates the role of IL-15 in the pathogenesis of septic shock. Endogenous IL-15 doesn’t play an acute role in aggravating septic mortality but is able to facilitate sepsis-induced systemic inflammation, hypothermia, acute organ injuries and death by maintaining the NK cell pool. Exogenous IL-15 (IL-15 SA), at higher doses, potently activates NK cells and increases the susceptibility of mice to endotoxin shock. Results of this study and others also indicate that IFNγ contributes significantly to NK cell-mediated inflammation and injury during septic shock as well as in IL-15-induced exacerbation of sepsis-associated pathobiology.
Supplementary Material
Acknowledgments
IL-15 neutralizing antibody M96 was generously provided by Amgen (Thousand oaks, CA).
Footnotes
This study was supported by NIH Grant R01 GM66885 and R01 GM104306.
N.K.P is supported by American Heart Association Postdoctoral Grant (16POST29920007).
References
- 1.Puzanov IJ, Bennett M, Kumar V. IL-15 can substitute for the marrow microenvironment in the differentiation of natural killer cells. Journal of immunology. 1996;157:4282–4285. [PubMed] [Google Scholar]
- 2.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo JP. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. The Journal of experimental medicine. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. The Journal of experimental medicine. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 5.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. Journal of immunology. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 6.Malamut G, El Machhour R, Montcuquet N, Martin-Lanneree S, Dusanter-Fourt I, Verkarre V, Mention JJ, Rahmi G, Kiyono H, Butz EA, Brousse N, Cellier C, Cerf-Bensussan N, Meresse B. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. The Journal of clinical investigation. 2010;120:2131–2143. doi: 10.1172/JCI41344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. The Journal of experimental medicine. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 10.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 11.Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. Journal of immunology. 2001;167:1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad R, Ennaciri J, Cordeiro P, El Bassam S, Menezes J. Herpes simplex virus-1 up-regulates IL-15 gene expression in monocytic cells through the activation of protein tyrosine kinase and PKC zeta/lambda signaling pathways. Journal of molecular biology. 2007;367:25–35. doi: 10.1016/j.jmb.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 13.Blaser BW, Schwind NR, Karol S, Chang D, Shin S, Roychowdhury S, Becknell B, Ferketich AK, Kusewitt DF, Blazar BR, Caligiuri MA. Trans-presentation of donor-derived interleukin 15 is necessary for the rapid onset of acute graft-versus-host disease but not for graft-versus-tumor activity. Blood. 2006;108:2463–2469. doi: 10.1182/blood-2006-04-019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castillo EF, Stonier SW, Frasca L, Schluns KS. Dendritic cells support the in vivo development and maintenance of NK cells via IL-15 trans-presentation. Journal of immunology. 2009;183:4948–4956. doi: 10.4049/jimmunol.0900719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han KP, Zhu X, Liu B, Jeng E, Kong L, Yovandich JL, Vyas VV, Marcus WD, Chavaillaz PA, Romero CA, Rhode PR, Wong HC. IL-15:IL-15 receptor alpha superagonist complex: high-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine. 2011;56:804–810. doi: 10.1016/j.cyto.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherwood ER, Enoh VT, Murphey ED, Lin CY. Mice depleted of CD8+ T and NK cells are resistant to injury caused by cecal ligation and puncture. Laboratory investigation; a journal of technical methods and pathology. 2004;84:1655–1665. doi: 10.1038/labinvest.3700184. [DOI] [PubMed] [Google Scholar]
- 18.Enoh VT, Fairchild CD, Lin CY, Varma TK, Sherwood ER. Differential effect of imipenem treatment on wild-type and NK cell-deficient CD8 knockout mice during acute intra-abdominal injury. American journal of physiology. Regulatory, integrative and comparative physiology. 2006;290:R685–R693. doi: 10.1152/ajpregu.00678.2005. [DOI] [PubMed] [Google Scholar]
- 19.Tao W, Enoh VT, Lin CY, Johnston WE, Li P, Sherwood ER. Cardiovascular dysfunction caused by cecal ligation and puncture is attenuated in CD8 knockout mice treated with anti-asialoGM1. American journal of physiology. Regulatory, integrative and comparative physiology. 2005;289:R478–R485. doi: 10.1152/ajpregu.00081.2005. [DOI] [PubMed] [Google Scholar]
- 20.Orinska Z, Maurer M, Mirghomizadeh F, Bulanova E, Metz M, Nashkevich N, Schiemann F, Schulmistrat J, Budagian V, Giron-Michel J, Brandt E, Paus R, Bulfone-Paus S. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat Med. 2007;13:927–934. doi: 10.1038/nm1615. [DOI] [PubMed] [Google Scholar]
- 21.Chung KP, Chang HT, Lo SC, Chang LY, Lin SY, Cheng A, Huang YT, Chen CC, Lee MR, Chen YJ, Hou HH, Hsu CL, Jerng JS, Ho CC, Huang MT, Yu CJ, Yang PC. Severe lymphopenia is associated with elevated plasma interleukin-15 levels and increased mortality during severe sepsis. Shock. 2015;43:569–575. doi: 10.1097/SHK.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 22.Kimura A, Ono S, Hiraki S, Takahata R, Tsujimoto H, Miyazaki H, Kinoshita M, Hatsuse K, Saitoh D, Hase K, Yamamoto J. The postoperative serum interleukin-15 concentration correlates with organ dysfunction and the prognosis of septic patients following emergency gastrointestinal surgery. J Surg Res. 2012;175:e83–e88. doi: 10.1016/j.jss.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Herzig DS, Luan L, Bohannon JK, Toliver-Kinsky TE, Guo Y, Sherwood ER. The role of CXCL10 in the pathogenesis of experimental septic shock. Critical care. 2014;18:R113. doi: 10.1186/cc13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebrec H, Horner MJ, Gorski KS, Tsuji W, Xia D, Pan WJ, Means G, Pietz G, Li N, Retter M, Shaffer K, Patel N, Narayanan PK, Butz EA. Homeostasis of human NK cells is not IL-15 dependent. J Immunol. 2013;191:5551–5558. doi: 10.4049/jimmunol.1301000. [DOI] [PubMed] [Google Scholar]
- 25.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 26.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Luan L, Rabacal W, Bohannon JK, Fensterheim BA, Hernandez A, Sherwood ER. IL-15 Superagonist-Mediated Immunotoxicity: Role of NK Cells and IFN-gamma. Journal of immunology. 2015;195:2353–2364. doi: 10.4049/jimmunol.1500300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orinska Z, Maurer M, Mirghomizadeh F, Bulanova E, Metz M, Nashkevich N, Schiemann F, Schulmistrat J, Budagian V, Giron-Michel J, Brandt E, Paus R, Bulfone-Paus S. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nature medicine. 2007;13:927–934. doi: 10.1038/nm1615. [DOI] [PubMed] [Google Scholar]
- 29.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nature reviews. Cancer. 2016;16:7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 30.Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annual review of immunology. 2013;31:163–194. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- 31.Etogo AO, Nunez J, Lin CY, Toliver-Kinsky TE, Sherwood ER. NK but not CD1-restricted NKT cells facilitate systemic inflammation during polymicrobial intra-abdominal sepsis. Journal of immunology. 2008;180:6334–6345. doi: 10.4049/jimmunol.180.9.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emoto M, Miyamoto M, Yoshizawa I, Emoto Y, Schaible UE, Kita E, Kaufmann SH. Critical role of NK cells rather than V alpha 14(+)NKT cells in lipopolysaccharide-induced lethal shock in mice. Journal of immunology. 2002;169:1426–1432. doi: 10.4049/jimmunol.169.3.1426. [DOI] [PubMed] [Google Scholar]
- 33.Christaki E, Diza E, Giamarellos-Bourboulis EJ, Papadopoulou N, Pistiki A, Droggiti DI, Georgitsi M, Machova A, Lambrelli D, Malisiovas N, Nikolaidis P, Opal SM. NK and NKT Cell Depletion Alters the Outcome of Experimental Pneumococcal Pneumonia: Relationship with Regulation of Interferon-gamma Production. Journal of immunology research. 2015;2015:532717. doi: 10.1155/2015/532717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badgwell B, Parihar R, Magro C, Dierksheide J, Russo T, Carson WE., 3rd Natural killer cells contribute to the lethality of a murine model of Escherichia coli infection. Surgery. 2002;132:205–212. doi: 10.1067/msy.2002.125311. [DOI] [PubMed] [Google Scholar]
- 35.Goldmann O, Chhatwal GS, Medina E. Contribution of natural killer cells to the pathogenesis of septic shock induced by Streptococcus pyogenes in mice. The Journal of infectious diseases. 2005;191:1280–1286. doi: 10.1086/428501. [DOI] [PubMed] [Google Scholar]
- 36.Barkhausen T, Frerker C, Putz C, Pape HC, Krettek C, van Griensven M. Depletion of NK cells in a murine polytrauma model is associated with improved outcome and a modulation of the inflammatory response. Shock. 2008;30:401–410. doi: 10.1097/SHK.0b013e31816e2cda. [DOI] [PubMed] [Google Scholar]
- 37.Herzig DS, Driver BR, Fang G, Toliver-Kinsky TE, Shute EN, Sherwood ER. Regulation of lymphocyte trafficking by CXC chemokine receptor 3 during septic shock. American journal of respiratory and critical care medicine. 2012;185:291–300. doi: 10.1164/rccm.201108-1560OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bohannon J, Guo Y, Sherwood ER. The role of natural killer cells in the pathogenesis of sepsis: the ongoing enigma. Critical care. 2012;16:185. doi: 10.1186/cc11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herzig DS, Guo Y, Fang G, Toliver-Kinsky TE, Sherwood ER. Therapeutic efficacy of CXCR3 blockade in an experimental model of severe sepsis. Critical care. 2012;16:R168. doi: 10.1186/cc11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herzig D, Fang G, Toliver-Kinsky TE, Guo Y, Bohannon J, Sherwood ER. STAT1-deficient mice are resistant to cecal ligation and puncture-induced septic shock. Shock. 2012;38:395–402. doi: 10.1097/SHK.0b013e318265a2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherwood ER, Lin CY, Tao W, Hartmann CA, Dujon JE, French AJ, Varma TK. Beta 2 microglobulin knockout mice are resistant to lethal intraabdominal sepsis. Am J Respir Crit Care Med. 2003;167:1641–1649. doi: 10.1164/rccm.200208-950OC. [DOI] [PubMed] [Google Scholar]
- 42.Hu CK, Venet F, Heffernan DS, Wang YL, Horner B, Huang X, Chung CS, Gregory SH, Ayala A. The role of hepatic invariant NKT cells in systemic/local inflammation and mortality during polymicrobial septic shock. Journal of immunology. 2009;182:2467–2475. doi: 10.4049/jimmunol.0801463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura A, Ono S, Hiraki S, Takahata R, Tsujimoto H, Miyazaki H, Kinoshita M, Hatsuse K, Saitoh D, Hase K, Yamamoto J. The postoperative serum interleukin-15 concentration correlates with organ dysfunction and the prognosis of septic patients following emergency gastrointestinal surgery. The Journal of surgical research. 2012;175:e83–e88. doi: 10.1016/j.jss.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Patil NK, Luan L, Bohannon JK, Guo Y, Hernandez A, Fensterheim B, Sherwood ER. IL-15 Superagonist Expands mCD8+ T, NK and NKT Cells after Burn Injury but Fails to Improve Outcome during Burn Wound Infection. PloS one. 2016;11:e0148452. doi: 10.1371/journal.pone.0148452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, Osborne DF, Clark AT, Coopersmith CM, McDunn JE, Hotchkiss RS. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 2010;184:1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fink MP. Animal models of sepsis. Virulence. 2014;5:143–153. doi: 10.4161/viru.26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.