Abstract
Positive parenting has been related both to lower cortisol reactivity and more adaptive temperament traits in children, whereas elevated cortisol reactivity may be related to maladaptive temperament traits, such as higher negative emotionality (NE) and lower positive emotionality (PE). However, no studies have examined whether hypothalamic-pituitary-adrenal axis activity, as measured by cortisol reactivity, moderates the effect of the quality of the parent-child relationship on changes in temperament in early childhood. In this study, 126 3-year olds were administered the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith et al., 1995) as a measure of temperamental NE and PE. Salivary cortisol was collected from the child at 4 time points during this task. The primary parent and the child completed the Teaching Tasks battery (Egeland et al., 1995), from which the quality of the relationship was coded. At age 6, children completed the Lab-TAB again. From age 3 to 6, adjusting for age 3 PE or NE, a better quality relationship with their primary parent predicted decreases in NE for children with elevated cortisol reactivity and predicted increases in PE for children with low cortisol reactivity. Results have implications for our understanding of the interaction of biological stress systems and the parent-child relationship in the development of temperament in childhood.
Keywords: Cortisol, parenting, temperament, negative emotionality, positive emotionality
In the past few decades, three independent, but conceptually related, lines of research have developed pertaining to the interrelationships between parenting practices, children’s biological stress systems, and children’s temperament. There are now large bodies of literature on three specific relationships between these variables. First, much literature has established the effect of parenting behaviours on the development of the regulation of the hypothalamic-pituitary-adrenal (HPA) axis, one of the major stress-response systems, in children (see Gunnar & Quevedo, 2007 for a review). Second, evidence has also confirmed an effect of the quality of the parent-child relationship on the development of children’s temperament, such as positive emotionality (PE) and negative emotionality (NE; see Kiff et al., 2011; Lippscomb et al., 2011). Third, numerous studies have linked children’s temperament to alterations in HPA-axis reactivity (e.g., Gunnar and Donzella, 2002). Most research, however, has examined HPA axis reactivity as an outcome of either parenting or child temperament (e.g., Blair et al., 2008; Davis et al., 1999; Dettling et al., 1999; Gunnar et al., 1989; Smeekens et al., 2007; see Belsky & Pluess, 2009 for a discussion of this point). Individual differences in HPA-axis reactivity should also provide an index of children’s sensitivity to the effects of environmental factors on important aspects of psychological development, such as temperament. We are not aware of research that has conceptualized HPA-axis reactivity, as measured by variations in cortisol reactivity during a lab-based task, as a moderator of the effects of environmental experiences, such as parenting, on the development of temperament traits in children. As such, we examine whether HPA-axis regulation in three-year-old children during a lab-based task moderates the effect of the quality of the parent-child relationship on change in temperamental PE and NE over a period of three years during childhood, from age 3 to 6. Early childhood is a particularly important period in which to examine these effects, as children are highly dependent on their primary caregiver, making the parent-child relationship the most salient component of their environment (e.g., Maccoby, 1992), and this is the period during which temperament begins to stabilize (Caspi & Shiner, 2006; Roberts & DelVecchio, 2000). Moreover, focusing on the development of PE and NE is important given they are generally acknowledged to be core aspects of temperament or personality (see Klein et al., 2011), and are robustly associated with symptoms of psychopathology and other indices of psychosocial maladjustment (see Kotov et al., 2010).
Parenting, stress-reactivity, and childhood temperament
As noted above, all available research linking parenting and biological stress reactivity of which we are aware has examined cortisol reactivity as an outcome of parenting, with evidence supporting the contention that negative parenting practices dysregulate the HPA axis in children (see Gunnar & Quevedo, 2007, for a review). For instance, poorer quality of the child’s attachment to the parent, lower levels of caring maternal behaviours, disruptions to the parent-child relationship, family conflict, and parental maltreatment have all been related to elevations of the HPA stress response in childhood and adolescence (Albers et al., 2008; Ali & Pruessner, 2012; Blair et al., 2006; Blair et al., 2011; Dougherty et al., 2011; Gunnar et al., 2009; Luecken, 2000; Luecken & Lemery, 2004; Loman & Gunnar, 2010; Roisman et al., 2009; for reviews see Andrews et al., 2013; Gunnar & Quevedo, 2007; Hunter et al., 2011).
There are also robust relationships between parenting and child temperament. Temperament is broadly defined as individual differences in emotional and behavioural style that appear early in life, and is largely genetic in origin but is also influenced by environmental experiences (Rothbart & Bates, 2006). NE and PE are generally agreed to represent higher-order, core facets of child temperament (Caspi & Shiner, 2006; Zentner & Bates, 2008), and are respectively associated with the higher-order personality traits of neuroticism and extraversion later in life (Watson et al., 2005). NE is characterized by a tendency towards fear, anger, and sadness, whereas PE reflects exuberance, reward sensitivity, and engagement with the environment (Caspi & Shiner, 2006; Rothbart & Bates, 2006). NE and PE have important consequences for the social and psychological health and well-being of children (Compas et al., 2004; Sanson et al., 2004; Vitaro et al., 2006); as such, understanding factors that influence their development is highly important. Although children’s temperament traits often elicit different responses from parents (Kiff et al., 2011; Kopala-Sibley et al., 2012; Lengua & Kovacs, 2005), there is also evidence that parenting influences the subsequent development of temperament. For example, more negative parenting predicts increases in NE (Lengua & Kovacs, 2005; Lippscomb et al., 2011; see Kiff et al., 2011 for a review), whereas contingent responding and involvement predicts increases in positive emotionality (Belsky, Fish, & Isabella, 1991; Malatesta & Haviland, 1982).
Finally, numerous studies have established a link between child temperament and markers of HPA axis activity, although results have not always been consistent (Gunnar & Donzella et al., 2002). However, the majority of studies have reported positive associations between NE and cortisol levels. For instance, greater levels of NE predict greater increases in cortisol during the first day of kindergarten in three- to six-year-old children (Dettling et al., 1999). Although the literature on cortisol reactivity, or cortisol levels in stressful situations, and PE is much scarcer, a few studies have found decreased cortisol levels in infants or children who showed high positive affect or high PE (Dougherty et al., 2009; Fortunato et al., 2008; Hertsgaard et al., 1992). It is also worth noting that most studies of NE, PE, and cortisol have used single measures of cortisol levels during specific activities, situations, or times of day, rather than assessing cortisol reactivity over several time points during one task or set of tasks. One important exception is Donzella et al. (2000), who found greater increases in cortisol secretion 3- to 5-year-olds who had higher parent-reported surgency (a construct that overlaps with PE) after a competitive challenge relative to a baseline sample taken earlier in the day, although they found no effects of NE. In the current study, we examined cortisol reactivity by sampling cortisol four times during one lab visit.
A related issue is that the overlap between temperamental traits, such as NE, and physiological reactivity, such as HPA-axis activity, is unclear. Although research typically finds a significant correlation between the two, the size of this correlation is such that one does not appear to be only a manifestation of the other (Dettling et al., 1999; Dougherty et al., 2009; Gunnar & Quevedo, 2007). That is, the two may be linked via environmental moderators, such as parenting, as tested in this paper.
Although much literature has linked HPA-axis activity, temperament, and parenting, no research has examined cortisol as a moderator of the effects of parenting on change in temperament in childhood. HPA-axis reactivity has typically been conceptualized as a biomarker and/or outcome of temperament (e.g., Dougherty et al., 2009; Gunnar & Quevedo, 2007). That is, researchers have linked parenting to temperament via HPA-axis activity, or parenting to HPA-axis activity via temperament (i.e., mediation), but they have not examined HPA-axis activity as a moderator.
An alternative possibility is that greater cortisol reactivity in early childhood reflects higher susceptibility to the deleterious effects of environmental stressors (i.e., negative parenting) on the development of temperamental traits. This would be consistent with both the biological sensitivity to context (Boyce & Ellis, 2005; Ellis & Boyce, 2008) and differential susceptibility (Belsky & Pluess, 2009) models. For example, the biological sensitivity to context model proposes that cortisol reactivity is a trait measure of a heritable diathesis that interacts with context (e.g., parenting) to affect various developmental outcomes, such that highly reactive individuals suffer more in negative contexts, but benefit more in supportive environments than less reactive individuals (Boyce & Ellis, 2005; Ellis & Boyce, 2008). Similarly, the differential susceptibility model posits that there are stable individual differences in children’s characteristics, be they genetic, neurochemical, hormonal, or temperamental, which represent predispositions to responsiveness to the environment, and that children with these specific dispositions suffer more in negative, but benefit more in positive, environments than other children (Belsky & Pluess, 2009). Interestingly, Belsky & Pluess (2009) explicitly suggest that cortisol reactivity may represent a biological differential susceptibility factor. A handful of studies have supported this possibility, finding that individual differences in stress reactivity moderate the effects both of negative and positive contexts (e.g., parenting) on later psychopathological outcomes, such as internalizing and externalizing symptoms (e.g., Badanes, Watamura, & Hankin, 2011; Boyce et al., 2006; Hastings et al., 2011; Obradovic, Bush, Stamperdahl, Adler, & Boyce, 2010).
Although there are no extant data testing the possibility that individual differences in early childhood cortisol reactivity may also moderate the effects of context on the development of temperament traits, these theories converge in that both imply that children with greater cortisol reactivity should show change in temperament in what tends to be a relatively adaptive direction (i.e., decreased NE and increased PE), in the context of positive parenting, but should show temperamental change in a relatively less adaptive direction (i.e., increased NE and decreased PE), in the context of negative parenting.
Overview and hypotheses
The overarching aim of this study was to examine whether the effect of the quality of the parent-child relationship on change in children’s temperamental PE and NE is moderated by the child’s cortisol reactivity. Based on the biological sensitivity to context and differential susceptibility models (Belsky & Pluess, 2009; Boyce & Ellis, 2005), we expected that among children with greater cortisol reactivity at age 3, a poorer quality relationship with their parent would predict increases in NE and decreases in PE from age 3 to age 6. In contrast, among children with lower cortisol reactivity at age 3, the quality of their relationship with their parent should not be associated with change in NE or PE. These hypotheses were tested in a sample of 126 3 year-olds and their parents who completed observational measures of parenting and child temperament. They also completed a laboratory-based paradigm to assess cortisol stress reactivity, and were followed up three years later with a second laboratory observation of temperament.
Method
Participants
Participants were 160 3-year old children (80 males, 80 females) and their primary caregiver (155 mothers, 96.9%). The sample was randomly selected for a cortisol reactivity assessment from a larger sample (N = 559) enrolled in a longitudinal study (see Olino et al., 2010 for details). Participants were recruited through a commercial mailing list and screened by phone. Eligible children for the larger study had no significant medical problems or developmental disabilities, and at least one English-speaking biological participant who could participate. Children taking corticosteroid or anti-allergy medications were also excluded. All of the 160 children completed laboratory observation measures of childhood temperament and parent-child relationship quality at age 3. Subsequently, 126 (62 females) completed a second laboratory temperament assessment at age 6. Thus, there were 126 youth in the sample for these analyses. The mean age of our effective sample at the first assessment was 43.4 months (SD = 2.90), and 75.4 months (SD = 3.72) at the second assessment. Of the parents participating in the parent-child interaction portion of the study in the initial assessment, 121 were mothers (96.0%). These 126 children did not differ from the original 160 with cortisol reactivity data on any variable relevant to this study (all ps > .20).
Of these 126 children, most were White (96.0%) and came from two-parent (98.1%), middle-class families, as measured by the Four Factor Index of Social Status (M = 45.82; SD = 10.6; Hollingshead, 1975). Children were of average cognitive ability, as indexed by the Peabody Picture Vocabulary Test (M = 105.0, SD = 14.1; Dunn & Dunn, 1997). The 126 children with complete data did not differ significantly from other children in the larger sample of 559 on PE, NE, parent-child relationship quality, age, or sex. See Dougherty et al. (2011) for further details regarding recruitment methods and demographics.
Procedure
At age 3 and 6, children participated in the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith, Reilly, Lemery, Longley, & Prescott, 1995) as a measure of temperamental NE and PE. Cortisol levels were assessed at four time points during the Lab-TAB in the initial assessment wave. At age 3, the parent and their child returned to the laboratory 1–2 weeks later for the Teaching Tasks Battery (Egeland et al., 1995), during which the quality of the parent-child relationship was assessed.
Measures
Child NE and PE
At ages 3 and 6, child NE and PE were coded based on videotapes of the Lab-TAB. At age 3, 12 age-appropriate tasks were used, while at age 6, nine different age-appropriate tasks were used (Dyson et al., in press; Olino et al., 2010). Most were adapted from tasks used in the developmental literature, and were designed to elicit a range of temperament-relevant behaviours and emotions from the child (Gagne, Van Hulle, Aksan, Essex, & Goldsmith, 2011; Goldsmith et al., 1995). For a full description of all episodes at each assessment wave, see Dyson et al. (in press).
During each task, at age 3, each instance of children’s bodily, vocal, and facial expressions of positive affect, sadness, anger and fear, were rated on a three-point scale, while interest/engagement, was rated on a three-point scale based on the entire episode. At age 6, children’s reward sensitivity was also rated on a three-point scale. Ratings of sadness, anger, and fear were averaged to create a total NE scale at both age 3 and 6. Ratings of positive affect and interest/engagement at age 3 were averaged to create a PE scale. At age 6, ratings of interest/engagement, positive affect, and reward sensitivity were averaged to create a PE scale. Because ratings were averaged, rather than summed, PE scores at age 3 and 6 are directly comparable. At age 3, coefficient alphas for NE and PE were both .82, and the intraclass correlation coefficient (ICC) for interrater reliability (n = 35) for NE and PE was .74 and .89, respectively. At age 6, NE (a = .70) and PE (a = .83) respectively had ICCs of .81 and .88. At age 3, male and female children were not significantly different in NE, t (158) = −0.67, p = .50, or PE, t (158) = .41, p = .68. There were also no sex differences in NE, t (124) = −.55, p = .59, or PE, t (124) = −1.43, p = .15, at age 6.
Cortisol collection
Cortisol secretion was measured during the age 3 Lab-TAB, which took place during participants’ initial laboratory visit, at either 10:00 a.m. (n = 82) or 2:00 p.m. (n = 44). Parents were asked to refrain from feeding their child for 1 hr prior to coming to the laboratory and from giving their child caffeinated products for 2 hr prior to the session, as these factors are known to alter cortisol values (Gunnar & Talge, 2008). As in other studies employing laboratory stress assessments in preschool-age children, we used sequential measures of salivary cortisol to capture the continuous activity of the HPA axis in response to challenges (Luby et al., 2003; Talge, Donzella, & Gunnar, 2008). The timing of samples was determined on the basis of findings that salivary cortisol levels reflect the degree of stress experienced in the prior 20–40 min (Dickerson & Kemeny, 2004). The first cortisol sample (baseline; Time 1) was collected 20 min after adaptation to the laboratory. The second sample was collected 30 min after the Stranger Approach task (baseline + 60 min; Time 2). In this task, the child, who has been left alone in an empty room, is slowly approached by an unknown male research assistant who speaks to the child in a neutral voice. The third sample was taken 30 min after the Transparent Box task (baseline + 90 min; Time 3). In Transparent Box, the child is asked to pick an attractive toy, which is then placed inside a locked transparent box. The child is given a set of keys which s/he is told will unlock the box so the child can play with the toy; however, the keys do not fit the lock. The final sample was collected 20 min after the Box Empty task (baseline + 130 min; Time 4). In this task, the child is given a large, attractively wrapped, box to open, but rather than containing a present, the child finds that the box is empty. These tasks were selected because they were the most stressful in the battery and have been previously used to evoke individual differences in cortisol reactivity in children (Luby et al., 2003).
Saliva for cortisol determination was obtained by having children dip a 2-in. cotton dental roll into 0.025 g of cherry Kool-Aid mix. Children placed the cotton in their mouths until saturated. The wet cotton was then expressed into vials for storage at −20° C until assayed. Samples were shipped to the biochemistry laboratory at the University of Trier and assayed in duplicate, using a time-resolved fluorescence immunoassay with flourometric end-point detection (Dissociation-Enhanced Lanthanide Fluorescent Immunoassay, or DELFIA, PerkinElmer, Boston, MA). The use of the oral stimulant was monitored across all samples. These procedures have been shown to yield little to no effect on cortisol concentrations (Talge, Donzella, Kryzer, Gierens, & Gunnar, 2005). Inter- and intra-assay coefficients of variation were between 7.1% and 9.0% and 4.0% and 6.7%, respectively.
Parent-child relationship quality
The quality of the relationship between the primary parent and the child was coded while both participated in the Teaching Tasks Battery (Egeland et al., 1995) during the age 3 assessment. Different coders observed the Teaching Tasks Battery from those who observed the Lab-TAB. The battery consisted of 6 standardized parent–child interaction tasks lasting a total of 25–30 min. The tasks, which occurred in the order listed here, were designed to elicit a variety of parent and child behaviors, and, at age 3, consisted of book reading, naming objects with wheels, block building, matching shapes, completing a maze using an etch-a-sketch, and receiving a gift. Relationship quality in each of the 6 tasks was coded on a 5-point scale ranging from “very low” to “very high” and averaged across tasks to yield a total score (α = .86). Ratings were rated based on affective and verbal sharing between the child and parent, contingent responding to each other, sensitivity of the parent to the child’s distress, and effective conflict resolution. A high score indicates a strong sense of relatedness and mutual engagement between the parent and his or her child, with both explicitly acknowledging and responding to the other. The ICC (n = 35) for quality of the parent-child relationship was .79.
Data Analyses
We calculated the area under the curve (AUC) with respect to ground (AUCg) and the increase (AUCi) in cortisol (Pruessner et al., 2003). The AUCg is an estimate of total cortisol secretion, and the AUCi is an estimate of the increase in cortisol. We had no a priori hypotheses regarding whether only one or both of these measures would moderate the effect of the parent-child relationship on change in temperament, and so analyses were conducted separately for each. Primary data analyses consisted of multiple linear regressions. Analyses initially adjusted for child age, sex, and the time of day at which participants participated in the stress tasks. However, these variables were unrelated to NE or PE at age 6, or to parent-child relationship quality, AUCi, or AUCg at age 3. Moreover, when including time of day in our models, all results were significant at levels reported below. Child age, sex, and time of day were thus excluded from our final models, although these results are available upon request. We also repeated analyses after excluding the five fathers in our sample. All results remained significant at levels reported here, so results are reported with fathers retained in the sample. Finally, although not described above, we also assessed parent-child relationship quality at age 6 and repeated the analyses after adjusting for its effects on PE and NE at age 6. Age 6 parent-child relationship quality was not significantly related to NE or PE in any model and effects remained significant at levels reported here, so it is not included in any of the models reported here.
Although we did not hypothesize that there would be sex differences in the interaction between cortisol reactivity and parent-child relationship quality on change in temperament, we examined all 2 and 3 way interactions of sex with AUC and parent-child relationship quality, as well as two-way interactions of sex and age 3 temperament. None of these interactions were significant, indicating that effects did not vary by sex, so interactions with sex were dropped from our models.
We examined change in NE and PE in separate models. Each model controlled for levels of the corresponding variable at age 3. Dependent variables therefore represent residuals; that is, the effects of the predictors on the dependent variables reflect change in that variable from one time point to the next. A positive relationship between parent-child relationship quality and age 6 PE would, for instance, indicate that higher levels of relationship-quality predict increases in PE from age 3 to 6. In each model, temperament at age 6 was regressed first upon the same temperamental variable at the previous time point. Main effects of AUCi or AUCg were then added, as well as a main effect of quality of the parent-child relationship. The interaction between quality of relationship and either AUCi or AUCg was then added. All predictor variables were centered, as recommended by Aiken & West (1991). Standardized regression coefficients are presented for all regression effects. We also report the variance explained (R2) in age 6 temperament for each model. Interactions were interpreted by comparing simple slopes at high and low levels (±1 SD) of the AUCg or AUCi.
Results
Descriptive statistics and bivariate correlations
Zero-order correlations and descriptive statistics are presented in Table 1. AUCg and AUCi were uncorrelated. Higher levels of both were each associated with greater child NE at age 6, although higher AUCi was associated with lower levels of NE at age 3, and trended towards being related to a lower quality parent-child relationship. Better quality parent-child relationships were associated with higher PE at age 3, and trended towards being associated with lower NE at age 6.
Table 1.
Descriptive statistics for and bivariate correlations between all variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. AUCg | – | .02 | −.05 | −.02 | .08 | .09 | .29** |
| 2. AUCi | – | −.16+ | −.02 | −.21* | .12 | .23* | |
| 3. Relationship | – | .25** | .08 | .11 | −.16+ | ||
| Quality | |||||||
| 4. Age 3 PE | – | −.16+ | .34** | −.02 | |||
| 5. Age 3 NE | – | .03 | .11 | ||||
| 6. Age 6 PE | – | .23* | |||||
| 7. Age 6 NE | – | ||||||
|
| |||||||
| Mean | 483.12 | 11.03 | 3.97 | .006 | .56 | .001 | −.0003 |
| SD | 431.33 | 385.66 | .60 | 1.80 | .26 | .81 | .56 |
p < .01,
p < .05,
p < .10.
Note: PE = Positive Emotionality; NE = Negative Emotionality; AUCg = Area under the curve with respect to ground; AUCi = Area under the curve with respect to increase.
Predicting change in NE
Results (Table 2) from our model predicting change in NE from age 3 to 6, with AUCg as the moderator, showed that NE at age 3 was not significantly related to NE at age 6. Quality of relationship was also unrelated to age 6 NE, although greater levels of AUCg predicted increases in NE. However, this was qualified by a significant interaction between the quality of the parent-child relationship and AUCg (Figure 1, top panel). Inspection of this interaction revealed that a better quality parent-child relationship predicted decreases in NE in children who showed a high AUCg, β = −.12, t = −3.16, p = .002, but that there was no effect of relationship quality on age 6 NE in children who showed a low AUCg, β = .06, t = 1.33, p = .19. The full model accounted for 23.57% of variance in age 6 NE.
Table 2.
Results of regression models predicting age 6 NE or PE (N = 126).
| β | T | R2 | |
|---|---|---|---|
| Model 1: AUCg + QoR + AUCg*QoR | .24 | ||
|
| |||
| Age 3 NE | .06 | 1.64 | |
| AUCg | 09** | 2.86 | |
| QoR | −.03 | .77 | |
| AUCg*QoR | −0.09** | −4.42 | |
|
| |||
| Model 2: AUCi + QoR + AUCi*QoR ➔ NE | .21 | ||
|
| |||
| Age 3 NE | .08* | 2.05 | |
| AUCi | .02 | .78 | |
| QoR | −.02 | −.57 | |
| AUCi*QoR | −.10** | −4.17 | |
|
| |||
| Model 3: AUCg + QoR + AUCg*QoR ➔ PE | .19 | ||
|
| |||
| Age 3 PE | .30** | 4.06 | |
| AUCg | .05 | .83 | |
| QoR | .08 | 1.15 | |
| AUCg*QoR | −.12** | −3.20 | |
|
| |||
| Model 4: AUCi + QoR + AUCi*QoR ➔ PE | .22 | ||
|
| |||
| Age 3 PE | .30 | 4.13 | |
| AUCi | −.01 | −.14 | |
| QoR | .10 | 1.45 | |
| AUCg*QoR | −.16** | −3.71 | |
p < .01,
p < .05,
p < .10.
Note: Positive Emotionality; NE = Negative Emotionality; AUCg = Area under the curve with respect to ground; AUCi = Area under the curve with respect to increase. QoR = Quality of parent-child relationship.
Figure 1.
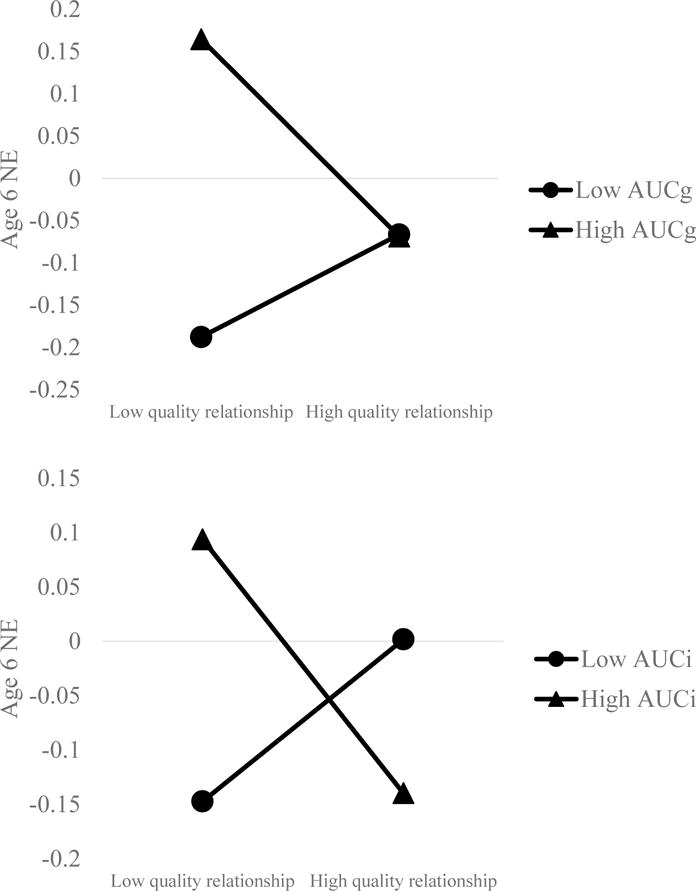
Relationship between quality of parent-child relationship at age 3 and change in negative affect from age 3 to 6. AUC = Area under the curve (i = with respect to increase, g = with respect to ground). AUC and parent-child relationship assessed at age 3. Note: Only slope at high AUCi or AUCg is significant.
Results (Table 2) from the same model but in which AUCi was treated as the moderator, showed that greater levels of NE at age 3 predicted greater levels of NE at age 6; over and above this effect, there was no main effect of either quality of relationship or AUCi. However, there was a significant interaction between quality of the parent-child relationship and AUCi (Figure 1, bottom panel). Inspection of this interaction revealed that a better quality parent-child relationship predicted decreases in NE in children who showed a high AUCi, β = −.12, t = −3.01, p = .003, but that there was no effect of relationship quality on NE in children who showed a low AUCi, β = .07, t = 1.54, p = .13. The full model accounted for 20.75% of variance in age 6 NE.
Predicting change in PE
Results (Table 2) from our model predicting change in PE from age 3 to 6, with AUCg as the moderator, showed that greater levels of PE at age 3 predicted greater levels of PE at age 6. Over and above this effect, there was no main effect of either quality of parent-child relationship or AUCg, but there was a significant interaction between quality of relationship and AUCg (Figure 2, top panel). Inspection of this interaction revealed that a better quality parent-child relationship predicted increases in PE in children who showed a low AUCg, β = .21, t = 2.31, p = .02, but that there was no effect of relationship quality on PE in children who showed an elevated AUCg, β = −.04, t = −.55, p = .58. The full model accounted for 19.1% of variance in age 6 PE.
Figure 2.
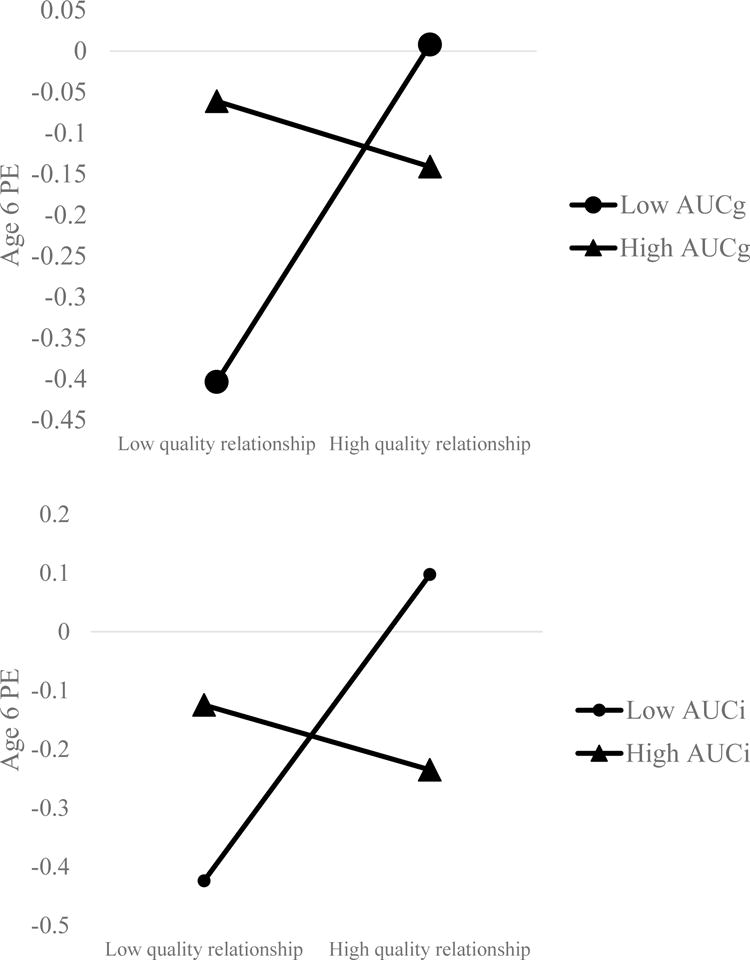
Relationship between quality of parent-child relationship and change in positive emotionality from age 3 to 6. AUC = Area under the curve (i = with respect to increase, g = with respect to ground). AUC and parent-child relationship assessed at age 3. Note: Only slopes at low AUC are significant.
Results (Table 2) were similar in the model in which AUCi was treated as the moderator. Greater levels of PE at age 3 predicted greater levels of PE at age 6. Over and above this effect, there was no main effect of either quality of the parent-child relationship or AUCi, but there was a significant interaction between quality of relationship and AUCi (Figure 2, bottom panel). Inspection of this interaction revealed that a better quality parent-child relationship predicted increases in PE in children who showed a low AUCi, β = .26, t = 2.87, p = .005, but that there was no effect of relationship quality on PE in children who showed an elevated AUCi, β = −.06, t = −.74, p = .46. The full model accounted for 22.04% of variances in age 6 PE.1
Discussion
This study is the first, to our knowledge, to examine whether early childhood cortisol reactivity moderates the effects of the quality of the parent-child relationship on the development of temperamental PE and NE, two traits that are closely linked to psychosocial well-being and functioning throughout the life span. Results were partially consistent with the biological sensitivity to context (Boyce & Ellis, 2005) and differential susceptibility (Belsky & Pluess, 2009) models in that children who showed greater cortisol secretion, either overall (i.e., with respect to ground) or in terms of individual changes during lab-based stressors (i.e., with respect to increase), developed higher levels of NE from age 3 to 6 in the context of a poor quality relationship with their parent. However, our second set of findings is inconsistent with these influential theories – children who showed lower cortisol reactivity exhibited increases in PE from age 3 to 6 in the context of a better quality parent-child relationship. Taken together, results suggest that high levels of cortisol reactivity may exacerbate the deleterious effects of poor quality of the parent-child relationship on the development of temperamental NE; however, low cortisol reactivity in childhood increases the likelihood that a better-quality parent-child relationship will lead to increases in PE. Importantly, the results were virtually identical using two different and distinct measures of cortisol reactivity, supporting the robustness of these findings. Effects were also over and above any effects of the concurrent parent-child relationship quality. Overall, the results provide only partial support for differential susceptibility and biological sensitivity to context theories. In contrast to these theories, lower cortisol reactivity, rather than high, appeared to enhance the ameliorative effects of a positive parent-child relationship quality on the development of PE, while blunting the deleterious effects of a poorer quality parent-child relationship on the development of NE. Results suggest the effects of the interaction of cortisol reactivity and environmental contexts on psychosocial outcomes may be more complicated that proposed by either differential susceptibility or biological sensitivity to context theories.
HPA Axis regulation and the development of temperament
Although the effects of parenting on temperament and the development of the HPA system as well as the relationship between childhood temperament and HPA activity have been well-studied (Gunner & Quevedo, 2007; Kiff et al., 2011), the current results offer a novel perspective on the interrelationships of these factors. Research has begun to examine HPA axis reactivity as a moderator of the relationship between environmental context and youths’ internalizing or externalizing symptoms (Badanes et al., 2011; Boyce et al., 2006; Hastings et al., 2011; Obradovic et al., 2010). However, as noted elsewhere (e.g., Belsky & Pluess, 2009), research into the development of temperament and personality has largely examined cortisol reactivity, as well as other measures of cortisol (e.g basal, awakening response), as an outcome of either temperament or developmental experiences (i.e., parenting) (although see Gunnar et al., 1997 for a study examining the relationship between cortisol and subsequent temperament traits). In contrast, surprisingly little research has considered the possibility that individual differences in stress reactivity render the child more susceptible to the effects of environmental experiences (e.g., parent-child relationship) on temperament or personality development over time.
Our results support this proposition. It appears that, on one hand, a poorer quality relationship with the parent may result in an increase in levels of NE, but only if the child shows elevated cortisol reactivity early on. These children, who are more biologically reactive to stress, may be more likely to internalize messages of being unloved or unworthy if their parent exhibits low levels of care, support, and sensitivity. Similarly, a poor quality relationship may be indicative of the parent being unavailable to help her child regulate their emotions during times of distress; this lack of external emotional regulation may be particularly detrimental to children who are already more reactive to stress. It is also worth noting that low levels of biological reactivity to stress appear to protect the child against the potentially deleterious effects of a poor-quality parent-child relationship on temperamental NE. In contrast to children with more highly reactive endocrine systems, for whom the development of temperament appears to be closely intertwined with the parent-child relationship, children with lower endocrine reactivity may be inherently more emotionally stable, and their temperamental NE may therefore be less dependent on environmental contexts, such as the parent-child relationship.
In contrast, a good quality relationship with the parent predicted increases in PE over time, but only in children who showed lower cortisol reactivity. Less reactive children may be more emotionally stable to begin with, and so are better able to internalize the parent’s modeling of adaptive emotional regulation during times of distress, and are also better able to attend to and internalize the parent’s caring, supportive, or sensitive behaviours. Although it is plausible that relatively more or less reactive children may elicit more or less negative parenting, this possibility is mitigated by the lack of associations in our data between the parent-child relationship and child cortisol reactivity.
It is also conceivable that variations in HPA axis activity alter the effects of the parent-child relationship on the development of neural regions and networks associated with the cognitive, behavioural, and affective components of temperament. Thus, there is evidence that adverse environmental experiences, such as poor quality parenting, lead to long-term changes both in the volume of and connectivity between brain regions, such as the amygdala, anterior cingulate, hippocampus, ventral striatum, and orbitofrontal cortex, associated with NE and PE (Lupien, McEwen, Gunnar, & Heim, 2009; Ormel et al., 2013; White et al., 2012; Whittle et al., 2006). These changes appear to be mediated, at least in part, by chronic up-regulation of the HPA axis (Chrousos, 2009; Lupien et al., 2009). Our results extend this perspective by suggesting that there are individual differences in children’s HPA axis reactivity that may also moderate these processes. That is, some children may be more sensitive to particular environmental influences than others, and these individual differences in cortisol reactivity may moderate the effects of the parent-child relationship on neurodevelopment, in turn influencing the development of temperament traits. This, admittedly, is speculative, and should be tested in future research. Regardless, our results suggest that elevated cortisol reactivity appears to exacerbate the effects of a lower-quality parent-child relationship on the development of NE, whereas lower cortisol reactivity appears to enhance the effects of a better quality parent-child relationship on the development of PE.
Finally, a persistent issue in biological stress-reactivity research is that, in some studies, a “blunted,” or lowered stress response appears to associated with maladaptive outcomes, while in other studies, a hyper-reactive stress response appears maladaptive (see Hankin et al., 2010). It is important to note prior studies have addressed psychopathology, whereas the current study focuses on temperament; cortisol reactivity may be differentially related to psychopathology compared to temperament. Our results suggest that a heightened stress response confers vulnerability to the effects of negative contexts (i.e., a poor quality parent-child relationship), but that a lowered stress response enhances the effects of positive experiences. As such, our results provide further evidence that a heightened stress response is maladaptive. Unfortunately, our results do little to clarify why, in other studies, a lowered stress response is maladaptive. Future research will likely benefit from attending to various methodological issues, including the age and nature of the sample, the method and timing of assessing cortisol, the context of the cortisol collection, and analytic approaches, and their role in determining whether a lowered or heighted cortisol response is adaptive or maladaptive.
Limitations and future directions
This study had several notable strengths, including laboratory-based measures of child temperament and parent-child relationship quality, which remove concerns about associations being due to shared method variance or biased reporting, as well as a three-year longitudinal design which allowed us to adjust for baseline levels of temperament and therefore examine change in temperament over time. However, several limitations should be noted.
At the broadest level, it is difficult to disentangle the overlap between HPA-axis reactivity and temperamental NE, which is, in part, defined by high mood reactivity to stress (Klein et al., 2011). It is unclear from the current study and the extant literature to what extent temperament and HPA-axis reactivity are different constructs versus similar constructs assessed at different levels. Another broad issue is the overlap between temperament and the parent-child relationship. Some literature has shown that children’s temperament influences the parenting behaviours they receive (e.g., Kopala-Sibley, Zuroff, & Koestner, 2012; Putnam et al., 2002). While our analyses adjust for the independent effects of child temperament at age 3 and the parent-child relationship quality, future research would benefit from genetically-informed designs to disentangle the effects of baseline temperament and parenting behaviours on the subsequent development of temperament.
Otherwise, there are several more specific limitations that should be noted. First, we do not know if the more highly or less reactive children in our sample exhibited elevated, normative, or blunted cortisol secretion in absolute terms; we can only conclude that they showed high or low cortisol levels relative to our specific sample. Second, we also do not know if or to what point in subsequent development our results would extend, or whether the results apply to other aspects of parenting, specific parenting behaviours, or other environmental stressors; future research should examine whether cortisol reactivity moderates the effects of a range of environmental experiences on change in temperament and personality in later childhood, adolescence, and adulthood. Third, our sample of parents consisted primarily of mothers; thus, we cannot address whether these findings extend to the quality of father-child relationships. Fourth, cortisol levels on the initial collection when children arrive to the lab could represent reactivity to arriving to the lab, which may explain the parallel findings across our measures of area under the curve with respect to increase and to ground, although it would not explain our overall pattern of findings. Fifth, our final sample of cortisol was collected 20-minutes post-task, so we cannot extend our results to cortisol recovery from stress. Sixth, we did not measure cortisol reactivity at age 6. Seventh, while laboratory observations of temperament and parenting behaviours are an improvement in many ways over parent-reports, it would be useful to know if results extend to those methodologies. Finally, our sample was moderately sized, which raises issues of generalizability to the population.
Summary
In contrast to prior research which has typically examined HPA axis activity as a developmental outcome of either temperament or parenting, this was the first study to examine whether early childhood cortisol reactivity moderated the effect of the parent-child relationship on the development of temperamental NE and PE from age 3 to 6 years. Results showed that a poorer quality parent-child relationship predicted increases in laboratory-based measures of NE, but only in children who showed elevated cortisol reactivity at baseline, and that a better quality parent-child relationship predicted increases in PE, but only in children who showed lowered cortisol reactivity at baseline. Results provide a novel perspective regarding the role of HPA axis activity in psychological development in childhood and suggest it may be fruitful in future research to examine HPA axis regulation as a moderator of a wider range of environmental influences on psychosocial development.
Research Highlights.
No research has examined whether early childhood cortisol reactivity moderates the effects of the parent-child relationship quality on change in temperamental positive and negative emotionality during early childhood.
A poorer quality parent-child relationship predicted increases in negative emotionality in children who showed elevated cortisol reactivity at baseline.
A better quality parent-child relationship predicted increases in positive emotionality in children who showed lowered cortisol reactivity at baseline.
Results suggest early childhood cortisol reactivity represents a biological marker of susceptibility to the effects of environmental influences on psychosocial development in childhood.
Footnotes
Results were reanalyzed after excluding reward sensitivity from our measure of age 6 PE. The pattern and significance of results were highly similar to those reported here.
Contributor Information
Daniel C. Kopala-Sibley, Department of Psychology, Stony Brook University, Stony Brook, NY
Lea R. Dougherty, Department of Psychology, University of Maryland, College Park, MD
Margret W. Dyson, Department of Psychiatry, University of California, San Diego, San Diego, CA
Rebecca S. Laptook, Departments of Psychiatry and Pediatrics, Warren Alpert Medical School of Brown University, Providence, RI
Thomas M. Olino, Department of Psychology, Temple University, Philadelphia, PA
Sara J. Bufferd, Department of Psychology, California State University San Marcos, San Marcos, CA
Daniel N. Klein, Department of Psychology, Stony Brook University, Stony Brook, NY
References
- Aiken LS, West SG, Reno RR. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Albers EM, Marianne Riksen-Walraven J, Sweep FC, Weerth CD. Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. Journal of Child Psychology and Psychiatry. 2008;49(1):97–103. doi: 10.1111/j.1469-7610.2007.01818.x. [DOI] [PubMed] [Google Scholar]
- Ali N, Pruessner JC. The salivary alpha amylase over cortisol ratio as a marker to assess dysregulations of the stress systems. Physiology & Behavior. 2012;106(1):65–72. doi: 10.1016/j.physbeh.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Andrews J, Ali N, Pruessner JC. Reflections on the interaction of psychogenic stress systems in humans: the stress coherence/compensation model. Psychoneuroendocrinology. 2013;38(7):947–961. doi: 10.1016/j.psyneuen.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: Associations with family risk and internalizing disorders. Development and Psychopathology. 2011;23(03):881–896. doi: 10.1017/S095457941100037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Fish M, Isabella R. Continuity and discontinuity in infant negative and positive emotionality: Family antecedents and attachment consequences. Developmental Psychology. 1991;27:421–431. [Google Scholar]
- Blair C, Granger DA, Kivlighan KT, Mills-Koonce R, Willoughby M, Greenberg MT, Fortunato CK. Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology. 2008;44(4):1095. doi: 10.1037/0012-1649.44.4.1095. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger D, Willoughby M, Kivlighan K. Maternal sensitivity is related to hypothalamic-pituitary-adrenal axis stress reactivity and regulation in response to emotion challenge in 6-month-old infants. Annals of the New York Academy of Sciences. 2006;1094(1):263–267. doi: 10.1196/annals.1376.031. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, Fortunato CK. Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development. 2011;82(6):1970–1984. doi: 10.1111/j.1467-8624.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17(02):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Alkon A, Goldsmith HH, Kraemer HC, Kupfer DJ. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(12):1510–1520. doi: 10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- Bruce J, Davis EP, Gunnar MR. Individual differences in children’s cortisol response to the beginning of a new school year. Psychoneuroendocrinology. 2002;27(6):635–650. doi: 10.1016/s0306-4530(01)00031-2. [DOI] [PubMed] [Google Scholar]
- Caspi A, Shiner RL. Personality development. In: Damon W, Lerner R, Eisenberg N, editors. Handbook of child psychology: Vol. 3. Social, emotional, and personality development. 6th. New York: Wiley; 2006. pp. 300–364. [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews Endocrinology. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Compas BE, Connor-Smith J, Jaser SS. Temperament, stress reactivity, and coping: Implications for depression in childhood and adolescence. Journal of Clinical Child and Adolescent Psychology. 2004;33(1):21–31. doi: 10.1207/S15374424JCCP3301_3. [DOI] [PubMed] [Google Scholar]
- Davis EP, Donzella B, Krueger WK, Gunnar MR. The start of a new school year: Individual differences in salivary cortisol response in relation to child temperament. Developmental Psychobiology. 1999;35(3):188–196. doi: 10.1002/(sici)1098-2302(199911)35:3<188::aid-dev3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- De Pauw SSW, Mervielde I. Temperament, personality and developmental psychopathology: A review based on the conceptual dimensions underlying childhood traits. Child Psychiatry and Human Development. 2010;41:313–329. doi: 10.1007/s10578-009-0171-8. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Gunnar MR, Donzella B. Cortisol levels of young children in full-day childcare centers: Relations with age and temperament. Psychoneuroendocrinology. 1999;24(5):519–536. doi: 10.1016/s0306-4530(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Donzella B, Gunnar MR, Krueger WK, Alwin J. Cortisol and vagal tone responses to competitive challenge in preschoolers: Associations with temperament. Developmental Psychobiology. 2000;37(4):209–220. doi: 10.1002/1098-2302(2000)37:4<209::aid-dev1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Rose S, Laptook RS. Hypothalamic-pituitary-adrenal axis reactivity in the preschool-age offspring of depressed parents: moderation by early parenting. Psychological Science. 2011;2(5):650–658. doi: 10.1177/0956797611404084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Olino TM, Dyson M, Rose S. Increased waking salivary cortisol and depression risk in preschoolers: The role of maternal history of melancholic depression and early child temperament. Journal of Child Psychology and Psychiatry. 2009;50(12):1495–1503. doi: 10.1111/j.1469-7610.2009.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson MW, Olino TM, Durbin CE, Goldsmith HH, Bufferd SJ, Miller AR, Klein DN. The structural and rank-order stability of temperament in young children based on a laboratory-observational measure. Psychological Assessment. doi: 10.1037/pas0000104. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland B, Weinfield N, Hiester M, Lawrence C, Pierce S, Chippendale K. Teaching tasks administration and scoring manual. Minneapolis, MN: University of Minnesota; 1995. [Google Scholar]
- Egger HL, Ascher BH, Angold A. The preschool age psychiatric assessment: Version 1.1. Durham, NC: Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center; 1999. [Google Scholar]
- Ellis BJ, Boyce WT. Biological sensitivity to context. Current Directions in Psychological Science. 2008;17(3):183–187. [Google Scholar]
- Fortunato CK, Dribin AE, Granger DA, Buss KA. Salivary alpha-amylase and cortisol in toddlers: Differential relations to affective behavior. Developmental Psychobiology. 2008;50(8):807–818. doi: 10.1002/dev.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JR, Van Hulle CA, Aksan N, Essex MJ, Goldsmith HH. Deriving childhood temperament measures from emotion-eliciting behavioral episodes: Scale construction and initial validation. Psychological Assessment. 2011;23:337–353. doi: 10.1037/a0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley S, Prescott A. Unpublished manuscript. Department of Psychology, University of Wisconsin; Madison WI: 1995. Laboratory Temperament Assessment Battery: Preschool version. [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009;34(1):62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Mangelsdorf S, Larson M, Hertsgaard L. Attachment, temperament, and adrenocortical activity in infancy: A study of psychoendocrine regulation. Developmental Psychology. 1989;25(3):355. [Google Scholar]
- Gunnar MR, Talge NM. Neuroendocrine measures in developmental research. In: Schmidt LA, Segalowitz SJ, editors. Developmental psychophysiology: Theory, systems, and methods. New York, NY: Cambridge University Press; 2008. pp. 343–366. [Google Scholar]
- Gunnar MR, Tout K, de Haan M, Pierce S, Stanbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Developmental Psychobiology. 1997;31(1):65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JR, Watamura SE. Hypothalamic–pituitary–adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological Psychiatry. 2010;68(5):484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Shirtcliff EA, Klimes-Dougan B, Allison AL, Derose L, Kendziora KT, Zahn-Waxler C. Allostasis and the development of internalizing and externalizing problems: Changing relations with physiological systems across adolescence. Development and Psychopathology. 2011;23(04):1149–1165. doi: 10.1017/S0954579411000538. [DOI] [PubMed] [Google Scholar]
- Hertsgaard L, Gunnar M, Larson M, Brodersen L, Lehman H. First time experiences in infancy: when they appear to be pleasant, do they activate the adrenocortical stress response? Developmental Psychobiology. 1992;25(5):319–333. doi: 10.1002/dev.420250503. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Department of Sociology, Yale University; New Haven, CT: 1975. [Google Scholar]
- Hunter AL, Minnis H, Wilson P. Altered stress responses in children exposed to early adversity: a systematic review of salivary cortisol studies. Stress. 2011;14(6):614–626. doi: 10.3109/10253890.2011.577848. [DOI] [PubMed] [Google Scholar]
- Kiff CJ, Lengua LJ, Zalewski M. Nature and nurturing: Parenting in the context of child temperament. Clinical Child and Family Psychology Review. 2011;14(3):251–301. doi: 10.1007/s10567-011-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Kotov R, Bufferd SJ. Personality and depression: explanatory models and review of the evidence. Annual Review of Clinical Psychology. 2011;7:269. doi: 10.1146/annurev-clinpsy-032210-104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopala-Sibley DC, Zuroff DC, Koestner R. The determinants of negative maternal parenting behaviours: maternal, child, and paternal characteristics and their interaction. Early Child Development and Care. 2012;182(6):683–700. [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychological Bulletin. 2010;136:768. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Lengua LJ, Kovacs EA. Bidirectional associations between temperament and parenting and the prediction of adjustment problems in middle childhood. Journal of Applied Developmental Psychology. 2005;26(1):21–38. [Google Scholar]
- Loman MM, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience & Biobehavioral Reviews. 2010;34(6):867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb ST, Leve LD, Harold GT, Neiderhiser JM, Shaw DS, Ge X, Reiss D. Trajectories of parenting and child negative emotionality during infancy and toddlerhood: A longitudinal analysis. Child Development. 2011;82(5):1661–1675. doi: 10.1111/j.1467-8624.2011.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Archives of General Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- Luecken LJ. Parental caring and loss during childhood and adult cortisol responses to stress. Psychology and Health. 2000;15(6):841–851. [Google Scholar]
- Luecken LJ, Lemery KS. Early caregiving and physiological stress responses. Clinical Psychology Review. 2004;24(2):171–191. doi: 10.1016/j.cpr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior, and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maccoby EE. The role of parents in the socialization of children: An historical overview. Developmental Psychology. 1992;28:1006–1017. [Google Scholar]
- Malatesta CZ, Haviland JM. Learning display rules: The socialization of emotion expression in infancy. Child development. 1982;53:991–1003. [PubMed] [Google Scholar]
- Obradović J, Bush NR, Boyce WT. The interactive effect of marital conflict and stress reactivity on externalizing and internalizing symptoms: The role of laboratory stressors. Development and Psychopathology. 2011;23(01):101–114. doi: 10.1017/S0954579410000672. [DOI] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: associations in a large community sample. Journal of Abnormal Psychology. 2010;119(3):468. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Bastiaansen A, Riese H, Bos EH, Servaas M, Ellenbogen M, Aleman A. The biological and psychological basis of neuroticism: current status and future directions. Neuroscience & Biobehavioral Reviews. 2013;37(1):59–72. doi: 10.1016/j.neubiorev.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Sanson AV, Rothbart MK. Child temperament and parenting. In: Bornstein M, editor. Handbook of parenting. Mahwah: Lawrence Erlbaum; 2002. pp. 255–277. [Google Scholar]
- Roberts BW, DelVecchio WF. The rank-order consistency of personality from childhood to old age: A quantitative review of longitudinal studies. Psychological Bulletin. 2000;136:3–25. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament in children’s development. In: Damon W, Lerner R, Eisenberg N, editors. Handbook of child psychology, 6th edition: Social, emotional, and personality development. Vol. 3. New York: Wiley; 2006. pp. 99–166. [Google Scholar]
- Sanson A, Hemphill SA, Smart D. Connections between temperament and social development: A review. Social Development. 2004;13(1):142–170. [Google Scholar]
- Smeekens S, Marianne Riksen-Walraven J, Van Bakel HJ. Cortisol reactions in five-year-olds to parent–child interaction: the moderating role of ego-resiliency. Journal of Child Psychology and Psychiatry. 2007;48(7):649–656. doi: 10.1111/j.1469-7610.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- Talge NM, Donzella B, Gunnar MR. Fearful temperament and stress reactivity among preschool-aged children. Infant and Child Development. 2008;17:427–445. doi: 10.1002/icd.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talge NM, Donzella B, Kryzer EM, Gierens A, Gunnar MR. It’s not that bad: Error introduced by oral stimulants in salivary cortisol research. Developmental Psychobiology. 2005;47:369–376. doi: 10.1002/dev.20097. [DOI] [PubMed] [Google Scholar]
- Vitaro F, Barker ED, Boivin M, Brendgen M, Tremblay RE. Do early difficult temperament and harsh parenting differentially predict reactive and proactive aggression? Journal of Abnormal Child Psychology. 2006;34(5):681–691. doi: 10.1007/s10802-006-9055-6. [DOI] [PubMed] [Google Scholar]
- Watson D, Gamez W, Simms LJ. Basic dimensions of temperament and their relation to anxiety and depression: A symptom-based perspective. Journal of Research in Personality. 2005;39(1):46–66. [Google Scholar]
- White LK, Lamm C, Helfinstein SM, Fox NA. Neurobiology and neurochemistry of temperament in children. In: Zentner M, Shiner R, editors. Handbook of Temperament. New York: Guilford Press; 2012. pp. 347–367. [Google Scholar]
- Whittle S, Allen NB, Lubman DI, Yücel M. The neurobiological basis of temperament: towards a better understanding of psychopathology. Neuroscience & Biobehavioral Reviews. 2006;30(4):511–525. doi: 10.1016/j.neubiorev.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Zentner M, Bates JE. Child temperament: An integrative review of concepts, research programs, and measures. European Journal of Developmental Science. 2008;2:7–37. [Google Scholar]


