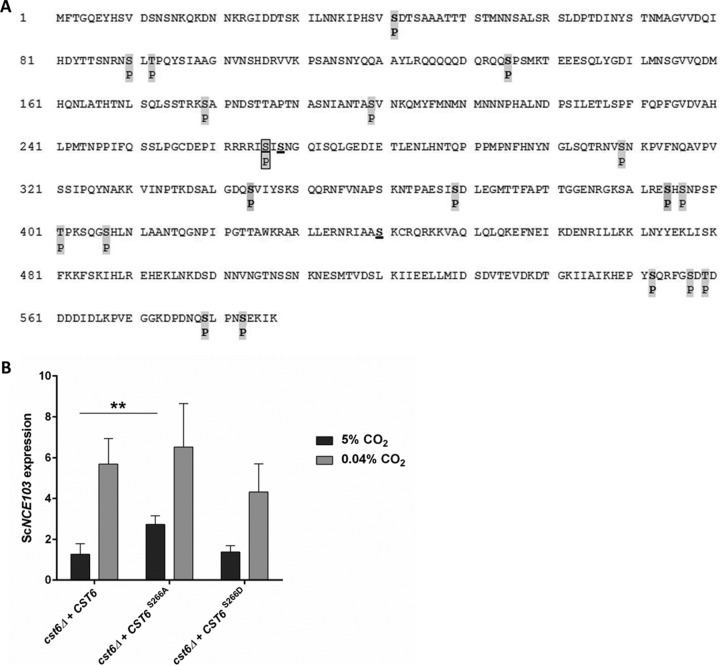
FIG 4 .
ScCst6 phosphorylation at position S266 is crucial for ScNCE103 regulation. (A) ScCst6-His, overexpressed in the WT under 5% CO2, was affinity purified and subjected to SDS-PAGE. Corresponding bands were excised and digested with trypsin-LysC, AspN, GluC, or LysargiNase. Additionally, in-solution digestion for trypsin-LysC and GluC was performed. Phosphorylated peptides were enriched and analyzed by LC-MS/MS. Nineteen different phosphorylation sites, indicated by “P,” were identified in at least 2 of 10 independent experiments. Newly identified phosphorylation sites are shown in boldface, and previously known sites are not in boldface. Underlined are the 3 serine residues that were conserved in S. cerevisiae, C. glabrata, and C. albicans. Among them, only S266 was found to be phosphorylated in the WT, noted within a box. (B) Site-specific mutation of S266 was performed to investigate direct influence on ScNCE103 expression under changing CO2 conditions. Expression levels were normalized to WT ScNCE103 expression in 5% CO2. Changing S266 to alanine (cst6Δ + CST6S266A) showed a significant upregulation of ScNCE103CO2, while ScNCE103air levels were unaltered. This is comparable to expression changes of the sch9Δ mutant and proves the critical role of Cst6 S266 for CA regulation. The bars show means ± standard deviations from 5 independent experiments. Significance against the cst6Δ + CST6 strain, calculated by a two-sided t test for unpaired samples, was defined as P ≤ 0.01 (**).