Summary
Lymphocytes circulate through lymph nodes (LN) in search for antigen in what is believed to be a continuous process. Here, we show that lymphocyte migration through lymph nodes and lymph occurred in a non-continuous, circadian manner. Lymphocyte homing to lymph nodes peaked at night onset, with cells leaving the tissue during the day. This resulted in strong oscillations in lymphocyte cellularity in lymph nodes and efferent lymphatic fluid. Using lineage-specific genetic ablation of circadian clock function, we demonstrated this to be dependent on rhythmic expression of promigratory factors on lymphocytes. Dendritic cell numbers peaked in phase with lymphocytes, with diurnal oscillations being present in disease severity after immunization to induce experimental autoimmune encephalomyelitis (EAE). These rhythms were abolished by genetic disruption of T cell clocks, demonstrating a circadian regulation of lymphocyte migration through lymph nodes with time-of-day of immunization being critical for adaptive immune responses weeks later.
keywords: circadian rhythm, leukocyte trafficking, adaptive immune response, lymph node homing, lymph node egress, immunization
Graphical Abstract
Highlights
-
•
Lymphocyte numbers in lymph nodes and lymph oscillate over the course of the day
-
•
Rhythmic Ccr7 and S1pr1 expression drives rhythmic lymphocyte homing and egress
-
•
Adaptive immune responses to immunization and pathogens are time-of-day dependent
-
•
Loss of circadian clocks in lymphocytes ablates rhythmic adaptive immune responses
Lymphocyte trafficking through lymph nodes and lymph is an important immune surveillance mechanism of the body. Druzd et al. (2017) demonstrate that this trafficking occurs in a circadian manner and that adaptive immune responses are also time-of-day dependent and are ablated when circadian clock function is lost in T cells.
Introduction
Lymphocytes survey antigen by circulating through blood, lymph nodes (LNs) and lymph and shape specific immune responses in LNs. To enter LNs, lymphocytes must undergo extensive interactions with high endothelial cell venules (HEVs) (Butcher, 1991, Förster et al., 2008, Ley et al., 2007, Muller, 2011, Springer, 1994, Vestweber and Blanks, 1999, von Andrian and Mempel, 2003, Wagner and Frenette, 2008). Lymphocytes initially tether on peripheral nodal addressin (PNAd) expressed on HEVs using L-selectin (CD62L) as a ligand. Lymphocytes roll along the vascular endothelium and become activated via interactions of the chemokine receptors CCR7 and CXCR4 with their respective ligands CCL21 and CXCL12. Activated leukocytes use the integrin LFA-1 (CD11a) to bind to ICAM-1 to promote adhesion and finally emigrate into the LN parenchyma.
After LN entry, lymphocytes interact with dendritic cells in order to scan presented antigen (Gasteiger et al., 2016), and finally emigrate into efferent lymphatic vessels. For this egress, expression of the sphingosine-1-phosphate-receptor 1 (S1P1, encoded by S1pr1) on lymphocytes is critical, recognizing the chemoattractant phospholipid sphingosine-1-phosphate (S1P) (Matloubian et al., 2004). S1P concentrations are high in blood and lymph but low in tissues, thus providing a gradient that guides lymphocytes out of the LN and into efferent lymph (Cyster and Schwab, 2012). This mechanism is therapeutically exploited for treating multiple sclerosis patients by antagonizing S1P1 function with the drug FTY720 (fingolimod) to keep autoreactive T cells from exiting LNs and entering the central nervous system (Massberg and von Andrian, 2006).
Lymphocyte trafficking into LNs is believed to occur in a continuous fashion, and not to be influenced by time-of-day variables. Moreover, it is generally unclear whether circadian rhythms regulate overall cellularity in these tissues (Arjona and Sarkar, 2005, Esquifino et al., 1996, Fortier et al., 2011, Hemmers and Rudensky, 2015).
Circadian rhythms are important drivers for most physiological processes as they align the body with rhythmically occurring daily changes in the environment (Dibner et al., 2010). They normally rely on an intricate interplay of cell-intrinsic clock genes driving circadian responses (Mohawk et al., 2012). Daily oscillations of lymphocyte counts in blood have been described (Arjona et al., 2012, Curtis et al., 2014, Haus and Smolensky, 1999, Labrecque and Cermakian, 2015, Scheiermann et al., 2013) and cells of the adaptive immune system such as T and B cells, as well as dendritic cells, possess the components of the molecular clock machinery (Bollinger et al., 2011, Hemmers and Rudensky, 2015, Silver et al., 2012a). In contrast to monocytes of the innate immune system (Nguyen et al., 2013), however, the functional relevance of these cell-intrinsic oscillations for lymphocytes is unclear (Hemmers and Rudensky, 2015). Stimulated by previous findings, which described periodic oscillations in innate immune cell function (Gibbs et al., 2014, Nguyen et al., 2013, Scheiermann et al., 2012) and T helper-17 (Th17) cell differentiation (Yu et al., 2013), we postulated that the migration of lymphocytes through murine LNs might be regulated in a circadian manner with direct relevance for the mounting of adaptive immune responses.
Results
Lymphocyte Numbers Exhibit Circadian Oscillations in Lymph Nodes
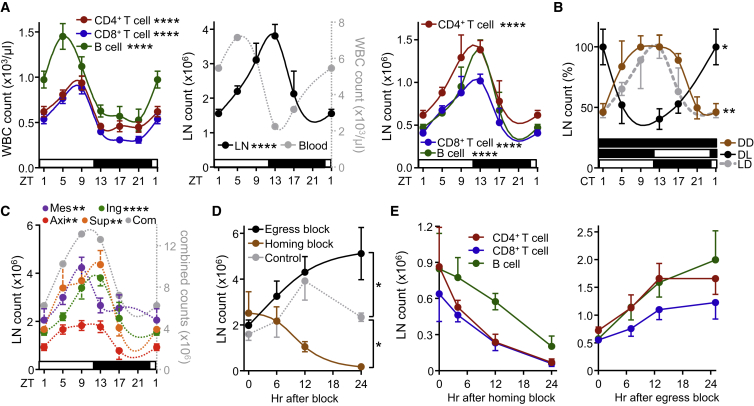
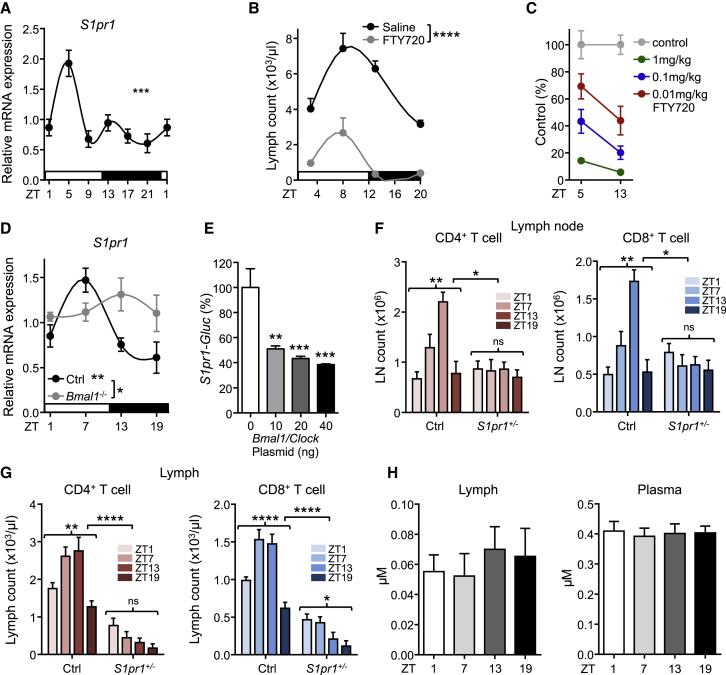
In contrast to circulating blood lymphocyte numbers, which peak during the day in mice around Zeitgeber time (ZT) 5 (i.e., 5 hr after light onset) (Figure 1A), numbers for CD4+ and CD8+ T cells as well as B cells showed delayed oscillations (by ∼8 hr) in inguinal lymph nodes (iLNs), with highest counts occurring at the beginning of the dark phase (ZT13, i.e., 1 hr after lights off) (Figure 1A). These rhythms were consistently observed for naive and central memory T cells, demonstrating a broad phenomenon also affecting T lymphocyte subpopulations (Figures S1A–S1C). Oscillations were not only observed in the rhythmic environment represented by 12 hr light:12 hr dark conditions (LD) but were sustained in constant darkness (dark:dark, DD), indicating their bona fide endogenous circadian nature (Figure 1B). Light exposure was an important entrainment factor, since rhythms were inverted when the light regime was reversed (DL) (Figure 1B). Rhythms were furthermore detected across various types of LNs (Figure 1C and Figures S1D–S1F), indicating a relevant phenomenon across the LN compartment. To investigate the underlying mechanisms driving these oscillations, we focused on the cellular LN input and output pathways by blocking lymphocyte homing or egress, both critical determinants of LN cellularity (Lo et al., 2005). Blocking homing with anti-integrin antibodies dramatically decreased LN cellularity over 24 hr while blocking lymphocyte egress with FTY720 increased LN cellularity over the same time frame, confirming the temporally highly dynamic cellular nature of this tissue (Figures 1D and 1E). Both treatments ablated rhythmicity, indicating that lymphocyte homing and egress—but not intranodal proliferation (Figures S1G and S1H)—were the central determinants of circadian oscillatory cellularity. These data demonstrate a striking circadian oscillation in lymph node cellularity, peaking at night onset.
Figure 1.
Lymphocyte Numbers Exhibit Circadian Oscillations in Lymph Nodes
(A) Lymphocyte oscillations in blood (left panel) and inguinal lymph node (middle and right panels) over 24 hr. Zeitgeber time (ZT, time after light onset) 1 is double-plotted to facilitate viewing; n = 4–49 mice, one-way ANOVA, WBC: white blood cells.
(B) Lymph node oscillations under light-dark (LD), dark-dark (DD) and inverted, dark-light (DL) conditions, normalized to peak times; CT, circadian time in constant darkness conditions; n = 3–15 mice, one-way ANOVA.
(C) Oscillations across multiple lymph nodes, axi: axillary, sup: superficial cervical, ing: inguinal, mes: mesenteric, com: combined counts; n = 3–19 mice, one-way ANOVA, counts are plotted per single lymph node.
(D) Lymph node counts after treatment with FTY720 (egress block) or integrin-blocking antibodies (homing block); n = 3–5 mice, one-way ANOVA with Tukey’s multiple comparisons test.
(E) Lymphocyte subpopulations after homing block (left) and egress block (right); n = 3 mice. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001. All data are represented as mean ± SEM. See also Figure S1.
Lymphocyte Homing Is Dependent on Oscillations in Lymphocytes and Microenvironment
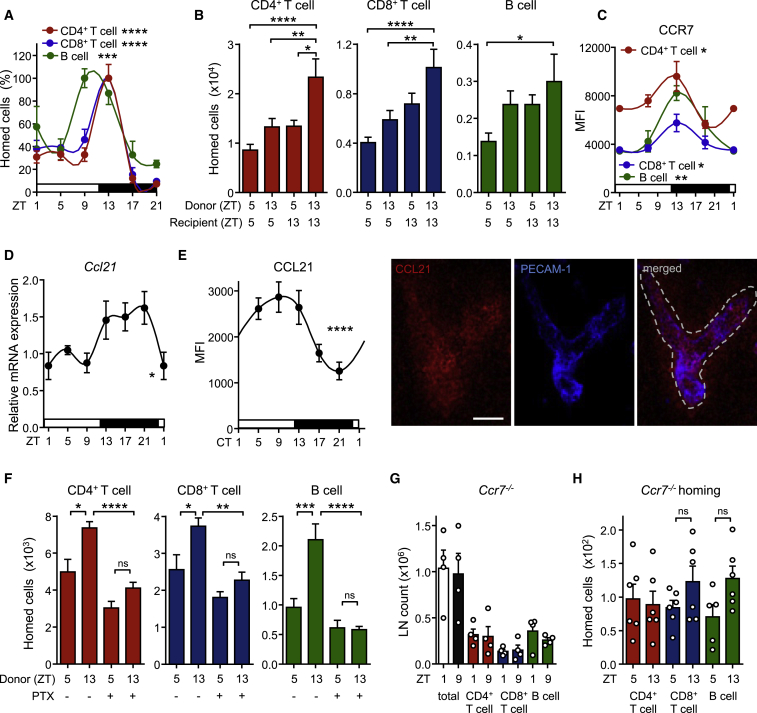
We next used adoptive transfer techniques to determine whether lymphocyte homing to the LN was occurring in a rhythmic manner. LN infiltration of lymphocyte subpopulations peaked around night onset and remained low during the day (Figure 2A). To define whether oscillations were determined by lymphocyte-intrinsic and/or microenvironmental signals, we adoptively transferred cells harvested at ZT5 (“day”) or ZT13 (“night”) into LD-entrained recipients at either ZT5 or ZT13. While “day” (cells) into “day” (recipient) transfers exhibited the lowest homing capacity and “night” into “night” transfers the highest, a mixed contribution of both lymphocyte and microenvironment timing was observed in the “day” into “night” and “night” into “day” chimeras (Figure 2B). A screen for oscillations of promigratory factors on T and B cells revealed that expression of the chemokine receptor CCR7 exhibited rhythmicity peaking at ZT13 (Figure 2C) while the adhesion molecules CXCR4, CD11a, and L-selectin showed either no oscillations or not for all lymphocyte subpopulations (Figures S2A and S2B). In addition, expression analyses of whole lymph node mRNA and extracellular protein on HEVs revealed oscillatory amounts of the chemokine CCL21, a ligand for CCR7—but not CXCL12 (not shown)—being high around night onset (Figures 2D and 2E). HEVs also exhibited rhythmic expression of ICAM-1 but not of PNAd (Figures S2C and S2D). Oscillations in lymphocyte chemokine receptors were critical for rhythmic homing because a titrated, short pretreatment of adoptively transferred cells with pertussis toxin (PTX) (Lo et al., 2005), an inhibitor of chemokine receptor signaling, ablated rhythmicity (Figure 2F). To investigate the involvement of CCR7 in this process, we analyzed total lymph node cellularity of CCR7-deficient mice, as well as the rhythmic homing capacity of isolated CCR7-deficient cells. Ccr7−/− mice exhibited no oscillations in lymph node cell counts while also exhibiting the expected lower overall numbers (Förster et al., 1999) (Figure 2G). In addition, Ccr7−/− cells failed to show rhythmic lymph node homing (Figure 2H). These data demonstrated that lymphocyte recruitment to LNs is determined by rhythms in leukocytes and the microenvironment, along with in-phase expression of the CCR7-CCL21 receptor-ligand axis.
Figure 2.
Rhythmic Lymphocyte Homing Is Dependent on Oscillations in Both Lymphocytes and Microenvironment
(A) Lymph node homing of lymphocyte populations over the course of the day, normalized to peak times; n = 3–17 mice, one-way ANOVA.
(B) Adoptive transfer of lymphocyte populations using donor and recipient mice kept at ZT5 or ZT13; n = 6–17 mice, one-way ANOVA with Tukey’s multiple comparisons test.
(C) Oscillations of CCR7 surface expression on lymphocyte subpopulations in LN; n = 3–5 mice, one-way ANOVA.
(D) Q-PCR analysis of LN Ccl21 amounts over 24 hr; n = 3–5 mice, one-way ANOVA.
(E) Quantification and images of expression of CCL21 on HEV over 24 hr in constant darkness (CT, circadian time: the corresponding light and dark phase are indicated); n = 3–18 mice, one-way ANOVA. Scale bar represents 50 μm.
(F) LN homing of lymphocytes harvested at ZT5 or ZT13 and treated with or without pertussis toxin (PTX); n = 5–11 mice, one-way ANOVA with Tukey’s multiple comparisons test.
(G) Lack of oscillations in LN cellularity of Ccr7−/− mice; n = 4 mice, unpaired Student’s t test.
(H) Lack of rhythmic LN homing of Ccr7−/− cells into WT hosts; n = 5–6 mice, unpaired Student’s t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. All data are represented as mean ± SEM. See also Figure S2.
Circadian Clocks Control Cellular Oscillations in Lymph Nodes
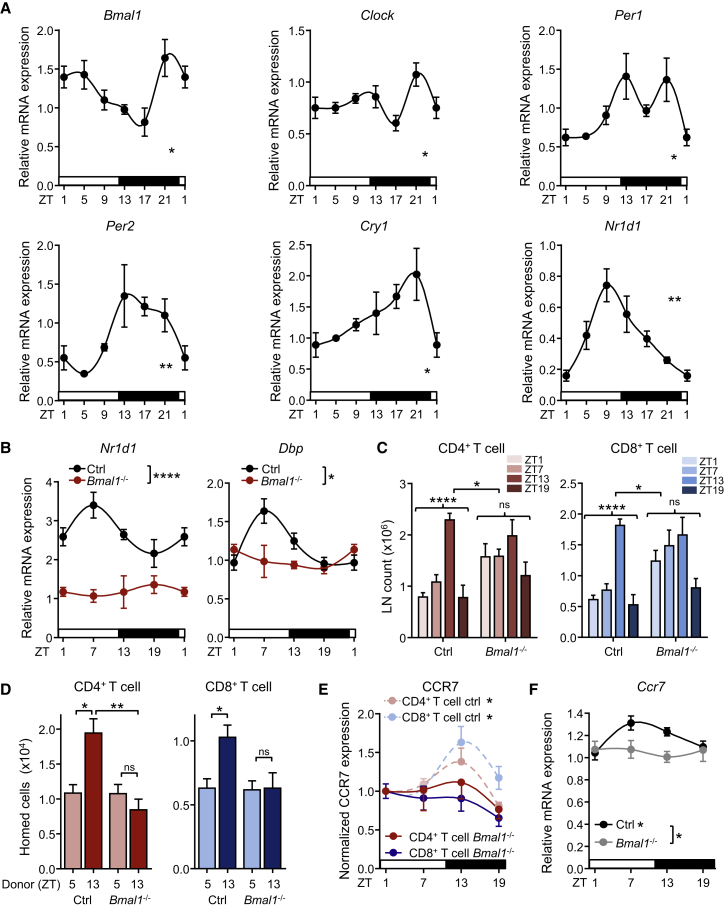
LNs exhibit oscillations of clock genes (Figure 3A), prompting us to investigate the role of lymphocyte clocks in their migratory behavior. We generated mice in which the core clock gene Bmal1 (also known as Arntl) was deleted in T cells (Bmal1flox/floxxCd4-cre) or B cells (Bmal1flox/floxxCd19-cre) (Figure 3B and Figures S2E and S2F). Remarkably, loss of lymphocyte BMAL1 ablated the overall rhythmicity of T and B cell numbers in lymph nodes (Figure 3C and Figure S2G). In addition, rhythmic homing of Bmal1flox/floxxCd4-cre T cells into WT recipients was ablated (Figure 3D). In agreement with these findings, rhythmic expression of CCR7 surface protein (Figure 3E) and mRNA (Figure 3F) was absent in BMAL1-deficient CD4+ T cells, indicating the regulation of the molecule at the transcriptional level by the circadian clock. Together, these data provide evidence for a functional role of cell-autonomous clocks in lymphocyte migration.
Figure 3.
Oscillations of Circadian Clock Genes in Lymph Nodes Control Cellularity
(A) Q-PCR analysis of circadian clock genes in LN over 24 hr; n = 3–5 mice, one-way ANOVA.
(B) Circadian clock gene mRNA profiles in sorted CD4+ T cells from Bmal1flox/floxxCd4-cre and control animals; n = 3–10 mice, two-way ANOVA.
(C) Lymph node CD4 and CD8 T cell counts in control and T cell specific Bmal1−/− mice, n = 3–9 mice, one-way and two-way ANOVA.
(D) LN homing of lymphocytes harvested from control or T cell-specific Bmal1−/− mice at ZT5 or ZT13 into WT hosts; n = 10–34 mice, one-way ANOVA with Tukey’s multiple comparisons test.
(E) CCR7 surface expression on T lymphocyte subpopulations in LN of control and T cell-specific Bmal1−/− mice; n = 3–5 mice, one-way ANOVA.
(F) Q-PCR analysis of CD4+ T cell Ccr7 over 24 hr in control and T cell-specific Bmal1−/− mice; n = 4–8 mice, one-way and two-way ANOVA. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001. All data are represented as mean ± SEM. See also Figure S2.
Lymphatic Egress from Lymph Nodes Is under Circadian Control
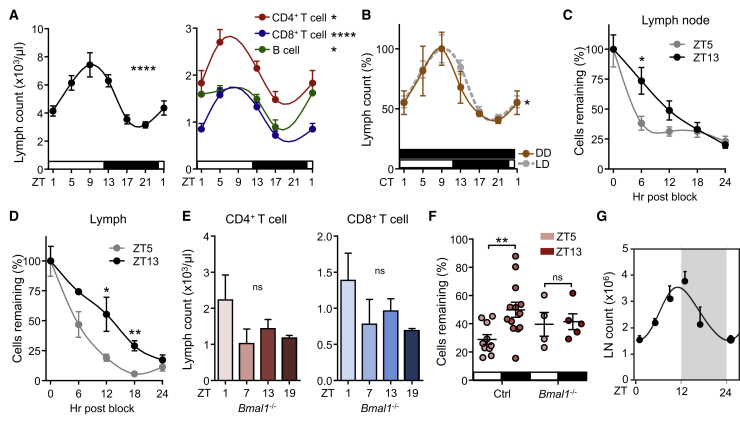
Because our data indicated that, in addition to a rhythmic homing component, lymphocyte egress might counterbalance LN oscillations (Figures 1D and 1E), we quantified lymphocyte numbers in lymph fluid by cannulating efferent lymphatic vessels. Prominent rhythms in cellular counts were detected, peaking at ZT9 and exhibiting a low at ZT21 (Figure 4A). These oscillations were observed for different lymphocyte populations (Figure 4A and Figures S2H and S2I) and were bona fide circadian in nature as they persisted in constant darkness (Figure 4B). Rhythms were not due to higher lymph volume or flow rates at different times of the day (Figure S2J). To verify whether oscillations in lymph cellularity were truly attributable to rhythmic egress and not secondary to rhythmic input into LNs (Figure 2A), we transferred lymphocytes at different times of the day, blocked subsequent LN entry and quantified their transit through the LN into lymph over time (Mandl et al., 2012). A higher LN retention capacity of cells injected at ZT13 was observed compared to ZT5 and a less rapid accumulation of cells in lymph (Figures 4C and 4D and Figure S3), demonstrating lymphocyte egress to be highly rhythmic. This effect resulted in longer LN half-lives of cells injected at ZT13 (CD4: 12 hr, CD8: 12 hr, B cells: 16 hr) compared to ZT5 (CD4: 9 hr, CD8: 9 hr, B cells: 13.5 hr) (Figures 4C and 4D and Figure S3). T- and B cell-specific BMAL1-deletion ablated oscillations in lymph, indicating the importance of cell-autonomous clocks also for lymphocyte egress (Figure 4E and data not shown). Of importance, adoptively transferred BMAL1-deficient CD4+ T cells exhibited no time-of-day variations in their LN half-life (Figure 4F), demonstrating the relevance of T cell clocks in their rhythmic trafficking behavior.
Figure 4.
Circadian Oscillations in Lymphocyte Egress from LNs
(A) Oscillations of total leukocyte (left panel) and lymphocyte (right panel) counts in efferent lymph over 24 hr; n = 6–33 mice, one-way ANOVA.
(B) Lymph leukocyte count oscillations under light-dark (LD) and dark-dark (DD) conditions; n = 3–37 mice, one-way ANOVA.
(C and D) Remaining cellular numbers (in %) in lymph node (C) and lymph (D) over 24 hr after block of leukocyte homing. Lymph node: n = 3–10 mice; lymph: n = 3–6 mice, unpaired Student’s t test.
(E) Lymph CD4+ and CD8+ T cell counts in T cell-specific Bmal1−/− mice; n = 3–8 mice, one-way ANOVA.
(F) Remaining cells (in %) in lymph nodes of adoptively transferred control and BMAL1-deficient CD4+ T cells 12 hr after transfer; n = 4–13, unpaired Student’s t test.
(G) Mathematical model of leukocyte homing and egress. The model is expressed as a line based on the indicated data points. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001. All data are represented as mean ± SEM. See also Figures S2–S4.
Using a mathematical approach, we assessed whether oscillatory LN counts could be modeled with only either homing or egress to be rhythmic or whether both components needed to oscillate. Although oscillations were also observed when only one component was rhythmic, the best fit was achieved when both homing and egress were assumed to oscillate, thus supporting our experimental data (Figure 4G and Figure S4). In summary, lymphocyte clocks and the time-of-day entry of cells into LNs have functional consequences for LN transit and egress into lymph.
Rhythmic Lymphocyte Egress Depends on Oscillatory S1pr1 Expression
S1P-receptors are critical in regulating lymph node egress (Cyster and Schwab, 2012). We therefore investigated whether expression of S1P-receptor family members exhibited oscillations using quantitative PCR (Q-PCR). All S1P receptors exhibited robust diurnal oscillations peaking between ZT1 and ZT9 (Figure 5A and Figure S5A), which coincided with high lymphocyte egress. In addition, FTY720, as well as the S1P1-specific functional antagonist SEW2871 strongly down-modulated lymphatic egress in a time- and concentration-dependent manner (Figures 5B and 5C and Figure S5B). The observation that FTY720-treated animals exhibited reduced but still rhythmic lymph cellularity indicated a daytime-sensitive role for S1P1 in mediating lymphocyte exit. Rhythmic expression of S1pr1 was ablated in BMAL1-deficient CD4+ T cells, pointing toward a regulation of the gene by the circadian clock (Figure 5D). To investigate this in more detail, we performed an in vitro assay, in which the promoter region of S1pr1 was cloned in front of the luciferase (luc) reporter gene. Luciferase activity in HEK293 cells transfected with the S1pr1-luc reporter was decreased after co-transfection of increasing amounts of Bmal1 and Clock expression plasmids (Figure 5E). This demonstrated that expression of S1pr1 is regulated by BMAL1 and CLOCK.
Figure 5.
Rhythmic Lymphocyte Egress Depends on Oscillatory S1pr1 Expression
(A) Q-PCR analysis of LN S1pr1 over 24h. n = 3–5 mice, one-way ANOVA.
(B) Lymph counts after blockade of S1P-receptor function using FTY720 at the indicated times; n = 3–33 mice, two-way ANOVA.
(C) FTY720 titration and respective lymph counts at two time points. n = 3–5 mice.
(D) Q-PCR analysis of CD4+ T cell S1pr1 over 24 hr in control and T cell-specific Bmal1−/− mice; n = 4–9 mice, one-way and two-way ANOVA.
(E) Normalized activity (in %) of Gaussia Luciferase driven by murine S1pr1 promoter (S1pr1-GLuc) after co-transfection with various doses of Clock and Bmal1 plasmids in HEK293 cells (n = 6). Data shown are pooled from two independent experiments, one-way ANOVA with Tukey’s multiple comparisons test.
(F) LN CD4+ and CD8+ T cell counts in control and T cell-specific S1pr1 heterozygous mice; n = 3–6 mice, one-way and two-way ANOVA.
(G) Lymph CD4+ and CD8+ T cell counts in control and T cell-specific S1pr1 heterozygous mice; n = 3–12 mice, one-way and two-way ANOVA.
(H) Mass spectrometric analysis of sphingosine-1-phosphate (S1P) in lymph and blood plasma; n = 9–11 mice. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. All data are represented as mean ± SEM. See also Figure S5.
To confirm the role of S1P1 in the time-of-day-dependent egress genetically, we generated T cell-specific mice that were heterozygous for S1pr1 in order not to completely block lymphocyte egress (Matloubian et al., 2004) but to titrate S1P1 amounts, as loss of one allele had been demonstrated to result in haploinsufficiency (Lo et al., 2005). S1pr1+/floxxCd4-cre mice exhibited no more oscillations in LN counts and altered lymph rhythmicity, demonstrating the importance of S1P1 in the proper timing of lymphocyte egress (Figures 5F and 5G and Figure S5C). Importantly, no diurnal oscillations were observed in amounts of S1P in efferent lymph (Figure 5H) or in S1P synthesizing or degrading enzymes in lymph node (Figure S5D), suggesting that oscillatory expression of the receptor (S1P1) and not its ligand (S1P) was the driver for rhythmic lymphocyte egress. Together, these data demonstrate a critical role for S1P1 in mediating circadian lymphocyte egress from lymph nodes into efferent lymph.
Relevance of Circadian Oscillations in Lymph Node Cellularity
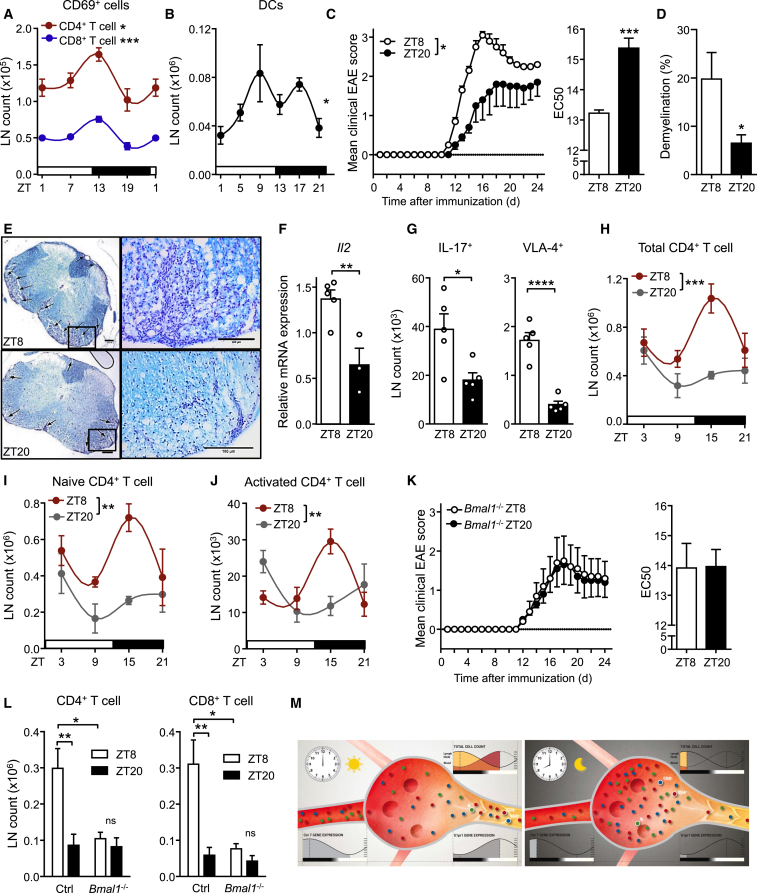
We hypothesized that oscillatory lymphocyte counts in LNs might have functional consequences in a potential time-of-day dependence of adaptive immune responses. We therefore tested whether the activation status of lymphocytes in LNs varied over the course of the day. More activated T cells were present in LNs at night onset as assessed by CD69 staining, coinciding with higher overall lymphocyte counts at this time (Figure 6A). Because dendritic cells (DCs) are key antigen-presenting cells critical in the activation of lymphocytes and the generation of adaptive immune responses (Girard et al., 2012), we next investigated whether these cells also exhibited oscillatory LN counts. Migratory DC cellularity showed strong oscillations peaking in phase with lymphocytes (Figure 6B and Figure S6A). These data point to the existence of a concerted circadian migration pattern of antigen-bearing (DCs) and antigen-recognizing (T cells) cells in LNs.
Figure 6.
T Cell Clock Function Regulates Disease Severity in EAE
(A) Oscillations of CD69+ T cell numbers in lymph node; n = 3–5 mice, one-way ANOVA.
(B) Oscillations of migratory dendritic cells (DCs) in lymph node; n = 6–12 mice, one-way ANOVA.
(C) EAE disease scores of mice immunized at ZT8 or ZT20. Disease score EC50 comparisons show accelerated symptom progression in ZT8-immunized mice; n = 5 mice, two-way ANOVA (left panel) and unpaired Student’s t test (right panel).
(D) Quantification of demyelination in lumbar spinal cord sections; n = 5 mice, unpaired Student’s t test.
(E) Luxol Fast Blue staining of lumbar spinal cord sections of mice immunized at ZT8 (top) or ZT20 (bottom) at the peak of the disease showing demyelinated areas (arrows), scale bar represents 200 μm (left images), 100 μm (right images).
(F) LN IL-2 mRNA after EAE induction; n = 3–5 mice, unpaired Student’s t test.
(G) LN counts of IL-17+ and VLA-4+ CD4+ T cells after EAE induction; n = 5 mice, unpaired Student’s t test.
(H–J) Diurnal profiles of inguinal lymph node counts of total CD4+ T cells (H), naive CD4+ T cells (I), and activated CD4+ T cells (J) on day 2 after EAE induction; n = 4 mice, two-way ANOVA with Bonferroni’s post hoc test.
(K) EAE disease scores and EC50 values in T cell-specific Bmal1−/− mice immunized at ZT8 or ZT20; n = 5 mice.
(L) Inguinal lymph node counts of CD4+ T cells, and CD8+ T cells in T cell-specific Bmal1−/− mice at ZT15 on day 2 after EAE induction; n = 4–5 mice, unpaired Student’s t test.
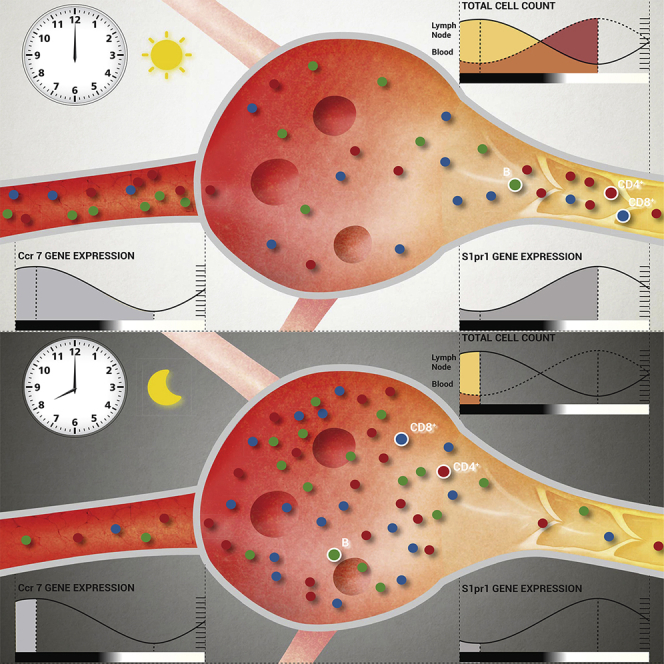
(M) Schematic diagram of circadian lymphocyte migration through lymph nodes. At night onset, increased homing due to higher CCR7 amounts leads to enhanced lymphocyte counts in the lymph node. During the day, higher S1pr1 expression induces the egression of lymphocytes into efferent lymph. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. All data are represented as mean ± SEM. See also Figure S6 and Movie S1.
Recent evidence indicates that the immune system can respond to challenges in a rhythmic fashion (Fortier et al., 2011, Gibbs et al., 2014, Nguyen et al., 2013, Scheiermann et al., 2012, Silver et al., 2012b). We therefore investigated the pathophysiological significance of circadian oscillatory LN counts in the autoimmunity model of EAE. Mice immunized during the late light phase (ZT8, when cell counts are high in LNs, Figures 1A and 1C) showed a dramatically accelerated disease progression 2 weeks later, with higher clinical scores compared to late night-immunized animals (ZT20, when LN counts trough) (Figure 6C). Differences in disease scores were associated with higher immune cell infiltration and demyelination in the spinal cord at the peak of the disease (Figures 6D and 6E). We detected elevated interleukin-2 (Il2) mRNA amounts (Figure 6F) and a higher number of IL-17 producing as well as very-late antigen (VLA)-4 integrin positive CD4+ T cells in LNs of ZT8-immunized mice, subtypes that have been shown to be critical for the induction of EAE (Kawakami et al., 2012) (Figure 6G). This indicated that circadian regulation of immunization occurred at a very early phase of the process when T cells are activated in draining lymph nodes (Figure S6B). Two days after induction of EAE, an increase of both naive and activated CD4+ and CD8+ T cells was detected in draining lymph nodes of ZT8-immunized animals, while in ZT20-immunized animals T cell numbers remained relatively low (Figures 6H–6J and Figure S6C). Thus, oscillations in the numbers of CD4+ T cells in lymph nodes during initial encounter with antigen appear to be pivotal for the severity of EAE. To investigate whether T cell autonomous clocks regulate this response, we genetically deleted T cell circadian clock function. Although in control animals disease development depended on the time of immunization, in T cell specific Bmal1−/− mice it did not (Figure 6K). Two days after immunization, total and T cell counts in draining lymph nodes were different at ZT15 between day- and night-immunized control, but not in T cell specific Bmal1−/− mice (Figure 6L and Figure S6D). Hence, T cell clocks determine time-of-day function and, after challenge, development of autoimmune sequelae. We finally investigated whether adaptive immune responses to pathogens exhibited similar circadian rhythmicity. Mice were infected with the gastric bacterial pathogen Helicobacter pylori at three different time points during the day, and lymph node counts were quantified 3 weeks later. Also in this chronic infection model, LN counts showed strong circadian responsiveness to the initial infection with highest numbers present at ZT7, analogous to the EAE immunization experiments (Figure S6E). In addition, acute viral infection with influenza A virus led to stronger pulmonary infiltration of CD8+IFN-γ+ T cells when animals were infected at ZT8 compared to ZT20, 8 days post infection (Figure S6F). Together, these data strongly indicate that immunization reactions and the adaptive immune responses to various pathogens follow a circadian rhythm (Figure 6M and Movie S1).
Discussion
We have described here the mechanisms that govern a circadian rhythmicity in the capacity of lymphocytes to enter and exit lymph nodes, which depend on cell-autonomous, clock-gene-controlled expression of promigratory factors. Lymphocytes entered LNs most prominently at the onset of the night phase and egressed from the tissue during the day. This resulted in oscillatory cell counts in lymph nodes and lymph and time-of-day differences in the adaptive immune response weeks after immunization. In addition, DCs were found to be present in LN in highest numbers around night onset, peaking in phase with the lymphocyte populations. Our data reveal that T cell-autonomous circadian oscillations are critical in regulating adaptive immunity.
It is surprising to note that lymph nodes exhibit circadian differences in their cellularity, given that they represent such a central tissue of the immune system and, accordingly, have thus been intensely studied. Since we observed oscillations in all investigated lymph nodes, the phenomenon appears to be broad and robust and not restricted to specific body locations. It is noteworthy that other lymphoid organs such as the thymus (data not shown) and the bone marrow do not exhibit overt circadian oscillations in absolute numbers. At least the latter, however, still displays circadian activity in cellular trafficking as hematopoietic stem and progenitor cells (HSPCs) are mobilized into blood (Lucas et al., 2008, Méndez-Ferrer et al., 2008) and recruited back into the bone marrow (Scheiermann et al., 2012) at different times. The fraction of mobilized and homed cells might be small compared with the overall numbers, though, which might explain why overt oscillations of total cells in the BM are not observed. In contrast to the BM, lymph node total cellularity is highly dynamic over 24 hr, as seen when homing or egress is blocked. Still, homing of leukocytes to lymph nodes and bone marrow occurs predominantly at night, while egress (or mobilization in the case of the bone marrow) occurs predominantly during the day. Thus, rhythmic egress of lymphocytes via efferent lymph is a major mechanism underlying the oscillatory leukocyte numbers in blood. Whether other egress routes for lymphocytes, from the thymus or the spleen, occur in a circadian manner is currently unclear.
Our data point to a critical role of cell-intrinsic clock-dependent mechanisms in the regulation of T and B lymphocyte trafficking. While global BMAL1 deficiency results in a diverse array of phenotypes (Bunger et al., 2000), such as altered B cell numbers (Sun et al., 2006), few studies have focused on cell-type specific deletion of BMAL1 in the immune system. Lineage-specific ablation of BMAL1 in myeloid cells results in a pro-inflammatory state (Nguyen et al., 2013), yet a similar approach targeting lymphocytes yielded no obvious phenotype (Hemmers and Rudensky, 2015). The latter finding might be due to mice with clock-deficient lymphocytes exhibiting phenotypes only at specific times, so that only when tested over multiple time points across the day can alterations be detected. Our data showing that lack of BMAL1 in T cells ablated oscillations in EAE disease scores indicates an important role for T cell clocks in the adaptive immune response. This is in line with a previous observation that T cells harvested from Clock mutant mice exhibited altered proliferation responses at specific times (Fortier et al., 2011).
We demonstrated the critical regulators of lymphocyte trafficking, Ccr7 and S1pr1, to be under circadian control. Ccr7 and S1pr1 oscillations showed opposite phases, being high in the evening for the former and high during the day for the latter. This agrees with the additional role of CCR7 as a retention factor for T cells in lymph nodes that can antagonize S1P1 function (Pham et al., 2008). In addition, more T cells expressed the activation marker CD69 at night onset, a negative regulator of S1P1 (Shiow et al., 2006). These inverted oscillations of retention and egress factors appeared to be responsible for keeping lymphocytes retained in the node at night and promoting daytime egress. In addition to these lymphocyte intrinsic signals, the microenvironmental fluctuation of CCL21 indicated an additional rhythmic component that was non cell-autonomous. Whether direct autonomic innervation governs these oscillations—as has been shown for the bone marrow and skeletal muscle (Méndez-Ferrer et al., 2008, Scheiermann et al., 2012)—and/or humoral factors as has been shown for the lung (Gibbs et al., 2014), is outside the scope of this manuscript and should be investigated in the future.
Lymph nodes act as the body’s immunological sieve, capturing lymph-borne antigens and antigen-presenting cells (APCs) and bringing these components in close contact with lymphocytes. Our data show that not only T cells migrate to the lymph node in a circadian manner but that also dendritic cells, the major APCs, are present more prominently around night onset, peaking at the same time as lymphocytes. Both cell types need to interact to produce a functional adaptive immune response (Gasteiger et al., 2016). It therefore appears likely that having them present at the same time in the confined environment of the lymph node enhances the likelihood of antigen encounter by the very few specific T cell clones, as opposed to cells entering and exiting at random times. This process of more effective cellular interactions appears to be further helped by the fact that during the night fewer cells exit the tissue than during the day, thus giving APC-T cell pairs more time to interact functionally. Whether also antigen encounter with APCs at peripheral surfaces is occurring in a rhythmic manner, which could ultimately be driving a circadian migratory DC process, should be the subject of future investigations.
Compared to the immediate response of the innate immune system, which is under circadian control (Gibbs et al., 2014, Keller et al., 2009, Nguyen et al., 2013, Scheiermann et al., 2012), it is remarkable that also the adaptive immune system appears to be temporally regulated, because these responses take much longer to be mounted. How can the time-of-day dependent differences encountered during the initial stimulus be preserved to exhibit circadian gating of immune responses weeks later? It appears that the initial hours in the generation of the adaptive immune response and the number of cells present in the lymph node are critical in regulating the strength of the response (Moon et al., 2007), which cannot be compensated for by the next circadian cycle.
In steady state, critical factors of the immune system oscillate in mice and humans with opposite phases according to their inverted rest-activity times (Arjona et al., 2012, Curtis et al., 2014, Labrecque and Cermakian, 2015, Scheiermann et al., 2013). Circulating leukocyte counts are high during the respective resting phases, which occur during the day in mice and at night in humans. In addition, also under stimulated conditions timing matters, as administration of granulocyte colony-stimulating factor (G-CSF) to patients in order to mobilize hematopoietic stem cells from bone marrow into blood yields higher numbers at different times than in mice (Lucas et al., 2008). These data suggest that also adaptive immune responses in humans could be under circadian control. Since in our EAE model, immunization close to the onset of the activity phase produced a higher immune response in mice, in humans the early morning hours should produce a higher adaptive immune reactivity. Indeed, this appears to be the case as recent studies have unveiled that administration of hepatitis A (Phillips et al., 2008) and flu vaccines (Long et al., 2016) in the morning yielded highest antibody titers compared to other times. Together, our data provide mechanistic insights for a time-of-day difference in lymphocyte trafficking and adaptive immune responses, thus warranting further investigations into time-of-day optimization of immune therapies and vaccination programs.
Experimental Procedures
Animals
Cd4cre, Lckcre, Cd19cre, Bmal1flox/flox, and S1pr1flox/flox mice were obtained from Jackson Laboratories and crossbred to target T cells and B cells, respectively. Ccr7−/− mice were a gift from Reinhold Förster. 7- to 8-week-old wild-type C57BL/6 mice were purchased from Charles River and Janvier. Mice were housed under a 12 hr:12 hr light-dark cycle with food and water ad libitum. To induce changes in light regime, we placed mice in a light cycler (Park Bioservices) with a 12 hr-inverted light cycle for a minimum of 2 weeks to completely establish an inverse light cycle or kept in constant darkness. All animal experimental procedures were carried out in accordance with the German Law of Animal Welfare and approved by the Regierung of Oberbayern or the ethics committee of the Schleswig-Holstein State Ministry of Energy, Agriculture, Environment and Rural Areas.
Flow Cytometry
Blood was collected into EDTA-coated capillary tubes (Microvete 300). Counts were obtained using an IDEXX ProCyte DX cell counter or a Hemavet Hematology Analyzer 950FS (Drew Scientific). Erythrocytes were lysed by incubation in 0.8% NH4Cl. Lymph was collected as described below. Spleens, lymph nodes, or thymi were harvested from animals and processed through a cell strainer (40–70 μM pore size) and resuspended in PBS. For quantification of dendritic cells, lymph nodes were first chopped into small pieces and incubated in collagenase IV (1 mg/ml, C5138, Sigma) and DNase I (0.2 mg/ml, Roche) for 30 min at 37°C with gentle agitation. Following digestion, cells were passed through a 40 μm strainer and resuspended in PBS supplemented with 2% fetal bovine serum (GIBCO). A 20 min blocking step using anti-CD16/32 antibody (2.4G2; BD Biosciences) was performed on ice prior to fluorescent staining. Single-cell suspensions were stained with fluorescence-conjugated antibodies and analyzed by flow cytometry using a Gallios Flow Cytometer (Beckman Coulter) or a FACSCanto II flow cytometer (BD Biosciences). Prior to staining of intracellular cytokines, cells were restimulated for 5 hr with PMA and ionomycine (cell stimulation cocktail with protein transport inhibitors, eBioscience). Data were analyzed with FlowJo software (Tree Star).
Adoptive Transfer Studies
Lymphocytes from spleen and peripheral lymph nodes (ratio 80:20) were labeled with 1.5 μM of carboxyfluorescein succinimidyl ester (CFSE, Thermo Fisher Scientific) or 2.5 μM CellTracker Red CMTPX (Thermo Fisher Scientific) in PBS containing 0.2% BSA and 2 mM EDTA for 20 min at 37°C and washed 4 times. 20 × 106 cells were then injected i.v. into recipient mice. In some experiments, lymph node entry was blocked two and 12 hr later by intraperitoneal (i.p.) administration of 100 μg anti-αL (CD11a, clone M17/4; BioXCell) and anti-α4 (CD49d, clone PS/2; BioXCell) integrin antibodies in PBS. In some experiments, cells were pre-incubated with a 200 ng/ml pertussis toxin (PTX) pulse of 10 min at 37°C and washed 4 times before injection together with control-treated cells (Lo et al., 2005). The subsets of adoptively transferred cells in the recipient mice were analyzed via flow cytometry. Dyes were switched to avoid dye-specific effects.
S1pr1 Promoter Luciferase Reporter Assay
The S1pr1-Gaussia Luciferase (S1pr1-GLuc) GLuc-ON promoter clone containing a ∼1 kb fragment upstream of the transcription start site and a ∼0.3 kb fragment of the exon 1 of murine S1pr1 in pEZX-PG04 vector (also containing a constitutively expressed secreted alkaline phosphatase (SEAP) secondary reporter as an internal control) was obtained from GeneCopoeia. HEK293AAV cells (Cellbiolabs) were plated onto 96-well plates and maintained in DMEM with 2 mM stable glutamine supplemented with 10% fetal bovine serum (FBS) and 10,000 U penicillin/streptomycin at 37°C with 5% CO2. Upon 80%–90% confluence, cells were transfected with the following expression plasmids: 20 ng S1pr1-luc with various combinations of the following clock gene constructs: HA-Clock, HA-Bmal1, and pcDNA3.1 (mock transfection) as indicated per well using Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. Medium was changed once on the next day and was collected for measurement 48 hr after transfection. The secreted GLuc and SEAP activities were measured with the Secrete-Pair Dual Luminescence Assay Kit (GeneCopoeia) according to the manufacturer’s protocol using the Berthold TriStar LB 941 plate reader (Berthold Technologies). Normalized promoter activity was calculated as the ratio of GLuc to SEAP activities.
Lymph Cannulation
40 min prior to cannulation, 200 μl olive oil was administered intragastrally to identify mesenteric lymph vessels that are located just upstream of the thoracic duct. Mice were anesthetized (100 mg/kg ketamine, 20 mg/kg xylazine, 1% acepromazin i.p.), vessels were cannulated and lymph was drawn via a fine bore polythene tubing (Smiths Medical) that had previously been flushed with PBS containing EDTA. In some experiments, mice were injected i.p. with 1 mg/kg (or less) of FTY720 or 10 mg/kg of SEW2871 1 or 2 hours, respectively, before cannulation. Cell numbers were determined using an IDEXX ProCyte DX cell counter.
Immunofluorescence Microscopy and Quantitative Imaging Analysis
Harvested lymph nodes were embedded in OCT (TissueTec), frozen and sectioned with a thickness of 10 μm on a cryostat (Leica). Sections were fixed in methanol or left unfixed, incubated with fluorescently coupled antibodies and imaged on an Axio Examiner.D1 microscope (Zeiss) equipped with LEDs of 405 nm, 488 nm, 561 nm, and 642 nm excitation wavelengths or equipped with a 4-color laser stack and a confocal spinning-disk head (Intelligent Imaging Innovations). For investigations of protein expression in HEVs, all quantifications were performed using mask analysis (Zeiss software) based on PECAM-1 expression and quantifying expression of other fluorescent channels within the mask containing PECAM+-pixels delineating HEV vascular structures as previously detailed (Scheiermann et al., 2012).
EAE Induction
For EAE induction the MOG35-55-CFA Emulsion PTX Kit (EK-2110, Hooke Labs) was used according to the manufacturer’s protocol. Mice were anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine i.p.) and bilaterally s.c. injected with 200 μg myelin oligodendrocyte glycoprotein (MOG) 35–55 in 200 μL Complete Freund’s Adjuvant (CFA) at the indicated time points, followed by two i.p. injections of 200 ng pertussis toxin (PTX) in phosphate buffered saline (PBS), immediately after immunization and 24 hr later. From day 8 on, paralysis was assessed using the clinical scoring system: 0, no obvious signs of disease; 0.5, distal paralysis of the tail; 1, complete tail paralysis; 1.5, mild paresis of one or both hind legs; 2, severe paresis of both hind legs; 2.5, complete paralysis of one hind leg; 3, complete paralysis of both hind legs; 3.5, complete paralysis of hind legs and paresis of one front leg. Animals with scores of 2 and up were provided access to food and water on the bottom of the cage.
T and B Cell Isolation
CD4+ T cells for Q-PCR analysis were purified from lymph nodes of C57BL/6 WT, × S1pr1+/floxxCd4-cre, Bmal1flox/floxxCd4-cre or littermate control mice using the EasySep Mouse CD4+ T cell enrichment kit (StemCell Technologies). Purity was confirmed via flow cytometry and was generally > 96%. B220+ B cells for western blot analysis were purified from spleens of Bmal1flox/floxxCd19-cre or littermate control mice using the EasySep Mouse B cell enrichment kit (StemCell Technologies). The Pan T Cell Isolation Kit (Miltenyi Biotec) was used to purify total T cells from mouse splenocytes. Splenocytes were labeled with a mixture of biotin-conjugated monoclonal antibodies directed against cells other than T cells and followed by conjugate binding with anti-biotin microbeads. Magnetically labeled non-T cells were depleted with autoMACS (Miltenyi Biotec) and the negative fraction was collected, yielding an average 90% purity of (CD3+) T cells.
Antibodies, Western Blotting, Mass Spectrometry Analyses, Quantitative Real-Time PCR, Histology, T Cell Proliferation Analyses, Helicobacter Pylori Infection and Influenza A Virus Infection
Statistical Analyses
All data are represented as mean ± SEM. Comparisons between two samples were performed using the paired and unpaired Student’s t tests or Mann-Whitney test. One-way ANOVA analyses followed by Tukey’s multiple comparison test, two-way ANOVA analyses followed by Bonferroni’s post hoc test were used for multiple group comparisons. Statistical analyses were performed with GraphPad Prism 6 software. Statistical significance was assessed as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
Author Contributions
D.D. and O.M. designed and performed experiments, analyzed results, and wrote the manuscript; L.I., U.H., R.H., W.H., A.H.T., A.L., M.E., C.-S.C., K.K., A.d.J., S.M.H., N.K., O.U., L.Y., and L.E.S. performed experiments and provided valuable inputs on the manuscript; C. Schmal and H.H. performed modeling analyses; B.K. provided access to mass spectrometers; W.S. and H.O. designed and supervised experiments, discussed data, and wrote the manuscript; C. Scheiermann conceived and supervised the study, designed and performed experiments, discussed data, and wrote the manuscript.
Acknowledgments
We thank Reinhold Förster and Leandro Moschovakis for providing Ccr7−/− mice, Steffen Massberg, Markus Sperandio, and Martha Merrow for critical comments on the manuscript and Victor Lavilla for designing the video. This work was supported by the German Research Foundation (DFG) (Emmy-Noether grant (SCHE 1645/2-1) and SFB914 projects B09 and Z03 to C. Scheiermann, SFB914 project B05 to R.H., SFB654 project C08 to W.S. and H.O., TR128 project B10 and a Heisenberg fellowship to N.K., SFB-TR84 project C08 to L.E.S., C. Schmal and H.H. are supported by BO 3612/2-1. C. Scheiermann holds a European Research Council (ERC) starting grant (635872, CIRCODE). H.O. is a Lichtenberg fellow of the Volkswagen Foundation. L.E.S. and L.Y. are supported by the Else Kröner Fresenius Foundation (EKFS, 2012_A56 to L.E.S.).
Published: January 10, 2017
Footnotes
Supplemental Information includes six figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2016.12.011.
Supplemental Information
Movie showing the circadian migration of lymphocytes through lymph nodes. At night onset, increased homing due to higher amounts of lymphocyte CCR7 leads to enhanced lymphocyte counts in the lymph node. During the day, higher S1pr1 expression induces the egression of lymphocytes into efferent lymph. Related to Figure 6.
References
- Arjona A., Sarkar D.K. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. Journal of Immunology. 2005;174:7618–7624. doi: 10.4049/jimmunol.174.12.7618. [DOI] [PubMed] [Google Scholar]
- Arjona A., Silver A.C., Walker W.E., Fikrig E. Immunity’s fourth dimension: approaching the circadian-immune connection. Trends Immunol. 2012;33:607–612. doi: 10.1016/j.it.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger T., Leutz A., Leliavski A., Skrum L., Kovac J., Bonacina L., Benedict C., Lange T., Westermann J., Oster H., Solbach W. Circadian clocks in mouse and human CD4+ T cells. PLoS ONE. 2011;6:e29801. doi: 10.1371/journal.pone.0029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger M.K., Wilsbacher L.D., Moran S.M., Clendenin C., Radcliffe L.A., Hogenesch J.B., Simon M.C., Takahashi J.S., Bradfield C.A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E.C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Curtis A.M., Bellet M.M., Sassone-Corsi P., O’Neill L.A. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Cyster J.G., Schwab S.R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- Dibner C., Schibler U., Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Esquifino A.I., Selgas L., Arce A., Maggiore V.D., Cardinali D.P. Twenty-four-hour rhythms in immune responses in rat submaxillary lymph nodes and spleen: effect of cyclosporine. Brain Behav. Immun. 1996;10:92–102. doi: 10.1006/brbi.1996.0010. [DOI] [PubMed] [Google Scholar]
- Förster R., Schubel A., Breitfeld D., Kremmer E., Renner-Müller I., Wolf E., Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Förster R., Davalos-Misslitz A.C., Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat. Rev. Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- Fortier E.E., Rooney J., Dardente H., Hardy M.P., Labrecque N., Cermakian N. Circadian variation of the response of T cells to antigen. Journal of Immunology. 2011;187:6291–6300. doi: 10.4049/jimmunol.1004030. [DOI] [PubMed] [Google Scholar]
- Gasteiger G., Ataide M., Kastenmüller W. Lymph node - an organ for T-cell activation and pathogen defense. Immunol. Rev. 2016;271:200–220. doi: 10.1111/imr.12399. [DOI] [PubMed] [Google Scholar]
- Gibbs J., Ince L., Matthews L., Mei J., Bell T., Yang N., Saer B., Begley N., Poolman T., Pariollaud M. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat. Med. 2014;20:919–926. doi: 10.1038/nm.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J.P., Moussion C., Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 2012;12:762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- Haus E., Smolensky M.H. Biologic rhythms in the immune system. Chronobiol. Int. 1999;16:581–622. doi: 10.3109/07420529908998730. [DOI] [PubMed] [Google Scholar]
- Hemmers S., Rudensky A.Y. The Cell-Intrinsic Circadian Clock Is Dispensable for Lymphocyte Differentiation and Function. Cell Rep. 2015;11:1339–1349. doi: 10.1016/j.celrep.2015.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami N., Bartholomäus I., Pesic M., Mues M. An autoimmunity odyssey: how autoreactive T cells infiltrate into the CNS. Immunol. Rev. 2012;248:140–155. doi: 10.1111/j.1600-065X.2012.01133.x. [DOI] [PubMed] [Google Scholar]
- Keller M., Mazuch J., Abraham U., Eom G.D., Herzog E.D., Volk H.D., Kramer A., Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrecque N., Cermakian N. Circadian Clocks in the Immune System. J. Biol. Rhythms. 2015;30:277–290. doi: 10.1177/0748730415577723. [DOI] [PubMed] [Google Scholar]
- Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Lo C.G., Xu Y., Proia R.L., Cyster J.G. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 2005;201:291–301. doi: 10.1084/jem.20041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.E., Drayson M.T., Taylor A.E., Toellner K.M., Lord J.M., Phillips A.C. Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine. 2016;34:2679–2685. doi: 10.1016/j.vaccine.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D., Battista M., Shi P.A., Isola L., Frenette P.S. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell. 2008;3:364–366. doi: 10.1016/j.stem.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl J.N., Liou R., Klauschen F., Vrisekoop N., Monteiro J.P., Yates A.J., Huang A.Y., Germain R.N. Quantification of lymph node transit times reveals differences in antigen surveillance strategies of naive CD4+ and CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2012;109:18036–18041. doi: 10.1073/pnas.1211717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S., von Andrian U.H. Fingolimod and sphingosine-1-phosphate--modifiers of lymphocyte migration. N. Engl. J. Med. 2006;355:1088–1091. doi: 10.1056/NEJMp068159. [DOI] [PubMed] [Google Scholar]
- Matloubian M., Lo C.G., Cinamon G., Lesneski M.J., Xu Y., Brinkmann V., Allende M.L., Proia R.L., Cyster J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S., Lucas D., Battista M., Frenette P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Mohawk J.A., Green C.B., Takahashi J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J.J., Chu H.H., Pepper M., McSorley S.J., Jameson S.C., Kedl R.M., Jenkins M.K. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W.A. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K.D., Fentress S.J., Qiu Y., Yun K., Cox J.S., Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T.H., Okada T., Matloubian M., Lo C.G., Cyster J.G. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28:122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A.C., Gallagher S., Carroll D., Drayson M. Preliminary evidence that morning vaccination is associated with an enhanced antibody response in men. Psychophysiology. 2008;45:663–666. doi: 10.1111/j.1469-8986.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- Scheiermann C., Kunisaki Y., Lucas D., Chow A., Jang J.E., Zhang D., Hashimoto D., Merad M., Frenette P.S. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C., Kunisaki Y., Frenette P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013;13:190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow L.R., Rosen D.B., Brdicková N., Xu Y., An J., Lanier L.L., Cyster J.G., Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Silver A.C., Arjona A., Hughes M.E., Nitabach M.N., Fikrig E. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain Behav. Immun. 2012;26:407–413. doi: 10.1016/j.bbi.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver A.C., Arjona A., Walker W.E., Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36:251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Sun Y., Yang Z., Niu Z., Peng J., Li Q., Xiong W., Langnas A.N., Ma M.Y., Zhao Y. MOP3, a component of the molecular clock, regulates the development of B cells. Immunology. 2006;119:451–460. doi: 10.1111/j.1365-2567.2006.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D., Blanks J.E. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- von Andrian U.H., Mempel T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Wagner D.D., Frenette P.S. The vessel wall and its interactions. Blood. 2008;111:5271–5281. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Rollins D., Ruhn K.A., Stubblefield J.J., Green C.B., Kashiwada M., Rothman P.B., Takahashi J.S., Hooper L.V. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342:727–730. doi: 10.1126/science.1243884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie showing the circadian migration of lymphocytes through lymph nodes. At night onset, increased homing due to higher amounts of lymphocyte CCR7 leads to enhanced lymphocyte counts in the lymph node. During the day, higher S1pr1 expression induces the egression of lymphocytes into efferent lymph. Related to Figure 6.