Abstract
Background
Tumor cell lines are commonly used as experimental tools in cancer research, but their relevance for the in vivo situation is debated. In a series of 11 microsatellite stable (MSS) and 9 microsatellite unstable (MSI) colon cancer cell lines and primary colon carcinomas (25 MSS and 28 MSI) with known ploidy stem line and APC, KRAS, and TP53 mutation status, we analyzed the promoter methylation of the following genes: hMLH1, MGMT, p16INK4a (CDKN2A α-transcript), p14ARF (CDKN2A β-transcript), APC, and E-cadherin (CDH1). We compared the DNA methylation profiles of the cell lines with those of the primary tumors. Finally, we examined if the epigenetic changes were associated with known genetic markers and/or clinicopathological variables.
Results
The cell lines and primary tumors generally showed similar overall distribution and frequencies of gene methylation. Among the cell lines, 15%, 50%, 75%, 65%, 20% and 15% showed promoter methylation for hMLH1, MGMT, p16INK4a, p14ARF, APC, and E-cadherin, respectively, whereas 21%, 40%, 32%, 38%, 32%, and 40% of the primary tumors were methylated for the same genes. hMLH1 and p14ARF were significantly more often methylated in MSI than in MSS primary tumors, whereas the remaining four genes showed similar methylation frequencies in the two groups. Methylation of p14ARF, which indirectly inactivates TP53, was seen more frequently in tumors with normal TP53 than in mutated samples, but the difference was not statistically significant. Methylation of p14ARF and p16INK4a was often present in the same primary tumors, but association to diploidy, MSI, right-sided location and female gender was only significant for p14ARF. E-cadherin was methylated in 14/34 tumors with altered APC further stimulating WNT signaling.
Conclusions
The present study shows that colon cancer cell lines are in general relevant in vitro models, comparable with the in vivo situation, as the cell lines display many of the same molecular alterations as do the primary carcinomas. The combined pattern of epigenetic and genetic aberrations in the primary carcinomas reveals associations between them as well as to clinicopathological variables, and may aid in the future molecular assisted classification of clinically distinct stages.
Keywords: APC, colon cancer cell lines, colorectal carcinomas, CpG methylation, E-cadherin, hMLH1, KRAS, MGMT, MSI, MSS, p14, p16, TP53
Background
During the last decade, epigenetic changes have been reported in many cancers and they are now recognized to be at least as common as genetic changes [1]. Aberrant methylation of cytosine located within the dinucleotide CpG is by far the best-categorized epigenetic change. The genome of the cancer cell demonstrates global hypomethylation [2,3] as well as regional promoter hypermethylation of several tumor suppressor genes [4]. Hypermethylation of selected CpG sites within CpG islands in the promoter region of genes is associated with loss of gene expression and is observed in both physiological conditions, such as X chromosome inactivation [5], and neoplasia [6]. By inactivating various tumor suppressor genes, this epigenetic modification can affect many important cellular processes, such as the cell cycle (RB, p15INK4b, p16INK4a), the TP53 pathway (p14ARF), the WNT signaling pathway (APC, E-cadherin), DNA repair (MGMT, hMLH1, BRCA1), apoptosis (DAPK), and the metastasizing process (E-cadherin, TIMP3) (reviewed in [1,7,8]).
Development of colorectal cancer through various morphological stages has been linked to several genetic and epigenetic changes. The majority of carcinomas have several chromosomal aberrations, a phenotype often referred to as chromosomal instability. Approximately 15% of the tumors are near diploid but exhibit microsatellite instability (MSI), seen as genome-wide short nucleotide insertions and deletions [9]. This phenotype is caused by a defect DNA mismatch repair system [9]. Subgroups of both types of colorectal carcinomas reveal aberrant methylation of tumor suppressor genes leading to lack of expression [10,11].
Human cancer cell lines are important tools in cancer research. Their commercial availability and unrestrained growth make them well suited for in vitro studies. Although many of the known genetic aberrations in colon cancer cell lines have been comprehensively described [12], several of these cell lines have not been analyzed for methylation status of pathogenetically important target genes.
The frequencies of both methylation and gene mutation differ among various studies of cell lines and primary tumors. The genome characteristics, profiles of gene mutations, and methylation status are rarely reported in the same samples, let alone in large series. In the present report we address these potentially connected pathogenetic mechanisms by presenting methylation profiles of a set of genes in a series of MSI and microsatellite stable (MSS) colon cancer cell lines and primary colorectal carcinomas. The methylation profiles are compared with various known genetic and clinicopathological features of the same series.
Results
Methylation status of target genes in colon cancer cell lines
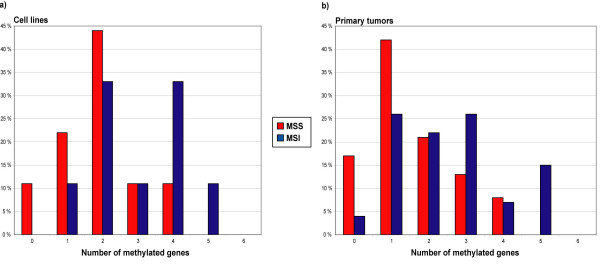
The colon cancer cell line methylation-specific PCR (MSP) results are summarized in Table 1 and Figure 1a. Among the MSI cell lines 3/9, 5/9, 7/9, 8/9, 2/9, and 2/9 showed promoter hypermethylation of hMLH1, MGMT, p16INK4a, p14ARF, APC, and E-cadherin, respectively, whereas 0/11, 5/11, 8/11, 5/11, 2/11, and 1/11 of the MSS cell lines were hypermethylated for the same genes (Table 2). Hence, the cell lines with MSI generally showed higher methylation frequencies than did the MSS cell lines (Figures 1a, 2a). In most cases, methylation of the target genes was biallelic, but in 10 of the 20 cell lines, monoallelic methylation (detection of both methylated and unmethylated MSP gel bands) was found for one or more of the genes (Table 1). The MSS V9P was the only cell line unmethylated for all six genes analyzed.
Table 1.
Promoter methylation of colon cancer cell lines. MSI, microsatellite instable; MSS, microsatellite stable; U, unmethylated; M, methylated. The references give results in agreement with our own data except when the reference is underlined. Note that reference 15 does not use the category monoallelic methyaltion, but reports the promoters only as methylated or unmethylated.
| Cell line | hMLH1 | MGMT | p16INK4a | p14ARF | APC | E-Cadherin | |
| MSI | |||||||
| Co115 | M12 | M | M12 | M | U/M | U | |
| HCT15 | U12,13,14,15 | U/M15,16 | M12,14,15 | M14,15,17 | U | U15 | |
| HCT116 | U12,13,15,18,19,20,21,22 | U/M15,20 | U/M12,15,20,21,22,23 | U/M15,17,21,24 | U/M | U15 | |
| LoVo | U12,13,14,15,18,22,26 | U15,31 | M12,14,15,22 | M14,15,24,25 | U | U15 | |
| LS174T | U12,13,18,22 | U/M | U12,22 | U/M | U | U | |
| RKO | M15,18,19,20,22,26 | U15,20 | M15,20,22,27 | M15,24 | U | M15 | |
| SW48 | M12,13,14,15,18,20,22,26,28,29 | M15,20,31 | M12,14,15,20,22,27,29 | M14,15,24 | U | U15 | |
| TC7 | U12 | U | U12 | U/M | U | U | |
| TC71 | U12 | U | M12 | U | U | U/M | |
| MSS | |||||||
| ALA | U12 | U | M12 | U | M | U | |
| Colo320 | U12,14,18,30 | M | M12,14,27 | U14 | U30 | M | |
| EB | U12 | M | M12 | U | U | U | |
| FRI | U12 | U/M | U12 | U/M | U | U | |
| HT29 | U12,13,14,15,18,21,22,26,30 | U15,31,32,33 | M12,14,15,21,22,27 | U14,15,21,24 | U30 | U15 | |
| IS1 | U12,21 | U | M12,21 | M21 | U | U | |
| IS2 | U12 | U | U/M12 | M | U | U | |
| IS3 | U12 | U | U12 | M | U | U | |
| LS1034 | U12,13 | U/M | U/M12 | M | U/M | U | |
| SW480 | U12,14,15,19,21,22,26,30 | U/M15 | M12,14,15,21,22,27 | U14,15,21,24,25 | U30 | U15 | |
| V9P | U12 | U | U12 | U | U | U |
Figure 1.
Distribution of simultaneously methylated promoters in MSS and MSI colon cancer cell lines and colorectal carcinomas. The two panels illustrate the percentage of MSS and MSI samples displaying methylation of zero to all of the promoters analyzed in the present study in a) cell lines and b) primary colorectal tumors. Abbreviations: MSS, microsattelite stable; MSI, microsattelite instable.
Table 2.
Methylation frequencies among MSS and MSI colon cancer cell lines and primary colorectal tumors. Abbreviations; MSS, microsatellite stable; MSI, microsatellite instable; CRC, colorectal cancer; U, unmethylated;M, methylated. Note that the calculated methylation frequencies of the MSS cell lines includes results from three cell lines derived from the same patient.
| MSS | MSI | Total | ||||
| Gene | Cell lines | CRCs | Cell lines | CRCs | Cell lines | CRCs |
| hMLH1 | 0/11 (0%) | 0/25 (0%) | 3/9 (33%) | 11/28 (39%) | 3/20 (15%) | 11/53 (21%) |
| MGMT | 5/11 (45%) | 10/25 (40%) | 5/9 (56%) | 11/28 (39%) | 10/20 (50%) | 21/53 (40%) |
| p16INK4a | 8/11 (73%) | 7/25 (28%) | 7/9 (78%) | 10/28 (36%) | 15/20 (75%) | 17/53 (32%) |
| p14ARF | 5/11 (45%) | 3/24 (12%) | 8/9 (89%) | 17/28 (61%) | 13/20 (65%) | 20/52 (38%) |
| APC | 2/11 (18%) | 7/25 (28%) | 2/9 (22%) | 10/28 (36%) | 4/20 (20%) | 17/53 (32%) |
| E-cadherin | 1/11 (9%) | 10/24 (42%) | 2/9 (22%) | 11/28 (39%) | 3/20 (15%) | 21/52 (40%) |
Figure 2.
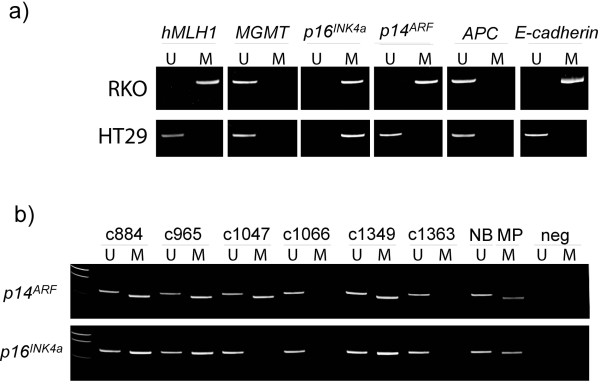
Promoter hypermethylation in colon cancer cell lines and colorectal primary tumors. Methylation was evaluated by methylation-specific PCR (MSP). A visible PCR product in Lanes U indicates the presence of unmethylated alleles whereas a PCR product in Lanes M indicates the presence of methylated alleles. The upper panel (a) illustrates the methylation status of all the loci analyzed in a MSI cell line (RKO) and a MSS cell line (HT29). The lower panel (b) shows the methylation status of representative primary colorectal tumors. Abbreviations: NB, normal blood (positive control for unmethylated samples); MP, methylated placenta (positive control for methylated samples); neg, negative control (containing water as template); U, lane for unmethylated MSP product; M, lane for methylated MSP product.
Methylation status of target genes in primary colorectal carcinomas. Comparison with colon cancer cell lines
Methylation status was assessable in more than 99% of the total number of analyses (53 tumors × 6 genes = 318 analyses).
The results of the methylation analyses of 53 primary colorectal carcinomas (25 MSS and 28 MSI) are shown in Table 2 and illustrated in Figures 1b and 2b. All the methylated primary tumors examined showed an unmethylated band in addition to the methylated one, probably due to the presence of normal cells. The methylation frequencies varied from 0% among MSS tumors at the hMLH1 promoter to 61% among the MSI tumors for the p14ARF gene (Table 2).
Several of the primary tumor samples displayed widespread CpG island methylation (Figure 1b). Eighteen of 52 tumors (35%) were methylated in 3 or more of the 6 genes analyzed. Only 5/52 (10%) of the tumor samples did not show hypermethylation in any of the genes analyzed. We saw no statistical difference in the number of methylated target genes in colon cancer cell lines versus colorectal primary tumors (Mean Rank 32 for primary tumors versus 38 for cell lines, P = 0.231, Mann-Whitney test).
Methylation profiles compared with genetic characteristics
The methylation status of the primary tumors was compared with genetic characteristics of the same tumors (Table 3). In general, higher frequencies of gene methylation were found among diploid than among aneuploid tumors, reflecting the MSI status, but the differences reached statistical significance only for p14ARF (P < 0.001) and hMLH1 (P = 0.015). Sixteen of 49 primary tumors harbored TP53 mutations, and all of the tumors with TP53 mutations also harbored unmethylated hMLH1 (P = 0.009). p14ARF hypermethylation was less common in tumors with mutated TP53 than in tumors with wild type TP53, although this was not statistically significant (P = 0.127). Four tumors displayed a G:C to A:T TP53 mutation and three of them simultaneously harbored a methylated MGMT gene. Four of 11 tumors with G:C to A:T KRAS (KRAS2) mutations were methylated at the MGMT promoter. Overall, the presence of KRAS mutations was not associated with the methylation status of the genes analyzed. Among the 20 tumors with p14ARF methylation, 10 were also methylated at the adjacent p16INK4a gene (P = 0.067). Finally, the APC promoter was methylated in 17/53 (32%) tumors, and 8/17 (47%) tumors displayed both APC mutation and methylation.
Table 3.
CpG island methylation of selected genes compared with the patients clinicopathological features and tumor genetics. Abbreviations: Gen. Characteristics, Genetic Characteristics; MSI, microsatellite instability; MSS, microsatellite stable; NS, not significant; Clin. and Path. Features, Clinical and Pathological Features. Comparison of different groups were tested with Fisher exact test or Pearsons χ2 test, P values are two sided and are considered statistically significant when P ≤ 0.05. The table is based on primary tumors (53) and not patients (52) *Statistically significant Pearsons χ2 tests with expected count less than 5.
| hMLH1 | MGMT | p16INK4a | p14ARF | APC | E-cadherin | |||||||||||||
| M | U | M | U | M | U | M | U | M | U | M | U | |||||||
| Individuals | ||||||||||||||||||
| No | 11/53 | 42/53 | 21/53 | 32/53 | 17/53 | 36/53 | 20/52 | 32/52 | 17/53 | 36/53 | 21/52 | 31/52 | ||||||
| Gen. Characteristics | ||||||||||||||||||
| Ploidy | ||||||||||||||||||
| Diploid | 10 | 20 | 10 | 20 | 10 | 20 | 18 | 12 | 11 | 19 | 13 | 17 | ||||||
| Aneuploid | 1 | 22 | 11 | 12 | 7 | 16 | 2 | 20 | 6 | 17 | 8 | 14 | ||||||
| P value | 0.02 | NS | NS | < 0.001 | NS | NS | ||||||||||||
| MSI-status | ||||||||||||||||||
| MSI | 11 | 17 | 11 | 17 | 10 | 18 | 17 | 11 | 10 | 18 | 11 | 17 | ||||||
| MSS | 0 | 25 | 10 | 15 | 7 | 18 | 3 | 21 | 7 | 18 | 10 | 14 | ||||||
| P value | < 0.001 | NS | NS | 0.001 | NS | NS | ||||||||||||
| TP53 | ||||||||||||||||||
| Wild type | 11 | 22 | 12 | 21 | 11 | 22 | 16 | 16 | 8 | 25 | 13 | 19 | ||||||
| Mutation | 0 | 16 | 7 | 9 | 5 | 11 | 4 | 12 | 7 | 9 | 7 | 9 | ||||||
| P value | 0.01 | NS | NS | NS | NS | NS | ||||||||||||
| wt+non G-A mutation | 11 | 33 | 15 | 29 | 14 | 30 | 18 | 25 | 13 | 31 | 17 | 26 | ||||||
| G-A mutation | 0 | 4 | 3 | 1 | 1 | 3 | 1 | 3 | 1 | 3 | 2 | 2 | ||||||
| P value | NS | NS | NS | NS | NS | NS | ||||||||||||
| K-Ras | ||||||||||||||||||
| Wild type | 8 | 19 | 13 | 14 | 9 | 18 | 12 | 15 | 7 | 20 | 9 | 18 | ||||||
| Mutation | 1 | 14 | 6 | 9 | 3 | 12 | 2 | 12 | 6 | 9 | 6 | 8 | ||||||
| P value | NS | NS | NS | 0.08 | NS | NS | ||||||||||||
| wt+non G-A mutation | 8 | 23 | 15 | 16 | 9 | 22 | 13 | 18 | 8 | 23 | 10 | 21 | ||||||
| G-A mutation | 1 | 10 | 4 | 7 | 3 | 8 | 1 | 9 | 5 | 6 | 5 | 5 | ||||||
| P value | NS | NS | NS | NS | NS | NS | ||||||||||||
| APC | ||||||||||||||||||
| Wild type | 7 | 19 | 12 | 14 | 10 | 16 | 9 | 17 | 9 | 17 | 12 | 14 | ||||||
| Mutation | 3 | 23 | 8 | 18 | 7 | 19 | 10 | 15 | 8 | 18 | 9 | 16 | ||||||
| P value | NS | NS | NS | NS | NS | NS | ||||||||||||
| Clin. and Path. Features | ||||||||||||||||||
| Sex | ||||||||||||||||||
| Male | 2 | 23 | 9 | 16 | 8 | 17 | 6 | 19 | 10 | 15 | 8 | 17 | ||||||
| Female | 9 | 19 | 12 | 16 | 9 | 19 | 14 | 13 | 7 | 21 | 13 | 14 | ||||||
| P value | 0.04 | NS | NS | 0.05 | NS | NS | ||||||||||||
| Age (years) | ||||||||||||||||||
| <68 | 2 | 21 | 10 | 13 | 4 | 19 | 7 | 16 | 8 | 15 | 9 | 14 | ||||||
| ≥68 | 9 | 21 | 11 | 19 | 13 | 17 | 13 | 16 | 9 | 21 | 12 | 17 | ||||||
| P value | 0.09 | NS | 0.07 | NS | NS | NS | ||||||||||||
| Location | ||||||||||||||||||
| Right | 10 | 8 | 7 | 11 | 7 | 11 | 12 | 6 | 7 | 11 | 7 | 11 | ||||||
| Left | 1 | 19 | 8 | 12 | 9 | 11 | 5 | 14 | 6 | 14 | 8 | 11 | ||||||
| Rectum | 0 | 14 | 6 | 8 | 1 | 13 | 2 | 12 | 4 | 10 | 5 | 9 | ||||||
| P value | < 0.001* | NS | 0.05 | 0.01 | NS | NS | ||||||||||||
| Histologic grade | ||||||||||||||||||
| Poorly differentiated | 4 | 8 | 7 | 5 | 6 | 6 | 7 | 4 | 5 | 7 | 4 | 7 | ||||||
| Moderately differentiated | 7 | 30 | 13 | 24 | 11 | 26 | 12 | 25 | 11 | 26 | 14 | 23 | ||||||
| Well differentiated | 0 | 3 | 1 | 2 | 0 | 3 | 0 | 3 | 1 | 2 | 2 | 1 | ||||||
| P value | NS | NS | NS | NS | NS | NS | ||||||||||||
| Dukes' classification | ||||||||||||||||||
| A | 2 | 2 | 3 | 1 | 1 | 3 | 2 | 2 | 0 | 4 | 1 | 3 | ||||||
| B | 5 | 22 | 10 | 17 | 8 | 19 | 9 | 17 | 13 | 14 | 12 | 14 | ||||||
| C | 2 | 13 | 4 | 11 | 4 | 11 | 4 | 11 | 3 | 12 | 5 | 10 | ||||||
| D | 2 | 5 | 4 | 3 | 4 | 3 | 5 | 2 | 1 | 6 | 3 | 4 | ||||||
| P value | NS | NS | NS | NS | 0.07 | NS | ||||||||||||
Among the tumors with widespread methylation (3 or more methylated genes), 13/18 (72%) tumors demonstrated MSI, whereas 5/24 (21%) were MSS (P = 0.080). We found no statistically significant associations between tumors with widespread methylation and presence of TP53, KRAS, or APC mutations.
Methylation profiles and clinicopathological features
The clinicopathological features and methylation status of the primary tumors are summarized in Table 3. We saw more methylation among tumors from females than in those from males for both hMLH1 (P = 0.043) and p14ARF (P = 0.050). Tumors from patients younger than the mean age (68 years) had a lower methylation frequency for p16INK4a than did tumors from older patients, although this was not statistically significant (P = 0.074). There was a strong association between methylation and right-sided tumor location as 10/11 (91%) tumors methylated in hMLH1 and 12/19 (63%) of the tumors methylated in p14ARF were located in the right side of the colon (P < 0.001 and P = 0.005, respectively). There was no statistically significant association between methylation and histological grade. Most of the tumors with APC methylation (13/17, 76%) belonged to the Dukes' B group, but the differences were not statistically significant (P = 0.068).
Tumors with widespread methylation (≥ 3 loci) are associated with right-sided localization; 10/17 (59%), versus 5/17 (29%) left-sided (P = 0.035). We saw no statistically significant associations between presence of widespread methylation and the remaining clinicopathological variables included in the present study.
Discussion
Tumor cell lines are commonly used as experimental tools in cancer research, including studies designed to assess epigenetic changes. But whereas the genetic aberrations of colon cancer cell lines have been comprehensively described [12], the methylation profiles of potential target genes in the same or similar cell lines are often described only sparingly. A literature survey of the 20 colon cancer cell lines and their methylation status analyzed in this study showed that some cell lines and genes had been extensively studied, whereas others were left undescribed (Table 1). For half of the cell lines included in the present study, both methylated and unmethylated alleles have been found for one or more of the genes studied. As non-neoplastic cells are not found in cultured cancer cell lines, this can not be caused by the presence of normal cells, and although several biological and technical explanations may exist, allele specific methylation seems the most likely interpretation [23,34]. In contrast, admixture of normal cells, tumor heterogeneity and/or monoallelic methylation may explain the coexistence of unmethylated and methylated bands in primary tumors.
It has been debated for some time whether cell lines are more frequently methylated than primary tumors [35]. Regarding overall CpG island hypermethylation, cancer cell lines have in general demonstrated an increased frequency of hypermethylation compared with primary tumors [15]. However, only a limited number of the genes analyzed have shown a statistically significant difference in methylation frequency [15]. Among several cancer types examined, colon cancer cell lines have been shown to resemble the most their respective primary tumor in this respect [36]. For the cell lines and primary tumors included in this study, the fraction of MSI and MSS samples was about the same and we saw no statistical difference in the overall number of methylated target genes in colon cancer cell lines versus colorectal primary tumors. Seemingly, large methylation percentage differences for individual genes were seen (Table 2) but they were statistically significant only for p16INK4a methylation, independent of MSI stratification. Comparisons of in vitro tumor cells with primary tumors of each subtype (MSS and MSI) have also shown similar frequencies of TP53, KRAS and APC mutations [12] and ploidy stem line [37], which further supports the conclusion that the in vitro system is a suitable experimental tool that closely reflect the in vivo situation.
Previously reported variations in promoter hypermethylation frequencies of different tumor suppressor genes in colorectal cancer can be explained by various ratios of MSI versus MSS samples in the series analyzed, different methods for analyzing methylation, the inter-individual variation in scoring of methylated samples, incomplete bisulphite modification, tumor heterogeneity, and the fact that different parts of the gene promoter region in question have been analyzed. In the present study, we used primer sets known to only detect methylation in tumor cells, never in normal tissues from the same patients [24,31,38-42]. The promoter hypermethylation in these areas has also shown an impressive correlation with lack of protein expression, confirming that these are essential regions for gene expression [24,31,38-42]. The hMLH1 primers we designed amplify a region of the promoter, in which methylation invariably correlates with the lack of hMLH1 expression [18,43,44]. Methylation of this region has only been detected in tumor cells and not in normal mucosa [18,43,44].
As expected, the MSI primary tumors showed more methylation overall than did the MSS group. However, this was only significant for the hMLH1 and p14ARF genes, whereas the four additional genes analyzed revealed similar methylation frequencies in the MSS and MSI groups. Promoter methylation of the hMLH1 gene was, not surprisingly, found only in tumors and cell lines with MSI, not in the MSS samples. The MSS tumors and cell lines per definition contain functional hMLH1 protein, and transcriptional silencing of hMLH1 by hypermethylation is known to be the main cause of MSI in sporadic CRC [26,28,45]. Also p14ARF methylation may have a specific role in MSI tumors, since it seems to be most often inactivated in tumors with wild type TP53 (see below). However, the relatively high methylation frequencies of the remaining analyzed genes, and also their overall similar frequency in MSI and MSS samples, imply that they are important in colorectal carcinogenesis independently of tumor site and MSI status.
Inactivation of tumor suppressor genes by promoter hypermethylation has been recognized to be at least as common as gene disruption by mutation in tumorigenesis [1]. Indeed, most types of primary tumors harbor several genes inactivated in this way and some genes, like p16INK4a, have been reported to be methylated consistently in most tumor types analyzed [46]. In colorectal carcinomas, the reported p16INK4a methylation frequencies vary from 18% [47] to 50 % [48] with most of the observations centered around 36–40% [11,27,46,49-51], i.e., slightly higher than our result. Both p16INK4a and p14ARF are more commonly methylated in tumors with MSI than in MSS [10,11,51-53], although we found that the methylation frequency of p14ARF is higher than that for p16INK4a in MSI colorectal carcinomas.
The DNA repair protein MGMT is able to remove promutagenic alkyl groups from O6-guanine by an irreversible transfer to an internal cysteine residue [54]. Left unrepaired, the alkylated O6-guanine has a tendency to base pair with thymine during replication, thereby introducing a G:C to A:T transition mutation in the DNA [55]. Inactivating promoter hypermethylation of the MGMT gene has previously been reported to be associated with G:C to A:T mutations in the tumor suppressor gene TP53 [56] and the proto-oncogene KRAS [57,58]. Our data support this assumption for TP53 but seemingly not for KRAS, although no certain conclusions can be drawn from the limited number of samples with G:C to A:T mutations.
The p14ARF protein interacts in vivo with the MDM2 protein, neutralizing MDM2's inhibition of TP53 [59]. Less hypermethylation of p14ARF in tumors with mutated TP53 than in tumors with wild type TP53 has been reported previously [24]. Additionally, several reports have described an inverse relationship between MSI and TP53 mutation in colorectal carcinomas [60-62]. The frequent methylation we report for the p14ARF gene in MSI tumors with few TP53 mutations is in agreement with a recent study [53] and supports the existence of this alternative pathway for TP53 inactivation.
Inactivation of the APC gene is frequent in colorectal and other gastrointestinal carcinomas, usually by truncating mutations [63,64]. An alternative mechanism to inactivate the gene in colorectal tumors is by promoter methylation, and we report a frequency of APC methylation in the upper range of what has been seen in previous studies [51,65,66]. Somatic mutations in APC are common in colorectal cancer [67,68] and, similar to what has been seen by others [12,22,69], almost half of the tumors displaying APC mutations in our study were also methylated. We have not looked at allele-specific mutation, but methylation and mutation in the same tumor might reflect one mutated allele and methylation of the other, in accordance with Knudson's two hit hypothesis. This has previously been demonstrated for APC in colorectal cancer samples by Esteller et. al [65]. APC has a central role in the WNT signaling pathway, which is suggested to play a part in colorectal carcinogenesis by its constitutive activation. Activation of this pathway results in increased transcription levels of genes like MYC and CCND1 (cyclin D1) further stimulating cell proliferation [63]. Among the 52 successfully analyzed primary tumors in this study, 35 had altered APC caused by methylation (n = 17) and/or gene mutation (n = 26). The E-cadherin gene was also methylated in 14/34 tumors with altered APC, presumably further stimulating WNT signaling [63]. Interestingly, APC methylation seemed to be more common in Dukes B stage tumors.
The present study confirms that methylation of hMLH1 in sporadic carcinomas is associated with proximal tumor location in the large bowel [14,21,45,70], as above 90% of the primary tumors harboring a methylated hMLH1 promoter were taken from the right side of the colon. An association between sporadic proximal colon carcinomas and methylation has also been reported for p16INK4a and p14ARF [14,21,45]. Among our 53 primary tumors, we can only confirm this statistically for p14ARF. However, p16INK4a demonstrated the same tendency. Both hMLH1 and p14ARF are strongly associated with MSI and MSI is in turn strongly associated with proximal tumor location [71,72], hence, it is not unexpected that the methylation of both genes is associated with proximal location.
When it comes to gene methylation and its association with other clinicopathological features, contradictory results have been reported. Our observation that methylation of p14ARF does not exclude p16INK4a methylation, is in accordance with previous studies [21,24]. Correlation of p16INK4a or p14ARF methylation with female gender and increased age has been described in some studies [14,47] but not in others [11,21,24]. We found such an association between female gender and methylation of p14ARF and hMLH1, but not of p16INK4a. We also found a weak association between p16INK4a methylation and increasing age. This potential age-specific methylation was not confirmed for any of the other genes studied. The gender-associated methylation of hMLH1 has previously been described [73,74] and might explain the increased prevalence of colorectal tumors of the MSI type in the female patient group [74].
Like Toyota et. al [51], we found no statistically significant associations between tumors with widespread methylation and age, gender, or stage of the colorectal cancer.
Conclusions
The data presented here demonstrate that multiple genes are methylated in colorectal carcinomas. This underlines the important role epigenetic inactivation of tumor suppressor genes plays during the process of tumor development. Epigenetic changes in colon cancer cell lines are overall comparable with those of primary carcinomas of the large bowel, which make the cell lines relevant models for the in vivo situation. The methylation profile of specific genes, in particular hMLH1 and p14ARF, has strong associations with genetic and clinicopathological features and might be related to biologically distinct subsets of colorectal tumors.
Methods
Patients and cell lines
Fifty-three primary colorectal carcinomas from 52 patients, including 25 MSS tumors and 28 MSI tumors, were submitted to methylation analyses. One of the tumors was from a patient with hereditary non-polyposis colorectal cancer (HNPCC), whereas the rest of the cases were sporadic [71]. The tumors have known DNA ploidy pattern [75], MSI status [76], as well as mutation status for TP53, KRAS and APC [62,64,77]. The genetic and clinicopathological variables are found in Table 3. Twenty colon cancer cell lines, 11 MSS and 9 MSI, were also included in the study. These cell lines have previously been characterized for MSI status [61,78-80], 31 different genetic alterations [12], and total genome profiles by Kleivi et. al [37]. The primary tumors included in the present study are from a series of carcinomas evaluated to contain a mean number of 84% tumor cells [81]. The DNA was extracted by standard phenol -chloroform procedure.
Methylation-specific PCR (MSP)
Promoter methylation was studied in hMLH1, MGMT, p16INK4a, p14ARF, APC and E-cadherin by MSP, a method that distinguishes unmethylated from methylated alleles of a given gene [38]. After bisulphite treatment of DNA, which converts unmethylated but not methylated cytosines to uracil, DNA is amplified by PCR using primers specific to methylated and unmethylated sequences.
One or two μg DNA from each sample was modified as described [82]. Previously reported primer sets were used for amplification of the MGMT [31,82], p16INK4a [38,82], p14ARF [24], APC [39,40] and E-cadherin fragments [41] (island 3). The primers for amplifying unmethylated and methylated hMLH1 fragments were designed in accordance with hMLH1 promoter methylation and gene expression studies [18,44]. All primer sets (see Additional file 1) were purchased from Medprobe AS (Oslo, Norway).
All the PCRs were carried out in a total volume of 25 μl containing 1 × PCR Buffer (15mM MgCl2 or no MgCl2; QIAGEN Inc., Valencia, CA), 200 μM dNTP (Amersham Pharmacia Biotech Products Inc., Piscataway, NJ), and 0.625 U HotStarTaq DNA Polymerase (QIAGEN). PCR products were loaded onto 7.5% polyacrylamide gels, stained with ethidium bromide, and visualized by UV illumination. An independent second "methylated reaction" of the MSP was performed for all the samples included in the present study. In cases with diverging results from the two rounds of MSP, we did a third independent MSP round.
Human placental DNA (Sigma Chemical Co., St. Louis, MO) treated in vitro with SssI methyltransferase (New England Biolabs Inc., Beverly, MA) was used as a positive control for MSP of methylated alleles, whereas DNA from normal lymphocytes was used as a control for unmethylated alleles. Water was used as a negative PCR control in both reactions.
Statistics
All 2 × 2 contingency tables were analyzed using Fisher's exact test. Three × 2 tables were analyzed by the Pearson χ2 test. Two of the statistically significant cross-tables analyzed by the Pearson χ2 had cells with expected count less than 5, with a minimum count of 2.96 (Table 3). The Mann -Whitney test was in addition performed when appropriate. All P values are derived from two tailed statistical tests using the SPSS 11.5 software.
Authors' contributions
GEL cultured and isolated DNA from all cell lines and carried out the MSP analyses of these and of the patient samples. GEL interpreted the results, performed the statistics and drafted the manuscript. LT participated in the study design, scored the MSP results independently of author 1, and contributed to manuscript preparation. TL was responsible for the update of the APC mutation status in the cohort. GIM and TOR have collected the series of human primary tumors and provided all clinical and pathological information. RH provided all cell lines and information about them. ME contributed with scientific discussions of the results and participated in the writing of the manuscript. RAL conceived the study, was responsible for its design and coordination, and contributed in the evaluation of the results and in preparation of the manuscript. All authors have read and approved of the final manuscript.
Supplementary Material
Additional file 1 lists the MSP primers used in the present study.
Acknowledgments
Acknowledgements
We thank Professor Sverre Heim for critically reading the manuscript. GEL is a Research Fellow and LT a Post-Doctoral Fellow of the Norwegian Cancer Society. TL is Post-Doctoral Fellow of the Norwegian Foundation for Health and Rehabilitation. The study was funded by grants from the Norwegian Cancer Society (A95068 and D97127 RAL).
Contributor Information
Guro E Lind, Email: guroli@ulrik.uio.no.
Lin Thorstensen, Email: l.c.thorstensen@medisin.uio.no.
Tone Løvig, Email: tlovig@labmed.uio.no.
Gunn I Meling, Email: gi@meling.net.
Richard Hamelin, Email: richard.hamelin@cephb.fr.
Torleiv O Rognum, Email: t.o.rognum@labmed.uio.no.
Manel Esteller, Email: mesteller@cnio.es.
Ragnhild A Lothe, Email: rlothe@radium.uio.no.
References
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg962. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Gehrke CW, Kuo KC, Ehrlich M. Reduced genomic 5-methylcytosine content in human colonic neoplasia. Cancer Res. 1988;48:1159–1161. [PubMed] [Google Scholar]
- Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/S0168-9525(99)01971-X. [DOI] [PubMed] [Google Scholar]
- Latham KE. X chromosome imprinting and inactivation in the early mammalian embryo. Trends Genet. 1996;12:134–138. doi: 10.1016/0168-9525(96)10017-2. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- Esteller M, Herman JG. Cancer as an epigenetic disease: DNA methylation and chromatin alterations in human tumours. J Pathol. 2002;196:1–7. doi: 10.1002/path.1024. [DOI] [PubMed] [Google Scholar]
- Esteller M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene. 2002;21:5427–5440. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- Rajagopalan H, Nowak MA, Vogelstein B, Lengauer C. The significance of unstable chromosomes in colorectal cancer. Nat Rev Cancer. 2003;3:695–701. doi: 10.1038/nrc1165. [DOI] [PubMed] [Google Scholar]
- Shannon BA, Iacopetta BJ. Methylation of the hMLH1, p16, and MDR1 genes in colorectal carcinoma: associations with clinicopathological features. Cancer Lett. 2001;167:91–97. doi: 10.1016/S0304-3835(01)00431-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Min Y, Itoh F, Imsumran A, Horiuchi S, Yoshida M, Iku S, Fukushima H, Imai K. Differential involvement of the hypermethylator phenotype in hereditary and sporadic colorectal cancers with high-frequency microsatellite instability. Genes Chromosomes Cancer. 2002;33:322–325. doi: 10.1002/gcc.10010. [DOI] [PubMed] [Google Scholar]
- Gayet J, Zhou XP, Duval A, Rolland S, Hoang JM, Cottu P, Hamelin R. Extensive characterization of genetic alterations in a series of human colorectal cancer cell lines. Oncogene. 2001;20:5025–5032. doi: 10.1038/sj.onc.1204611. [DOI] [PubMed] [Google Scholar]
- Wheeler JM, Beck NE, Kim HC, Tomlinson IP, Mortensen NJ, Bodmer WF. Mechanisms of inactivation of mismatch repair genes in human colorectal cancer cell lines: the predominant role of hMLH1. Proc Natl Acad Sci U S A. 1999;96:10296–10301. doi: 10.1073/pnas.96.18.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Chen P, McMillan A, Lafuente A, Lafuente MJ, Ballesta A, Trias M, Wiencke JK. Correlations of partial and extensive methylation at the p14(ARF) locus with reduced mRNA expression in colorectal cancer cell lines and clinicopathological features in primary tumors. Carcinogenesis. 2000;21:2057–2064. doi: 10.1093/carcin/21.11.2057. [DOI] [PubMed] [Google Scholar]
- Paz MF, Fraga MF, Avila S, Guo M, Pollan M, Herman JG, Esteller M. A systematic profile of DNA methylation in human cancer cell lines. Cancer Res. 2003;63:1114–1121. [PubMed] [Google Scholar]
- Liu L, Schwartz S, Davis BM, Gerson SL. Chemotherapy-induced O(6)-benzylguanine-resistant alkyltransferase mutations in mismatch-deficient colon cancer. Cancer Res. 2002;62:3070–3076. [PubMed] [Google Scholar]
- Robertson KD, Jones PA. The human ARF cell cycle regulatory gene promoter is a CpG island which can be silenced by DNA methylation and down-regulated by wild-type p53. Mol Cell Biol. 1998;18:6457–6473. doi: 10.1128/mcb.18.11.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Chen A, Hong J, Chae HS, Kim YS. Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res. 1999;59:2029–2033. [PubMed] [Google Scholar]
- Veigl ML, Kasturi L, Olechnowicz J, Ma AH, Lutterbaugh JD, Periyasamy S, Li GM, Drummond J, Modrich PL, Sedwick WD, Markowitz SD. Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Natl Acad Sci U S A. 1998;95:8698–8702. doi: 10.1073/pnas.95.15.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Shen L, Issa JP. Critical role of histone methylation in tumor suppressor gene silencing in colorectal cancer. Mol Cell Biol. 2003;23:206–215. doi: 10.1128/MCB.23.1.206-215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri N, Shaw P, Bouzourene H, Sordat I, Sordat B, Gillet M, Schorderet D, Bosman FT, Chaubert P. Methylation silencing and mutations of the p14ARF and p16INK4a genes in colon cancer. Lab Invest. 2001;81:217–229. doi: 10.1038/labinvest.3780230. [DOI] [PubMed] [Google Scholar]
- Suter CM, Norrie M, Ku SL, Cheong KF, Tomlinson I, Ward RL. CpG island methylation is a common finding in colorectal cancer cell lines. Br J Cancer. 2003;88:413–419. doi: 10.1038/sj.bjc.6600699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myöhänen SK, Baylin SB, Herman JG. Hypermethylation can selectively silence individual p16ink4A alleles in neoplasia. Cancer Res. 1998;58:591–593. [PubMed] [Google Scholar]
- Esteller M, Tortola S, Toyota M, Capella G, Peinado MA, Baylin SB, Herman JG. Hypermethylation-associated inactivation of p14(ARF) is independent of p16(INK4a) methylation and p53 mutational status. Cancer Res 2000. 2000;60:129–133. [PubMed] [Google Scholar]
- Esteller M, Cordon-Cardo C, Corn PG, Meltzer SJ, Pohar KS, Watkins DN, Capella G, Peinado MA, Matias-Guiu X, Prat J, Baylin SB, Herman JG. p14ARF silencing by promoter hypermethylation mediates abnormal intracellular localization of MDM2. Cancer Res. 2001;61:2816–2821. [PubMed] [Google Scholar]
- Herman JG, Umar A, Polyak K, Graff JR, Ahuja N, Issa JP, Markowitz S, Willson JK, Hamilton SR, Kinzler KW, Kane MF, Kolodner RD, Vogelstein B, Kunkel TA, Baylin SB. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci U S A. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JG, Merlo A, Mao L, Lapidus RG, Issa JP, Davidson NE, Sidransky D, Baylin SB. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55:4525–4530. [PubMed] [Google Scholar]
- Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, Jessup JM, Kolodner R. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57:808–811. [PubMed] [Google Scholar]
- Arnold CN, Goel A, Boland CR. Role of hMLH1 promoter hypermethylation in drug resistance to 5-fluorouracil in colorectal cancer cell lines. Int J Cancer. 2003;106:66–73. doi: 10.1002/ijc.11176. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Kitazawa R, Maeda S, Kitazawa S. Methylation of CpG loci in 5'-flanking region alters steady-state expression of adenomatous polyposis coli gene in colon cancer cell lines. J Cell Biochem. 2001;80:415–423. doi: 10.1002/1097-4644(20010301)80:3<415::AID-JCB150>3.3.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59:793–797. [PubMed] [Google Scholar]
- Danam RP, Qian XC, Howell SR, Brent TP. Methylation of selected CpGs in the human O6-methylguanine-DNA methyltransferase promoter region as a marker of gene silencing. Mol Carcinog. 1999;24:85–89. doi: 10.1002/(SICI)1098-2744(199902)24:2<85::AID-MC2>3.3.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Qian XC, Brent TP. Methylation hot spots in the 5' flanking region denote silencing of the O6-methylguanine-DNA methyltransferase gene. Cancer Res. 1997;57:3672–3677. [PubMed] [Google Scholar]
- Yeager TR, DeVries S, Jarrard DF, Kao C, Nakada SY, Moon TD, Bruskewitz R, Stadler WM, Meisner LF, Gilchrist KW, Newton MA, Waldman FM, Reznikoff CA. Overcoming cellular senescence in human cancer pathogenesis. Genes Dev. 1998;12:163–174. doi: 10.1101/gad.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antequera F, Boyes J, Bird A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell. 1990;62:503–514. doi: 10.1016/0092-8674(90)90015-7. [DOI] [PubMed] [Google Scholar]
- Smiraglia DJ, Rush LJ, Fruhwald MC, Dai Z, Held WA, Costello JF, Lang JC, Eng C, Li B, Wright FA, Caligiuri MA, Plass C. Excessive CpG island hypermethylation in cancer cell lines versus primary human malignancies. Hum Mol Genet. 2001;10:1413–1419. doi: 10.1093/hmg/10.13.1413. [DOI] [PubMed] [Google Scholar]
- Kleivi K, Teixeira MR, Eknæs M, Diep CB, Jakobsen KS, Hamelin R, Lothe RA. Genome signatures of colon carcinoma cell lines. Cancer genetics and Cytogenetics. 2004. [DOI] [PubMed]
- Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M, Sparks A, Toyota M, Sanchez-Cespedes M, Capella G, Peinado MA, Gonzalez S, Tarafa G, Sidransky D, Meltzer SJ, Baylin SB, Herman JG. Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res. 2000;60:4366–4371. [PubMed] [Google Scholar]
- Virmani AK, Rathi A, Sathyanarayana UG, Padar A, Huang CX, Cunnigham HT, Farinas AJ, Milchgrub S, Euhus DM, Gilcrease M, Herman J, Minna JD, Gazdar AF. Aberrant methylation of the adenomatous polyposis coli (APC) gene promoter 1A in breast and lung carcinomas. Clin Cancer Res. 2001;7:1998–2004. [PubMed] [Google Scholar]
- Graff JR, Herman JG, Myöhänen S, Baylin SB, Vertino PM. Mapping patterns of CpG island methylation in normal and neoplastic cells implicates both upstream and downstream regions in de novo methylation. J Biol Chem. 1997;272:22322–22329. doi: 10.1074/jbc.272.35.22322. [DOI] [PubMed] [Google Scholar]
- Tamura G, Yin J, Wang S, Fleisher AS, Zou T, Abraham JM, Kong D, Smolinski KN, Wilson KT, James SP, Silverberg SG, Nishizuka S, Terashima M, Motoyama T. E-Cadherin gene promoter hypermethylation in primary human gastric carcinomas. J Natl Cancer Inst. 2000;92:569–573. doi: 10.1093/jnci/92.7.569. [DOI] [PubMed] [Google Scholar]
- Deng G, Peng E, Gum J, Terdiman J, Sleisenger M, Kim YS. Methylation of hMLH1 promoter correlates with the gene silencing with a region-specific manner in colorectal cancer. Br J Cancer. 2002;86:574–579. doi: 10.1038/sj.bjc.6600148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakura Y, Sugano K, Konishi F, Ichikawa A, Maekawa M, Shitoh K, Igarashi S, Kotake K, Koyama Y, Nagai H. Extensive methylation of hMLH1 promoter region predominates in proximal colon cancer with microsatellite instability. Gastroenterology. 2001;121:1300–1309. doi: 10.1053/gast.2001.29616. [DOI] [PubMed] [Google Scholar]
- Kuismanen SA, Holmberg MT, Salovaara R, de la Chapelle A, Peltomäki P. Genetic and epigenetic modification of MLH1 accounts for a major share of microsatellite-unstable colorectal cancers. Am J Pathol. 2000;156:1773–1779. doi: 10.1016/S0002-9440(10)65048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61:3225–3229. [PubMed] [Google Scholar]
- Wiencke JK, Zheng S, Lafuente A, Lafuente MJ, Grudzen C, Wrensch MR, Miike R, Ballesta A, Trias M. Aberrant methylation of p16INK4a in anatomic and gender-specific subtypes of sporadic colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:501–506. [PubMed] [Google Scholar]
- Hibi K, Nakayama H, Koike M, Kasai Y, Ito K, Akiyama S, Nakao A. Colorectal cancers with both p16 and p14 methylation show invasive characteristics. Jpn J Cancer Res. 2002;93:883–887. doi: 10.1111/j.1349-7006.2002.tb01333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijnsoever M, Grieu F, Elsaleh H, Joseph D, Iacopetta B. Characterisation of colorectal cancers showing hypermethylation at multiple CpG islands. Gut. 2002;51:797–802. doi: 10.1136/gut.51.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M, Gonzalez S, Risques RA, Marcuello E, Mangues R, Germa JR, Herman JG, Capella G, Peinado MA. K-ras and p16 aberrations confer poor prognosis in human colorectal cancer. J Clin Oncol. 2001;19:299–304. doi: 10.1200/JCO.2001.19.2.299. [DOI] [PubMed] [Google Scholar]
- Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;20:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja N, Mohan AL, Li Q, Stolker JM, Herman JG, Hamilton SR, Baylin SB, Issa JP. Association between CpG island methylation and microsatellite instability in colorectal cancer. Cancer Res. 1997;57:3370–3374. [PubMed] [Google Scholar]
- Shen L, Kondo Y, Hamilton SR, Rashid A, Issa JP. P14 methylation in human colon cancer is associated with microsatellite instability and wild-type p53. Gastroenterology. 2003;124:626–633. doi: 10.1053/gast.2003.50102. [DOI] [PubMed] [Google Scholar]
- Pegg AE. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990;50:6119–6129. [PubMed] [Google Scholar]
- Coulondre C, Miller JH. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977;117:577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Esteller M, Risques RA, Toyota M, Capella G, Moreno V, Peinado MA, Baylin SB, Herman JG. Promoter hypermethylation of the DNA repair gene O(6)-methylguanine-DNA methyltransferase is associated with the presence of G:C to A:T transition mutations in p53 in human colorectal tumorigenesis. Cancer Res. 2001;61:4689–4692. [PubMed] [Google Scholar]
- Esteller M, Toyota M, Sanchez-Cespedes M, Capella G, Peinado MA, Watkins DN, Issa JP, Sidransky D, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is associated with G to A mutations in K-ras in colorectal tumorigenesis. Cancer Res. 2000;60:2368–2371. [PubMed] [Google Scholar]
- Whitehall VL, Walsh MD, Young J, Leggett BA, Jass JR. Methylation of O-6-methylguanine DNA methyltransferase characterizes a subset of colorectal cancer with low-level DNA microsatellite instability. Cancer Res. 2001;61:827–830. [PubMed] [Google Scholar]
- Pomerantz J, Schreiber-Agus N, Liegeois NJ, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee HW, Cordon-Cardo C, DePinho RA. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/S0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- Kahlenberg MS, Stoler DL, Basik M, Petrelli NJ, Rodriguez-Bigas M, Anderson GR. p53 tumor suppressor gene status and the degree of genomic instability in sporadic colorectal cancers. J Natl Cancer Inst. 1996;88:1665–1670. doi: 10.1093/jnci/88.22.1665. [DOI] [PubMed] [Google Scholar]
- Cottu PH, Muzeau F, Estreicher A, Flejou JF, Iggo R, Thomas G, Hamelin R. Inverse correlation between RER+ status and p53 mutation in colorectal cancer cell lines. Oncogene. 1996;13:2727–2730. [PubMed] [Google Scholar]
- Børresen-Dale AL, Lothe RA, Meling GI, Hainaut P, Rognum TO, Skovlund E. TP53 and long-term prognosis in colorectal cancer: mutations in the L3 zinc-binding domain predict poor survival. Clin Cancer Res. 1998;4:203–210. [PubMed] [Google Scholar]
- Thorstensen L, Lothe RA. The WNT signaling pathway and its role in human solid tumors. Atlas of Genetics and Cytogenetics in Oncology and Haematology. 2003. http://www.infobiogen.fr/services/chromcancer/Deep/WNTSignPathID20042.html
- Løvig T, Meling GI, Diep CB, Thorstensen L, Norheim AS, Lothe RA, Rognum TO. APC and CTNNB1 mutations in a large series of sporadic colorectal carcinomas stratified by the microsatellite instability status. Scand J Gastroenterol. 2002;37:1184–1193. doi: 10.1080/003655202760373407. [DOI] [PubMed] [Google Scholar]
- Esteller M, Fraga MF, Guo M, Garcia-Foncillas J, Hedenfalk I, Godwin AK, Trojan J, Vaurs-Barriere C, Bignon YJ, Ramus S, Benitez J, Caldes T, Akiyama Y, Yuasa Y, Launonen V, Canal MJ, Rodriguez R, Capella G, Peinado MA, Borg A, Aaltonen LA, Ponder BA, Baylin SB, Herman JG. DNA methylation patterns in hereditary human cancers mimic sporadic tumorigenesis. Hum Mol Genet. 2001;10:3001–3007. doi: 10.1093/hmg/10.26.3001. [DOI] [PubMed] [Google Scholar]
- Eads CA, Danenberg KD, Kawakami K, Saltz LB, Danenberg PV, Laird PW. CpG island hypermethylation in human colorectal tumors is not associated with DNA methyltransferase overexpression. Cancer Res. 1999;59:2302–2306. [PubMed] [Google Scholar]
- Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, Aoki T, Miki Y, Mori T, Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1:229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- Hawkins N, Norrie M, Cheong K, Mokany E, Ku SL, Meagher A, O'Connor T, Ward R. CpG island methylation in sporadic colorectal cancers and its relationship to microsatellite instability. Gastroenterology. 2002;122:1376–1387. doi: 10.1053/gast.2002.32997. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Konishi F, Masubuchi S, Shitoh K, Nagai H, Tsukamoto T. Densely methylated MLH1 promoter correlates with decreased mRNA expression in sporadic colorectal cancers. Genes Chromosomes Cancer. 2002;35:1–10. doi: 10.1002/gcc.10100. [DOI] [PubMed] [Google Scholar]
- Lothe RA, Peltomäki P, Meling GI, Aaltonen LA, Nyström-Lahti M, Pylkkänen L, Heimdal K, Andersen TI, Møller P, Rognum TO, Fosså SD, Haldorsen T, Langmark F, Brøgger A, de la Chapelle A, Børresen AL. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993;53:5849–5852. [PubMed] [Google Scholar]
- Thibodeau SN, French AJ, Cunningham JM, Tester D, Burgart LJ, Roche PC, McDonnell SK, Schaid DJ, Vockley CW, Michels VV, Farr GH, Jr, O'Connell MJ. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res. 1998;58:1713–1718. [PubMed] [Google Scholar]
- Malkhosyan SR, Yamamoto H, Piao Z, Perucho M. Late onset and high incidence of colon cancer of the mutator phenotype with hypermethylated hMLH1 gene in women. Gastroenterology. 2000;119:598. doi: 10.1053/gast.2000.16154. [DOI] [PubMed] [Google Scholar]
- Duval A, Iacopetta B, Thorstensen L, Meling GI, Lothe RA, Thuille B, Suraweera N, Thomas G, Hamelin R. Gender difference for mismatch repair deficiency in human colorectal cancer. Gastroenterology. 2001;121:1026–1027. doi: 10.1053/gast.2001.28585. [DOI] [PubMed] [Google Scholar]
- Meling GI, Lothe RA, Børresen AL, Graue C, Hauge S, Clausen OP, Rognum TO. The TP53 tumour suppressor gene in colorectal carcinomas. II. Relation to DNA ploidy pattern and clinicopathological variables. Br J Cancer. 1993;67:93–98. doi: 10.1038/bjc.1993.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep CB, Thorstensen L, Meling GI, Skovlund E, Rognum TO, Lothe RA. Genetic tumor markers with prognostic impact in Dukes' stages B and C colorectal cancer patients. J Clin Oncol. 2003;21:820–829. doi: 10.1200/JCO.2003.05.190. [DOI] [PubMed] [Google Scholar]
- Breivik J, Meling GI, Spurkland A, Rognum TO, Gaudernack G. K-ras mutation in colorectal cancer: relations to patient age, sex and tumour location. Br J Cancer. 1994;69:367–371. doi: 10.1038/bjc.1994.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang JM, Cottu PH, Thuille B, Salmon RJ, Thomas G, Hamelin R. BAT-26, an indicator of the replication error phenotype in colorectal cancers and cell lines. Cancer Res. 1997;57:300–303. [PubMed] [Google Scholar]
- da Costa LT, Liu B, El-Deiry W, Hamilton SR, Kinzler KW, Vogelstein B, Markowitz S, Willson JK, de la Chapelle A, Downey KM, So AG. Polymerase delta variants in RER colorectal tumours. Nat Genet. 1995;9:10–11. doi: 10.1038/ng0195-10. [DOI] [PubMed] [Google Scholar]
- Eshleman JR, Lang EZ, Bowerfind GK, Parsons R, Vogelstein B, Willson JK, Veigl ML, Sedwick WD, Markowitz SD. Increased mutation rate at the hprt locus accompanies microsatellite instability in colon cancer. Oncogene. 1995;10:33–37. [PubMed] [Google Scholar]
- Meling GI, Lothe RA, Børresen AL, Hauge S, Graue C, Clausen OP, Rognum TO. Genetic alterations within the retinoblastoma locus in colorectal carcinomas. Relation to DNA ploidy pattern studied by flow cytometric analysis. Br J Cancer. 1991;64:475–480. doi: 10.1038/bjc.1991.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Sørensen B, Lind GE, Skotheim RI, Fosså SD, Fodstad Ø, Stenwig AE, Jakobsen KS, Lothe RA. Frequent promoter hypermethylation of the O6-Methylguanine-DNA Methyltransferase (MGMT) gene in testicular cancer. Oncogene. 2002;21:8878–8884. doi: 10.1038/sj.onc.1205978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 lists the MSP primers used in the present study.