Abstract
Objective
To identify roles physicians assumed as part of new health care delivery models and related strategies that facilitated physician engagement across 21 Health Care Innovation Award (HCIA) programs.
Data Sources
Site‐level in‐depth interviews, conducted from 2014 to 2015 (N = 672) with program staff, leadership, and partners (including 95 physicians) and direct observations.
Study Design
NORC conducted a mixed‐method evaluation, including two rounds of qualitative data collected via site visits and telephone interviews.
Data Collection/Extraction Methods
We used qualitative thematic coding for data from 21 programs actively engaging physicians as part of HCIA interventions.
Principal Findings
Establishing physician champions and ensuring an innovation‐values fit between physicians and programs, including the strategies programs employed, facilitated engagement. Among engagement practices identified in this study, tailoring team working styles to meet physician preferences and conducting physician outreach and education were the most common successful approaches.
Conclusions
We describe engagement strategies derived from a diverse range of programs. Successful programs considered physicians' values and engagement as components of process and policy, rather than viewing them as exogenous factors affecting innovation adoption. These types of approaches enabled programs to accelerate acceptance of innovations within organizations.
Keywords: Physician engagement, health care innovation, care coordination
Background
New models of care delivery alter practice operations and workflow, creating both benefits and challenges for physicians. Physicians are often the last members of care teams to embrace new methods of service delivery or enhanced patient experience efforts (Reinertsen et al. 2007), but their support is essential for innovation success (Caverzagie et al. 2009; Lieberhaber, Draper, and Cohen 2009). For this study, physician engagement is defined as “active support for a project” (AHRQ 2011). Compared with other professionals, physicians, especially those providing primary and critical care, are more likely to suffer from burnout (Shanafelt et al. 2012; Peckham 2015), making it difficult to engage them in innovations. Physicians' top concerns about their jobs include uncertainty about the impact of the Affordable Care Act, lower reimbursement, administrative communication difficulties, time involved in using electronic health records (EHR) and meeting new regulatory requirements, new demands to take on leadership roles, and difficulties achieving work–life balance (Punke 2013). Physicians may be initially reluctant to delegate patient care responsibilities to other care team members (Yee, Lechner, and Carrier 2012). Integration of these team members, however, may relieve some burdens when they practice at the top of their licenses, freeing physicians to spend more time providing clinical care (Reinertsen et al. 2007). Failure to address physicians' concerns systematically, however, may exacerbate burdens by demanding that physicians fill new leadership roles and increasing documentation and communication requirements that appear unrelated to patient care. Physicians' work intensity may also increase if other care team members manage lower‐intensity cases (Friedberg et al. 2015).
Multiple factors play a role in the success of physician engagement efforts (Reinertsen et al. 2007). These include organizational culture (Williams et al. 2007; Gregory et al. 2009); physician specialty (Kumar, Sherwood, and Sutaria 2013); support of hospital leadership (Spaulding, Gamm, and Menser 2014), administration (Coulam et al. 2016), and physician supervisors (Shanafelt et al. 2015); relationships with local organizations; and market structure (Rodriguez et al. 2009). Successful engagement strategies have included enhancing administrative support, creating physician leaders, and educating physicians on programmatic goals (Sears 2011; Bleser et al. 2014; Govender 2015; Swensen, Kabcenell, and Shanafelt 2015). Financial incentives (e.g., capitation payments, production‐based compensation) have not proven particularly effective (Kralewski et al. 1996; Conrad et al. 1998; Reschovsky, Hadley, and Landon 2006; Scott et al. 2011). This may be because physicians' concerns usually relate to increased time and responsibility needed to generate the same compensation, rather than inadequate base salaries (Shanafelt et al. 2012; Punke 2013). In addition, financial incentives can distort providers' motivations; thus, there may be better, nonfinancial means that build physicians' desire to engage in innovations (Berenson and Rice 2015).
The goal of this study was to illustrate how organizations and programs can foster physician engagement to effectively implement programs and achieve innovation success. In 2012, the Center for Medicare & Medicaid Innovation (CMMI) invested $900 million in 108 U.S. health care provider organizations and other stakeholders through its Health Care Innovation Awards (HCIA). The intent of HCIA was to test new care delivery models, including those that leverage technology, workforce training, and ongoing improvements informed by rapid‐cycle feedback (CMS 2016). The 21 programs in this study were selected from a subset of HCIA awards evaluated by NORC at the University of Chicago; these 21 programs dedicated specific efforts toward garnering buy‐in and had components that relied on physician engagement.
Most studies of innovation implementation and physician engagement have a narrow focus, considering only one innovation, setting, health condition, health plan, or geographic region (e.g., Helfrich et al. 2007; Caverzagie et al. 2009; Lieberhaber, Draper, and Cohen 2009; Lemak et al. 2013; Bleser et al. 2014; Spaulding, Gamm, and Menser 2014). The diversity of the 21 HCIA programs in this study provides a unique opportunity to understand the factors that influence implementation effectiveness across a variety of conditions, geographic locations, facilities, and institutional cultures. HCIA interventions encompassed eight different health conditions (including patients with multiple chronic conditions), 15 states, and five different types of organizations (e.g., clinics, hospitals, community‐based nonprofits); physicians were also members of multidisciplinary teams participating in innovation implementation. Organizational expertise levels also differed, although programs included evidence‐based components. About half of the programs in our sample did not have prior experience implementing their specific interventions; thus, implementation required substantial resources and physician outreach was an important part of these efforts. We identified roles and responsibilities that physicians assumed as part of new health care delivery models in these programs and studied how programs engaged physicians. Our findings include strategies that facilitated establishing physician champions, garnered physicians' buy‐in to the values of the innovation, and contributed to implementation effectiveness.
Conceptual Framework
We organize our findings using the validated Framework for Complex Innovation Implementation (Helfrich et al. 2007) (hereafter “the framework”). As shown in Figure 1, implementation climate—a shared perception of the innovation's value across the organization—is influenced by innovation champions, alignment between innovation users' values and the innovation, called innovation‐values fit, and implementation policies and practices that are driven by the organization's management support and resource availability (Helfrich et al. 2007). Within the context of this study, innovation‐values fit occurs when there is alignment between the innovation and physicians' priorities and beliefs. By extension, values of the organization also affect this fit, which additionally impacts the implementation climate.
Figure 1.
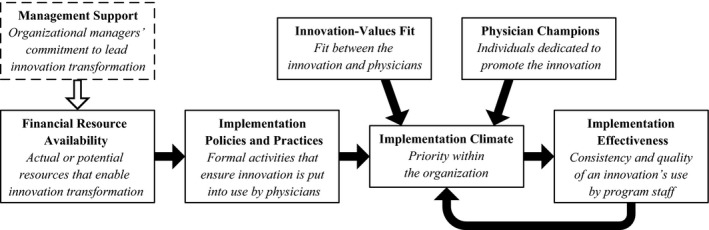
Adapted Conceptual Framework of Complex Innovation Implementation (Helfrich et al. 2007)
The framework suggests that innovations depending on physician participation will necessarily succeed or fail depending on champions—leaders that are dedicated to promoting the innovation and overcoming resistance or differences (Helfrich et al. 2007). The need for physician champions reinforces the model's assertion that in order to be successful, innovations should correspond with physicians' values, which generates a positive innovation climate.
We exclude the “management support” domain from our results because our observations were largely limited to internal program leaders (subsequently referred to as “managers,” “management,” “leadership,” etc.) rather than managers at an organizational level as specified in the framework. Instead, we include references to intervention managers (e.g., site directors, supervisors) and leaders (e.g., principal investigators, program managers) from internal teams. In addition, we note that “management” had different meanings across programs depending on their structure and number of sites. Management might occur at a high level where individuals oversee multiple sites, and/or at a site level or include workforce supervisory roles; as a result, we could not detect larger trends across the programs due to differences in management roles.
Methods
Setting
Targeted settings included outpatient physician practices (both primary care and specialty care), hospitals, patient homes, emergency rooms, community health centers, skilled nursing facilities, and rehabilitation hospitals. Table 1 provides an overview of the 21 programs included in this study. Physicians assumed varying roles and levels of involvement in innovations, which entailed managing patient care across several conditions, populations, workforce models, and settings.
Table 1.
Context of HCIA Innovation Programs
| Disease Focus | Innovation Program | Intervention Type | Organization Running the Program | Central Staff |
|---|---|---|---|---|
| Asthma | Health Resources in Action, Inc. | Chronic disease management | Not‐for‐profit community health and medical foundation with seven sites | CHWs |
| Le Bonheur Community Health and Well‐Being | Chronic disease management | Not‐for‐profit hospital | Physician, RN/RT, CHWs | |
| Nemours Foundation | Chronic disease management | Not‐for‐profit integrated health system | CHWs | |
| Cancer | Innovative Oncology Business Solutions, Inc. | Symptom management and care coordination | Entity created for HCIA, partnering with seven private community oncology practices | Triage RNs |
| University of Alabama at Birmingham | CHW navigation for all cancer stages | Not‐for‐profit hospital system with partners across five states | CHWs | |
| University of Virginia | Symptom management for advanced cancer | Private university health system | RNs | |
| Cardiovascular disease | Christiana Care Health Services, Inc. | Postacute care and long‐term care coordination | Not‐for‐profit private hospital system | Care coordinators (RN) |
| St. Francis Healthcare Foundation of Hawaii | Postacute care and telehealth intervention | Not‐for‐profit hospital system | RNs | |
| Chronic pain | Mountain Area Health Education Center, Inc. | Case management and care coordination for chronic condition | Not‐for‐profit integrated health center with four sites | NP |
| CHF, COPD, AMI | Pittsburgh Regional Health Initiative | Intensive coordination and disease management | Not‐for‐profit regional health improvement collaborative in six participating hospitals | RN, pharmacist, and care manager |
| Dementia | University of California, Los Angeles | Comprehensive dementia care | Not‐for‐profit medical clinic part of university system | Care coordinator (NP) |
| Diabetes | Southeastern Diabetes Initiative | Chronic disease management | Federally qualified health centers | Nurses (RNs) and CHWs |
| Multiple chronic conditions | North Carolina Community Network | Care coordination for high‐risk pediatrics | Not‐for‐profit Medicaid vendor | Care coordinators (RN) |
| Palliative Care Consultants of Santa Barbara | In‐home emergency department diversion program | Small for‐profit private practice | Care coordinators (RN) | |
| Providence Portland | Comprehensive care management | Not‐for‐profit managed care organization serving three counties | Licensed clinical social workers | |
| Sutter Health | End‐of‐life care coordination intervention | Not‐for‐profit hospital system | RNs | |
| University Emergency Medical Services | Emergency department diversion program using CHWs | Not‐for‐profit medical clinic | CHWs | |
| University of Iowa | Comprehensive care coordination | Private hospital system with 10 critical access hospitals | RNs | |
| University of New Mexico | Specialist care comanagement | Public university health system | Care coordinators (NP/PA) and CHWs | |
| Vanderbilt University Medical Center | Coordination of care transitions and longitudinal care management | Not‐for‐profit university hospital system with three affiliated practices | Care coordinators (RN) | |
| Courage Kenny Rehabilitation Institute | Comprehensive care planning, patient education | Private not‐for‐profit hospital system (rehabilitation institute) | Care coordinators (RN) |
Credentials in parentheses denote education levels of care coordinators.
AMI, acute myocardial infarction; CHF, congestive heart failure; CHW, community health worker; COPD, chronic obstructive pulmonary disease; NP, nurse practitioner; PA, physician assistant; RN, registered nurse; RT, respiratory therapist.
Study Design and Sample
This qualitative study took place within a larger, 4‐year mixed‐methods evaluation conducted by NORC at the University of Chicago under contract with CMMI. This analysis focuses on 21 programs that actively sought to engage physicians in their interventions.
Data Collection
From March 2014 to December 2015, NORC staff conducted two rounds of site‐based data collection. Site visits included semistructured interviews with representative program staff (e.g., frontline staff, site managers), program leaders, and partners (e.g., community groups, health departments) (NORC Disease‐Specific 2015a; NORC Complex/High‐Risk 2015b). NORC staff members visited all programs in person at least once. Direct observations and the majority of interviews occurred in person, but some occurred by phone to accommodate participants. In total, data from the 21 programs consist of interviews and direct observations with 672 individuals, including 95 physicians. Most interviews occurred with one to three individuals usually within one workforce category such as supervisors or care coordinators. Conversations with physicians were primarily one‐on‐one or in small groups with other program leaders.
Data Analysis
Following site visits and phone interviews, teams prepared a final set of transcripts by triangulating raw notes with interview recordings (Onwuegbuzie et al. 2009). Transcripts were coded with NVivo software (version 10; QSR International, Doncaster, Victoria, Australia). We developed coding schemas through a combination of inductive and deductive processes (Fereday and Muir‐Cochrane 2006) and reflected themes from research questions established by an implementation contractor and a meta‐evaluator for the entire HCIA program (Berry et al. 2013; CMS 2014). While the Framework for Complex Innovation Implementation did not inform codebook development, the evaluation research questions guiding the evaluation emphasized themes of implementation effectiveness, teamwork, and staff roles so that our findings conceptually fit under the framework's domains.
One or more members of each site visit team participated in coding. This best practice approach improved coding quality by leveraging coders' background knowledge about the programs (Barbour 2001; Bradley, Curry, and Devers 2007). After team training, individuals independently coded transcripts based on their particular expertise. An agreement rate of at least 95 percent was reached through practice, discussions, and code refinement (Garrison et al. 2006; Bradley, Curry, and Devers 2007). We used quality assurance measures such as random spot‐checks, consensus‐building discussions among coders and subject matter experts (Garrison et al. 2006; Bradley, Curry, and Devers 2007), methodological expert reviews, and an additional intervening round of reliability calculations.
For the analysis presented in this paper, two trained coders reviewed relevant codes, particularly those related to “provider buy‐in and interactions.” Coders also conducted word searches for “physician,” “doctor,” “hospitalist,” “PCP” (primary care physician), and related specialties (e.g., oncologist, pediatrician), and then reviewed surrounding text to determine whether it was relevant to this study. Coders reviewed relevant sections of raw interview data and inductively synthesized quotes into subthemes, reaching consensus on physicians' roles and what activities constituted engagement strategies (Thomas 2006; Williams 2008). As further quality assurance, subject matter experts from the NORC evaluation team assisted in verifying themes when interview data were unclear.
Physician engagement efforts were identified as “successful” through triangulation. For example, if at least two stakeholder groups, such as leadership and frontline workers, provided reinforcing observations about an intervention's engagement success, we described it as successful. When physician engagement appeared challenging, we probed to understand the extent to which physicians were engaged and what aspects of the innovation's values they embraced or rejected, and then looked for verification from two or more stakeholder groups to assess whether challenges persisted or were resolved.
Results
Physicians in the HCIA programs had significant opportunities to facilitate implementation effectiveness, as Helfrich et al. define it, “the consistency and quality of innovation use” (2007). Physicians participated in various implementation policies and practices by being members of interdisciplinary care teams, innovation champions (also called “physician champions”), and referral sources into programs. We summarize these three nonmutually exclusive roles in Table 2.
Table 2.
Physician Roles and Activities
| Major Roles (no. of programs) | Key Activities |
|---|---|
| Member of interdisciplinary care teams (11) | Acts as a source of clinical expertise; guides care planning; liaises with care coordinators and lay health workers |
| Referral source for programs (16) | Targets, identifies, and refers patients to programs; spreads awareness of programs to patients and other providers |
| Innovation champion who can influence institutional culture (14) | Serves as innovation and program architect; sets program priorities; provides guidance to staff to ensure fidelity; connects with peers both internal and external to the program to market and promote the program and explain its purpose |
Physicians in interdisciplinary teams participated in planning meetings with care coordinators, community health workers (CHWs), and palliative care teams, and often assumed leadership or program management roles. These physicians provided feedback about the program to staff and suggested improvements or communicated challenges. Programs also encouraged physicians to refer patients into HCIA‐funded interventions and enlisted them to help target and recruit prospective enrollees. Fourteen programs had at least one physician champion, a crucial factor in generating a positive innovation climate and ensuring that physician engagement policies and practices reflected appropriate physician values. Below we describe examples and strategies programs used to leverage physician champions to spread awareness among their peers regarding the program's value, helping to generate overall buy‐in for the program.
Determinants of Effective Physician Engagement
Leveraging physician champions and establishing innovation‐values fit between programs and physicians were critical parts of engagement. In addition to generating a positive innovation climate, these approaches informed innovation policies and procedures as well as how programs tried to prove their value to physicians, often through emphasizing increased workflow efficiency and minimal time investment. Table 3 shows physician engagement lessons learned, organized by domains from the Framework for Complex Innovation Implementation. The most common successful strategies that programs employed were tailoring teams' working styles to meet physician preferences and conducting outreach and education.
Table 3.
Physician Engagement Approaches and Techniques
| Framework Domain(s) | Key Lessons Learned |
|---|---|
| Physician champion | Enlist a physician champion to educate, encourage buy‐in among his/her peers, and ensure innovation‐values fit between physicians and the program |
| Innovation‐values fit | Craft policies and practices that take physician values into account and recognize physicians' specific resource concerns |
| Implementation policies and practices | Involve physicians early in implementation (e.g., working groups, protocol design) |
| Conduct formal outreach to physicians and deliver education about the program's aims | |
| Tailor team working styles to physician preferences or practice culture | |
| Share program data with physicians | |
| Resource availability | Explain how the program will improve physician workflows, save time, and improve efficiency |
| Offer financial incentives (e.g., overtime, finder fees) | |
| Implementation climate | Consider whether past history or experience of staff members, leaders, and the organization can facilitate physician buy‐in |
| Recognize value differences among internal and external physicians |
Our analysis did not yield any unsuccessful strategies; we observed that unsuccessful physician engagement seemed to stem either from contextual barriers or from a lack of any explicit strategy to engage physicians. Programs usually expected and encountered initial resistance to new ideas. As one medical director of a cancer care initiative noted,
Any time you introduce something new, there's always somebody who's unhappy. They always say, “This is too much work; this is different from what we signed up for at the beginning.” There's always something.
Innovation Champions
Physician champions played pivotal roles educating their peers and fostering engagement across 14 programs. Overall, they were critical to successful engagement efforts. For example, when an integrated delivery intervention faced challenges engaging physicians, the program's physician leadership personally explained the program to outpatient specialists in order to establish connections and foster a collegial environment. Other programs with multiple locations assigned physician champions to each site to build relationships. Physician champions also supported implementation practices that increased physician buy‐in and helped ensure that programs achieved innovation‐values fit between the program and other physicians.
Innovation‐Values Fit
Innovation‐values fit between programs and physicians informed effective engagement strategies. This usually meant that engagement efforts addressed physicians' concerns over the time and effort required of them by the innovations. Most programs continuously revised their processes to establish regular communication between physicians and care teams in order to adapt to physicians' needs and preferences. As outcomes data became available from the intervention, efforts that considered physicians' interests when trying to demonstrate program effectiveness were appreciated by physicians. Physicians' concerns also shaped how programs explained programs in terms of resources such as enhanced workflow efficiency.
Implementation Policies and Practices
Overall, we identified four common physician engagement practices: involving physicians early in program implementation; conducting formal physician outreach and education; tailoring working styles to physician preferences and prioritizing communication with physicians; and sharing program data with physicians.
Early Involvement
Involving physicians early in planning or implementation helped ensure that their priorities and values informed the innovation, making it easier to build and sustain physician engagement. Five programs involved physicians near the start of their respective interventions where physicians participated in working groups or designed protocols. One physician champion created a well‐received supportive care tumor board that brought in new members (e.g., CHWs, pharmacists, palliative care) to confer about cancer patients. Meetings took place early in the morning to accommodate physicians' schedules and ensure maximum attendance. A second program developed a working group that convened influential leaders from participating practices. This group strategized about how to reach other physicians and ultimately concluded that the best practice was for physicians to leverage their personal connections with peers by visiting community practices to present the program. At a different program, a panel of physician disease experts helped draft risk stratification thresholds and protocols for the intervention. In addition, early adopters at this medical center provided workflow feedback to enhance best practices. As a program manager described,
After the first couple of weeks, one hospitalist … asked us to send [biometrics data] once a month instead of every day. We compromised and now send notifications on a weekly basis.
Outreach and Education
Several programs held formal presentations for other physicians. Leaders at one program held 15–20 presentations for outside physician practices where they explained the program, distributed brochures, and described workforce roles. In another case, a physician champion and staff member explained the program to approximately 70 institutionally affiliated physicians, offering information on how to engage patients as well as elicit and deliver feedback. One program manager explained how extensive their outreach had to be:
I do the training for the staff. I meet with different groups. We do presentations for physician groups. The different networks have physician advisory boards, and I travel to meet with them.
As some programs identified potential participants, program staff personally contacted primary care providers to explain the program and ask physicians' permission to enroll patients. Additionally, a clinic‐based program arranged initial individual meetings with physicians who were not part of program implementation to explain how an algorithm assigned risk levels to individual patients.
Eight programs successfully engaged physicians by delineating and explaining staff roles. One program used multiple strategies to help hospital staff understand differences in existing roles for nurse navigators versus the roles of the program's new cancer care liaisons. That program's quality director explained:
When we onboard staff, we make sure they understand the differences between our services and navigators for when they work in the same space or work around them. … We have brochures — we took the navigation piece and incorporated the care liaisons as part of that overall navigation team and when it's side by side you can see the differences more clearly.
Although educational outreach helped mitigate some physicians' concerns over changing practice due to the interventions, such fears often persisted as a barrier to physician engagement. Across nine innovation programs, physicians feared they would be held liable for other care team members' decisions when protocols emphasized keeping patients at home rather than recommending an emergency department (ED) visit or hospitalization. Most commonly, physicians expressed concerns regarding whether a nurse care coordinator or CHW could adequately triage and make decisions that were safe for their patients. Aside from delivering education, programs did not identify effective ways to mitigate such concerns.
Education through Targeted Communication Policies
Among 21 programs that focused on carefully orchestrated communication with physicians, 17 successfully engaged physicians through this strategy. Innovation programs found that it was important to clarify who within the program would communicate with physicians and to define how program staff would streamline communication to minimize any effect on physician workflows. About half of the 21 programs tailored team communication styles to meet physicians “where they are” and offered physicians seamless access to patient‐level information obtained during care coordination to support clinical decisions.
Staff members tried to cater to specific physician preferences in terms of when and in what form they provided patient information, and five programs found it useful to share information about physician preferences with other team members. At a telemonitoring program, teams forwarded patient‐level reports to practices the day of patient appointments rather than risking inundating physicians with biometric data collected on a daily basis. Similarly, another initiative developed a process to distinguish between alerts that needed to be forwarded to physicians immediately and information that could be bundled in a weekly report.
Seven programs established a single point of contact who provided centralized, innovation‐specific knowledge to physicians. Physicians appreciated the ability to reach a designated individual who could address their concerns rather than having to coordinate conversations with multiple staff members.
Sharing Data
Tailored communication strategies extended to sharing data through health IT platforms in order to improve bidirectional communication and mitigate logistical challenges. Although most programs attempted to streamline communication this way, many did not achieve full‐fledged bidirectional communications systems due to interoperability challenges between EHR systems. In successful instances, effective use of EHR systems was key. Managers in a comprehensive dementia care program developed consolidated care plans based on all EHR data and provided the plan in an easily accessible part of the EHR, thereby saving physicians significant time. At one outpatient intervention, physicians left notes in the EHR indicating what information they found helpful; this created a feedback loop that enabled care coordinators to prioritize the information they shared with physicians.
Physicians also appreciated learning about program outcomes and the value of delivery system reforms for their patients; however, for data sharing to be effective, metrics had to be those that physicians believed they could influence. When programs focused on a specific chronic condition, related biometrics data (e.g., improved hemoglobin A1C levels in diabetics) were most useful. However, sharing data often proved challenging. Quality improvement programs usually could not yield results quickly enough to use in building relationships with community physicians. Similarly, interventions that stabilized high‐risk patient populations over time did not always show overall improvements soon enough to use in relationship building. For example, improvements in outcomes for cardiovascular patients, such as preventing a second heart attack or stroke, generally were not observable within the 3‐year innovation period. Although programs shared data on utilization and cost measures (e.g., hospitalizations, readmissions), most physicians felt that these measures were only marginally within their ability to address and did not automatically buy into programs based on these types of data. A program director at a palliative cancer care program noted:
I think that the question has to be asked and answered of the physicians who are looking at this data. … “Am I finding this information valuable in my decision making?” The question is, is [data] actually bettering the human condition? Or is it just data for data's sake?
Resource Availability
Successful engagement typically occurred when programs explained how innovations would positively impact physician resources such as workflow efficiency while not increasing administrative burden or taxing their time. Financial incentives to engage with programs were offered by only two programs and met with mixed results.
Increased Efficiency
A medical resident in a chronic pain initiative described how a nurse practitioner concentrated on pain management so that physicians could focus on other medical issues:
One of the best … and most helpful things is having a [nurse practitioner] there … it helps me as a provider when I'm overbooked and having to see three patients at a time and one also has 17 other health problems … I know I have that support from [the nurse practitioner]… so I can see that patient and focus on their COPD [instead].
Program staff at one cancer care initiative explained to physicians how engagement with the innovation would save them time, as program staff members would handle services such as patient education. A psychologist at a health system's asthma intervention observed:
I think that a qualm from physicians in primary care is that they're doing a lot of things … that take up a lot of their time, whether it be behavior, school. So I think … having folks that know a little bit about that can help with some more valuable resources.
Financial Incentives
One program gave physicians a “finder fee” for referrals but phased out the incentive as the program gained more traction and providers willingly referred patients. At a care coordination program, physicians resisted working weekends. Even though the intervention allocated funds toward paying overtime wages, physicians did not seem to value the increase in pay over their weekend time.
Thirteen programs noted that it was particularly difficult to engage physicians because they were not reimbursed for taking time to refer patients to a program, attend program presentations, or review materials regarding staff roles. However, most programs were unable to offer financial incentives to test the effectiveness of payments.
Implementation Climate
In addition to the strategies discussed above, physician and institutional past experiences as well as physicians' relationships to organizations housing the innovations also affected implementation climates. The effect of physician engagement policies and practices on implementation climates could vary depending on whether physicians were part of or associated with the organization housing the innovation (“internal” physicians) or part of an external entity (“external” physicians). Internal physicians were typically easier to engage and played more significant roles in implementation policies and practices aimed at garnering physician engagement. External physicians were primarily referral sources to programs but were often uninterested in or even distrustful of engagement efforts. Ten programs reported that external physicians were concerned that innovation programs would permanently “steal” their patients, thereby threatening their practices' incomes. Such fears reduced the likelihood of referrals, and only five programs ultimately convinced these physicians of the innovation's value. This challenge is understandable, as individuals working for innovations within organizations were better placed to identify physician values within that culture and mitigate their concerns as opposed to external physicians who may have had different values and priorities.
Staff reported that innovations were easier to implement when individuals or organizations had prior experience in implementing a similar program, if they had a reputation for treating certain diseases, or if programs were housed under academic teaching institutions that were invested in implementing innovation pilot programs. External physicians tended to trust organizations and individuals that had a proven record and reputation for treating specific populations or conditions. We observe that these factors also affected how willingly external physicians referred patients into programs based on their familiarity with the organization and its experience.
Discussion
Unlike previous efforts to explore physician engagement by a narrow range of settings, diseases, or intervention types, this paper offers a unique perspective of engagement across a broad variety of programs implemented across 15 states. Findings are consistent with the conceptual categories proposed by Helfrich et al. (2007), and we find a strong support for the need to develop alignment between implementation practices and physician values. Helfrich et al. (2007) propose innovation‐values fit as an independent factor necessary to innovation climate, but our data indicate that values interact with implementation policies and practices through a feedback loop, thus allowing values to shape policies and practices.
Despite programmatic and contextual differences among the 21 HCIA awardees included in this study, a number of specific policies and practices proved broadly successful. Targeted communication and education that leveraged physician champions and promoted program aspects valued by physicians emerged as the most widely used successful strategies. These strategies are consistent with alleviating sources of physician stress, particularly burdens related to administrative communication and documentation, taking on new leadership roles, and work–life balance (Shanafelt et al. 2012; Punke 2013; Peckham 2015). Innovation program staff spent considerable time and effort educating physicians, but well‐planned and organized efforts generally paid off. Because physicians' time is reimbursed at a high rate and because physicians resist increased time demands (Shanafelt et al. 2012), efforts by other staff to design workflows around what physicians value may increase the likelihood of generating a positive innovation climate.
Our study of HCIA programs corroborates prior studies' findings that effective physician engagement requires multiple approaches tailored to organizational contexts. Prior studies suggest strategies to promote an innovation‐values fit and a positive innovation climate, such as communicating one‐on‐one, managing program trade‐offs that use valuable physician time, offering financial incentives (in select cases), and creating trust‐building activities to align institutional goals with proposed initiatives (Guthrie 2005; Reinertsen et al. 2007; Taitz, Lee, and Sequist 2011; Lemak et al. 2013; Spaulding, Gamm, and Menser 2014).
In line with prior findings, we found that use of financial rewards played an insubstantial role in engaging physicians. HCIA programs tended to work best when they appealed to physicians' intrinsic motivations (Conrad et al. 2002; Conrad and Christianson 2004; Swensen, Kabcenell, and Shanafelt 2015) such as prioritizing high‐quality and efficient service provision (Reinertsen et al. 2007; Punke 2013). The tailored strategies highlighted in this analysis may offer alternative solutions to programs that lack financial resources to provide direct incentives.
We observed that common physician engagement strategies worked across a varied group of HCIA programs. This suggests that other innovators can establish a strong rapport with physicians as long as they cultivate champions and implement programs in a manner consistent with physician and organizational values. However, the selection process for HCIA was highly competitive. Of the 3,000 applications for Round One funding, CMS funded just over 100 awards that may not be representative of other health care institutions.
Still, the successful strategies among diverse HCIA programs provide useful lessons for institutions working toward team‐based care coordination and quality improvement initiatives, regardless of targeted condition or setting. Our study suggests integrating physicians into care teams by providing tailored education and effective communication can be major drivers of positive change. These successful practices address primary concerns of physicians in the contemporary universe of health care provision: administrative burden, communication challenges, lack of organizational support, being forced into leadership roles without preparation, and increased demands on time (Punke 2013). By considering physicians' values as components of process and policy, rather than assuming that physicians' values independently and exogenously affect innovation climate, organizations can accelerate adoption of innovations. The question of whether physician engagement actually leads to improved patient outcomes for certain chronic diseases is beyond the scope of this study but deserves more research attention.
Supporting information
Appendix SA1: Author Matrix.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: We are grateful to John Kralewski and Jon Christianson for sharing their expertise about the current landscape of physician engagement. We would like to thank Tianne Wu for contributing to the initial framing of this analysis, and we thank Melissa Atlas for assistance with citations as well as Health Care Innovation Award (HCIA) Disease‐Specific and High‐Risk NORC Evaluation Teams for their expertise and analysis across HCIA programs. This research was conducted under contract numbers HHSM‐500‐2011‐00002I and HHSM‐500‐2011‐00002H under the Center for Medicare and Medicaid Innovation.
An early version of findings in this paper was presented at the AcademyHealth Research Meeting on June 26, 2016.
Disclosures: None.
Disclaimer: The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the U.S. Department of Health and Human Services or any of its agencies.
The abstract for this paper was recognized as a winner at the 2016 AcademyHealth Annual Research Meeting and was invited to be published as part of the Health Services Research 2016 Best of ARM special section.
References
- Agency for Healthcare Research & Quality . 2011. “Physician Engagement (Slide Presentation)” [accessed on April 10, 2016]. Available at http://www.ahrq.gov/professionals/education/curriculum-tools/cusptoolkit/toolkit/contentcalls/physengagement-slides/physengageslides.html
- Barbour, R. S. 2001. “Checklists for Improving Rigour in Qualitative Research: A Case of the Tail Wagging the Dog?” British Medical Journal 322 (7294): 1115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson, R. A. , and Rice T.. 2015. “Beyond Measurement and Reward: Methods of Motivating Quality Improvement and Accountability.” Health Services Research 50 (S2): 2155–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry, S. H. , Concannon T. W., Gonzalez Morganti K., Auerbach D. I., Beckett M. K., Chen P. G., Farley D. O., Han B., Harris K. M., Jones S. S., Liu H., Lovejoy S. L., Marsh T., Martsolf G. R., Nelson C., Okeke E. N., Pearson M. L., Pillemer F., Sorbero M. E., Towne V., and Weinick R. M.. 2013. “CMS Innovation Center Health Care Innovation Awards Evaluation Plan.” RAND Corporation [accessed on April 11, 2016]. Available at http://www.rand.org/pubs/research_reports/RR376.html [PMC free article] [PubMed]
- Bleser, W. K. , Miller‐Day M., Naughton D., Bricker P. L., Cronholm P. F., and Gabbay R. A.. 2014. “Strategies for Achieving Whole‐Practice Engagement and Buy‐in to the Patient‐Centered Medical Home.” Annals of Family Medicine 12 (1): 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley, E. H. , Curry L. A., and Devers K. J.. 2007. “Qualitative Data Analysis for Health Services Research: Developing Taxonomy, Themes, and Theory.” Health Services Research 42 (4): 1758–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caverzagie, K. J. , Bernabeo E. C., Reddy S. G., and Holmboe E. S.. 2009. “The Role of Physician Engagement on the Impact of the Hospital‐Based Practice Improvement Module (PIM).” Journal of Hospital Medicine 4 (8): 466–70. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services . 2014. “Guidance for Front Line Evaluators: Health Care Innovation Awards.” Internal Report from the Meta‐Evaluator.
- Centers for Medicare & Medicaid Services . 2016. “Health Care Innovation Awards” [accessed on April 10, 2016]. Available at https://innovation.cms.gov/initiatives/Health-Care-Innovation-Awards/
- Conrad, D. A. , and Christianson J. B.. 2004. “Penetrating the ‘Black Box’: Financial Incentives for Enhancing the Quality of Physician Services.” Medical Care Research and Review 61 (3 Suppl): 37S–68S. [DOI] [PubMed] [Google Scholar]
- Conrad, D. A. , Maynard C., Cheadle A., Ramsey S., Marcus‐Smith M., Kirz H., Madden C. A., Martin D., Perrin E. B., Wickizer T., and Zierler B.. 1998. “Primary Care Physician Compensation Method in Medical Groups: Does It Influence the Use and Cost of Health Services for Enrollees in Managed Care Organizations?” Journal of the American Medical Association 279 (11): 853–8. [DOI] [PubMed] [Google Scholar]
- Conrad, D. A. , Sales A., Liang S. Y., Chaudhuri A., Maynard C., Pieper L., Weinstein L., Gans D., and Piland N.. 2002. “The Impact of Financial Incentives on Physician Productivity in Medical Groups.” Health Services Research 37 (4): 885–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulam, R. , Kralewski J., Dowd B., and Gans D.. 2016. “The Role of Medical Group Practice Administrators in the Adoption and Implementation of Medicare's Physician Quality Reporting System.” Health Care Management Review 41 (2): 145–54. [DOI] [PubMed] [Google Scholar]
- Fereday, J. , and Muir‐Cochrane E.. 2006. “Demonstrating Rigor Using Thematic Analysis: A Hybrid Approach of Inductive and Deductive Coding and Theme Development.” International Journal of Qualitative Methods 5 (1): 80–92. [Google Scholar]
- Friedberg, M. W. , Chen P. G., White C., Jung O., Raaen L., Hirshman S., Hoch E., Stevens C., Ginsburg P. B., Casalino L. P., Tutty M., Vargo C., and Lipinski L.. 2015. “Effects of Health Care Payment Models on Physician Practice in the United States.” RAND Corporation [accessed on April 6, 2016]. Available at http://www.rand.org/content/dam/rand/pubs/research_reports/RR800/RR869/RAND_RR869.pdf [PMC free article] [PubMed]
- Garrison, D. R. , Cleveland‐Innes M., Koole M., and Kappelman J.. 2006. “Revisiting Methodological Issues in Transcript Analysis: Negotiated Coding and Reliability.” The Internet and Higher Education 9 (1): 1–8. [Google Scholar]
- Govender, T. 2015. “Physician Engagement and Documentation Excellence.” Journal of Health Care Compliance 17 (4): 33–53. [Google Scholar]
- Gregory, B. T. , Harris S. G., Armenakis A. A., and Shook C. L.. 2009. “Organizational Culture and Effectiveness: A Study of Values, Attitudes, and Organizational Outcomes.” Journal of Business Research 62 (7): 673–9. [Google Scholar]
- Guthrie, M. 2005. “Engaging Physicians in Performance Improvement.” American Journal of Medical Quality 20 (5): 235–9. [DOI] [PubMed] [Google Scholar]
- Helfrich, C. D. , Weiner B. J., McKinney M. M., and Minasian L.. 2007. “Determinants of Implementation Effectiveness Adapting a Framework for Complex Innovations.” Medical Care Research and Review 64 (3): 279–303. [DOI] [PubMed] [Google Scholar]
- Kralewski, J. E. , Wingert T. D., Knutson D. J., Johnson C. E., and Veazie P. J.. 1996. “The Effects of Capitation Payment on the Organizational Structure of Medical Group Practices.” Journal of Ambulatory Care Management 19 (1): 1–16. [DOI] [PubMed] [Google Scholar]
- Kumar, P. , Sherwood A., and Sutaria S.. 2013. “Engaging Physicians to Transform Operational and Clinical Performance.” McKinsey & Company [accessed on April 11, 2016]. Available at http://healthcare.mckinsey.com/sites/default/files/MCK_Hosp_MDSurvey.pdf
- Lemak, C. H. , Cohen G. R., Erb N., and Dhawan A.. 2013. “Engaging Primary Care Physicians in Quality Improvement: Lessons from a Payer‐Provider Partnership.” Journal of Healthcare Management 58 (6): 429–43. [PubMed] [Google Scholar]
- Lieberhaber, A. , Draper D. A., and Cohen G. R.. 2009. “Hospital Strategies to Engage Physicians in Quality Improvement.” Issue Brief Center for Studying Health Systems Change 127: 1–4. [PubMed] [Google Scholar]
- NORC at the University of Chicago . 2015a. “Annual Report One: Health Care Innovation Awards Disease‐Specific Evaluation” [accessed on April 6, 2016]. Available at http://innovation.cms.gov/Files/reports/HCIA-DS-FirstEvalRpt.pdf
- NORC at the University of Chicago . 2015b. “HCIA Complex/High‐Risk Patient Targeting: First Annual Report” [accessed on April 6, 2016]. Available at https://innovation.cms.gov/Files/reports/HCIA-CHSPT-FirstEvalRpt.pdf
- Onwuegbuzie, A. J. , Dickinson W. B., Leech N. L., and Zoran A. G.. 2009. “A Qualitative Framework for Collecting and Analyzing Data in Focus Group Research.” International Journal of Qualitative Methods 8 (3): 1–21. [Google Scholar]
- Peckham, C. 2015. “Medscape Physician Lifestyle Report 2014.” Medscape [accessed July 29, 2016]. Available at http://www.medscape.com/features/slideshow/lifestyle/2015/public/overview#2
- Punke, H. 2013. “Top 10 Physician Complaints of 2013.” Becker's Hospital Review [accessed July 18, 2016]. Available at http://www.beckershospitalreview.com/hospital-physician-relationships/top-10-physician-complaints-of-2013.html
- Reinertsen, J. L. , Gosfield A. G., Rupp W., and Whittington J. W.. 2007. “Engaging Physicians in a Shared Quality Agenda.” Institute for Healthcare Improvement [accessed on April 25, 2016]. Available at http://www.ihi.org/resources/Pages/IHIWhitePapers/EngagingPhysiciansWhitePaper.aspx
- Reschovsky, J. D. , Hadley J., and Landon B. E.. 2006. “Effects of Compensation Methods and Physician Group Structure on Physicians' Perceived Incentives to Alter Services to Patients.” Health Services Research 41 (4p1): 1200–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, H. P. , Von Glahn T., Rogers W. H., and Safran D. G.. 2009. “Organizational and Market Influences on Physician Performance on Patient Experience Measures.” Health Services Research 44 (3): 880–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, A. , Sivey P., Ait Ouakrim D., Willenberg L., Naccarella L., Furler J., and Young D.. 2011. “The Effect of Financial Incentives on the Quality of Health Care Provided by Primary Care Physicians.” Cochrane Database of Systematic Reviews 9 (9): CD008451. [DOI] [PubMed] [Google Scholar]
- Sears, N. 2011. “5 Strategies for Physician Engagement: Taking a Close Look at Successful Physician Engagement Strategies Employed by Other Organizations Can Help Hospitals Develop an Effective Approach for Collaboration–and Improve Margins.” Healthcare Financial Management 65 (1): 78–83. [PubMed] [Google Scholar]
- Shanafelt, T. D. , Boone S., Tan L., Dyrbye L. N., Sotile W., Satele D., West C. P., Sloane J., and Oreskovich M.. 2012. “Burnout and Satisfaction with Work‐Life Balance among US Physicians Relative to the General US Population.” Archives of Internal Medicine 172 (18): 1377–85. [DOI] [PubMed] [Google Scholar]
- Shanafelt, T. D. , Gorringe G., Menaker R., Storz K. A., Reeves D., Buskirk S. J., Sloan J. A., and Swensen S. J.. 2015. “Impact of Organizational Leadership on Physician Burnout and Satisfaction.” Mayo Clinic Proceedings 90 (4): 432–40. [DOI] [PubMed] [Google Scholar]
- Spaulding, A. , Gamm L., and Menser T.. 2014. “Physician Engagement: Strategic Considerations among Leaders at a Major Health System.” Hospital Topics 92 (3): 66–73. [DOI] [PubMed] [Google Scholar]
- Swensen, S. , Kabcenell A., and Shanafelt T.. 2015. “Physician‐Organization Collaboration Reduces Physician Burnout and Promotes Engagement: The Mayo Clinic Experience.” Journal of Healthcare Management/American College of Healthcare Executives 61 (2): 105–27. [PubMed] [Google Scholar]
- Taitz, J. M. , Lee T. H., and Sequist T. D.. 2011. “A Framework for Engaging Physicians in Quality and Safety.” British Medical Journal of Quality and Safety 21 (9): 722–8. [DOI] [PubMed] [Google Scholar]
- Thomas, D. R. 2006. “A General Inductive Approach for Analyzing Qualitative Evaluation Data.” American Journal of Evaluation 27 (2): 237–46. [Google Scholar]
- Williams, J. P. 2008. “Emergent Themes” In The Sage Encyclopedia of Qualitative Research Methods, edited by Given L. M., pp. 248–9. Los Angeles, CA: Sage Publications. [Google Scholar]
- Williams, E. S. , Manwell L. B., Konrad T. R., and Linzer M.. 2007. “The Relationship of Organizational Culture, Stress, Satisfaction, and Burnout with Physician‐Reported Error and Suboptimal Patient Care: Results from the MEMO Study.” Health Care Management Review 32 (3): 203–12. [DOI] [PubMed] [Google Scholar]
- Yee, T. , Lechner A. E., and Carrier E.. 2012. “High‐Intensity Primary Care: Lessons for Physician and Patient Engagement.” National Institute for Health Care Reform 9: pp. 1–7 [accessed on April 11, 2016]. Available at http://nihcr.org/wp-content/uploads/2016/07/Research_Brief_No._9.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


