Abstract
Background: As the heterogeneity of CTCs is becoming increasingly better understood, it is clear that identifying particular subtypes of CTCs would be more relevant.
Methods: We detected folate receptor (FR)-positive circulating tumor cells (FR+-CTCs) by a novel ligand-targeted polymerase chain reaction (LT-PCR) detection technique.
Results: In the none-dynamic study, FR+-CTC levels of patients with lung cancer were significantly higher than controls (patients with benign lung diseases and healthy controls). With a threshold of 8.7 CTC units, FR+-CTC showed a sensitivity of 77.7% and specificity of 89.5% in the diagnosis of lung cancer. When compared with established clinical biomarkers including carcinoembryonic antigen (CEA), cytokeratin 19 fragment (CYFRA21-1), and neuron-specific enolase (NSE), FR+-CTC showed the highest diagnostic efficiency. Notably, the combination of FR+-CTC, CEA, NSE, and CYFRA21-1 could significantly improve the diagnostic efficacy in differentiating patients with lung cancer from benign lung disease. In our dynamic surveillance study, the CTC levels of 62 non-small cell lung cancer (NSCLC) patients decreased significantly after tumor resection.
Conclusion: We established a LT-PCR-based FR+-CTC detection platform for patients with lung cancer that exhibits high sensitivity and specificity. This platform would be clinical useful in lung cancer diagnosis and treatment response assessment.
Keywords: circulating tumor cells, folate receptor, ligand-targeted polymerase chain reaction, lung cancer.
Introduction
Lung cancer is the primary cause of cancer-related death worldwide 1. The majority of lung cancers are detected at an advanced stage in which treatments have limited efficacy and survival rates are dismally low. Therefore, detection and diagnosis of lung cancer at an early stage has the possibility of significantly reducing mortality with a greater chance of cure, which underscores the need for more effective, sensitive, and specific detection methods 2.
Circulating tumor cells (CTCs) were first observed from patients with metastatic malignancies more than 100 years ago 3; however, it was not until the past few years that the clinical and research potential of CTCs became widely recognized. Studies have demonstrated that CTCs are important potential biomarkers for diagnosis, evaluation of treatment, and prognosis in several cancers including lung cancer 4. More recently, accurate CTC detection has become increasingly important, especially when there is difficulty in obtaining sufficient tissue for diagnosis and molecular characterization in metastatic cancer.
While many challenges still exist, the detection of CTCs in blood is becoming increasingly feasible. To date, the majority of studies have employed the CellSearchTM System (Veridex LLC, Huntingdon Valley, PA, USA) which is a semi-automated system for CTC quantification by employing magnetic beads coated with anti-epithelial cell adhesion molecule (EpCAM) antibodies. Other techniques involve EpCAM antibody-coated microporous chips, microporous polycarbonate filters, and PCR. However, methods based on EpCAM analysis have shown limitations in detecting CTCs in patients without or low epithelial characteristics 5-7. Besides, CTC isolation by size may inadvertently omit cells less than 8 µm, thus requires further validation before clinical use 7. Reverse transcriptase polymerase chain reaction (RT-PCR) can detect CTCs with high sensitivity 8. However, post-transcriptional regulation that deregulates gene expression has been identified in numerous cancer cells 9, and it alters gene expression through modification of mRNA stability and/or transcriptional efficiency; thus protein content may possibly not correlate with mRNA levels.
Folate receptors (FRs) are cell-surface glycoproteins that are highly expressed in many cancers including, lung and ovarian cancer 10,11. Our previous study showed promising clinical significance quantification of FR+-CTCs by a novel ligand-targeted polymerase chain reaction (LT-PCR) method 12. Building on that previous study and in order to evaluate the efficacy and feasibility of FR+-CTC detection method, we conducted this large scale, prospective, single-center clinical trial.
Materials and Methods
Patients
From October 2013 to April 2014, we recruited 368 participants into this prospective, single-center clinical trial conducted at Shanghai Chest Hospital, Shanghai Jiaotong University, China. Among these patients, 197 patients with lung cancer, 119 patients with benign lung diseases and 52 healthy controls were enrolled in our none-dynamic study. In addition, 62 NSCLC patients among the 197 lung cancer patients who underwent surgical treatment were enrolled for our dynamic surveillance study. Lung cancer was defined on the basis of pathologic diagnosis, computed tomography (CT) scan, and laboratory examination according to the guidelines set forth by the National Comprehensive Cancer Network (NCCN). Benign lung diseases included pneumonia, tuberculosis, bronchiectasis or pneumothorax. The Research Ethics Committee of Shanghai Chest Hospital approved the clinical trial and an informed consent was obtained from each participant prior to study entry. The registration number of the clinical trial named “FR+-CTC detection in lung cancer patients” is LS1308.
CTC preparation and FA-oligonucleotide conjugation
CTC analysis was performed using CytoploRare® circulating lung cancer cell kit provided by GenoSaber Biotech Co. Ltd. (Shanghai, China), as previously described 12. We collected 3 ml of peripheral blood into EDTA-containing anticoagulant tubes from all study participants before treatment. Peripheral blood specimens were stored in 4-8°C and analyzed within 24 h.
Following the manufacturer's instruction manual, CTCs were enriched by lysis of erythrocytes and immuno-magnetic depletion of leukocytes. The enriched CTCs were incubated with 10 μl of labeling buffer that contained conjugates of a tumor-specific ligand, folic acid, and a synthesized oligonucleotide for 40 minutes at room temperature. Next, the unbound conjugates were washed off using 1 mL wash buffer and the cells were centrifuged at 500 rpm for 10 minutes at 4°C. The specific ligand-oligonucleotide conjugates were removed with 120 μL stripping buffer for 2 minutes at 4°C, collected by centrifugation, and neutralized by 24 μl of neutralization buffer for further RT-PCR analysis.
FR+-CTC LT-PCR
In the phase of PCR analysis, the specific conjugate was first annealed and extended on the RT primer before amplification. Then the extended conjugate was analyzed using a Taqman probe based on quantitative PCR method on ABI StepOneTM system (Life technologies). The primer sequences were listed as follows: reverse transcription (RT) primer (an oligonucleotide that is conjugated to the tumor-specific ligand folic acid), 5′ - CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAG- GGTTCTAA - 3′; forward primer, 5′-TATGATTATGAGGCATGA-3′; reverse primer, 5′-GGTGTCGTGGAGTCG-3′; TaqMan probe, 5′-FAM-CAGTTGAGGGTTC-MGB-3′. The following reaction conditions were used on the ABI StepOneTM instrument: denaturation at 95°C for 2 minutes, annealing at 40°C for 30 seconds, extension at 72°C for 30 seconds, and then cooling at 8°C for 5 minutes; 40 cycles of denaturation at 95°C for 10 seconds, annealing at 35°C for 30 seconds, and extension at 72°C for 10 seconds.
In this study, we used a self-defined CTC unit, which was the number of CTCs detected in 3 ml blood, thus one CTC unit represented one CTC in 3 ml blood. A serial of standards containing oligonucleotides (10-14 to 10-9 M, corresponding to 2 to 2 × 105 CTC units/3 mL blood) are used for CTC quantification. All patient samples were tested in duplicates with six standards and three quality controls.
Clinical Tumor Biomarker Analysis
Three milliliters of peripheral blood of all enrolled patients were withdrawn into coagulant tubes. After centrifuging at 800-1000 rpm for 10 min, the serum was collected for analysis of tumor markers (CEA, CYFRA21-1, NSE) by chemiluminescence method (Abbott Laboratories).
Statistical Analysis
Statistical analysis was performed using SPAA 18.0 software (SPSS Inc., Chicago, IL) or Prism 5.0 (GraphPad Software Inc., San Diego, CA). Wilcoxon test and Kruskal-Wallis test were used for comparison between groups, as appropriate. A receiver operating characteristics (ROC) curve was used to determine the threshold of specificity and sensitivity, and the area under the curves (AUC) was calculated for each index.
Results
Patient characteristics
We recruited 368 participants into this prospective, single-center clinical trial (Figure 1). Among these patients, 197 patients with lung cancer, 119 patients with benign lung diseases and 52 healthy controls were enrolled in our none-dynamic study (Table S1). In addition, 62 NSCLC patients among the 197 lung cancer patients who underwent surgical treatment were enrolled for our dynamic surveillance study. The clinical characteristics of the patients are presented in Table S2.
Figure 1.
Distribution of patients enrolled in the study.
Diagnostic value of FR+-CTC in patients with lung cancer
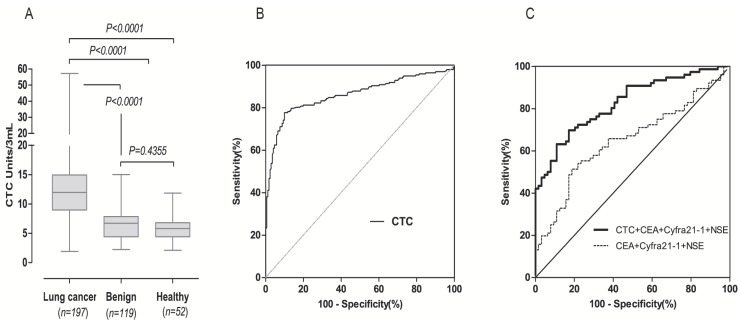
To investigate the diagnostic value of FR+-CTC, we compared CTC levels in patients with lung cancer, benign lung diseases and healthy donors. As shown in Figure 2A, the CTC levels in lung cancer patients (median 11.97 CTC units) was significantly higher than benign lung disease patients (median 6.72 CTC units, p<0.0001) and healthy donors (5.82 CTC units, p<0.0001). However, there was no significant difference between the two control groups (p = 0.4355). The diagnostic value of FR+-CTCs in lung cancer patients was evaluated, and the area under curve (AUC) for FR-positive CTCs in discriminating between patients with lung cancer and the other two groups was 0.8607 (Figure 2B). According to the ROC analysis, the cut-off threshold between control group (benign patients and healthy volunteers) and the lung cancer group was 8.7 CTC units, with a sensitivity of 77.7% and specificity of 89.5%. When compared with existing clinical biomarkers (CEA, CYFRA21-1, NSE), as shown in Table 1, the FR+-CTC detection method displayed the highest AUC and could satisfactorily discriminate lung cancer patients from benign lung disease and healthy controls. Notably, the combination of FR+-CTC, CEA, NSE, and CYFRA21-1 significantly improved the diagnostic efficacy in differentiating patients with lung cancer from the other two groups (Figure 2C), implying that a multi-marker strategy integrating FR+-CTC levels with established tumor markers might be more effective for clinical lung cancer diagnosis.
Figure 2.
Diagnostic value of FR+-CTCs in patients with lung cancer. (A) FR+-CTC levels in patients with lung cancer, benign lung disease and healthy controls. (B) ROC curve for FR+-CTC in discriminating patients with lung cancer from patients with benign lung disease and healthy controls. (C) ROC curve for FR+-CTC combined with tumor marker in discriminating patients with lung cancer from patients with benign lung disease and healthy controls.
Table 1.
Comparison of FR+-CTC with Established Tumor Biomarkers.
| Lung cancer vs. benign disease | AUC (95%CI) |
|---|---|
| FR+-CTC | 0.7956 (0.7205-0.8708) |
| CEA | 0.6289 (0.5371-0.7208) |
| NSE | 0.5090 (0.4120-0.6061) |
| CYFRA21-1 | 0.5507 (0.4551-0.6462) |
| CEA+NSE+CYFRA21-1 | 0.6495 (0.5583-0.7406) |
| CEA+NSE+CYFRA21-1+ FR+-CTC | 0.8267 (0.7601-0.8933) |
Association of FR+-CTC with TNM stage and histopathologic subtypes of lung cancer patients
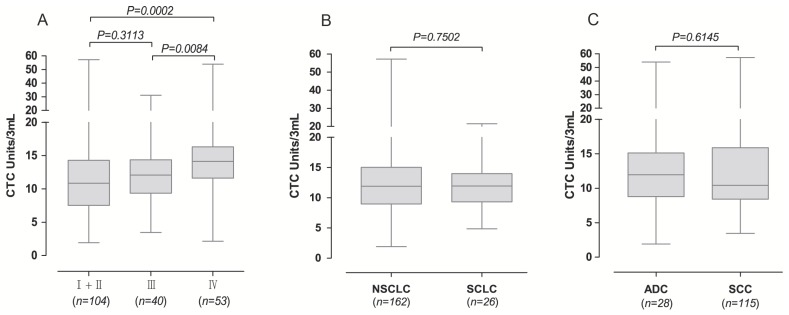
We next examined whether FR+-CTCs could stratify TNM stages and histopathologic subtypes. Lung cancer stages were based on the seventh edition of the American Joint Committee on Cancer (AJCC) staging manual 13. The CTC levels in patients with stage IV lung cancer were significantly higher than those with stage I and II lung cancer (p = 0.0002) and stage III lung cancer (p = 0.0084), while there was no significant difference among CTC levels in patients with stage I, II and III (p = 0.3113; Figure 3A). In assessing whether FR+-CTC could discern between NSCLC and SCLC, the median levels in 162 NSCLC patients, (median 11.90) were not significantly different from the 26 SCLC patients (median 11.93) (p = 0.7502, Figure 3B). Additionally, we did not find a significant difference in FR+-CTC levels in patients with adenocarcinoma (ADC) and squamous cell carcinoma (SCC) (p = 0.6145, Figure 3C).
Figure 3.
Association of FR+-CTC with TNM stage and histopathologic subtypes of lung cancer patients. (A) The FR+-CTC level in lung cancer patients with different TNM stages. (B) The FR+-CTC level between patients with NSCLC and SCLC. NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer. (C) The FR+-CTC level between patients with ADC and SCC. ADC, adenocarcinoma; SCC, squamous cell carcinoma.
In a more detailed analysis, we investigated the diagnostic efficiency of FR+-CTC in different histological subtypes and pathological stages of lung cancer. As shown in Table 2, the diagnostic sensitivity for patients with stage I-IV lung cancer was 69.8% (60/86), 72.2% (13/18), 80.0% (32/40), and 90.6% (48/53), respectively. In regard to the different histological subtypes of lung cancer, the diagnostic sensitivity of the method for NSCLC and SCLC patients was 77.2% (125/162) and 80.8% (21/26), respectively.
Table 2.
Diagnostic Efficiency of FR+-CTC in Lung Cancer Histopathologic Subtype.
| Characteristics |
≥8.70 FR+-CTC Units/3 mL | <8.70 FR+-CTC Units/3 mL | ||
|---|---|---|---|---|
| NO. | % | NO. | % | |
| Lung cancer (n = 197) | 153 | 77.7 | 44 | 22.3 |
| TNM stage | ||||
| I (n = 86) | 60 | 69.8 | 26 | 30.2 |
| II (n = 18) | 13 | 72.2 | 5 | 27.8 |
| III (n = 40) | 32 | 80 | 8 | 20 |
| I+II+III (n = 144) | 105 | 72.9 | 39 | 27.1 |
| IV (n = 53) | 48 | 90.6 | 5 | 9.4 |
| Histopathologic subtype | ||||
| NSCLC (n = 162) | 125 | 77.2 | 37 | 22.8 |
| ADC (n = 115) | 88 | 76.5 | 27 | 23.5 |
| SCC (n = 28) | 20 | 71.4 | 8 | 28.6 |
| Others of NSCLC (n = 19) | 17 | 89.5 | 2 | 10.5 |
| SCLC (n = 26) | 21 | 80.8 | 5 | 19.2 |
| Others of lung cancer (n = 9) | 7 | 77.8 | 2 | 22.2 |
| Benign diseases (n = 119) | 14 | 11.8 | 105 | 88.2 |
| Healthy donors (n = 52) | 4 | 7.7 | 48 | 92.3 |
These results suggested that patients with localized disease and those with metastatic lung cancer have distinct levels of CTC; however, the expression of folate receptor might be similar across all histological subtypes of lung cancer.
Dynamic changes of FR+-CTC in patients with resectable NSCLC
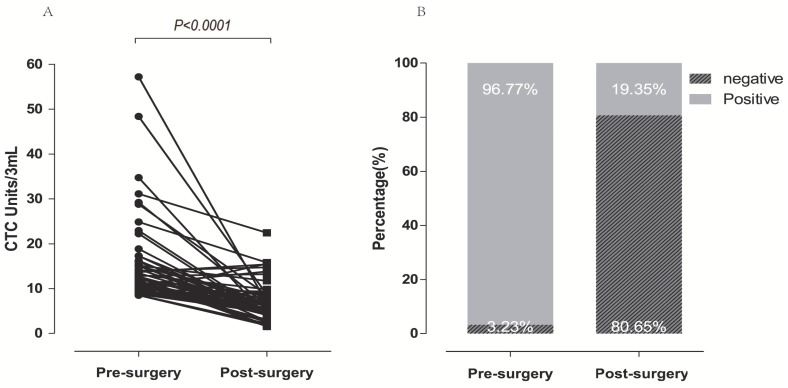
In a dynamic surveillance study, 62 NSCLC patients with CTC levels higher than 8.5 Units/3 ml before surgery were recruited to investigate the changes of FR+-CTCs during the peri-operative period. Blood samples were collected before and seven days after surgery (median 5.46 FR+-CTC Units/3ml for post-surgery; 12 FR+-CTC Units/3 ml for pre-surgery). As shown in Figure 4A and 4B, compared with pre-surgery, FR+-CTC load decreased significantly after resection (96.77% vs. 19.35%, p<0.0001). Among these patients, 48 patients with FR+-CTCs positive before resection achieved a negative status seven days after operation, 12 patients were still FR+-CTC positive, and two patients with FR+-CTCs negative before resection remained negative.
Figure 4.
Dynamic changes of FR+-CTC in patients with resectable NSCLC. Dynamic changes of FR+-CTC level in patients receiving curative resection. FR+-CTC positive rates of patients before and after curative resection.
Discussion
Although CTCs were first observed more than 100 years ago, their clinical significance has only recently become widely recognized. Quantification and characterization of CTCs may serve as a surrogate diagnostic test that could constitute a “liquid biopsy” and provide real-time information about the patient's disease state 14,15. Technology that can reliably identify CTCs in peripheral blood is becoming increasingly available. CellSearchTM System (Veridex LLC, Huntingdon Valley, PA, USA) has been by far the most commonly used method, and is the only technique thus far approved for routine use in the clinical setting by the FDA 16-18. A recent method of CTC detection is “CTC-Chip” has been investigated for use in lung, prostate, pancreatic, breast, and colon cancer, and has been shown to reliably detect CTCs in 99% of patients with metastatic disease with a purity of approximately 50% 19. Isolation of tumor cells by size is a non-immunological based, cytometric CTC isolation technique which has been used successfully to identify CTCs in a number of cancers such as breast, hepatocellular carcinoma, and NSCLC 20-22. Though widely used, the antigen-dependent immunological techniques may sacrifice sensitivity for the sake of specificity and vice versa for non-immunological techniques. The heterogeneity of CTCs is becoming increasingly better understood and it is clear that identifying particular subtypes of CTCs would be more relevant.
The folate receptor (FR), a cell-surface receptor glycoprotein, which is highly expressed in a variety of cancers, especially in ovarian and lung cancers, has become an important potential drug target for patients with NSCLC 10,23. Although FR has been identified in some normal tissues, including kidney, spleen and lung, no cells expressing FR have been identified in the circulatory system except for CTCs or activated monocytes 10,24,25. Preliminary studies found that these activated monocytes subpopulation is barely detectable in blood samples from healthy donors or patients with benign disease 24,25. Besides, FR expression is also found in tumor activated macrophages (TAMs) 27, and our previous study demonstrated that the depletion mechanism using anti-CD45 magnetic beads was sufficient to remove the impact of TAMs on CTC counts 12. A study found that FR expression was upregulated in about 75.7% of patients with NSCLC 28, therefore, FR may be a potential target for detecting CTCs in patient with lung cancers.
Our previous small sample study showed promising clinical value of detecting FR+-CTC by a novel ligand-targeted polymerase chain reaction (LT-PCR) method in NSCLC patients 12. LT-PCR is a sensitive, rapid, and cost-effective technique for molecular identification of CTC. The major drawback of this method is the relatively high false-positive rate due to contamination with leukocytes, which can be reduced by effective pre-enrichment of CTC. In order to validate our previous data and evaluate the feasibility of LT-PCR FR+-CTC detection method in the diagnosis of lung cancer, we conducted this large scale, prospective, clinical trial. We found that FR+-CTC levels were significantly higher in 197 patients with lung cancer compared with 119 patients with benign lung disease and 52 healthy donors (p<0.0001, Figure 2A). Using the marker of FR, our platform showed a significant diagnostic performance in discrimination between patients with lung cancer, patients with benign lung disease and healthy donors with an AUC of 0.8607 and a sensitivity of 77.7% and specificity of 89.5% (Figure 2B). In clinical practice, plasma tumor markers CEA, NSE, and CYFRA21-1 were used to help diagnose and surveil treatment efficiency of lung cancer. Comparing with these plasma tumor markers, FR+-CTC detection showed the highest AUC in the diagnosis of lung cancer (Table 1). Notably, the combination of FR+-CTC, CEA, NSE, and CYFRA21-1 could significantly improve the diagnostic efficacy in differentiating patients with lung cancer from benign lung disease (Figure 2C), implying that a multi-marker strategy integrating CTC levels with tumor markers might be a more effective method for clinical lung cancer diagnosis. Moreover, our data also demonstrated the clinical significance of FR+-CTC detection by our LT-PCR platform for surveillance of treatment efficiency in patients with NSCLC treated with surgery (Figure 4A, 4B).
Studies on CTC detection in NSCLC previously identified a correlation between disease stages and CTC numbers 7, and our results also found that the CTC levels in patients with stage IV lung cancer were significantly higher than those with stage I and II lung cancer (p = 0.0002) and stage III lung cancer (p = 0.0084), while there was no significant difference among CTC levels in patients with stage I, II and III (p = 0.3113; Figure 3A). Although, previous studies demonstrated that the FR expression was much lower in SCC than in ADC in primary tumors 7,10,28,29, our results showed no significant difference of FR+-CTC levels between patients with SCC and NSCLC (p = 0.6145, Figure 3B), partly because gene expression between primary tumors and CTC samples is often different 30, as previously demonstrated in breast cancer 31.
In this large-scale, prospective clinical trial, we established a LT-PCR-based FR+-CTC detection platform for patients with lung cancer that exhibits high sensitivity and specificity. Our results indicate that FR+-CTC detected by this platform would be clinically useful in lung cancer diagnosis and treatment response assessment. Further investigation on the prognostic value of CTC levels detected by LT-PCR is required.
Supplementary Material
Supplementary tables.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of China (2014DFA33010), the Science and Technology Commission of Shanghai Municipality (14411950800, 13441902200), the Hospital Development Center of Shanghai (SHDC22014011), Shanghai Chest Hospital (2014YZDC10100, YZ14-22).
References
- 1.Jemal A, Bray F, Center MM. et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Oak CH, Wilson D, Lee HJ. et al. Potential molecular approaches for the early diagnosis of lung cancer. Mol Med Rep. 2012;6(5):931–936. doi: 10.3892/mmr.2012.1042. [DOI] [PubMed] [Google Scholar]
- 3.Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med J Australia. 1869;14:146–149. [Google Scholar]
- 4.Plaks V, Koopman CD, Werb Z. Cancer, circulating tumor cells. Science. 2013;341(6151):1186–8. doi: 10.1126/science.1235226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young R, Pailler E, Billiot F. et al. Circulating tumor cells in lung cancer. Acta Cytol. 2012;56:655–660. doi: 10.1159/000345182. [DOI] [PubMed] [Google Scholar]
- 6.Gorges TM, Tinhofer I, Drosch M. et al. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178. doi: 10.1186/1471-2407-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krebs MG, Sloane R, Priest L. et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29:1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 8.Ignatiadis M, Xenidis N, Perraki M. et al. Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol. 2007;25:5194–5202. doi: 10.1200/JCO.2007.11.7762. [DOI] [PubMed] [Google Scholar]
- 9.Audic Y, Hartley RS. Post-transcriptional regulation in cancer. Biol Cell. 2004;96:479–498. doi: 10.1016/j.biolcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Parker N, Turk MJ, Westrick E. et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Christoph DC, Asuncion BR, Hassan B. et al. Significance of folate receptor alpha and thymidylate synthase protein expression in patients with non-small-cell lung cancer treated with pemetrexed. J Thorac Oncol. 2013;8:19–30. doi: 10.1097/JTO.0b013e31827628ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lou JT, Ben SQ, Yang GH. et al. Quantification of Rare Circulating Tumor Cells in Non-Small Cell Lung Cancer by Ligand-Targeted PCR. PloS One. 2013;8(12):e80458. doi: 10.1371/journal.pone.0080458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 14.Alix-Panabieres C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem. 2013;59:110–8. doi: 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- 15.Pantel K, Alix-Panabieres C. Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res. 2013;73:6384–8. doi: 10.1158/0008-5472.CAN-13-2030. [DOI] [PubMed] [Google Scholar]
- 16.Cristofanilli M, Budd GT, Ellis MJ. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 17.Cohen SJ, Punt CJ, Iannotti N. et al. (Pinzani et al., 2006) J Clin Oncol. 2008;26:3213–21. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 18.Bono JS, Scher HI, Montgomery RB. et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 19.Nagrath S, Sequist LV, Maheswaran S. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–9. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desitter I, Guerrouahen BS, Benali-Furet N. et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res. 2011;31:427–41. [PubMed] [Google Scholar]
- 21.Pinzani P, Salvadori B, Simi L. et al. Isolation by size of epithelial tumor cells in peripheral blood of patients with breast cancer: correlation with real-time reverse transcriptase-polymerase chain reaction results and feasibility of molecular analysis by laser microdissection. HumPathol. 2006;37:711–8. doi: 10.1016/j.humpath.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 22.Vona G, Estepa L, Beroud C. et al. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology. 2004;39:792–7. doi: 10.1002/hep.20091. [DOI] [PubMed] [Google Scholar]
- 23.Thomas A, Maltzman J, Hassan R. Farletuzumab in lung cancer. Lung Cancer. 2013;80:15–18. doi: 10.1016/j.lungcan.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He W, Kularatne SA, Kalli KR. et al. Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands. Int J Cancer. 2008;123:1968–1973. doi: 10.1002/ijc.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy JA, Haneline LS, Srour EF. et al. Expression and functional characterization of the beta-isoform of the folate receptor on CD34(+) cells. Blood. 1999;93:3940–3948. [PubMed] [Google Scholar]
- 26.He W, Wang H, Hartmann LC. et al. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. PNAS. 2007;104:11760–11765. doi: 10.1073/pnas.0703875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puig-Kroger A, Sierra-Filardi E, Dominguez-Soto A. et al. Folate receptor beta is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 2009;69:9395–9403. doi: 10.1158/0008-5472.CAN-09-2050. [DOI] [PubMed] [Google Scholar]
- 28.Nunez MI, Behrens C, Woods DM. et al. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR[corrected] mutation. J Thorac Oncol. 2012;7:833–840. doi: 10.1097/JTO.0b013e31824de09c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Shannessy DJ, Yu G, Smale R. et al. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget. 2012;3:414–425. doi: 10.18632/oncotarget.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao CG, Chianese D, Doyle GV. et al. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int J Oncol. 2005;27:49–57. [PubMed] [Google Scholar]
- 31.Ligthart ST, Bidard FC, Decraene C. et al. Unbiased quantitative assessment of Her-2 expression of circulating tumor cells in patients with metastatic and non-metastatic breast cancer. Ann Oncol. 2013;24:1231–1238. doi: 10.1093/annonc/mds625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.