Abstract
Objective
To evaluate the comparative safety of laparoscopic and open colectomy across surgeons varying in experience with laparoscopy.
Data Sources
National Medicare data (2008–2010) for beneficiaries undergoing laparoscopic or open colectomy.
Study Design
Using instrumental variable methods to address selection bias, we evaluated outcomes of laparoscopic and open colectomy. Our instrument was the regional use of laparoscopy in the year prior to a patient's operation. We then evaluated outcomes stratified by surgeons' annual volume of laparoscopic colectomy.
Principal Findings
Laparoscopic colectomy was associated with lower mortality (OR: 0.75, 95 percent CI: 0.70–0.78) and fewer complications than open surgery (OR: 0.82, 95 percent CI: 0.79–0.85). Increasing surgeon volume was associated with better outcomes for both procedures, but the relationship was stronger for laparoscopy. The comparative safety depended on surgeon volume. High‐volume surgeons had 40 percent lower mortality (OR: 0.60, 95 percent CI: 0.55–0.65) and 30 percent fewer complications (OR: 0.70, 95 percent CI: 0.67–0.74) with laparoscopy. Conversely, low‐volume surgeons had 7 percent higher mortality (OR: 1.07, 95 percent CI: 1.02–1.13) and 18 percent more complications (OR: 1.18, 95 percent CI: 1.12–1.24) with laparoscopy.
Conclusions
This population‐based study demonstrates that the comparative safety of laparoscopic and open colectomy is influenced by surgeon volume. Laparoscopic colectomy is only safer for patients whose surgeons have sufficient experience.
Keywords: Comparative safety, colectomy, instrumental variables
Laparoscopy is increasingly applied to common surgical procedures such as colectomy. Numerous randomized clinical trials and large observational studies demonstrate fewer complications and shorter hospital stays when compared to traditional open operations (Weeks et al. 2002; 2004; Fleshman et al. 2007; Gervaz et al. 2010; Bagshaw et al. 2012). The evidence favoring laparoscopy, coupled with increasing recognition of its benefits by patients and referring physicians, has amplified pressure on surgeons to provide this minimally invasive approach (Thaler et al. 2003). Many surgeons in practice are not formally trained to perform these procedures or have had limited experience since residency (American Society of Colon and Rectal Surgeons [ASCRS] et al. 2006; Ho et al. 2012). Nonetheless, the perceived benefits of laparoscopic colectomy encourage its diffusion into general practice, which has increased fivefold over the past decade (Kemp and Finlayson 2008; Rea et al. 2011).
However, it is unclear whether new laparoscopic procedures such as colectomy retain their benefits when implemented across diverse practice settings. Randomized clinical trials (i.e., efficacy trials) in surgery are often conducted at centers with the highest volume surgeons and may not reflect treatment outcomes among providers who differ in their proficiency performing the procedure. Although there is a well‐known relationship between volume and outcome for high‐risk surgeries, including colectomy, its implications for comparative effectiveness research have not been explored (Birkmeyer et al. 2002; Kiran et al. 2010a,b; Finks, Osborne, and Birkmeyer 2011). If the relationship between volume and outcomes is stronger for new, more technically complex procedures like laparoscopic colectomy, current information regarding the benefits of laparoscopy may not represent the outcomes achieved by lower volume surgeons.
In this context, we conducted a population‐based study using national Medicare data for patients undergoing laparoscopic or open colectomy. We employed an instrumental variable approach to address selection bias from unmeasured patient characteristics and illness severity common to administrative datasets (Xian et al. 2011; Tan et al. 2012). To assess for heterogeneity across providers, we stratified patients by their surgeon's annual procedure volume. If the benefits of laparoscopic colectomy were not related to surgeon volume, we would expect to see no difference in the benefits of laparoscopy between high‐ and low‐volume providers.
Methods
Data Source and Study Population
We used national data from the 100 percent Medicare Provider Analysis and Review (MEDPAR) files for the years 2008 through 2010. The Centers for Medicare & Medicaid Services (CMS) maintains this database using claims submitted by hospitals where Medicare beneficiaries receive care. Patient data included age, sex, race, comorbidities (including principal and secondary diagnosis codes), procedural codes, 30‐day complications and mortality, and information regarding length of hospital stay. We selected patients undergoing colon resection using International Classification of Disease, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes.1 We excluded patients with incomplete data in the Medicare files (<1 percent overall). We used the American Hospital Association (AHA) Annual Survey Database to assign patients to Hospital Referral Regions (HRR) using the Medicare provider identification number for the hospital in which they underwent operation that is common to both datasets.
Outcomes
Our primary outcomes of interest were the incidence of 30‐day complications and mortality. Complications were identified by ICD‐9‐CM codes.2 These complications represent a subset of ICD‐9 codes with the highest sensitivity and specificity as has been previously described (Iezzoni et al. 1994). Overall complication rates were consistent with previously published work using similar patient populations (Bilimoria et al. 2008).
Statistical Analysis
We first sought to evaluate the independent influence of laparoscopic colectomy on the incidence of postoperative complications and mortality using multilevel mixed‐effects logistic regression models. For all models, we adjusted for patient characteristics including age, race, principal diagnosis, and 29 Elixhauser comorbid diseases. This method has been previously tested and validated for risk adjustment when using administrative data (Elixhauser et al. 1998; Southern, Quan, and Ghali 2004). We also accounted for differences in case mix using categorical dummy variables for right, left, transverse, and total colectomy. We also accounted for clustering of outcomes within hospitals using a variable that uniquely identifies each hospital. This was performed for all analyses, including the IV models discussed below. We evaluated each model's discriminatory function by c‐statistic and assessed calibration across deciles of risk using the Hosmer–Lemeshow test. Because the robustness of our models may be influenced by differences in operative indication, we performed sensitivity analyses for each model using patients undergoing operations for cancer and benign indications separately.
We next employed an instrumental variable analysis to reduce selection bias not addressed by our multivariate analysis (Newhouse and McClellan 1998). We hypothesize that patients selected for laparoscopic operations are more commonly predisposed to better outcomes based on clinical characteristics (e.g., smaller tumors or more favorable anatomy). This would inflate the relative safety of laparoscopy over open surgery. Instrumental variable methods are a powerful econometric technique that can balance both measured and unmeasured patient characteristics between two comparison groups. An instrumental variable must be highly correlated with the exposure (laparoscopic vs. open approach), but not associated with the outcomes except through its relationship with the exposure (the instrumental variable is exogenous). Our instrumental variable was the regional use of the laparoscopic approach in the prior year. For this analysis, we calculated the proportion of colon resections performed laparoscopically for each HRR in the year prior to a given patient's operation. This instrument should not directly influence patient outcomes in the following year. HRR's are large enough that patients are not concentrating in certain HRR's for laparoscopic operations (the instrument is exogenous). Exogeneity in this regard is generally not testable by analytic means. Intuitively, some patients are more likely to receive laparoscopic colectomy simply because they were treated within a region performing a high proportion of these procedures. Our analysis accounts for this and explicitly compares laparoscopic to open colectomy in the marginal patient (i.e., a patient who would be considered a candidate for either approach). To evaluate our instrumental variable, we first confirmed its relationship with our exposure, the receipt of laparoscopic colectomy (F statistic = 240, indicating a “strong” instrument). Note that the first‐stage regression also controls for HRR fixed effects, meaning that the instrument is strong even after controlling for HRR‐level factors. Identification relies on within‐HRR variation in practice patterns over time.
Our instrumental variable is not designed to reduce bias associated with surgeon factors (e.g., a particular surgeon's skill or technique). It is not associated with a patient receiving an operation by a high‐volume laparoscopic provider. Thus, it does not meet strict criteria as an instrumental variable for this purpose. We also did not observe any significant association between the regional use of laparoscopy and the likelihood of operation by a high‐volume laparoscopic provider. One explanation is that many providers within a hospital or health system offer laparoscopic colon surgery. Intuitively, each surgeon will vary in his or her experience and application of this technology. Nonetheless, to account for the fact that regional differences may also be associated with important variation in overall surgeon skill, we included categorical dummy variables for each HRR as a fixed effect in both our first‐ and second‐stage models described below. The results did not differ when these variables were excluded. Similarly, we included a dummy variable for the year of operation to account for any possible time trends. This did not influence the outcomes from any of our models.
We employed a two‐stage residual inclusion (2SRI) method for our instrumental variable analysis of postoperative complications and mortality (Terza, Basu, and Rathouz 2008). We elected to use a residual inclusion model because it has been shown to provide less biased estimates from nonlinear models (Terza, Bradford, and Dismuke 2008). Our first‐stage model (logistic regression) assessed the association between receipt of laparoscopic colectomy and our instrumental variable, while also adjusting for known patient‐level covariates identical to those used in our conventional logistic regression analysis and including dummy variables for each HRR. From this model, we predicted the raw residuals for each patient as the difference between the model‐predicted probability of receiving laparoscopic colectomy and the actual treatment received. This is our exogeneity test. The coefficient for the residuals was statistically significant for both mortality (−0.45, z = −28.1, p < .01) and morbidity (−.079, z = −17.9, p < .01). This indicates an endogeneity problem addressed by our IV approach. These values were then used as a covariate in our second‐stage logistic regression model, which assessed the association between laparoscopic colectomy and the incidence of postoperative complications or mortality. In this model we also adjusted for patient age, race, diagnosis, HRR, and comorbidities in a manner identical to our logistic regression models. We generated average outcome rates for each procedure using marginal means. Finally, from the second‐stage model, we report odds ratios and average treatment effects (ATE) for laparoscopy relative to open surgery for each outcome (Ghislandi, Torbica, and Boriani 2013). The average treatment effect was calculated using the following method, where X p is a binary variable for laproscopic (1) or open (0) colectomy. Furthermore, μ(X p, X oi, X u; τ) is the predicted logit probability for the ith sample member for procedure X p, X o is a vector of control variables, τ is the logit estimate of the model parameters. The second‐stage estimates were obtained using the logit and logistic functions in STATA version 13.1.
We used bootstrapping to generate confidence intervals and the corresponding z‐statistics. The z‐statistics were generated from normal‐based confidence intervals derived from bootstrapping with 1,000 replications, where draws were made at the hospital level to deal with clustering at the hospital level.
To study the influence of surgeon volume, we calculated each surgeon's annual number of laparoscopic and open colectomy procedures in Medicare beneficiaries. To do this, we first identified physicians using the unique provider identification number from the inpatient file. We selected those providers listed as the primary operator using a method that has been previously described and validated (Miller, Welch, and Welch 1996). We were unable to identify certain surgeons who were not compensated by Medicare and this group represented 31 percent of our patient population. However, patient characteristics and outcome rates were not different between these patients and those whose surgeon was identifiable. We then grouped patients into quartiles based on their surgeon's annual volume of laparoscopic and open colectomy separately (i.e., there is a low‐volume laparoscopic group and a low‐volume open group of mostly different surgeons). We combined results for the middle two quartiles for reporting to improve generalizability.
Using the 2SRI model described above, we calculated estimates (predicted probabilities) of complications and mortality for laparoscopic and open operations separately, stratified by quintiles of surgeons' annual volume for each type of procedure. We then conducted our evaluation of surgeon volume and the relative safety of laparoscopic colectomy in two ways using the instrumental variable models. The methods are identical to those described above for our main effects analysis. First, we created an interaction term between the categorical dummy variable for procedure and strata of surgeons' procedure volume, using marginal means to calculate outcome rates. This interaction term in the low‐volume group, for example, would be the category of low‐volume laparoscopic surgeons times the dichotomous variable for laparoscopy or open surgery. We compared these results to an alternative approach in which we restricted the model to only those patients represented by annual procedural volume. We found the results to be consistent between both methods.
All statistical analyses were performed using STATA statistical software version 13 (College Station, TX, USA). We employed a two‐sided approach at the 5 percent significance level for all hypothesis testing. This study was deemed exempt by the Institutional Review Board at the University of Michigan.
Results
Patients were similar in age, race, and comorbid disease burden when stratified by operative approach. However, there were significant differences in primary diagnosis and procedure priority (elective or emergent) between patients undergoing laparoscopic and open colectomy (Table 1). When stratified by the instrumental variable, however, all patient characteristics, including operative indications, procedure priority, and the probability of adverse events, were well balanced (Table 1). This effect persisted when comparing patient characteristics between those hospitals performing the most (top quartile) and least (bottom quartile) laparoscopy.
Table 1.
Patient Characteristics by Type of Procedure and the Regional Use of Laparoscopic Colectomy
| Type of Procedure | Regional Use of Laparoscopya | |||
|---|---|---|---|---|
| Laparoscopic (n = 68,394) | Open (n = 189,353) | <25% (n = 128,492) | ≥25% (n = 129,255) | |
| Age, year | ||||
| Mean (SD) | 73.9 (9.0) | 74.3 (10.3) | 74.1 (10.0) | 74.3 (9.9) |
| Median (IQR) | 74 (68–80) | 75 (68–82) | 74 (67–81) | 75 (68–82) |
| Race, n (%) | ||||
| White | 59, 321 (86.7) | 162,161 (85.6) | 111,976 (87.1) | 109,506 (84.8) |
| Black | 6,031 (8.8) | 19,145 (10.1)b | 12,312 (9.6) | 12, 864 (9.9) |
| Other | 3,042 (4.5) | 8,047 (4.3) | 4,204 (3.3) | 6885 (5.3%) |
| Comorbid conditions, # | ||||
| Mean (SD) | 2.0 (1.3) | 2.1 (1.3) | 2.0 (1.3) | 2.1 (1.3) |
| Median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| Specific comorbidities, n (%) | ||||
| Congestive heart failure | 4,253 (6.2) | 17,636 (9.3)b | 11,507 (9.0) | 10,382 (8.0) |
| Pulmonary circulatory disease | 1,072 (1.5) | 4,007 (2.1)b | 2,579 (2.0) | 2,500 (1.9) |
| Diabetes mellitus | 12,106 (17.7) | 26,144 (13.7)b | 19,391 (15.1) | 18,859 (14.6) |
| Diabetes with complications | 1,148 (12.9) | 2,787 (12.0) | 1,930 (1.5) | 2,005 (1.5) |
| Liver disease | 837 (1.2) | 2,177 (1.1)b | 1,450 (1.1) | 1,564 (1.2) |
| Renal failure | 3,378 (4.9) | 12,827 (6.7)b | 8,107 (6.3) | 8,098 (6.2) |
| Metastatic cancer | 7,340 (10.7) | 28,112 (14.8)b | 17,864 (13.6) | 17,588 (13.9) |
| Obesity | 4,087 (5.9) | 7,974 (4.2) | 6,018 (4.7) | 6,043 (4.7) |
| Depression | 3,345 (4.8) | 6,886 (3.6) | 5,148 (4.0) | 5,083 (3.9) |
| Operative indication, n (%) | ||||
| Malignancy | 50,377 (73.7) | 93,523 (49.4)b | 71,243 (55.4) | 72,657 (56.2) |
| Diverticular disease/fistula | 12,183 (17.8) | 58,766 (31.0)b | 35,657 (27.7) | 35,292 (27.3) |
| Inflammatory bowel disease | 872 (1.3) | 6,667 (3.5)b | 3,749 (7.8) | 3,790 (7.0) |
| Vascular insufficiency | 1,056 (1.5) | 18,072 (9.5)b | 10,032 (2.9) | 9,096 (2.9) |
| Obstruction/hernia/volvulus | 16,536 (24.1) | 54,952 (29.0)b | 35,315 (27.4) | 36,173 (27.9) |
| Presentation, n (%) | ||||
| Elective | 53,387 (78.1) | 84,089 (44.4)b | 67,762 (52.7) | 69,714 (53.9) |
| Preoperative probability, % | ||||
| Complications | 25.2 | 33.5b | 31.4 | 31.2 |
| Mortality | 6.2 | 10.0b | 9.0 | 9.0 |
Instrumental variable—the proportion of colectomies performed laparoscopically within each hospital referral region.
Denotes significant differences between treatment groups (p < 0.05).
We first assessed the comparative effectiveness of laparoscopic versus open colectomy using conventional multivariable logistic regression. Compared to open surgery, we observed that laparoscopic colectomy was associated with lower complication rates (23.5 percent vs. 33.4 percent; OR: 0.55, 95 percent CI: 0.53–0.56; p < .01) and mortality (4.3 percent vs. 9.4 percent; OR: 0.38, 95 percent CI: 0.35–0.40; p < .01) (Table 2). In the instrumental variable analysis, the comparative safety of laparoscopy was attenuated, likely reflecting the ability of this method to account for unmeasured patient characteristics. In this analysis, laparoscopic colectomy was also associated with lower complication rates (27.6 percent vs. 30.5 percent; OR: 0.82, 95 percent CI: 0.79–0.85; p < .01) and mortality (5.9 percent vs. 8.2 percent; OR: 0.75, 95 percent CI: 0.70–0.78; p < .01) in patients considered candidates for either operation. The average treatment effect of laparoscopy decreased complications by 2.9 percent and mortality by 2.3 percent. Sensitivity analyses for patients undergoing colectomy for cancer or benign diagnoses showed similar estimates (Table 2). Our estimates did not change significantly when including hospital characteristics in the models.
Table 2.
Comparison of Outcomes Following Laparoscopic versus Open Colectomy Using Logistic Regression and Instrumental Variable Methods
| Odds of Adverse Outcome Associated with Laparoscopic versus Open Approach (95% CI) | Average Treatment Effect (ATE)a | z‐Statisticb | ||
|---|---|---|---|---|
| Logistic Regression Analysis | Instrumental Variable Analysis | |||
| All operations | ||||
| Complications | 0.55 (0.53–0.56) | 0.82 (0.79–0.85) | −0.17 | −14.8 |
| Mortality | 0.38 (0.35–0.40) | 0.75 (0.70–0.78) | −0.22 | −20.9 |
| Cancer operations | ||||
| Complications | 0.69 (0.67–0.72) | 0.89 (0.86–0.93) | −0.13 | −20.3 |
| Mortality | 0.53 (0.49–0.58) | 0.83 (0.80–0.85) | −0.18 | −18.3 |
| Benign operations | ||||
| Complications | 0.44 (0.43–0.49) | 0.77 (0.75–0.79) | −0.23 | −30.7 |
| Mortality | 0.27 (0.24–0.29) | 0.70 (0.68–0.72) | −0.26 | −36.4 |
Average treatment effect indicates the relative decrease (or increase) in the incidence of complications or mortality ascribed to laparoscopy compared with open surgery.
All above are significant to p < .05. Z‐statistics computed from bootstrapped standard errors.
Average treatment effects are reported for the second‐stage 2SRI model.
Next, we evaluated the relationship between surgeon volume and outcomes for open and laparoscopic operations separately (i.e., the volume groupings are unique for each approach) (Figure 1). For laparoscopy, median volume thresholds for low‐, medium‐, and high‐volume surgeons were 2 (IQR: 1–3), 8 (IQR: 4–12), and 34 (IQR: 25–43), respectively. For open surgery, median volume thresholds for low‐, medium‐, and high‐volume surgeons were 2 (IQR: 1–3), 7 (IQR: 5–9), and 17 (IQR: 13–20), respectively. Increasing surgeon volume was associated with better outcomes for both open and laparoscopic colectomy. However, we observed a stronger volume effect (i.e., more difference between high‐ and low‐volume surgeons) for laparoscopic procedures. For example, the absolute difference in complication rates between high‐ and low‐volume surgeons was 9.6 percent for laparoscopic operations and only 4.9 percent for open operations (p < .01). Surgeons included in the low‐volume laparoscopic group were evenly distributed across low‐ (27 percent), medium‐ (44 percent), and high‐volume (29 percent) categories for open surgery.
Figure 1.
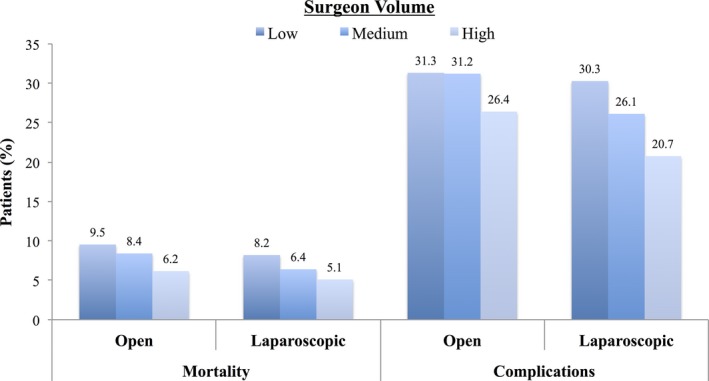
Risk‐Adjusted Rates of Complications and 30‐day Mortality for Patients Following Open and Laparoscopic Operations
- Notes. Outcomes are stratified by surgeons' annual volume for each procedure separately (i.e., outcomes following open operations stratified by surgeon volume for open operations).
We then explored the relationship between surgeon volume and comparative effectiveness for laparoscopic colectomy. We found laparoscopy to be safer than open operations across most surgeon volume categories. High‐volume surgeons had lower complication rates (20.7 percent vs. 28.4 percent; OR: 0.70, 95 percent CI: 0.67–0.74; p < .01) and mortality (5.1 percent vs. 8.5 percent; OR: 0.60, 95 percent CI: 0.55–0.65; p < .01) with laparoscopy (Figure 2A and B). Average treatment effects for laparoscopy were greatest for these surgeons (Table 3). In candidates for either operation, the incidence of complications decreased by 7.7 percent and mortality by 3.4 percent for high‐volume surgeons. Medium volume surgeons also had better outcomes with laparoscopy, though the magnitude of its benefit was lower. However, low‐volume surgeons had higher complication rates (30.3 percent vs. 26.3 percent; OR: 1.18, 95 percent CI: 1.12–1.24; p < .01) and mortality (8.2 percent vs. 7.7 percent; OR: 1.07, 95 percent CI: 1.02–1.13; p < .01) with laparoscopy. Similarly, the average treatment effect of laparoscopy indicated a 4 percent higher incidence of complications and 0.5 percent higher incidence of mortality.
Figure 2.
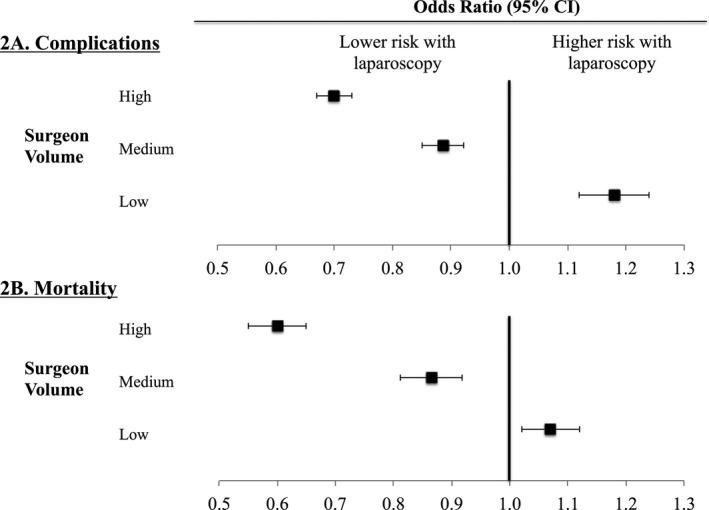
(A and B) Odds Ratios for Each Outcome Stratified by Surgeon's Annual Experience with Laparoscopic Colectomy
- Notes. Odds ratios greater than 1.0 convey higher risk of complications (2A) or mortality (2B) with laparoscopy compared to open operations.
Table 3.
Comparison of Outcomes for Laparoscopic and Open Operations Stratified by Surgeon Volume
| Complications | Mortality | |||
|---|---|---|---|---|
| Average Treatment Effecta | t‐Statisticb | Average Treatment Effecta | z‐Statisticb | |
| Surgeon volume | ||||
| High | −0.28 | −34.6 | −0.034 | −14.3 |
| Medium | −0.08 | −10.2 | −0.019 | −9.6 |
| Low | 0.11 | 6.5 | 0.03 | 3.2 |
Average treatment effect indicates the relative decrease (or increase) in the incidence of complications or mortality ascribed to laparoscopy compared with open surgery.
All above are significant to p < .05. Z‐statistics computed from bootstrapped standard errors.
Average treatment effects are reported for the second‐stage 2SRI model.
Discussion
In this study, we evaluated the comparative effectiveness of laparoscopic and open colectomy in the Medicare population. Because we use a national population inclusive of a heterogeneous group of providers, we were also able to explore how the comparative effectiveness of this intervention is influenced by surgeon volume. We observed that among high‐ and medium‐volume surgeons, laparoscopic colectomy was associated with lower complication and mortality rates when compared to open surgical techniques. However, among low‐volume surgeons, the use of laparoscopy was actually associated with a higher risk of complications and mortality. We also observed a stronger relationship between volume and outcomes for laparoscopic (vs. open) colectomy, the more technically complicated procedure. Within the broader context of comparative effectiveness research, these findings illustrate why provider proficiency should be an important consideration when evaluating the comparative outcomes of different procedures.
Numerous prior studies highlight the advantages of laparoscopic colectomy over traditional open surgery. For example, several well‐designed studies observed 30–70 percent reductions in the incidence of postoperative complications and shorter average hospitalizations by 2 days (Braga et al. 2002, 2010; Weeks et al. 2002; 2004; Veldkamp et al. 2005; Fleshman et al. 2007; Bilimoria et al. 2008; Gervaz et al. 2010; Bagshaw et al. 2012; McKay et al. 2012). Surgeons and other physicians have been critical of outcomes reported in these randomized trials of laparoscopic colectomy, citing a lack of generalizability. Specifically, these trials are often conducted by centers with the highest volume surgeons, which may overestimate the benefits of laparoscopy. Larger population‐based studies, which include both high‐ and low‐volume surgeons, are conducted with administrative data and are prone to selection bias from unmeasured clinical information (Southern, Quan, and Ghali 2004; Stukel et al. 2007; Lawson et al. 2012). In the present study, we specifically address both of these issues. We used national Medicare data to study a diverse group of surgeons and employed instrumental variable methods to address problems with selection bias.
There are also several well‐known studies suggesting a correlation between higher surgeon volumes and better outcomes for laparoscopic colectomy (Birkmeyer et al. 2002; Fox et al. 2012). When looking at individual surgeons' volume, studies vary in their estimation of the “learning curve” for proficiency in laparoscopy from 10 to 50 colectomies (Tekkis et al. 2005; Maeda et al. 2010; Waters et al. 2010). This prior work on the volume‐outcome effect addresses a different question than ours, asking whether outcomes are different between providers with varying levels of experience. In contrast, we evaluated whether surgeon volume influences the relative outcomes of two different approaches to colectomy, laparoscopy versus the traditional open procedure. This study brings together two areas of inquiry that are often only considered in isolation—variations in provider proficiency and comparative effectiveness. Provider proficiency (i.e., how well a procedure is performed) is often assumed to be constant in comparative effectiveness studies. However, as discussed above, we found that the relative safety of laparoscopic versus open colectomy is entirely dependent on who is performing the operation.
There are several limitations to this study. Because we use Medicare data for this analysis, our results may not be generalizable to all patients. However, colon cancer is more common in elderly populations, and we would not expect the comparative safety or effectiveness of procedures to differ significantly in an aged population. Furthermore, the use of administrative data for observational studies is limited by unreliable coding of comorbidities and complications. We have addressed this in several ways. First, we used established methods for determining the presence of comorbid conditions and incidence of postoperative complications with administrative data (Iezzoni et al. 1994; Elixhauser et al. 1998). Selection bias is also a limitation of studies using administrative data. However, a successful instrumental variable analysis (as explained in our methods) balances patient‐level covariates—both measured and unmeasured. Some may also be concerned that our instrumental variable is a surrogate for hospital or surgeon quality. For example, patients living in areas where more laparoscopic procedures are performed may receive care in better, more technologically advanced hospitals. We have addressed this by showing that the instrument itself is not considerably associated with postoperative outcomes. Others have shown that controlling for provider characteristics is important in comparative effectiveness studies that employ instrumental variables (Garabedian et al. 2014). However, these studies do not focus on procedural interventions where separating provider characteristics from the intervention itself may be problematic. It is possible that we have not addressed issues of surgeon quality and technical skill. Data on surgeon training and practice experience are not available for this national sample. Furthermore, measures of this kind are likely related to procedural volume. For example, a surgeon with special training in colorectal surgery does more colon resections than a general surgeon with a more diverse practice. However, we have addressed possible confounding from overall surgeon skill within a region by incorporating HRR dummy variables as fixed effects in our IV regression models. Our evaluation of procedural volume for surgeons also does not account for the possibility that surgeons' clinical practices include other laparoscopic cases. However, this information would generally bias our results towards the null hypothesis that provider volume does not influence the relative effectiveness of laparoscopic versus open colectomy. Finally, our volume calculations likely underestimate how many colectomies surgeons perform annually as we studied only Medicare patients. As a result, these thresholds should not be used for establishing minimum safety standards for laparoscopic colectomy.
This study has several important practical implications. For patients seeking laparoscopic colon resection, it is safer to have a higher volume surgeon. It is also important for patients to consider whether their surgeon's experience aligns with the treatment he or she is recommending. For surgeons, they must carefully examine their experience with new, more technically complex procedures such as laparoscopic colectomy, before incorporating them into their practice. Presently, surgeons may rely on didactics and short weekend “hands‐on” courses taught with cadavers or in animal laboratories. These techniques are often then applied to practice without oversight or proctoring (Davis et al. 1999; Committee 2009). Finally, for hospital leaders, these results should be considered within the context of surgeon credentialing. New procedures and techniques may be invisible to hospital credentialing committees because they fall under a broad category of procedures for which a surgeon already has clinical privileges (Dent 1992). Our study demonstrates that advanced laparoscopic approaches to existing operations require different skill sets. Many hospitals already require minimal volume standards for bariatric surgery, but no such standards exist for other procedures (Committee 2009).
This study also has broader implications for comparative effectiveness research for surgery and other procedures. First, our results underscore how unmeasured confounding can cause us to overestimate the benefits of a new procedure. We observed an attenuation of the benefits of laparoscopy in our instrumental variable analysis, highlighting this important design feature in comparative effectiveness studies prone to selection bias. Second, it is important to consider the proficiency of the provider when assessing the comparative outcomes of procedures. Unlike medical treatments (e.g., pharmaceuticals) where the intervention is generally standardized across providers, the relative benefits of surgical interventions are inherently linked to the proficiency of the surgeon. We have shown that a heterogeneous group of providers will appreciate varying degrees of benefit from a new, presumably better, operative technique. In other words, the comparative effectiveness of a specific therapy cannot be divorced entirely from considerations of who is performing the intervention.
Supporting information
Appendix SA1: Author Matrix.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The authors acknowledge the University of Michigan and the Center for Healthcare Outcomes and Policy for providing working space and infrastructure to facilitate the completion of this work.
Disclosures: None.
Disclaimers: None.
Notes
45.73, 17.33, 17.32, 45.75, 45.76, 17.35, 17.36, 45.74, 17.34, 45.82, 45.83, 45.81, 48.50, 48.51, 48.52, 48.53.
Pulmonary failure (518.81, 518.4, 518.5, 518.8), pneumonia (481, 482.0–482.9, 483, 484, 485, 507.0), myocardial infarction (410.00–410.91), deep venous thrombosis/pulmonary embolism (415.1, 451.11, 451.19, 451.2, 451.81, 453.8), renal failure (584), surgical site infection (958.3, 998.3, 998.5, 998.59, 998.51), gastrointestinal bleeding (530.82, 531.00–531.21, 531.40, 531.41, 531.60, 531.61, 532.00–532.21, 532.40, 532.41, 532.60, 532.61, 533.00–533.21, 533.40, 533.41, 533.60, 533.61, 534.00–534.21, 534.40, 534.41, 534.60, 534.61, 535.01, 535.11, 535.21, 535.31, 535.41, 535.51, 535.61, 578.9), and hemorrhage (998.1).
References
- American Society of Colon and Rectal Surgeons [ASCRS] ; Gastrointestinal and Endoscopic Surgeons [SAGES] , Fleshman J., Marcello P., Stamos M. J., and Wexner S. D.. 2006. “Focus Group on Laparoscopic Colectomy Education as Endorsed by the American Society of Colon and Rectal Surgeons (ASCRS) and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES): Guidelines for Laparoscopic Colectomy Course.” Surgical Endoscopy 20 (7): 1162–7. [DOI] [PubMed] [Google Scholar]
- Bagshaw, P. F. , Allardyce R. A., Frampton C. M., Frizelle F. A., Hewett P. J., McMurrick P. J., Rieger N. A., Smith J. S., Solomon M. J., and Stevenson A. R.. 2012. “Long‐Term Outcomes of the Australasian Randomized Clinical Trial Comparing Laparoscopic and Conventional Open Surgical Treatments for Colon Cancer: The Australasian Laparoscopic Colon Cancer Study Trial.” Annals of Surgery 256 (6): 915–9. [DOI] [PubMed] [Google Scholar]
- Bilimoria, K. Y. , Bentrem D. J., Merkow R. P., Nelson H., Wang E., Ko C. Y., and Soper N. J.. 2008. “Laparoscopic‐Assisted vs. Open Colectomy for Cancer: Comparison of Short‐Term Outcomes from 121 Hospitals.” Journal of Gastrointestinal Surgery 12 (11): 2001–9. [DOI] [PubMed] [Google Scholar]
- Birkmeyer, J. D. , Siewers A. E., Finlayson E. V., Stukel T. A., Lucas F. L., Batista I., Welch H. G., and Wennberg D. E.. 2002. “Hospital Volume and Surgical Mortality in the United States.” New England Journal of Medicine 346 (15): 1128–37. [DOI] [PubMed] [Google Scholar]
- Braga, M. , Vignali A., Gianotti L., Zuliani W., Radaelli G., Gruarin P., Dellabona P., and Di Carlo V.. 2002. “Laparoscopic versus Open Colorectal Surgery: A Randomized Trial on Short‐Term Outcome.” Annals of Surgery 236 (6): 759–66; disscussion 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga, M. , Frasson M., Zuliani W., Vignali A., Pecorelli N., and Di Carlo V.. 2010. “Randomized Clinical Trial of Laparoscopic versus Open Left Colonic Resection.” British Journal of Surgery 97 (8): 1180–6. [DOI] [PubMed] [Google Scholar]
- Clinical Outcomes of Surgical Therapy Study Group . 2004. “A Comparison of Laparoscopically Assisted and Open Colectomy for Colon Cancer.” New England Journal of Medicine 350 (20): 2050–9. [DOI] [PubMed] [Google Scholar]
- Committee, S. G. 2009. “SAGES Guideline for Clinical Application of Laparoscopic Bariatric Surgery.” Surgery for Obesity and Related Disorders 5 (3): 387–405. [DOI] [PubMed] [Google Scholar]
- Davis, D. , O'Brien M. A., Freemantle N., Wolf F. M., Mazmanian P., and Taylor‐Vaisey A.. 1999. “Impact of Formal Continuing Medical Education: Do Conferences, Workshops, Rounds, and Other Traditional Continuing Education Activities Change Physician Behavior or Health Care Outcomes?” Journal of the American Medical Association 282 (9): 867–74. [DOI] [PubMed] [Google Scholar]
- Dent, T. L. 1992. “Training, Credentialing, and Evaluation in Laparoscopic Surgery.” Surgical Clinics of North America 72 (5): 1003–11. [DOI] [PubMed] [Google Scholar]
- Elixhauser, A. , Steiner C., Harris D. R., and Coffey R. M.. 1998. “Comorbidity Measures for Use with Administrative Data.” Medical Care 36 (1): 8–27. [DOI] [PubMed] [Google Scholar]
- Finks, J. F. , Osborne N. H., and Birkmeyer J. D.. 2011. “Trends in Hospital Volume and Operative Mortality for High‐Risk Surgery.” New England Journal of Medicine 364 (22): 2128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman, J. , Sargent D. J., Green E., Anvari M., Stryker S. J., Beart R. W. Jr., Hellinger M., Flanagan R. Jr., Peters W., and Nelson H.. 2007. “Laparoscopic Colectomy for Cancer is Not Inferior to Open Surgery Based on 5‐Year Data from the COST Study Group Trial.” Annals of Surgery 246 (4): 655–62; discussion 62–4. [DOI] [PubMed] [Google Scholar]
- Fox, J. P. , Desai M. M., Krumholz H. M., and Gross C. P.. 2012. “Hospital‐Level Outcomes Associated with Laparoscopic Colectomy for Cancer in the Minimally Invasive Era.” Journal of Gastrointestinal Surgery 16 (11): 2112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian, L. F. , Chu P., Toh S., Zaslavsky A. M., and Soumerai S. B.. 2014. “Potential Bias of Instrumental Variable Analyses for Observational Comparative Effectiveness Research.” Annals of Internal Medicine 161 (2): 131–8. [DOI] [PubMed] [Google Scholar]
- Gervaz, P. , Inan I., Perneger T., Schiffer E., and Morel P.. 2010. “A Prospective, Randomized, Single‐Blind Comparison of Laparoscopic versus Open Sigmoid Colectomy for Diverticulitis.” Annals of Surgery 252 (1): 3–8. [DOI] [PubMed] [Google Scholar]
- Ghislandi, S. , Torbica A., and Boriani G.. 2013. “Assessing the Outcomes of Implantable Cardioverter Defibrillator Treatment in a Real World Setting: Results from Hospital Record Data.” BMC Health Services Research 13: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, V. P. , Trencheva K., Stein S. L., and Milsom J. W.. 2012. “Mentorship for Participants in a Laparoscopic Colectomy Course.” Surgical Endoscopy 26 (3): 722–6. [DOI] [PubMed] [Google Scholar]
- Iezzoni, L. I. , Daley J., Heeren T., Foley S. M., Fisher E. S., Duncan C., Hughes J. S., and Coffman G. A.. 1994. “Identifying Complications of Care Using Administrative Data.” Medical Care 32 (7): 700–15. [DOI] [PubMed] [Google Scholar]
- Kemp, J. A. , and Finlayson S. R.. 2008. “Nationwide Trends in Laparoscopic Colectomy from 2000 to 2004.” Surgical Endoscopy 22 (5): 1181–7. [DOI] [PubMed] [Google Scholar]
- Kiran, R. P. , El‐Gazzaz G. H., Vogel J. D., and Remzi F. H.. 2010a. “Laparoscopic Approach Significantly Reduces Surgical Site Infections after Colorectal Surgery: Data from National Surgical Quality Improvement Program.” Journal of the American College of Surgeons 211 (2): 232–8. [DOI] [PubMed] [Google Scholar]
- Kiran, R. P. , Kirat H. T., Ozturk E., Geisler D. P., and Remzi F. H.. 2010b. “Does the Learning Curve during Laparoscopic Colectomy Adversely Affect Costs?” Surgical Endoscopy 24 (11): 2718–22. [DOI] [PubMed] [Google Scholar]
- Lawson, E. H. , Louie R., Zingmond D. S., Brook R. H., Hall B. L., Han L., Rapp M., and Ko C. Y.. 2012. “A Comparison of Clinical Registry versus Administrative Claims Data for Reporting of 30‐Day Surgical Complications.” Annals of Surgery 256 (6): 973–81. [DOI] [PubMed] [Google Scholar]
- Maeda, T. , Tan K. Y., Konishi F., Tsujinaka S., Mizokami K., Sasaki J., and Kawamura Y. J.. 2010. “Accelerated Learning Curve for Colorectal Resection, Open versus Laparoscopic Approach, Can Be Attained with Expert Supervision.” Surgical Endoscopy 24 (11): 2850–4. [DOI] [PubMed] [Google Scholar]
- McKay, G. D. , Morgan M. J., Wong S. K., Gatenby A. H., Fulham S. B., Ahmed K. W., Toh J. W., Hanna M., and Hitos K.. 2012. “Improved Short‐Term Outcomes of Laparoscopic versus Open Resection for Colon and Rectal Cancer in an Area Health Service: A Multicenter Study.” Diseases of the Colon and Rectum 55 (1): 42–50. [DOI] [PubMed] [Google Scholar]
- Miller, M. E. , Welch W. P., and Welch H. G.. 1996. “The Impact of Practicing in Multiple Hospitals on Physician Profiles.” Medical Care 34 (5): 455–62. [DOI] [PubMed] [Google Scholar]
- Newhouse, J. P. , and McClellan M.. 1998. “Econometrics in Outcomes Research: The Use of Instrumental Variables.” Annual Review of Public Health 19: 17–34. [DOI] [PubMed] [Google Scholar]
- Rea, J. D. , Cone M. M., Diggs B. S., Deveney K. E., Lu K. C., and Herzig D. O.. 2011. “Utilization of Laparoscopic Colectomy in the United States before and after the Clinical Outcomes of Surgical Therapy Study Group Trial.” Annals of Surgery 254 (2): 281–8. [DOI] [PubMed] [Google Scholar]
- Southern, D. A. , Quan H., and Ghali W. A.. 2004. “Comparison of the Elixhauser and Charlson/Deyo Methods of Comorbidity Measurement in Administrative Data.” Medical Care 42 (4): 355–60. [DOI] [PubMed] [Google Scholar]
- Stukel, T. A. , Fisher E. S., Wennberg D. E., Alter D. A., Gottlieb D. J., and Vermeulen M. J.. 2007. “Analysis of Observational Studies in the Presence of Treatment Selection Bias: Effects of Invasive Cardiac Management on AMI Survival Using Propensity Score and Instrumental Variable Methods.” Journal of the American Medical Association 297 (3): 278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, H. J. , Norton E. C., Ye Z., Hafez K. S., Gore J. L., and Miller D. C.. 2012. “Long‐Term Survival Following Partial vs Radical Nephrectomy among Older Patients with Early‐Stage Kidney Cancer.” Journal of the American Medical Association 307 (15): 1629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekkis, P. P. , Senagore A. J., Delaney C. P., and Fazio V. W.. 2005. “Evaluation of the Learning Curve in Laparoscopic Colorectal Surgery: Comparison of Right‐Sided and Left‐Sided Resections.” Annals of Surgery 242 (1): 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terza, J. V. , Basu A., and Rathouz P. J.. 2008. “Two‐Stage Residual Inclusion Estimation: Addressing Endogeneity in Health Econometric Modeling.” Journal of Health Economics 27 (3): 531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terza, J. V. , Bradford W. D., and Dismuke C. E.. 2008. “The Use of Linear Instrumental Variables Methods in Health Services Research and Health Economics: A Cautionary Note.” Health Services Research 43 (3): 1102–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler, K. , Dinnewitzer A., Mascha E., Arrigain S., Weiss E. G., Nogueras J. J., and Wexner S. D.. 2003. “Long‐Term Outcome and Health‐Related Quality of Life after Laparoscopic and Open Colectomy for Benign Disease.” Surgical Endoscopy 17 (9): 1404–8. [DOI] [PubMed] [Google Scholar]
- Veldkamp, R. , Kuhry E., Hop W. C., Jeekel J., Kazemier G., Bonjer H. J., Haglind E., Pahlman L., Cuesta M. A., Msika S., Morino M., and Lacy A. M.. 2005. “Laparoscopic Surgery versus Open Surgery for Colon Cancer: Short‐Term Outcomes of a Randomised Trial.” Lancet Oncology 6 (7): 477–84. [DOI] [PubMed] [Google Scholar]
- Waters, J. A. , Chihara R., Moreno J., Robb B. W., Wiebke E. A., and George V. V.. 2010. “Laparoscopic Colectomy: Does the Learning Curve Extend Beyond Colorectal Surgery Fellowship?” Journal of the Society of Laparoendoscopic Surgeons 14 (3): 325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks, J. C. , Nelson H., Gelber S., Sargent D., and Schroeder G.. 2002. “Short‐Term Quality‐of‐Life Outcomes Following Laparoscopic‐Assisted Colectomy vs Open Colectomy for Colon Cancer: A Randomized Trial.” Journal of the American Medical Association 287 (3): 321–8. [DOI] [PubMed] [Google Scholar]
- Xian, Y. , Holloway R. G., Chan P. S., Noyes K., Shah M. N., Ting H. H., Chappel A. R., Peterson E. D., and Friedman B.. 2011. “Association between Stroke Center Hospitalization for Acute Ischemic Stroke and Mortality.” Journal of the American Medical Association 305 (4): 373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


