Abstract
Rs1344706 in the the zinc finger protein 804A (ZNF804A) gene has been identified to be associated with schizophrenia and bipolar disorder (BD) in Europeans. However, whether rs1344706 is associated with schizophrenia in Chinese populations remains inconclusive; furthermore, the association between rs1344706 and BD in Chinese populations has been rarely explored. To explore the association between rs1344706 and schizophrenia/BD in Chinese populations, we genotyped rs1344706 among 1128 Chinese subjects (537 patients with BD and 591 controls) and found that rs1344706 showed marginal allelic association with BD (P = 0.028) with T-allele being more prevalent in cases than that in controls (OR = 1.19, 95% CI 1.03–1.37). Meta-analysis of rs1344706 by pooling all available data showed that rs1344706 was significantly associated with BD (P = 0.001). Besides, positive association of rs1344706 with schizophrenia was observed in Northern Chinese (P = 0.005). Furthermore, ZNF804A is highly expressed in human and mouse brains, especially in prenatal stage.
Schizophrenia and bipolar disorder (BD) are two severe neuropsychiatric disorders, with each affecting approximately 1% populations worldwide1. Evidence from family, twin and adoption studies highlights the pivotal role of genetic components in the two disorders, with a heritability estimated at 80–85% for schizophrenia2 and 85–89% for BD3.
Though schizophrenia and BD have been defined as separate mental disorders over the past 100 years, both disorders share some clinical symptoms with each other, i.e., psychosis in BD and affective disruption in schizophrenia as observed in clinical practice4, suggesting shared pathophysiological mechanisms between schizophrenia and BD. Corresponding to this observation, increasing number of studies have revealed an overlap of risk variants between schizophrenia and BD5.
Till now, several dozens of risk single nucleotide polymorphisms (SNP) and genomic regions which showed significant association with both schizophrenia and BD have been identified through candidate gene association study (rs1006737 in CACNA1C and rs10994336 in ANK3)6,7, and genome-wide association studies (GWAS) (rs1109803 in NDST3)8.
Another promising common risk variant for schizophrenia and BD is rs1344706 in the zinc finger protein 804A (ZNF804A) gene, which was first identified by a GWAS in samples of Caucasian ancestry9. Follow-up studies conducted in different European populations have replicated this finding, suggestive that rs1344706 is probably an authentic risk SNP for schizophrenia and BD in Europeans10,11. In Chinese populations, several groups have reported the association between rs1344706 and schizophrenia, subjects from which were all from northern China (Shanxi, Xinxiang and Shandong)12,13,14. However, more studies with subjects recruited from central and Southern China (i.e., Jiangsu, Yunnan, Sichuan and Guangxi province) failed to replicate this association15,16,17,18. Whether rs1344706 in the ZNF804A gene is associated with schizophrenia therefore remains inconclusive. To address this issue, meta-analyses were performed in Chinese populations and Asian populations by multiple groups; however, the results from different groups remain still elusive, due to limited sample size and different inclusion criteria of eligible studies19,20. Moreover, in contrast to the extensive exploration of the association between rs1344706 and schizophrenia in Chinese populations, whether rs1344706 confers risk to BD in Chinese remains largely unknown, although nominal association between rs1344706 and BD has been observed recently by Zhang et al. in Shanghai (P = 0.034, OR = 1.19), with a relatively small sample (746 patients of BD and 762 controls)21.
In this study, we first explored the association between rs1344706 and BD in our Chinese sample consisting of 537 cases and 591 controls, and then conducted a meta-analysis to test the association of rs1344706 with either schizophrenia (11573 cases and 15321 controls, as well as 101 schizophrenia trios) or BD (1274 cases and 1343 controls) in Chinese populations with the largest sample size available to date. Moreover, temporal and spatial expression patterns of ZNF804A in different human brain regions were investigated using available online database.
Results
Rs 1344706 T-allele was marginally associated with BD in our samples
The genotypic distribution of rs1344706 confirmed to HWE in both control (χ2 = 0.29, P = 0.59) and case (χ2 = 2.41, P = 0.12) groups. As shown in Table 1, rs1344706 showed marginal allelic association with BD in our samples (χ2 = 4.85, P = 0.028), with T-allele more prevalent in patients of BD than that in healthy subjects (OR = 1.21, 95% CI 1.02–1.43), which survived after adjusting for age and gender (P = 0.047). Moreover, there was no significant difference in genotype frequencies of rs1344706 between the case and control group (χ2 = 5.62, P = 0.060). We further analyzed the association of rs1344706 with BD by gender, which revealed significant allelic association between rs1344706 T-allele and BD in males (χ2 = 4.48, P = 0.034, OR = 1.33, 95% CI 1.02–1.74) but not in females (P > 0.05). The association of rs1344706 with disease characteristics, i.e., age of onset, depressive or manic episodes, were summarized in Table S1.
Table 1. Allelic and genotypic association of rs1344706 with BD in our samples.
| Group | Sample (N) | Allele frequency (%) | P-value | OR (95% CI) | Genotype frequency (%) | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | T | G | TT | TG | GG | |||||
| BD | 528 | 581 (55.0) | 475 (45.0) | 0.028 | 1.21 (1.02–1.43) | 151 (28.6) | 279 (52.8) | 98 (18.6) | 0.060 | |
| Control | 581 | 585 (50.3) | 577 (49.7) | 144 (24.8) | 297 (51.1) | 140 (24.1) | ||||
| Male | ||||||||||
| BD | 210 | 239 (56.9) | 181 (43.1) | 0.034 | 1.33 (1.02–1.74) | 66 (31.4) | 107 (51.0) | 37 (17.6) | 0.091 | |
| Control | 231 | 230 (49.8) | 232 (50.2) | 58 (25.1) | 114 (49.4) | 59 (25.5) | ||||
| Female | ||||||||||
| BD | 318 | 344 (54.1) | 292 (45.9) | 0.218 | 1.15 (0.92–1.42) | 87 (27.3) | 170 (53.5) | 61 (19.2) | 0.412 | |
| Control | 350 | 355 (50.7) | 345 (49.3) | 86 (24.6) | 183 (52.3) | 81 (23.1) | ||||
The present sample size (537 BD cases and 591 controls) revealed approximately 32.6% power of detecting a significant association for rs1344706 with the following assumptions: P = 0.05, OR = 1.20 corresponding to a “weak to moderate” effect, and a given MAF of 0.453 according to the 1000 Genomes project (www.1000genomes.org/) (the same bellow).
Meta-analysis of the association of rs1344706 with BD and schizophrenia
To obtain a more comprehensive interpretation of the association of rs1344706 with psychiatric disorders, mainly BD and schizophrenia, we performed a meta-analysis by pooling our data and those from published studies conducted in Chinese populations. Following our literature search strategy, a total of 11 studies involving 15 independent samples which encompass 11573 cases and 15321 controls, as well as 101 schizophrenia trios, were identified for schizophrenia9,12,13,14,15,16,17,18,22,23,24, and 2 studies (including the present study) consisting of 1274 patients of BD and 1343 healthy controls was identified for BD21. Detailed information concerning the sample size and association results for each study was listed in Table 2. As shown in Fig. S1, there was no evidence implying publication bias checked by the Egger regression test (P = 0.596) and the Begg–Mazumdar test (P = 0.235).
Table 2. Characteristics of included studies of rs1344706 with schizophrenia in Chinese populations.
| Author, year | Diagnostic method | Region | Case |
control |
P-value | OR | 95(%) CI | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample (N)1 | Male (%) | Age (mean, SD) | Sample (N) | Male (%) | Age (mean, SD) | ||||||
| Schizophrenia | |||||||||||
| O’Donovan9 | DSM-IV | Shanghai | 1034 | 55.1 | 38.8 (14.1) | 1034 | 50.5 | 30.0 (8.7) | 0.166 | 1.06 | 0.94–1.20 |
| Zhang12 | DSM-IV | Shanxi | 566 | 52.1 | 34.0 (12.8) | 574 | 57.3 | 29.1 (13.8) | 8.30E-04 | 1.32 | 1.12–1.56 |
| DSM-IV | Shanxi | 101 schizophrenia trios | 0.058 | 1.20 | 0.91–1.57 | ||||||
| Li15 | ICD-10 | Yuxi | 488 | 53.1 | 38.5 (10.4) | 694 | 53.5 | 37.1 (6.8) | 0.876 | 1.01 | 0.86–1.19 |
| DSM-IV | Kunming | 403 | 44.4 | 36.3 (8.7) | 604 | 44.4 | 36.6 (7.0) | 0.489 | 0.94 | 0.79–1.12 | |
| Yue22 | DSM-IV | Beijing | 746 | 53.1 | 34.5 (8.7) | 1599 | 52.9 | 35.8 (7.8) | NA | 1.11 | 0.96–1.29 |
| Shi23 | DSM-IV | Shanghai and Anhui | 1238 | 55.9 | 36.2 (12.4) | 2856 | 35.5 | 60.9 (12.2) | 0.250 | 1.06 | 0.96–1.16 |
| DSM-IV | Beijing and Shandong | 1578 | 69.8 | 36.9 (9.3) | 1592 | 50.3 | 30.8 (11.1) | 0.100 | 0.92 | 0.83–1.02 | |
| DSM-IV | Guangdong and Guangxi | 934 | 58.4 | 36.3 (16.6) | 2020 | 47.7 | 56.1 (13.5) | 0.420 | 0.95 | 0.85–1.07 | |
| Wei40 | DSM-IV | Guangdong | 100 | 53.0 | 26.5 (6.9) | 69 | 56.5 | 25.4 (5.7) | 0.064 | 0.66 | 0.43–1.02 |
| Chen14 | ICD-10 | Shandong | 570 | 61.5 | 28.2 (7.8) | 448 | 65.1 | 23.0 (7.0) | 0.013 | 1.25 | 1.05–1.49 |
| Liou24 | DSM-IV | Taiwan | 522 | 55.4 | 44.1 (9.1) | 806 | 47.5 | 67.6 (9.4) | 0.570 | 1.05 | 0.89–1.22 |
| Yang13 | DSM-IV | Northern China | 1024 | 51.4 | 27.3 (8.0) | 975 | 49.0 | 27.7 (8.0) | 0.087 | 1.12 | 0.98–1.27 |
| Wong16 | DSM-IV | Sichuan | 1086 | NA | NA | 1060 | NA | NA | NA | 0.91 | 0.81–1.02 |
| Wang, 2016 | DSM-IV | Jiangsu | 1284 | 63.0 | 45.8 (11.5) | 990 | 55.4 | 44.9 (10.1) | 0.497 | 0.96 | 0.85–1.08 |
| Bipolar disorder | |||||||||||
| Zhang21 | DSM-IV | Shanghai | 746 | NA | NA | 762 | NA | NA | 0.034 | 1.19 | 1.03–1.37 |
| Current study | DSM-IV | Shanghai | 528 | 39.7 | 42.2 ± 10.4 | 581 | 40.3 | 31.9 ± 11.3 | 0.028 | 1.21 | 1.02–1.43 |
Note: the studies in bold were GWASs. NA, not available; OR, odds ratio; 95% CI, 95% confidence interval.
1The N represents the number of individuals having genotyping data for rs1344706.
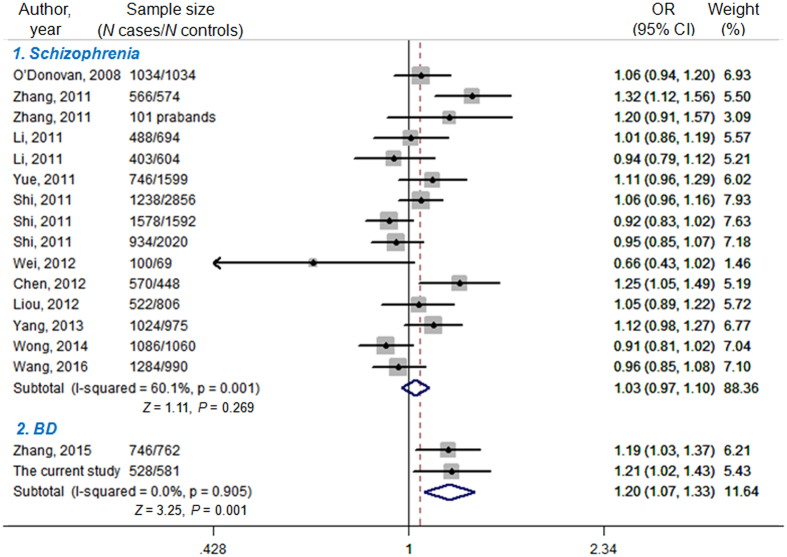
Before the pooling procedure, statistical power was assessed given the P of 0.05, OR of 1.20 and MAF of 0.453. For schizophrenia, the pooled sample size revealed 100% power to detect a significant association of rs1344706; for BD, the pooled sample size could provide 64.2% power to detect a significant association. Meta-analysis showed that there was significant association between rs1344706 T-allele and BD (P = 0.001, OR = 1.20, 95% CI 1.07–1.33) by the fixed-effect model, since no significant evidence of between-study heterogeneity was observed (I2 = 0.0%, P = 0.905). However, we observed no significant difference in T-allele distribution of rs1344706 between schizophrenia and controls (P = 0.269, OR = 1.03, 95% CI 0.97–1.10) using the random-effect model due to significant evidence of between-study heterogeneity (I2 = 60.1%, P = 0.001) (Fig. 1).
Figure 1. Meta-analysis for rs1344706 T-allele of the ZNF804A gene in schizophrenia and BD.
The sources of the published data were listed in Table 2. The random-effect model was applied to pool the data for association rs1344706 and schizophrenia, while the fixed-effect model for BD.
After careful examination of the regional distributions of recruited populations, we found that most of them resided in Northern China, followed by Southern and Central China. Moreover, the MAF of rs1344706 in controls was slightly different among Northern (0.489)13, Central (0.41)9 and Southern (0.491)15 Chinese populations, which might cause between-study heterogeneity. Therefore, we conducted meta-analysis using studies from northern, central, and southern China respectively. As shown in Fig. S2, significant association between rs1344706 T-allele and schizophrenia (P = 0.005, OR = 1.09, 95% CI 1.03–1.15) was observed in Northern Chinese populations by the random-effect model, but not in Central (P = 0.357) or Southern (P = 0.132) Chinese populations by the fixed-effect model.
Rs1344706 showed dramatic allelic difference in global populations
Recent studies suggest that genetic variants conferring risk of psychiatric disorders show significant frequency differences among worldwide populations, such as rs2709373 in CREB125 and rs6001946 in MKL126. Therefore, we detailed the global allele frequency distributions of rs1344706 in 53 world populations using the HGDP database27 and 1000 Genomes project28. Intriguingly, the derived allele (C-allele) of rs1344706 showed dramatic frequency differences among world populations, with the highest frequencies in most Asian populations, followed then by Middle East and Europe. At the extremes, we found that rs1344706 is nearly fixed for ancestral allele (A-allele) in most African populations (Fig. S3).
Spatiotemporal expression patterns of ZNF804A in different human brain regions
To further test whether ZNF804A contributes risk to BD, we explored the expression profiling of ZNF804A in diverse human brain tissues using the BRAINEAC data29. We found that ZNF804A is expressed in various brain regions, with the highest transcript level in cerebellar cortex, followed by frontal cortex, occipital cortex and temporal cortex (Fig. 2A). Using the expression data from BioGPS30, we confirmed that ZNF804A is mainly expressed in brain tissues, with limited or no expression in peripheral tissues (Fig. S4). Besides, we examined the temporal expression pattern of ZNF804A in developing and adult DPFC of normal subjects using the BrainCloud31. The expression of ZNF804A is relatively high in prenatal period, especially the late developmental stage (>20 weeks) (Fig. 2B). After birth, ZNF804A expression level is decreased, suggesting that ZNF804A plays a crucial role in brain development and dysregulation of ZNF804A may then confer risk to BD.
Figure 2. Spatiotemporal expression pattern of the ZNF804A gene in human brain regions.
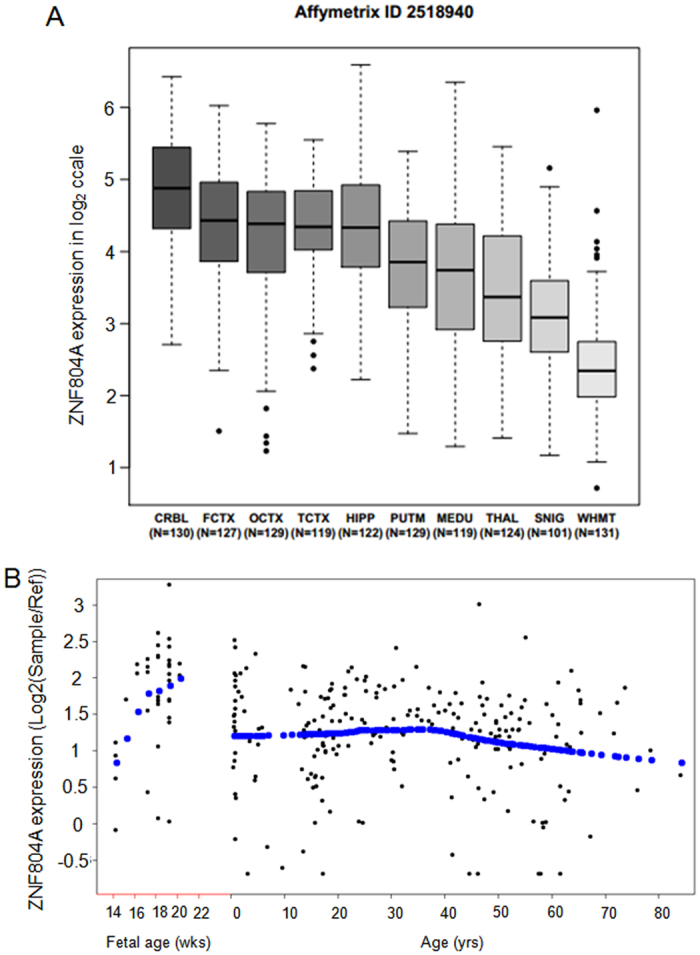
(A) ZNF804A is expressed in various brain regions, with the highest transcript level in cerebellar cortex (from the BRAINEAC). CRBL, cerebellar cortex; FCTX, frontal cortex; OCTX, occipital cortex; TCTX, temporal cortex; HIPP, hippocampus; PUTM, putamen (at the level of the anterior commissure); THAL, thalamus (at the level of the lateral geniculate nucleus); MEDU, inferior olivary nucleus (sub-dissected from the medulla); SNIG, substantia nigra; WHMT: intralobular white matter. (B) Temporal expression profiling of ZNF804A in the human PDFC of normal subjects across lifespan (from the BrainCloud).
Discussion
Recently, rs1344706 in the ZNF804A gene was implicated as one of the most compelling genetic loci that contribute to the susceptibility of both schizophrenia and BD in European populations, supported by the GWAS and follow-up replications. In the present study, we detected significant association between rs1344706 T-allele and BD in Chinese samples both in our case-control cohorts and subsequent meta-analysis. It should be noted that, as the sample size increased, this association between rs1344706 and BD was strengthened as indicated by sharply reduced P-value (seen in the meta-analysis), suggesting that rs1344706 may serve as an authentic risk SNP for BD in Chinese populations. Moreover, stratified analysis by gender showed that rs1344706 was associated with male BD (P = 0.034) but not with female BD (P = 0.218) in our samples, which was consistent with previous studies32. However, this association observed for rs1344706 with BD should be treated scrupulously, since the sample size used in the present study was underpowered which might inflate potential false positive associations and even drew an opposite conclusion. We noticed that our in-house samples (537 BD cases and 591 controls) revealed approximately 32.6% power of detecting a significant association for rs1344706, and even by combining all available data, the sample size (1274 patients with BD and 1343 controls) could only provide 64.2% power to detect a significant association.
Intriguingly, the allelic frequency of rs1344706 showed dramatic difference among worldwide populations, suggestive its implication in psychiatric disorders. From the aspect of evolution, the prevalence of specific disorders should decrease with time, however, this is not the case for psychiatric disorders which maintain a stable or more prevalence across all human cultures. One potential explanation for this paradox suggests positive selection as the driving force in maintaining risk loci of psychiatric disorders in the gene pool. In line with the hypothesis, genetic variants conferring risk of psychiatric disorders might show significant frequency differences among worldwide populations, and several risk loci of psychiatric disorders were thus identified, including rs2709373 in CREB125 and rs6001946 in MKL126.
In addition, our meta-analyses indicated significant association of rs1344706 with schizophrenia in Northern Chinese populations (P = 0.005, OR = 1.09, 95% CI 1.03–1.15), i.e., Shanxi, Xinxiang and Shandong12,13,14, which was inconsistent with most studies conducted in either Central or Southern Chinese populations, including Jiangsu, Yunnan, Sichuan and Guangxi province15,16,17,18. This inconsistence may originate from the following several reasons. First, divergent genetic backgrounds in different regions may result in differentiation in allele frequencies and linkage disequilibrium (LD) patterns, which can then lead to inconsistent associations. As reported in previous studies, the MAF of rs1344706 in controls was slightly different among Northern (0.489)13, Central (0.41)9 and Southern (0.491)15 Chinese populations. Second, differentiated environmental exposure, dietary and cultures might also contribute to inconsistent associations among Northern, Central and Southern Chinese, since incidence of schizophrenia can be greatly influenced by risk factors other than genetic variants. Given the relatively large sample size used, we thought that rs1344706 was a risk variant for schizophrenia in Northern Chinese populations, but not in Central or Southern Chinese populations.
ZNF804A, consisting of four exons and three introns (rs1344706 in intron 2), is located on chromosome 2q32.1 and potentially encodes a transcription factor. It has been shown that T-allele of rs1344706 can weaken the binding affinity of unidentified nuclear protein with ZNF804A in neural cells, indicating that rs1344706 may have a direct effect on ZNF804A expression and transcriptional regulation of its target genes33. Supporting it, Zhang et al. demonstrated that rs1344706 T-allele was significantly associated with elevated expression level in both the occipital cortex and hippocampus via the BRAINEAC database21. Besides, we noticed that ZNF804A has peak expression level during fetal developmental stage, suggesting that rs1344706 may confer susceptibility for BD by influencing early brain development. Finally, we found that ZNF804A was mainly expressed in cortical regions, followed by hypothalamus. In previous studies, abnormal cortical structures and functions have been extensively reported in BD patients. For example, BD patients exhibited significantly decreased thickness and increased activation in occipital cortex compared with healthy subjects, as demonstrated by MRI34.
However, there are several limitations to our interpretation of this study. First, the sample size in our initial case-control cohort was relatively small (528 patients of BD and 581 controls). Even when pooled with previously published data, the sample size (1274 cases and 1343 controls) was still modest. In order to exclude the possibility of false-positive, replication studies in more Chinese samples from different regions are required. Second, only one SNP, rs1344706, was analyzed in our samples, and SNPs located in other LD blocks and rare variants were not studied, which might miss some important genetic information.
In summary, we observed significant association of rs1344706 in the ZNF804A gene with BD and schizophrenia, especially in Northern Chinese populations. However, the association between rs1344706 and BD susceptibility should be interpreted with caution as a result of the limited sample size used in the present study and hereby produced false positive associations. Further investigations in larger samples are warranted to draw a solid conclusion.
Methods
Participants, DNA sampling and genotyping in our samples
In the present study, we recruited a total of 537 unrelated patients with BD type I or type II and 591 healthy subjects. All participants were of the Han Chinese origin and geographically came from the South of China. The patients who met DSM-IV criteria for BD type I or type II were recruited from outpatients that were admitted to the department of Psychiatry, the Affiliated Hospital of Southwest Medial University between June 2011 and January 2014.
All patients underwent the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I) -Patient Edition independently by two experienced psychiatrists. Meanwhile, the Hamilton Rating Scale for Depression-17 (HRSD-17) and Young Mania Rating Scale (YMRS) were used to assess depressive and manic features, respectively. Subjects with co-morbid diagnosis of other psychiatric disorders or severe organic disease were excluded from this study. The Extensive Clinical Interview contains items to assess demographics, ages at onset, episodes (depressive and manic) and mental status for the patients. Meanwhile, healthy controls were recruited from local communities. The detailed description of patients and controls were seen in Table S2.
Genomic DNA was extracted from peripheral blood leukocytes according to the standard phenol/chloroform procedure. Rs1344706 was genotyped by a TaqMan method as described in our previous study35. To assess the genotyping error rate, approximately 10% samples were randomly selected and retested, and the genotype concordance was 100%. The genotype call rate for rs1344706 was 98.3%.
Each subject provided written informed consent to attend this study
Meta-analysis of the association of rs1344706 with BD and schizophrenia
Meta-analysis was performed in agreement with previously described methods36,37,38. Eligible studies included in the analysis were selected from PubMed, Embase, Medline, ISI Web of Knowledge, ScienceDirect and SCOPUS database by searching the following keywords: zinc finger protein 804A gene or ZNF804A; rs1344706, SNP or common variants; schizophrenia; bipolar disorder, BD or BPD in varying combinations. Eligible studies included in our meta-analysis must meet the following criteria: 1) manuscripts written in English; 2) study design being case-control or family-based association studies; 3) subjects being of Chinese ancestry; 4) commonly acceptable diagnosis criteria for patients, such as the Diagnostic and Statistical Manual of Mental Disorders (DSM); 5) available odds ratio (OR) with 95% confidence interval (95% CI), or sufficient data to calculate these statistics; 6) the genotypic frequencies of rs1344706 in Hardy–Weinberg equilibrium (HWE) in healthy controls. Authors were contacted in case that the genotypic data was unavailable or there were other queries regarding their studies. Two independent authors extracted the following data from the main text: 1) author and publication year; 2) sample size, sample origin and genotyping method; 3) samples’ clinical status; 4) genotypic data of rs1344706 in both cases and controls; 5) P-value, OR and 95% CI.
For those samples used twice or more in different studies by the same group, only the studies with the largest sample size were included for analysis. Three samples39,40,41 were, therefore, excluded from the final meta-analysis as a result of partial overlap with other larger samples13,17. Besides, several genome-wide association studies (GWAS) of schizophrenia conducted with Chinese populations (Beijing, Bio-X in Shanghai and Taiwan) were also included in our meta-analysis16,22,23,24. We extracted the data of rs1344706 from Huang et al. study20.
Population genetic analysis of rs1344706
The allelic frequency distributions of rs1344706 in global populations were derived from the Human Genome Diversity Project (HGDP) selection browser (http://hgdp.uchicago.edu/cgi-bin/gbrowse/HGDP/)27, which contains the alelle frequency data of SNPs in 53 worldwide populations, and the 1000 Genomes Project (www.1000genomes.org/)28.
Spatiotemporal expression patterns of ZNF804A in different human brain regions
We detected the expression pattern of ZNF804A in diverse tissues and developmental stages of human brains using the following three data sets. 1) BRAINEAC (www.braineac.org/)29, a large exon-specific eQTL database covering ten brain regions from 134 post-mortem brains from individuals of European descent free from known neurological disorders. We used this database to explore spatial dynamics of ZNF804A expression in all covered brain regions. 2) BrainCloud31, which contains genome-wide genotyping data and whole transcriptome profiling data from postmortem dorsolateral prefrontal cortex (DPFC) of 269 healthy human subjects of different ages. This database aims to depict the temporal dynamics of gene expression across the lifespan, i.e., from 14 weeks at embryonic period through ageing. 3) BioGPS (http://biogps.org/)30, is a centralized gene portal, which contains information of gene expression data from 79 human and 61 mouse tissues. We explored the spatial expression pattern of ZNF804A in mouse. More detailed description of these data sets can be found in the original manuscripts.
Statistical methods
We applied the χ2 goodness-of-fit test to estimate the Hardy-Weinberg equilibrium (HWE) for the genotypic distributions of rs1344706 in our samples. Analysis of allelic and genotypic associations was performed by either the UNPHASED program (version 3.1.4) as used in our previous studies35. Regression analyses were applied to assess the association of rs1344706 allele distribution and disease characteristics. The odds ratio (OR) and 95% confidence interval (95% CI) were used to present the effect size of rs1344706 T-allele in all analyses. We applied the Power and Sample Size Program software to perform power analysis, and the Stata12.0 statistical software package (http://www.stata.com/) to conduct publication bias analysis and meta-analysis. Degree of potential publication bias was checked using the Egger regression test for a funnel plot and the Begg–Mazumdar test. Cochran’s χ2-based Q-statistic was performed to assess the heterogeneity between individual OR estimates, which is considered significant at P < 0.10. When heterogeneity was present, the random-effects model was used to combine the OR; otherwise, the fixed-effect model was used. The significance of the pooled OR was determined by the Z test and P < 0.05 was considered statistically significant.
The study was approved by the Institutional Reviewing Board of Southwest Jiaotong University. All the procedures were in compliance with the Declaration of Helsinki and other relevant national and international rules.
Additional Information
How to cite this article: Rao, S. et al. Genetic association of rs1344706 in ZNF804A with bipolar disorder and schizophrenia susceptibility in Chinese populations. Sci. Rep. 7, 41140; doi: 10.1038/srep41140 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank all the subjects who provided their DNA for this research. This work was supported by the National Natural Science Foundation of China (No. 81471364, to F.-Q. Z., and No. 81571319, to Y. X), the Fundamental Research Funds for the Central Universities of China (No. 2682016CX099, to X.-H.H.) and the Application and Basic Research Program from Science and Technology Department of Sichuan Province (No. 2016JY0113, to X.-H.H.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Author Contributions Rao S. designed the study and wrote the protocol. Rao S. and Yao Y. extracted DNA and performed SNP genotyping. Rao S., Yao Y., Wen Y., Ryan J., Mao C. and Jin C. managed systematic literature research and extracted data from eligible studies. Rao S., Huang X., Guo J. and Xu Y. performed bioinformatics analysis and statistical analysis. Rao S. wrote the article and Ryan J., Meyre D. and Zhang F. revised it. All authors have approved the submission.
References
- Ghaemi S. N. Paradigms of psychiatry: eclecticism and its discontents. Curr Opin Psychiatry 19, 619–624 (2006). [DOI] [PubMed] [Google Scholar]
- Cannon T. D., Kaprio J., Lonnqvist J., Huttunen M. & Koskenvuo M. The genetic epidemiology of schizophrenia in a Finnish twin cohort. A population-based modeling study. Arch Gen Psychiatry 55, 67–74 (1998). [DOI] [PubMed] [Google Scholar]
- McGuffin P. et al. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry 60, 497–502 (2003). [DOI] [PubMed] [Google Scholar]
- Murray R. M. et al. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res 71, 405–416 (2004). [DOI] [PubMed] [Google Scholar]
- International Schizophrenia C. et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460, 748–752 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E. K. et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry 15, 1016–1022 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A. et al. ANK3 as a risk gene for schizophrenia: new data in Han Chinese and meta analysis. Am J Med Genet B Neuropsychiatr Genet 159B, 997–1005 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang C. et al. A comprehensive analysis of NDST3 for schizophrenia and bipolar disorder in Han Chinese. Transl Psychiatry 6, e701 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan M. C. et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet 40, 1053–1055 (2008). [DOI] [PubMed] [Google Scholar]
- Steinberg S. et al. Expanding the range of ZNF804A variants conferring risk of psychosis. Mol Psychiatry 16, 59–66 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H. J. et al. Fine mapping of ZNF804A and genome-wide significant evidence for its involvement in schizophrenia and bipolar disorder. Mol Psychiatry 16, 429–441 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R. et al. Population-based and family-based association studies of ZNF804A locus and schizophrenia. Mol Psychiatry 16, 360–361 (2011). [DOI] [PubMed] [Google Scholar]
- Yang Y. et al. Evaluation of the relationship between the ZNF804A single nucleotide polymorphism rs1344706 A/C variant and schizophrenia subtype in Han Chinese patients. Int J Psychiatry Med 45, 269–278 (2013). [DOI] [PubMed] [Google Scholar]
- Chen M. et al. Evidence of IQ-modulated association between ZNF804A gene polymorphism and cognitive function in schizophrenia patients. Neuropsychopharmacology 37, 1572–1578 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. et al. Allelic differences between Han Chinese and Europeans for functional variants in ZNF804A and their association with schizophrenia. Am J Psychiatry 168, 1318–1325 (2011). [DOI] [PubMed] [Google Scholar]
- Wong E. H. et al. Common variants on Xq28 conferring risk of schizophrenia in Han Chinese. Schizophr Bull 40, 777–786 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q. et al. No association of ZNF804A rs1344706 with white matter integrity in schizophrenia: a tract-based spatial statistics study. Neurosci Lett 532, 64–69 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. No association between ZNF804A rs1344706 and schizophrenia in a case-control study of Han Chinese. Neurosci Lett 618, 14–18 (2016). [DOI] [PubMed] [Google Scholar]
- Zhang R. et al. Further evidence for the association of genetic variants of ZNF804A with schizophrenia and a meta-analysis for genome-wide significance variant rs1344706. Schizophr Res 141, 40–47 (2012). [DOI] [PubMed] [Google Scholar]
- Huang L. et al. A comprehensive meta-analysis of ZNF804A SNPs in the risk of schizophrenia among Asian populations. Am J Med Genet B Neuropsychiatr Genet 171B, 437–446 (2016). [DOI] [PubMed] [Google Scholar]
- Zhang C. et al. ZNF804A Genetic Variation Confers Risk to Bipolar Disorder. Mol Neurobiol (2015). [DOI] [PubMed] [Google Scholar]
- Yue W. H. et al. Genome-wide association study identifies a susceptibility locus for schizophrenia in Han Chinese at 11p11.2. Nat Genet 43, 1228–1231 (2011). [DOI] [PubMed] [Google Scholar]
- Shi Y. et al. Common variants on 8p12 and 1q24.2 confer risk of schizophrenia. Nat Genet 43, 1224–1227 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou Y. J. et al. Genome-wide association study of treatment refractory schizophrenia in Han Chinese. PLoS One 7, e33598 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. et al. Allelic differences between Europeans and Chinese for CREB1 SNPs and their implications in gene expression regulation, hippocampal structure and function, and bipolar disorder susceptibility. Mol Psychiatry 19, 452–461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X. J. et al. Common variants in the MKL1 gene confer risk of schizophrenia. Schizophr Bull 41, 715–727 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell J. K. et al. Signals of recent positive selection in a worldwide sample of human populations. Genome Res 19, 826–837 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genomes Project C. et al. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy A. et al. Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci 17, 1418–1428 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Jin X., Tsueng G., Afrasiabi C. & Su A. I. BioGPS: building your own mash-up of gene annotations and expression profiles. Nucleic Acids Res 44, D313–316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C. et al. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature 478, 519–523 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab S. G. et al. Association of rs1344706 in the ZNF804A gene with schizophrenia in a case/control sample from Indonesia. Schizophr Res 147, 46–52 (2013). [DOI] [PubMed] [Google Scholar]
- Hill M. J. & Bray N. J. Allelic differences in nuclear protein binding at a genome-wide significant risk variant for schizophrenia in ZNF804A. Mol Psychiatry 16, 787–789 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo I. K. et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord 8, 65–74 (2006). [DOI] [PubMed] [Google Scholar]
- Rao S., Ye N., Hu H., Shen Y. & Xu Q. Variants in TERT influencing telomere length are associated with paranoid schizophrenia risk. Am J Med Genet B Neuropsychiatr Genet 171B, 317–324 (2016). [DOI] [PubMed] [Google Scholar]
- Yao Y. et al. Meta-analysis indicates that SNP rs9939609 within FTO is not associated with major depressive disorder (MDD) in Asian population. J Affect Disord 193, 27–30 (2016). [DOI] [PubMed] [Google Scholar]
- Rao S. et al. Common variants in FKBP5 gene and major depressive disorder (MDD) susceptibility: a comprehensive meta-analysis. Sci Rep 6, 32687 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S. et al. Common variants in CACNA1C and MDD susceptibility: A comprehensive meta-analysis. Am J Med Genet B Neuropsychiatr Genet 171, 896–903 (2016). [DOI] [PubMed] [Google Scholar]
- Xiao B. et al. To the editor: association of ZNF804A polymorphisms with schizophrenia and antipsychotic drug efficacy in a Chinese Han population. Psychiatry Res 190, 379–381 (2011). [DOI] [PubMed] [Google Scholar]
- Wei Q. et al. Association of the ZNF804A gene polymorphism rs1344706 with white matter density changes in Chinese schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 36, 122–127 (2012). [DOI] [PubMed] [Google Scholar]
- Wei Q. et al. ZNF804A rs1344706 is associated with cortical thickness, surface area, and cortical volume of the unmedicated first episode schizophrenia and healthy controls. Am J Med Genet B Neuropsychiatr Genet 168B, 265–273 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.