Abstract
Malignant pleural effusion (MPE) is common in clinical practice, and despite the existence of studies to guide clinical decisions, it often poses diagnostic and therapeutic dilemmas. Once it is diagnosed, median survival does not usually exceed 6 months. The management of these patients focuses on symptom relief since no treatments have been shown to increase survival to date. Conversely, poor management can shorten survival. The approach must be multidisciplinary and allow for individualized care. Initial diagnostic procedures should be minimally invasive and, according to the results and other factors, procedures of increasing complexity will be selecting. Likewise, the treatment of MPEs should be individualized according to factors such as type of tumor, patient functional status, means available, benefits of each procedure, or life expectancy. Currently, treatment seems to tend toward less interventional approaches, in which patients can be managed on an outpatient basis, thus minimizing both the discomfort that more aggressive approaches involve and the costs of care associated with this disease. This article reviews the pleural procedures employed in the management of MPEs with special emphasis on the indication for each one, its usefulness, benefits, and complications.
Key words: Closed pleural biopsy, image-guided pleural biopsy, indwelling pleural catheter, intrapleural talc, malignant pleural effusion, medical pleuroscopy, pleural manometry, pleurodesis, thoracentesis, thoracic ultrasound, video-assisted thoracoscopic surgery
Malignant pleural effusion (MPE) is a common clinical problem. In Europe, only due to lung cancer, 125,000 people develop it each year,[1] and in the United States, about 150,000.[2] MPE accounts for 15%–35% of all pleural effusions (PEs) of each series.[3,4] Its appearance is key for the prognosis of a cancer patient because median survival from diagnosis does not usually exceed 6 months.[5,6] This means a management shift from a curative approach to palliative care, directed more specifically toward the MPE, trying to control the symptoms associated with it. Although no specific strategy for the management of MPE has been shown to improve survival time, erroneous treatment can aggravate the symptoms and shorten life expectancy.
A significant proportion of patients with neoplasia may present PE at some point that does not meet the conditions for malignancy (pleural infiltration). These so-called paraneoplastic effusions are usually caused by diseases that coexist with neoplasia, such as pulmonary embolism, thoracic duct obstruction, compression of the superior vena cava, pericardial infiltration, hypoalbuminemia, obstructive pneumonia, or atelectasis.[7] In these cases, the prognosis is more favorable, so it is very important to determine the etiology of the PE before considering it an MPE.
Once MPE is diagnosed, one has to consider factors such as type of primary tumor, estimated survival, functional status, associated symptoms, pulmonary elastance, and even patient preferences between different management options (observation, periodic PE drainage, pleurodesis, or indwelling pleural catheter [IPC] placement), which will determine the decisions to be taken in the management of MPE, so the care provided must be individualized.[8]
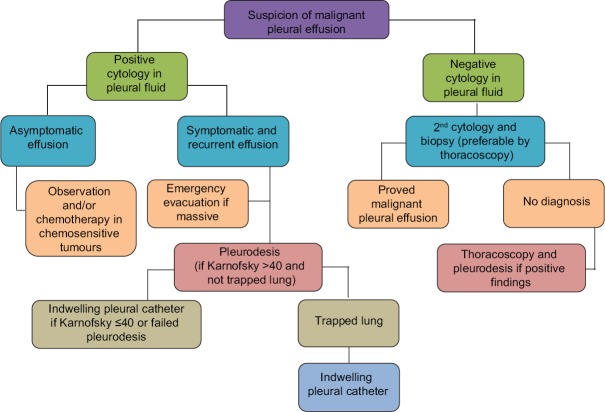
The aim of this review is to provide an update on the advances of pleural procedures used in clinical practice for the management of MPE. Throughout, we have followed the management algorithm recommended by the Spanish Society of Pneumology and Thoracic Surgery[4] [Figure 1].
Figure 1.
Algorithm for the management of malignant pleural effusion. MPE=malignant pleural effusion
Pathogeny
Knowing the mechanism by which MPE occurs informs us about what diagnostic tests are indicated and what benefit we can expect from each of them. The lymphatic system of the parietal pleura plays a key role in the reabsorption of pleural fluid (PF) so that any alteration in its entirety (between the parietal pleura and mediastinal lymph node) can give rise to PE. Several studies have shown that this is the main mechanism of PE production, and a strong relationship between the presence of PE and neoplastic infiltration of the mediastinal lymph nodes has been shown.[9,10] On the contrary, this relationship has not been found between metastatic infiltration of the pleura and the existence of PE.[10]
The tumors that most frequently metastasize to the pleura are cancers of the lung, breast, and lymphomas.[8] At first, the parietal pleura may be affected by neoplastic extension from the visceral pleura along pleural adhesions that may form and by neoplastic cells which are exfoliated from the visceral pleura. Metastases in the visceral pleura are caused by the invasion of the pulmonary artery and embolization.[9] If pleural metastases are bilateral, there is generally liver involvement with hematogenous spread to the contralateral lung.[10] In breast cancer, PE may be produced by lymphatic invasion of the chest wall, and if there is liver involvement, PE may be bilateral or contralateral.[11] In Hodgkin's disease, MPE is rare and lymphomatous invasion of the pleura is not usually seen until the final stages, occurring in 30% of cases. In these lymphomas, the primary mechanism appears to be associated with chest drainage. In previously untreated non-Hodgkin's lymphoma, MPE is common even in the absence of detectable intrathoracic lymphadenopathies and may be the only radiologic manifestation of the disease.[12] In these lymphomas, lymphomatous invasion of the pleura is the predominant mechanism.[13,14]
Imaging Techniques
Thoracic ultrasound
Thoracic ultrasound (US) serves as a guide for locating and puncturing a PE and allows visualizing pleural thickening or masses that suggest the existence of MPE. Thoracic US locates a PE from 5 mL and is more sensitive and specific than chest radiography (94% and 98%, respectively, vs. 51% and 91%, respectively).[15] Furthermore, it allows more accurate calculation of PE volume than radiography[16] as well as differentiation from solid pleural lesions and thickening.[17] The current recommendation is that all pleural procedures should be US guided[18] since they better identify the puncture site of thoracentesis[19] with less risk of pneumothorax[20,21] than physical examination and chest radiography.
The only US sign indicating malignancy, without being pathognomonic, is the presence of pleural nodules >1 cm (in the parietal, visceral, or diaphragmatic pleura) (sensitivity 73%, specificity 100%).[22] These malignant nodules are usually accompanied by PE which allows better US evaluation of the solid component.[17] In massive PE, often very malignant, a flattening and even inversion of the diaphragm can also be seen. Finally, a recent study has shown that chest US can identify an unexpandable lung[23] which has implications as discussed below.
Thoracic computed tomography
In the study of MPE, computed tomography (CT) of the chest has the same indications as US. Small studies have suggested that certain findings on chest CT are characteristic of malignant disease (thickening of the parietal pleura >1 cm and nodular or mediastinal pleural thickening) with good specificity (88%-100%) and poor sensitivity (36%–51%). Hallifax et al. retrospectively studied 370 patients with PE (202 with MPE and 168 with benign PE) and compared the histological results of thoracoscopy, with a presumptive diagnosis obtained with the previously performed chest CT. The sensitivity of CT was 68% and specificity 78%. However, perhaps, the most important aspect is that one out of three patients with PE and CT without evidence of malignancy had MPE.[24] The current guidelines recommend that if PF analysis does not reveal the etiology of a pleural exudate, chest CT should be performed.[25] However, these results suggest that if MPE is suspected and PF analysis does not reveal its etiology, chest CT should be combined with invasive pleural procedures.
Thoracentesis
Diagnostic thoracentesis is a simple, inexpensive technique, in which a small amount of PF is extracted.[26] A 60 mL sample of PF provides greater diagnostic yield for MPE than 10 mL, but more than 50 mL does not seem to improve diagnostic sensitivity.[27] Diagnostic yield depends on the type of tumor (higher for adenocarcinomas) and is about 60% (range, 40%-87%).[28] However, a second cytology may increase diagnostic sensitivity by 27%,[29] if several 100 mL is drained in the initial thoracentesis, since the number of fresh malignant cells exfoliated increases in the second.[7] The yield may also increase if both smears and cell blocks are prepared.[30] The sample of PF obtained by thoracentesis may also be used for immunohistochemistry (to differentiate between different types of tumors) or flow cytometry (if lymphoma is suspected).
Pneumothorax is the most common complication of thoracentesis, with an incidence of 4%–30% if performed without US guidance and 1.3%–6.7% if performed with it. Two large studies have directly compared the rates of pneumothorax in patients undergoing thoracentesis with or without guided US. In the first study, involving 342 patients, the rate of pneumothorax without US-guided thoracentesis was 13% versus 3% in those where the procedure was performed with US guidance.[31] In the second study, which included 450 patients, the percentages were 10.3% and 4.9%, respectively.[32] Because of these results, all thoracentesis should be guided by US. The use of mechanical ventilation does not increase the risk.[26] Less common complications include chest pain, hemothorax, pneumohemothorax, laceration of the spleen, abdominal or intrathoracic bleeding, catheter detachment, and anxiety.
Repeat therapeutic thoracentesis should be reserved for patients with a short life expectancy (approximately 1 month) and poor functional status to provide temporary relief of symptoms. It is performed with US guidance and the amount of liquid extracted will depend on the referring clinic but limited to no more than 1.5 L each time.[28]
Closed Pleural Biopsy
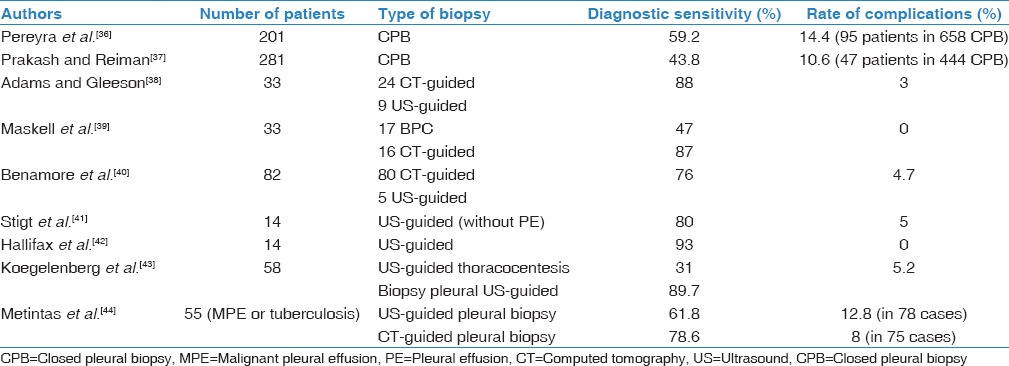
The use of closed pleural biopsy (CPB) has generated much controversy.[33,34] It is a simple, low-cost technique, well tolerated by patients, and its performance does not generally require hospital admission. Although diagnostic yield approaches 60%,[35,36] its main drawback is that it does not significantly increase the sensitivity of cytology when performed together,[37] probably because of the patchy and uneven distribution of tumor invasion of the pleura. Several studies have shown that the performance of percutaneous pleural biopsy increases significantly when guided by US or chest CT, possibly because it allows biopsy of abnormal focal areas and access to anatomical areas that are often avoided with CPB[36,37,38,39,40,41,42,43,44] [Table 1]. In all these studies, the reported rate of complications was low.
Table 1.
Diagnostic sensitivity and complications of nonpleuroscopic pleural biopsy techniques in malignant pleural effusions (since 2001)
Medical Pleuroscopy
A second sample of PF can increase the diagnostic yield of cytology, but a third does nothing to improve sensitivity.[2] If mesothelioma is suspected, the diagnostic yield of cytology is low (about 30%).[45] If cytology is negative, CPB improves performance very little and it should preferably be image-guided, especially if signs of malignancy are observed in the pleura. If imaging techniques do not show these signs, an alternative is to perform medical thoracoscopy under local anesthesia. Pleuroscopy allows draining large amounts of PF, direct visualization of the pleural cavity, performing multiple biopsies and if necessary, talc application to prevent recurrence of PE.[46]
In a review of 22 series evaluating the diagnostic yield of medical thoracoscopy in the diagnosis of MPE, sensitivity was 92.6%. On excluding patients undergoing CPB and pleuroscopy and the CPB was positive, the diagnostic yield of medical thoracoscopy remained high (90.1%).[47] Mortality in a series of 47 studies was 0.34%.[47] The deaths did not occur if only diagnostic pleuroscopy (0/2421 patients) was performed but occurred when powdered talc was used (16/2315). Nine of the 16 deaths occurred in a single trial where talc particle size was not taken into account.[48]
Pleuroscopy has several disadvantages. Obesity, lateral decubitus intolerance, and presence of cough due to uncontrolled pleural irritation are associated with complications; it is advisable to optimize the treatment of reversible diseases, and pleuroscopy may be contraindicated in situations such as highly loculated PF, inability of a lung to collapse due to an underlying disease, and existence of cardiovascular instability, pulmonary hypertension, or untreated hypoxemia. In addition, if the PE is small, it can be difficult to identify a safe place for pleuroscope insertion and a small pneumothorax may be necessary.[49] Finally, unlike CPB or image-guided pleural biopsy, patient hospitalization is needed.
An aspect that remains unresolved is the risk of tumor invasion through the tract created by the pleuroscope (9%–16% for unguided pleuroscopy and 0%–22% for image-guided pleural biopsy).[50] Three studies suggest that prophylactic radiotherapy provides no benefit.[51,52,53] However, until the results of new ongoing studies are known, the British Thoracic Society suggests that prophylactic radiation therapy may have a role in patients with good general condition.[54]
Video-assisted Thoracoscopic Surgery
Video-assisted thoracic surgery requires general anesthesia and is the “gold standard” for the diagnosis of MPE. Its diagnostic yield is 95%, but it is a relatively invasive technique often resorted to when cytology and CPB are negative,[55] and generally, when something more than a purely diagnostic procedure is needed (pleurectomy, decortication, etc.), it cannot be done using medical pleuroscopy. The procedure is relatively safe with a low rate of complications (<1%).[28]
Pleural Manometry
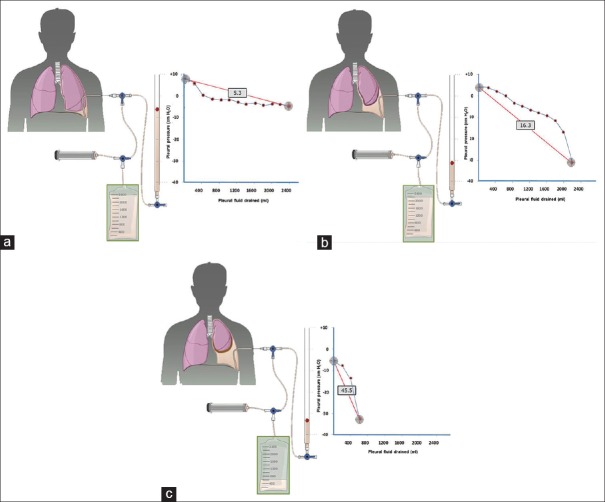
The measurement of pleural pressure helps to establish the diagnosis of unexpandable lung and predict the success of pleurodesis in the management of MPE. Unexpandable lung is a mechanical complication, in which the lung is unable to expand to the chest wall, thus impeding normal apposition between the visceral and parietal layers. The main mechanism involved is usually restriction of the visceral pleura. In the first phase, there is an active process of lung entrapment involving the visceral pleura which can be progressive or resolved with medical treatment. If it progresses, it will lead to a trapped lung that is the sequel to an old inflammatory process, in which a fibrous membrane in the visceral pleura prevents lung expansion during extraction of PF.[56] The measurement of pleural pressure and elastance (change in PF pressure measured in cm H2 O/L of fluid removed) allows us to distinguish a normal lung from one in the process of entrapment and a trapped lung [Figure 2].
Figure 2.
Curves obtained by manometry, with elastance value, in a normal lung (a), in a lung entrapment process (b), and in a trapped lung (c). When pleural effusion occurs in a normal lung (a), initial pleural pressure will be slightly positive. As fluid is withdrawn, pleural pressure will drop slowly and the lung will expand gradually. Once the entire effusion has been removed, the lung will make contact with the chest wall and the elastance obtained will be normal. In the lung entrapment process (b), the visceral pleura is slightly thickened and pleural pressure will be slightly positive, as in the normal lung. On fluid removal, initially, the lung will gradually expand and pleural pressure will drop slowly. At some point, the lung becomes trapped, unable to expand any further and the pressure will drop rapidly leading to high elastance with a bimodal pressure/volume curve. In the case of a trapped lung, the visceral pleura has a thicker layer of fibrin which prevents the lung from expanding, so the initial pressure is negative (c). The removal of liquid, on the one hand, and the rigidity of the lung, on the other hand, causes a rapid decrease in pleural pressure resulting in high elastance
The success of pleurodesis is determined by how good the apposition of the two pleural layers is.[57] Therefore, pleural pressure in an MPE should be determined before deciding on pleurodesis. This procedure is indicated if manometry shows that the lung expands normally. If, on the contrary, manometry suggests a trapped lung, an IPC should be inserted.[8]
Pleurodesis
Pleurodesis is iatrogenic-induced pleural fibrosis by a sclerosing agent to obliterate the pleural space and prevent the accumulation of PF. Talc is the most frequently used sclerosing agent since it is widely available, cheap, and effective.[58] It can be administered in two ways: Using a thoracoscope tube atomizer (talc poudrage) or an intercostal tube as a suspension (talc slurry). Other agents such as tetracycline derivatives, silver nitrate, povidone-iodine, and bleomycin have also been used. A recent meta-analysis of 62 randomized trials involving 3428 patients suggests that talc insufflation is the most effective method of pleurodesis in preventing re-accumulation of fluid but states that more data are needed to definitively confirm that it is more effective than talc slurry and doxycycline, due to the high clinical and statistical heterogeneity, and high risk of bias, of most of the studies included in the analysis.[59]
Regardless of whether the sclerosing agent is introduced through a chest tube or thoracoscopy, hospitalization is required. However, while the former can be done in the patient's room with analgesia, the second requires general anesthesia or conscious sedation. Randomized clinical trials have not demonstrated superiority of one technique over another,[48,60] and the British Thoracic Society indicates that the two approaches have similar efficacy.[8] The success of pleurodesis is usually determined by the nonre-accumulation of fluid within 30 days. However, this is not always easy to determine. Although one study showed a success rate of 91%,[61] no later studies have achieved these results. In a randomized trial that compared talc slurry versus powdered talc, success rates of pleurodesis were 71% and 78%, respectively. However, on including patients who had died before 30 days or had not achieved lung re-expansion, these rates fell to 53% and 60%, respectively.[48] There is controversy about the length of time the chest drain must be maintained after introducing the sclerosing agent. One trial suggests that once the lung has expanded, it is not necessary to wait for reduced fluid drain,[62] while another concludes that the withdrawal of the tube 24 h after introducing talc (instead of 72 h) does not compromise the results.[63] We recommend large particle talc (>15 microns) to avoid the possibility of acute respiratory distress syndrome and increased alveolar-arterial oxygen gradient.[64,65] Other complications associated with intrapleural talc are fever and chest pain.
Two aspects of pleurodesis are debatable: Drain tube gauge and rotation of patients once the sclerosing agent is administered. Several studies have shown a similar success rate with small-bore tubes (10°F–14°F) as with large caliber tubes[66,67,68] when a sclerosing agent is used. Further, no difference in the success rate of pleurodesis has been observed between rotated or nonrotated patients.[69]
Indwelling Pleural Catheter
IPCs are subcutaneously secured indwelling silicone tubes ending in a one-way valve. They are inserted to maintain lung expansion through continuous drainage of fluid rather than obliteration of the pleural space as occurs with pleurodesis. The goal is to control symptoms, and these usually improve rapidly after insertion, allowing patient management on an outpatient basis. IPCs have proven to be as effective as pleurodesis in the first-line treatment of MPE[70,71] and can also be used when pleurodesis fails or is contraindicated because of a trapped lung. As from the publication of the TIME-2 study,[70] IPC began to be considered a clear alternative to conventional sclerotherapy.[72,73] In a systematic review of 19 studies with 1370 patients which evaluated the safety and efficacy of IPCs in MPE, symptom improvement was achieved in 95% of cases.[74] The use of IPC can also lead to spontaneous pleurodesis in 46%-70% of patients presenting complete lung expansion through local inflammatory changes, induced either by the tumor itself or by the IPC.[73,74] Currently, IPCs also begin to be used to administer the sclerosing agent, with a high rate of success (92%; 22/24 patients).[75] To date, no technique has demonstrated superiority over the other (IPC vs. talc pleurodesis). IPC insertion requires shorter hospital stay and appears to offer advantages in patients with poor performance status who cannot tolerate pleurodesis or in those with trapped lung. Pleurodesis offers a greater chance of rapid resolution of PE with a shorter intervention time, but it is a more invasive procedure which may need to be repeated.[70,71] IPCs represent a solution for outpatients, but they do involve prolonged drainage and greater attention for those in whom pleurodesis is not indicated. Symptom relief and improved quality of life can be achieved with either approach, without significant differences between them. IPCs are associated with a greater number of complications (blocking or detachment of the catheter [<5%],[76] catheter rupture on removal,[77] and subcutaneous and pleural infection [0%-12%]);[78,79] however, generally, they are well tolerated and do not cause significant morbidity.[71] The cost of managing patients with an IPC versus talc pleurodesis has not been sufficiently studied since most studies are retrospective and use indirect comparisons with conventional treatments. Data from the TIME-2 study showed no difference in the total cost between the two techniques.[80] It has been suggested that IPCs are more cost-effective in patients with limited survival (<3 months), while talc pleurodesis is more cost-effective in those with longer survival.[81] However, to date, these data are not necessarily valid for all types of health care and have not been validated in clinical trials.
In summary, many questions remain unanswered in the current management of patients with MPE and often cause significant diagnostic and therapeutic dilemmas. All thoracentesis and CPBs should be performed under US guidance to improve diagnostic yield and decrease the rate of complications. After two negative cytologies and no signs of pleural malignancy on imaging tests, medical pleuroscopy provides clear advantages over CPB. However, if more than a purely diagnostic procedure is needed, video-assisted thoracic surgery may be considered. If pleural manometry rules out the existence of a trapped lung and the patient has no significant deterioration in functional status, it is advisable to perform pleurodesis (using either a chest tube or thoracoscopy). If, however, a trapped lung is confirmed, the treatment of choice is placement of an IPC. The approach must be multidisciplinary and begin with less invasive diagnostic procedures; if the results are negative, the complexity of such procedures should be gradually increased depending on factors such as patient's functional status, means available at each center, experience of the medical team, and even patient's own opinion. MPE treatment must be individualized, taking into account factors such as the type of tumor, symptoms, functional status, presence of a trapped lung, and life expectancy. The current treatment tends toward less interventional approaches, trying to manage patients as outpatients and thus minimizing both the discomfort caused by more aggressive actions and the cost associated with this disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mishra EK, Davies HE, Lee YC. Malignant pleural disease in primary lung cancer. In: Spiro SG, Janes SM, editors. Thoracic Malignancies. European Respiratory Monograph. Sheffield, UK: European Respiratory Society Journals Ltd; 2009. pp. 318–35. [Google Scholar]
- 2.American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987–2001. doi: 10.1164/ajrccm.162.5.ats8-00. [DOI] [PubMed] [Google Scholar]
- 3.Valdés L, Álvarez D, Valle JM, Pose A, San José E. The etiology of pleural effusions in an area with high incidence of tuberculosis. Chest. 1996;109:158–62. doi: 10.1378/chest.109.1.158. [DOI] [PubMed] [Google Scholar]
- 4.Villena Garrido V, Cases Viedma E, Fernández Villar A, de Pablo Gafas A, Pérez Rodríguez E, Porcel Pérez JM, et al. Recommendations of diagnosis and treatment of pleural effusion. Update. Arch Bronconeumol. 2014;50:235–49. doi: 10.1016/j.arbres.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez AV, Bezwada V, Beamis JF, Jr, Villanueva AG. Lung injury following thoracoscopic talc insufflation: Experience of a single North American center. Chest. 2010;137:1375–81. doi: 10.1378/chest.09-2020. [DOI] [PubMed] [Google Scholar]
- 6.Clive AO, Kahan BC, Hooper CE, Bhatnagar R, Morley AJ, Zahan-Evans N, et al. Predicting survival in malignant pleural effusion: Development and validation of the LENT prognostic score. Thorax. 2014;69:1098–104. doi: 10.1136/thoraxjnl-2014-205285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J. 1997;10:1907–13. doi: 10.1183/09031936.97.10081907. [DOI] [PubMed] [Google Scholar]
- 8.Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ. BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii32–40. doi: 10.1136/thx.2010.136994. [DOI] [PubMed] [Google Scholar]
- 9.Chernow B, Sahn SA. Carcinomatous involvement of the pleura: An analysis of 96 patients. Am J Med. 1977;63:695–702. doi: 10.1016/0002-9343(77)90154-1. [DOI] [PubMed] [Google Scholar]
- 10.Meyer PC. Metastatic carcinoma of the pleura. Thorax. 1966;21:437–43. doi: 10.1136/thx.21.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fentiman IS, Millis R, Sexton S, Hayward JL. Pleural effusion in breast cancer: A review of 105 cases. Cancer. 1981;47:2087–92. doi: 10.1002/1097-0142(19810415)47:8<2087::aid-cncr2820470830>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Castellino RA, Bellani FF, Gasparini M, Musumeci R. Radiographic findings in previously untreated children with non-Hodgkin's lymphoma. Radiology. 1975;117(3 Pt 1):657–63. doi: 10.1148/117.3.657. [DOI] [PubMed] [Google Scholar]
- 13.Xaubet A, Diumenjo MC, Marin A, Montserrat E, Estopá R, Llebaría C, et al. Characteristics and prognostic value of pleural effusions in non-Hodgkin's lymphomas. Eur J Respir Dis. 1985;66:135–40. [PubMed] [Google Scholar]
- 14.Sahn SA. State of the art. The pleura. Am Rev Respir Dis. 1988;138:184–234. doi: 10.1164/ajrccm/138.1.184. [DOI] [PubMed] [Google Scholar]
- 15.Yousefifard M, Baikpour M, Ghelichkhani P, Asady H, Shahsavari Nia K, Moghadas Jafari A, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran) 2016;4:1–10. [PMC free article] [PubMed] [Google Scholar]
- 16.Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hörmann MF, Grabenwöger F. Quantification of pleural effusions: Sonography versus radiography. Radiology. 1994;191:681–4. doi: 10.1148/radiology.191.3.8184046. [DOI] [PubMed] [Google Scholar]
- 17.Wernecke K. Ultrasound study of the pleura. Eur Radiol. 2000;10:1515–23. doi: 10.1007/s003300000526. [DOI] [PubMed] [Google Scholar]
- 18.Havelock T, Teoh R, Laws D, Gleeson F. BTS Pleural Disease Guideline Group. Pleural procedures and thoracic ultrasound: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii61–76. doi: 10.1136/thx.2010.137026. [DOI] [PubMed] [Google Scholar]
- 19.Diacon AH, Brutsche MH, Solèr M. Accuracy of pleural puncture sites: A prospective comparison of clinical examination with ultrasound. Chest. 2003;123:436–41. doi: 10.1378/chest.123.2.436. [DOI] [PubMed] [Google Scholar]
- 20.Cantey EP, Walter JM, Corbridge T, Barsuk JH. Complications of thoracentesis: Incidence, risk factors, and strategies for prevention. Curr Opin Pulm Med. 2016;22:378–85. doi: 10.1097/MCP.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: A 12-year experience. Thorax. 2015;70:127–32. doi: 10.1136/thoraxjnl-2014-206114. [DOI] [PubMed] [Google Scholar]
- 22.Qureshi NR, Rahman NM, Gleeson FV. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax. 2009;64:139–43. doi: 10.1136/thx.2008.100545. [DOI] [PubMed] [Google Scholar]
- 23.Salamonsen MR, Lo AK, Ng AC, Bashirzadeh F, Wang WY, Fielding DI. Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest. 2014;146:1286–93. doi: 10.1378/chest.13-2876. [DOI] [PubMed] [Google Scholar]
- 24.Hallifax RJ, Haris M, Corcoran JP, Leyakathalikhan S, Brown E, Srikantharaja D, et al. Role of CT in assessing pleural malignancy prior to thoracoscopy. Thorax. 2015;70:192–3. doi: 10.1136/thoraxjnl-2014-206054. [DOI] [PubMed] [Google Scholar]
- 25.Du Rand I, Maskell N. Introduction and methods: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii1–3. doi: 10.1136/thx.2010.137042. [DOI] [PubMed] [Google Scholar]
- 26.Sachdeva A, Shepherd RW, Lee HJ. Thoracentesis and thoracic ultrasound: State of the art in 2013. Clin Chest Med. 2013;34:1–9. doi: 10.1016/j.ccm.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Swiderek J, Morcos S, Donthireddy V, Surapaneni R, Jackson-Thompson V, Schultz L, et al. Prospective study to determine the volume of pleural fluid required to diagnose malignancy. Chest. 2010;137:68–73. doi: 10.1378/chest.09-0641. [DOI] [PubMed] [Google Scholar]
- 28.Hooper C, Lee YC, Maskell N. BTS Pleural Guideline Group. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii4–17. doi: 10.1136/thx.2010.136978. [DOI] [PubMed] [Google Scholar]
- 29.Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol. 1994;7:665–8. [PubMed] [Google Scholar]
- 30.Dekker A, Bupp PA. Cytology of serous effusions. An investigation into the usefulness of cell blocks versus smears. Am J Clin Pathol. 1978;70:855–60. doi: 10.1093/ajcp/70.6.855. [DOI] [PubMed] [Google Scholar]
- 31.Raptopoulos V, Davis LM, Lee G, Umali C, Lew R, Irwin RS. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156:917–20. doi: 10.2214/ajr.156.5.2017951. [DOI] [PubMed] [Google Scholar]
- 32.Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33:442–6. doi: 10.1002/jcu.20163. [DOI] [PubMed] [Google Scholar]
- 33.Silvestri GA, Strange C. Rest in peace: The decline in training and use of the closed pleural biopsy. J Bronchol. 2005;12:131–2. [Google Scholar]
- 34.Baumann MH. Closed pleural biopsy: Not dead yet! Chest. 2006;129:1398–400. doi: 10.1378/chest.129.6.1398. [DOI] [PubMed] [Google Scholar]
- 35.Tomlinson JR. Invasive procedures in the diagnosis of pleural disease. Semin Respir Med. 1987;9:30–60. [Google Scholar]
- 36.Pereyra MF, San-José E, Ferreiro L, Golpe A, Antúnez J, González-Barcala FJ, et al. Role of blind closed pleural biopsy in the management of pleural exudates. Can Respir J. 2013;20:362–6. doi: 10.1155/2013/731352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prakash UB, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: Analysis of 414 cases. Mayo Clin Proc. 1985;60:158–64. doi: 10.1016/s0025-6196(12)60212-2. [DOI] [PubMed] [Google Scholar]
- 38.Adams RF, Gleeson FV. Percutaneous image-guided cutting-needle biopsy of the pleura in the presence of a suspected malignant effusion. Radiology. 2001;219:510–4. doi: 10.1148/radiology.219.2.r01ma07510. [DOI] [PubMed] [Google Scholar]
- 39.Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: A randomised controlled trial. Lancet. 2003;361:1326–30. doi: 10.1016/s0140-6736(03)13079-6. [DOI] [PubMed] [Google Scholar]
- 40.Benamore RE, Scott K, Richards CJ, Entwisle JJ. Image-guided pleural biopsy: Diagnostic yield and complications. Clin Radiol. 2006;61:700–5. doi: 10.1016/j.crad.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Stigt JA, Boers JE, Groen HJ. Analysis of “dry” mesothelioma with ultrasound guided biopsies. Lung Cancer. 2012;78:229–33. doi: 10.1016/j.lungcan.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Hallifax RJ, Corcoran JP, Ahmed A, Nagendran M, Rostom H, Hassan N, et al. Physician-based ultrasound-guided biopsy for diagnosing pleural disease. Chest. 2014;146:1001–6. doi: 10.1378/chest.14-0299. [DOI] [PubMed] [Google Scholar]
- 43.Koegelenberg CF, Irusen EM, von Groote-Bidlingmaier F, Bruwer JW, Batubara EM, Diacon AH. The utility of ultrasound-guided thoracentesis and pleural biopsy in undiagnosed pleural exudates. Thorax. 2015;70:995–7. doi: 10.1136/thoraxjnl-2014-206567. [DOI] [PubMed] [Google Scholar]
- 44.Metintas M, Yildirim H, Kaya T, Ak G, Dundar E, Ozkan R, et al. CT scan-guided abrams' needle pleural biopsy versus ultrasound-assisted cutting needle pleural biopsy for diagnosis in patients with pleural effusion: A randomized, controlled trial. Respiration. 2016;91:156–63. doi: 10.1159/000443483. [DOI] [PubMed] [Google Scholar]
- 45.Renshaw AA, Dean BR, Antman KH, Sugarbaker DJ, Cibas ES. The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Chest. 1997;111:106–9. doi: 10.1378/chest.111.1.106. [DOI] [PubMed] [Google Scholar]
- 46.Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med. 2013;34:487–500. doi: 10.1016/j.ccm.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Rahman NM, Ali NJ, Brown G, Chapman SJ, Davies RJ, Downer NJ, et al. Local anaesthetic thoracoscopy: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii54–60. doi: 10.1136/thx.2010.137018. [DOI] [PubMed] [Google Scholar]
- 48.Dresler CM, Olak J, Herndon JE, nd, Richards WG, Scalzetti E, Fleishman SB, et al. Phase III intergroup study of talc poudrage vs. talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909–15. doi: 10.1378/chest.127.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loddenkemper R. Thoracoscopy - State of the art. Eur Respir J. 1998;11:213–21. doi: 10.1183/09031936.98.11010213. [DOI] [PubMed] [Google Scholar]
- 50.Lee C, Bayman N, Swindell R, Faivre-Finn C. Prophylactic radiotherapy to intervention sites in mesothelioma: A systematic review and survey of UK practice. Lung Cancer. 2009;66:150–6. doi: 10.1016/j.lungcan.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Bydder S, Phillips M, Joseph DJ, Cameron F, Spry NA, DeMelker Y, et al. A randomised trial of single-dose radiotherapy to prevent procedure tract metastasis by malignant mesothelioma. Br J Cancer. 2004;91:9–10. doi: 10.1038/sj.bjc.6601957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Rourke N, Garcia JC, Paul J, Lawless C, McMenemin R, Hill J. A randomised controlled trial of intervention site radiotherapy in malignant pleural mesothelioma. Radiother Oncol. 2007;84:18–22. doi: 10.1016/j.radonc.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 53.Clive AO, Taylor H, Dobson L, Wilson P, de Winton E, Panakis N, et al. Prophylactic radiotherapy for the prevention of procedure-tract metastases after surgical and large-bore pleural procedures in malignant pleural mesothelioma (SMART): A multicenter, open-label, phase 3, randomised controlled trial. Lancet Oncol. 2016;17:1094–104. doi: 10.1016/S1470-2045(16)30095-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.British Thoracic Society Standards of Care Committee. BTS statement on malignant mesothelioma in the UK, 2007. Thorax. 2007;62(Suppl 2):ii1–19. doi: 10.1136/thx.2007.087619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Page RD, Jeffrey RR, Donnelly RJ. Thoracoscopy: A review of 121 consecutive surgical procedures. Ann Thorac Surg. 1989;48:66–8. doi: 10.1016/0003-4975(89)90179-3. [DOI] [PubMed] [Google Scholar]
- 56.Pereyra MF, Ferreiro L, Valdés L. Unexpandable lung. Arch Bronconeumol. 2013;49:63–9. doi: 10.1016/j.arbres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Kennedy L, Rusch VW, Strange C, Ginsberg RJ, Sahn SA. Pleurodesis using talc slurry. Chest. 1994;106:342–6. doi: 10.1378/chest.106.2.342. [DOI] [PubMed] [Google Scholar]
- 58.Lee YC, Baumann MH, Maskell NA, Waterer GW, Eaton TE, Davies RJ, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries: Survey of pulmonologists. Chest. 2003;124:2229–38. doi: 10.1378/chest.124.6.2229. [DOI] [PubMed] [Google Scholar]
- 59.Clive AO, Jones HE, Bhatnagar R, Preston NJ, Maskell N. Interventions for the management of malignant pleural effusions: A network meta-analysis. Cochrane Database Syst Rev. 2016;5:CD010529. doi: 10.1002/14651858.CD010529.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yim AP, Chan AT, Lee TW, Wan IY, Ho JK. Thoracoscopic talc insufflation versus talc slurry for symptomatic malignant pleural effusion. Ann Thorac Surg. 1996;62:1655–8. [PubMed] [Google Scholar]
- 61.Kennedy L, Sahn SA. Talc pleurodesis for the treatment of pneumothorax and pleural effusion. Chest. 1994;106:1215–22. doi: 10.1378/chest.106.4.1215. [DOI] [PubMed] [Google Scholar]
- 62.Villanueva AG, Gray AW, Jr, Shahian DM, Williamson WA, Beamis JF., Jr Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax. 1994;49:23–5. doi: 10.1136/thx.49.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goodman A, Davies CW. Efficacy of short-term versus long-term chest tube drainage following talc slurry pleurodesis in patients with malignant pleural effusions: A randomised trial. Lung Cancer. 2006;54:51–5. doi: 10.1016/j.lungcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Maskell NA, Lee YC, Gleeson FV, Hedley EL, Pengelly G, Davies RJ. Randomized trials describing lung inflammation after pleurodesis with talc of varying particle size. Am J Respir Crit Care Med. 2004;170:377–82. doi: 10.1164/rccm.200311-1579OC. [DOI] [PubMed] [Google Scholar]
- 65.Janssen JP, Collier G, Astoul P, Tassi GF, Noppen M, Rodriguez-Panadero F, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: A prospective cohort study. Lancet. 2007;369:1535–9. doi: 10.1016/S0140-6736(07)60708-9. [DOI] [PubMed] [Google Scholar]
- 66.Seaton KG, Patz EF, Jr, Goodman PC. Palliative treatment of malignant pleural effusions: Value of small-bore catheter thoracostomy and doxycycline sclerotherapy. AJR Am J Roentgenol. 1995;164:589–91. doi: 10.2214/ajr.164.3.7532350. [DOI] [PubMed] [Google Scholar]
- 67.Morrison MC, Mueller PR, Lee MJ, Saini S, Brink JA, Dawson SL, et al. Sclerotherapy of malignant pleural effusion through sonographically placed small-bore catheters. AJR Am J Roentgenol. 1992;158:41–3. doi: 10.2214/ajr.158.1.1370073. [DOI] [PubMed] [Google Scholar]
- 68.Parker LA, Charnock GC, Delany DJ. Small bore catheter drainage and sclerotherapy for malignant pleural effusions. Cancer. 1989;64:1218–21. doi: 10.1002/1097-0142(19890915)64:6<1218::aid-cncr2820640609>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 69.Mager HJ, Maesen B, Verzijlbergen F, Schramel F. Distribution of talc suspension during treatment of malignant pleural effusion with talc pleurodesis. Lung Cancer. 2002;36:77–81. doi: 10.1016/s0169-5002(01)00475-5. [DOI] [PubMed] [Google Scholar]
- 70.Davies HE, Mishra EK, Kahan BC, Wrightson JM, Stanton AE, Guhan A, et al. Effect of an indwelling pleural catheter vs. chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: The TIME2 randomized controlled trial. JAMA. 2012;307:2383–9. doi: 10.1001/jama.2012.5535. [DOI] [PubMed] [Google Scholar]
- 71.Fysh ET, Waterer GW, Kendall PA, Bremmer PR, Dina S, Geelhoed E, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest. 2012;142:394–400. doi: 10.1378/chest.11-2657. [DOI] [PubMed] [Google Scholar]
- 72.Bhatnagar R, Maskell N. Indwelling pleural catheters for ambulatory out-patient care: A price worth paying? Respiration. 2013;86:181–2. doi: 10.1159/000354184. [DOI] [PubMed] [Google Scholar]
- 73.Tremblay A, Mason C, Michaud G. Use of tunnelled catheters for malignant pleural effusions in patients fit for pleurodesis. Eur Respir J. 2007;30:759–62. doi: 10.1183/09031936.00164706. [DOI] [PubMed] [Google Scholar]
- 74.Van Meter ME, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: A systematic review. J Gen Intern Med. 2011;26:70–6. doi: 10.1007/s11606-010-1472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahmed L, Ip H, Rao D, Patel N, Noorzad F. Talc pleurodesis through indwelling pleural catheters for malignant pleural effusions: Retrospective case series of a novel clinical pathway. Chest. 2014;146:e190–4. doi: 10.1378/chest.14-0394. [DOI] [PubMed] [Google Scholar]
- 76.Wrightson JM, Fysh E, Maskell NA, Lee YC. Risk reduction in pleural procedures: Sonography, simulation and supervision. Curr Opin Pulm Med. 2010;16:340–50. doi: 10.1097/MCP.0b013e32833a233b. [DOI] [PubMed] [Google Scholar]
- 77.Fysh ET, Wrightson JM, Lee YC, Rahman NM. Fractured indwelling pleural catheters. Chest. 2012;141:1090–4. doi: 10.1378/chest.11-0724. [DOI] [PubMed] [Google Scholar]
- 78.Fysh ET, Tremblay A, Feller-Kopman D, Mishra EK, Slade M, Garske L, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: An international multicenter study. Chest. 2013;144:1597–602. doi: 10.1378/chest.12-3103. [DOI] [PubMed] [Google Scholar]
- 79.Lui MM, Thomas R, Lee YC. Complications of indwelling pleural catheter use and their management. BMJ Open Respir Res. 2016;3:e000123. doi: 10.1136/bmjresp-2015-000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Penz ED, Mishra EK, Davies HE, Manns BJ, Miller RF, Rahman NM. Comparing cost of indwelling pleural catheter vs. talc pleurodesis for malignant pleural effusion. Chest. 2014;146:991–1000. doi: 10.1378/chest.13-2481. [DOI] [PubMed] [Google Scholar]
- 81.Puri V, Pyrdeck TL, Crabtree TD, Kreisel D, Krupnick AS, Colditz GA, et al. Treatment of malignant pleural effusion: A cost-effectiveness analysis. Ann Thorac Surg. 2012;94:374–9. doi: 10.1016/j.athoracsur.2012.02.100. [DOI] [PMC free article] [PubMed] [Google Scholar]