Abstract
Neuroendocrine tumors which have the potential to secrete catecholamines are either associated with sympathetic adrenal (pheochromocytoma) or nonadrenal (paraganglioma) tissue. Surgical removal of these tumors is always indicated to cure and prevent cardiovascular and other organ system complications associated with catecholamine excess. Some of these tumors have malignant potential as well. The diagnosis, localization and anatomical delineation of these tumors involve measurement of catecholamines and their metabolic end products in plasma and urine, 123I-metaiodobenzylguanidine scintigraphy, computed tomography, and/or magnetic resonance imaging. Before surgical removal of the tumors, the optimization of blood pressure, as well as intravascular volume, is an important measure to avoid and suppress perioperative adverse hemodynamic events. Preoperative preparation includes the use of alpha-adrenergic antagonists, beta-adrenergic antagonists with or without other antihypertensive agents, fluid therapy as well as insulin therapy for hyperglycemia if required. Due attention should be given to type and dose of alpha-receptor antagonists to be used and the duration of this therapy to achieve an optimal level of preoperative “alpha-blockade.” Despite this preoperative preparation, many patients will have hypertensive crises intraoperatively which need to be promptly and carefully managed by the anesthesia team which requires intensive and advanced monitoring techniques. The most common complication after tumor removal is hypotension which may require fluid therapy and vasopressor support for a few hours. With advancement in surgical and anesthetic techniques, the incidence of severe morbidity and mortality associated with the surgery is low in high volume centers.
INTRODUCTION
The 2004 WHO classification of endocrine tumors defines pheochromocytoma (PCC) as a catecholamine-producing intra-adrenal tumor arising from the chromaffin cells (intra-adrenal paraganglioma [PGL]). The related tumors of extra-adrenal sympathetic and parasympathetic paraganglia are classified as extra-adrenal PGLs.[1] The most important functional characteristics of the adrenal and extra-adrenal sympathetic tissue derived tumors is the production of various types of catecholamines and its related clinical attributes. In this review, the term PCC will be used for adrenal tumors, and the term PGL will be used for extra-adrenal tumors. PCCs form almost 80% to 85% of these tumors. The most common site of origin of PGLs is the organ of Zuckerkandl, the chromaffin tissue surrounding the inferior mesenteric artery and the bifurcation of aorta. Other sites of origin of PGL include infra-diaphragmatic para-aortic region, mediastinal thoracic sympathetic chain, and urinary bladder.[2] A few unusual sites reported are urethra, prostate, spermatic cord, genital tract, liver, and heart.[3,4] The site of origin of the catecholamine-secreting tumors is important to delineate not just to aid in surgical dissection but also to prognosticate the malignant potential of the tumors which is different according to the site of origin.
The classic association of 10% of PCCs being malignant, 10% being bilateral, 10% being extra-adrenal (of which 10% are extra-abdominal), 10% in nonhypertensive patients and 10% being hereditary does not hold true for these tumors anymore. Many of these traditionally associated numbers have changed as more and more facts about them have been unveiled by newer research. With more and more germline mutations being recognized, up to 40% of cases of PCC and PGL are now known to be attributed to these genetic alterations.[5] Hereditary syndromes have higher proportion of bilateral PCCs as compared to sporadic occurrences. The malignant potential of both PCC and PGL is confirmed by their metastasis rather than their histological features. Depending on the underlying mutation and the definition of malignant disease, various reports mention estimated rates of malignancy between 5% and 26% and even higher when associated with germline mutations.[6,7,8,9] Features suggestive of malignancy are seen 3–15 times more in PGL than in PCC.[10]
Surgery, wherever feasible, is the treatment of choice for these tumors and is curable for more than 90% of patients. The management of patients, however, requires a multi-disciplinary approach with involvement of endocrinologists, surgeons, and anesthesiologists. Improved awareness of the need for preoperative optimization of patients’ signs and symptoms in decreasing the perioperative complications has led to well-established protocols in various institutes which cater to these patients. Considerable improvement in surgical and anesthetic techniques also has a major role in decreasing the morbidity and mortality historically associated with patients undergoing surgical removal of these tumors.
The following review details the current practices prevalent in the perioperative management of patients undergoing surgery for removal of PCC and PGL.
CLINICAL PRESENTATION AND DIAGNOSIS
PCC and PGLs are associated with a gamut of symptomatology. About 10–40% of these tumors are found incidentally and may not be related to any clinical symptoms. The classic symptomatology, i.e. paroxysms of hypertension, palpitation with diaphoresis is present in only 40% of the patients. Effects of long standing and severe hypertension can be seen as damage to the end organs especially heart, kidney, eyes, and central nervous system.[11,12] Diabetes or deranged glucose metabolism may be present in up to 30–50% of the patients.[12,13]
The clinical features in the patients are somewhat related to the type and amount of catecholamine secreted by the tumor as well. These tumors may secrete epinephrine (E), norepinephrine (NE), or dopamine in either a continuous manner (leading to sustained hypertension) or episodic pattern (leading to paroxysms of symptoms).
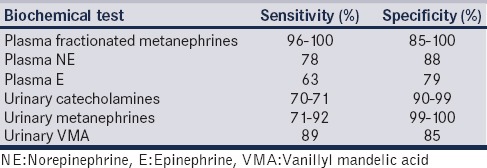
Biochemical tests are recommended and required for confirmation of the tumor. Within the chromaffin cells, NE and E are metabolized to normetanephrine and metanephrine. A rise in plasma metanephrines is thus indicative of increased tumoral production of catecholamines and forms the basis of the high sensitivity and specificity of this diagnostic test as compared to the measurement of parent catecholamine in the plasma which is dependent on their secretion from the tumor [Table 1].[14,15,16] Measurement of urinary fractionated metanephrines are also highly sensitive but offer low specificity.[17] Measurement of urinary and plasma vanillylmandelic acid (VMA) and catecholamine levels can also be used as a screening and diagnostic tests for these tumors. Measurement of urinary VMA is inexpensive and easy to perform by colorimetry and is, thus, useful as an initial screening test.[18]
Table 1.
Sensitivity and specificity of various biochemical tests useful in patients with pheochromocytoma and paraganglioma
Imaging studies are important for tumor localization and lineation of its extent. They are also important in diagnosing multiple primary tumors and/or metastatic lesions in patients with various genetic disorders. The approach to surgical removal may depend considerably on the location, extent, and the association of the tumor with nearby anatomical structures. Imaging studies, especially functional studies are also useful adjuncts in confirming diagnosis of the tumor when biochemical investigations are ambiguous or not available in patients with clinical suspicion of the tumor. Computed tomography (CT), contrast enhanced CT, and magnetic resonance imaging are now routinely complemented by functional imaging using various radiotracers such as 123I-metaiodobenzylguanidine (123I-MIBG) and 111In-DTPA-pentetreotide.[19,20,21]
PREOPERATIVE PREPARATION
The most important factor that has drastically reduced the perioperative morbidity and mortality in these patients is the meticulous preoperative preparation that is undertaken.[22] The effect of catecholamines, NE and E, is brought about by their action on various sympathetic receptors, alpha and beta. Preoperative preparation or optimization encompasses negation of the alpha-1 mediated vasoconstriction and beta-1 mediated tachycardia and inotropy. Both these effects lead to hypertension. A few authors have questioned the need for obligatory preoperative antihypertensive therapy.[23] Most of the recent evidence, however, points toward decreased intra- and post-operative cardiovascular complications in patients who have received some medical therapy for control of signs and symptoms of catecholamine excess.[24,25,26] Preoperative alpha-blockade, as well as fluid and salt intake, is recommended by the Endocrine Society Clinical Guidelines Subcommittee for patients undergoing PCC and PGL resection.[27] The strict protocol based preoperative antihypertensive therapy, however, has recently been criticized, and use of a more flexible approach modified according to patient characteristics has been advocated.[28] The course of treatment is well-delineated in patients who are symptomatic, however, controversy still exists on need for antihypertensive therapy in asymptomatic patients.[29]
In symptomatic patients, the mainstay of “preparation” or “optimization” includes control of hypertension and vascular expansion.
Control of hypertension
Hypertension in these patients may be either paroxysmal with baseline normal blood pressure, baseline elevated blood pressure with intermittent paroxysms or persistently increased blood pressure. The primary cause of hypertension is alpha-receptor activation by the catecholamines. Alpha-receptor antagonists are, thus, the initial choice of drugs for control of hypertension. Other drugs, such as beta-receptor antagonists and calcium channel blockers, also have their place in the preoperative control of symptoms.
Alpha-receptor antagonists
Nonselective, irreversible, alpha-receptor antagonists
Phenoxybenzamine is the prototype nonselective and noncompetitive alpha-receptor antagonist drug being used for this specific indication. It has been used for alpha blockade in patients with PCC and PGL as far back as early 50's ever since the advantages of preoperative preparation in decreasing perioperative morbidity and mortality were recognized. Phenoxybenzamine is initiated at doses of 10 mg every 6–12 h and increased to 30–40 mg every 6 h to a maximum dose of 240 mg/day. It causes irreversible inactivation of alpha-receptors (both alpha-1 and alpha-2) by covalently bonding to the receptor molecule. Receptors need to be formed anew for reversal of the effect of phenoxybenzamine which may take up to 24–48 h after stopping the drug. The mechanism of action of phenoxybenzamine implies that the intraoperative hemodynamics are better controlled during tumor manipulation when patients are prepared with this drug while the incidence of postoperative hypotension requiring vasopressor infusion is usually more as compared to patients prepared with selective alpha-1antagonists. Studies, however, disproving[30,31] and asserting[32,33] both the assumptions exist. The drug crosses blood-brain barrier and leads to inactivation of centrally located alpha-1 and alpha-2 receptors and causes side effects such as headache and drowsiness. The other side-effects of phenoxybenzamine such as orthostatic hypotension, tachycardia, dizziness, and syncope are also more morbid and more profound than that seen in patients on selective alpha-1 receptor antagonists. This, along with the fact that phenoxybenzamine has now been withdrawn from a few countries, has now resulted in physicians favoring selective alpha-1 receptor antagonists for preoperative preparation in patients with PCC and PGL.[28]
Selective alpha-1 receptor antagonists
The selective alpha-1 receptor antagonist drugs available are prazosin, doxazosin, and terazosin. The pharmacodynamics properties of all three drugs are given in Table 2. Selective alpha-1antagonists preferentially act on the alpha-1 receptors and cause vasodilatation. Since the alpha-2 receptors are spared the presynaptic release of NE is not enhanced, and thus severe tachycardia is avoided. The vasodilatation due to apha-1 receptor antagonism will also lead to tachycardia but a lesser degree than that seen with phenoxybenzamine. The antagonism by this class of drugs is reversible and depending on the pharmacodynamics of these drugs prolonged hypotension after tumor isolation is usually not seen. The commonest side effects seen with this class of drugs are vertigo, dizziness, malaise, mild headache, and gastrointestinal symptoms such as nausea, gastralgia, diarrhea, or vomiting. Postural hypotension can be quite severe especially with the initial doses; hence, the drug is usually started at bedtime and in low doses. Syncope, tachycardia, palpitations, fatigue, drowsiness, rash, flushes are the rarely encountered side-effects.[34]
Table 2.
Pharmacokinetic properties of alpha-receptor antagonists used for preoperative control of hypertension in patients with pheochromocytoma and paraganglioma

Prazosin is the most common used drug for this indication. The therapy with prazosin is usually initiated at 0.5–1 mg per dose every 4–6 h and titrated to a maximum of 20–24 mg/day. Many studies and reports have described good preoperative control of symptoms and adequate intraoperative alpha-blockade in patients prepared with prazosin preoperatively.[31,32,33]
Doxazosin is a longer acting drug and thus is usually required as once daily or twice daily dose. Despite being a longer acting drug refractory, hypotension after tumor removal requiring large amounts of intravenous fluids and vasopressor support is significantly less in patients pretreated with this drug as compared to patients who receive phenoxybenzamine.[35,36] Doxazosin is initiated at the dose of 1–2 mg/day and titrated to control of blood pressure up to a maximum dose of 16 mg/day.
Terazosin is also initiated at a dose of 1 mg/day and can be increased up to a maximum of 20 mg/day depending on goals of blood pressure control. It has a shorter half-life than doxazosin. Preoperative control of blood pressure for patients with PCC has been described in a few reports and it may be a suitable alternative to more commonly used prazosin and doxazosin.[37]
Beta-receptor antagonists
Beta-blockers should never be used before initiation of alpha-blockade in patients with functional tumors as suppression of beta-1 mediated cardiac sympathetic drive before adequate arteriolar dilatation can lead to acute cardiac insufficiency and pulmonary edema.[38,39] Added therapy with beta-receptor antagonists is required to counteract the tachycardia induced by nonselective alpha-blockade or due to vasodilatation induced increase in heart rate. Tachycardia is also seen commonly in patients with E secreting tumors. The presence of arrhythmias, features of myocardial ischemia and cardiomyopathy due to excessive catecholamine secretion also warrant their use. Cardioselective beta-antagonists are desirable and have less side-effect than nonselective beta-antagonists. Various beta-blockers used for this indication and their doses are given in Table 3. Labetalol has both α-and beta-receptor blocking activity and may be used in lieu of pure beta-antagonists (but never as an alternative to alpha-antagonists).[40] Labetalol reduces the uptake of 131I-MIBG and needs to be stopped 2 weeks before131 I-MIBG scintigraphy to avoid false negative test results.[41]
Table 3.
Commonly used beta-blockers for preoperative control of hypertension and tachycardia in patients with pheochromocytoma and paraganglioma

Calcium channel blockers
Calcium channel blockers have been used as primary therapy for blood pressure control[42,43] or as adjunct to alpha-antagonists[33,44] for preoperative optimization in patients with PCC and PGL. They are especially useful in normotensive patients and patients with paroxysmal hypertension with no elevation in baseline blood pressure.[26] Amlodipine (5–20 mg/day), nicardipine (60–90 mg/day), nifedipine (30–90 mg/day), verapamil (180–540 mg/day), and diltiazem (90–240 mg/day) are the commonly used calcium channel blockers.[40,44]
Vascular expansion
The catecholamines cause intense vasoconstriction through the alpha-1 receptors and initiation of alpha-blockade can lead to severe orthostatic hypotension. To counteract this hypotension patients are advised to increase fluid and salt intake. A patient may take 2–3 L of fluid (even more if acceptable to the patient) orally with 5–10 g of salt to increase the intravascular volume. If oral fluid and salt intake do not improve the orthostatic hypotension while the blood pressure of the patient still warrants antihypertensive therapy, crystalloids and colloids may be given intravenously. Serial hematocrit measurements give a guide to the effectiveness of volume expansion. Usually, a 5–10% fall in hematocrit is seen in well prepared patients. The fall in hematocrit is more a guide to the therapy rather than an end point for adequate volume expansion.
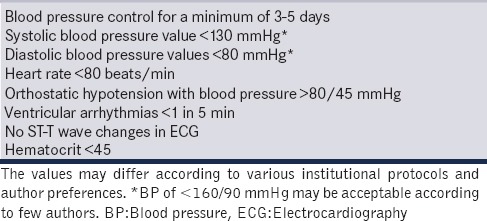
A patient may require 5–15 days of preoperative preparation with optimal alpha blocking drugs, increased oral fluids and salt intake and/or intravenous fluids before being “accepted” for surgery. The Endocrine Society Clinical Practice Guidelines also recommend a high-sodium diet and fluid intake to reverse catecholamine-induced blood volume contraction preoperatively and to prevent severe hypotension after tumor removal.[27] Monitoring and appropriate therapy for diabetes are also initiated in the preoperative period with oral hypoglycemic agents and/or insulin. Patients also need to undergo a battery of investigations to assess effect of catecholamine excess on end organs. The end organ most commonly affected is the heart, and due consideration should be paid to seek features of catecholamine or ischemia-induced cardiomyopathy. The goals of alpha-blockade have been arbitrarily defined by many authors [Table 4].[40,45,46] Clinically, however, these endpoints of alpha-blockade may not be clearly expressed due to the extremely variable features of the disease.
Table 4.
Goals of preoperative alpha-blockade
ANAESTHETIC MANAGEMENT
All patients will require general anesthesia with endotracheal intubation irrespective of the type of surgical approach. Epidural catheter is usually inserted in patients undergoing open surgical removal of tumor. Apart from the basic standards of monitoring as recommended by American Society of Anaesthesiologists, invasive blood pressure (IBP) monitoring is imperative in these patients. Normotensive patients and patients with incidentalomas also need IBP monitoring for immediate diagnosis and treatment of intraoperative hypertensive spikes. Hemodynamic instability, while customary in patients with overt PCC and PGLs, is not unusual in patients with adrenal incidentalomas either. In a retrospective study involving intraoperative hemodynamic behavior and postoperative outcome in patients with incidentalomas, almost half of the patients had hemodynamic instability during handling of the tumor and PCC was suspected in 26% of the patients.[47] A close watch on the blood pressure is thus warranted and mandatory in these patients.
A central venous access is also desirable in these patients. This should ideally be acquired in a large bore vein, internal jugular, axillary, or subclavian with a multi-lumen catheter. Central venous access helps in guiding the fluid therapy in these apparently vasoconstricted (explained above) patients as well as provides access to central vascular compartment for infusion of vasodilators and vasoconstrictors when required. Insertion of central venous catheter, however, may not be mandatory in all patients. Many authors have described the management of these patients without central venous catheterization as well.[33,48] On the other hand, measurement of pulmonary capillary wedge pressure using Swan-Ganz catheters or other accurate methods to estimate cardiac filling pressures and function may be needed in patients with catecholamine or hypertension induced severe cardiomyopathy.[26,49] Usefulness of noninvasive methods for cardiac output estimation and stroke volume variation to diagnose fluid deficit has also been revealed in patients with PCC recently.[50,51] Fluid therapy is a vital but complex component of perioperative management in patients with PCC and PGL. While under-hydration will lead to severe hypotension after tumor resection over-hydration can lead to pulmonary edema and congestive heart failure in an already compromised heart.
The main complication anticipated during surgery is the hemodynamic instability, hypertension before tumor removal and hypotension after tumor isolation. Management of hypertension should be done with short acting and potent vasodilators. NE secretion will lead to intense hypertension with either bradycardia or tachycardia, the former being more common. Epinephrine secretion usually causes severe tachycardia but hypertension of lesser magnitude. Sodium nitroprusside and nitroglycerine are the two drugs which are commonly used for intraoperative control of hypertension and have established safety profile. Esmolol, a short-acting beta-receptor antagonist, is a useful adjunct to vasodilators for control of intraoperative hypertension and tachycardia. Many reports have tried to elucidate the factors which can affect the number and severity of these hypertensive episodes.[48,52,53] Anesthetic drugs,[23,38] tumor size[52,54] and site,[55] associated genetic syndrome,[56] plasma catecholamine levels,[52,57] and type of surgical approach[54,55,58] may influence the intraoperative hemodynamic stability in these patients. Bleeding is another important concern especially during resection of PGL situated in the intra-aortocaval groove which may further confound the complex hemodynamic management of these patients.
POSTOPERATIVE MANAGEMENT
The postoperative management will usually require an intensive care or high dependency unit admission. Once the tumor is isolated the withdrawal of catecholamine effect will result in hypotension. Fluid loading along with vasopressor infusion is required to counteract the hypotension. The incidence of hypotension is variably described as 20–70% in various reports and may somewhat be dependent on the use of nature of preoperative alpha-antagonist and intraoperative hypotensive agents.[32] Vasopressor/s infusion is usually required for a short duration only although reports of refractory and prolonged vasopressor use in the postoperative period do exist. Sudden catecholamine withdrawal after tumor removal also leads to rebound hyperinsulinemia which along with already depleted glycogen stores can lead to severe hypoglycemia in the postoperative period. Hourly blood sugar monitoring, at least for the initial 12–24 h of the postoperative period, is mandatory after the surgery.
CONCLUSION
Surgical removal of PCC and PGL alleviates the signs, symptoms and probable end organ damage due to catecholamine hypersecretion. Preoperative optimization has played an important role in decreasing the high incidence of perioperative morbidity and mortality historically associated with these surgical procedures. Alpha-receptors blockade, especially with selective alpha-1 receptor antagonists, is thus vital in these patients preoperatively. Adjunct antihypertensive therapy includes beta-antagonists and calcium channel blockers. Adequate vascular volume replacement with oral fluids and salt is essential to avoid exaggerated fall in blood pressure perioperatively. Intensive hemodynamic monitoring instituted intraoperatively may need to be continued in the postoperative period as well to tide over the initial period of hypotension after tumor removal.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Pacak K, Eisenhofer G, Ahlman H, Bornstein SR, Gimenez-Roqueplo AP, Grossman AB, et al. Pheochromocytoma: Recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab. 2007;3:92–102. doi: 10.1038/ncpendmet0396. [DOI] [PubMed] [Google Scholar]
- 2.Baez JC, Jagannathan JP, Krajewski K, O’Regan K, Zukotynski K, Kulke M, et al. Pheochromocytoma and paraganglioma: Imaging characteristics. Cancer Imaging. 2012;12:153–62. doi: 10.1102/1470-7330.2012.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pacak K, Koch CA, Wofford MR, Ayala AR. In: Overview of endocrine hypertension. Endotext. De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, et al., editors. South Dartmouth, MA: MDText.com, Inc; 2000. [Last updated on 2009 Oct 21]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK278980 . [Google Scholar]
- 4.Liu S, Horne D, Freed DH, Sookhoo S, Strzelczyk J, Ravandi A, et al. Multimodality imaging of a cardiac pheochromocytoma. J Am Coll Cardiol. 2014;63:e189. doi: 10.1016/j.jacc.2013.06.075. [DOI] [PubMed] [Google Scholar]
- 5.Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: Learning from genetic heterogeneity. Nat Rev Cancer. 2014;14:108–19. doi: 10.1038/nrc3648. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein RE, O’Neill JA, Jr, Holcomb GW, 3rd, Morgan WM, 3rd, Neblett WW, 3rd, Oates JA, et al. Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229:755–64. doi: 10.1097/00000658-199906000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edström Elder E, Hjelm Skog AL, Höög A, Hamberger B. The management of benign and malignant pheochromocytoma and abdominal paraganglioma. Eur J Surg Oncol. 2003;29:278–83. doi: 10.1053/ejso.2002.1413. [DOI] [PubMed] [Google Scholar]
- 8.Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, et al. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63:5615–21. [PubMed] [Google Scholar]
- 9.Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NK, Dahia PL, de Krijger RR, et al. Malignant pheochromocytoma: Current status and initiatives for future progress. Endocr Relat Cancer. 2004;11:423–36. doi: 10.1677/erc.1.00829. [DOI] [PubMed] [Google Scholar]
- 10.Barakat MT, Meeran K, Bloom SR. Neuroendocrine tumours. Endocr Relat Cancer. 2004;11:1–18. doi: 10.1677/erc.0.0110001. [DOI] [PubMed] [Google Scholar]
- 11.Kudva YC, Young WF, Jr, Thompson GB, Grant CS, van Heerden JA. Adrenal incidentaloma: An important component of the clinical presentation spectrum of benign sporadic adrenal pheochromocytoma. Endocrinologist. 1999;9:77–80. [Google Scholar]
- 12.Baguet JP, Hammer L, Mazzuco TL, Chabre O, Mallion JM, Sturm N, et al. Circumstances of discovery of phaeochromocytoma: A retrospective study of 41 consecutive patients. Eur J Endocrinol. 2004;150:681–6. doi: 10.1530/eje.0.1500681. [DOI] [PubMed] [Google Scholar]
- 13.Pogorzelski R, Toutounchi S, Krajewska E, Fiszer P, Lykowski M, Zapala L, et al. The effect of surgical treatment of phaeochromocytoma on concomitant arterial hypertension and diabetes mellitus in a single-centre retrospective study. Cent European J Urol. 2014;67:361–5. doi: 10.5173/ceju.2014.04.art9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsiao RJ, Parmer RJ, Takiyyuddin MA, O’Connor DT. Chromogranin A storage and secretion: Sensitivity and specificity for the diagnosis of pheochromocytoma. Medicine (Baltimore) 1991;70:33–45. [PubMed] [Google Scholar]
- 15.Kudva YC, Sawka AM, Young WF., Jr Clinical review 164: The laboratory diagnosis of adrenal pheochromocytoma: The Mayo Clinic experience. J Clin Endocrinol Metab. 2003;88:4533–9. doi: 10.1210/jc.2003-030720. [DOI] [PubMed] [Google Scholar]
- 16.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, et al. Biochemical diagnosis of pheochromocytoma: Which test is best? JAMA. 2002;287:1427–34. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhofer G. Screening for pheochromocytomas and paragangliomas. Curr Hypertens Rep. 2012;14:130–7. doi: 10.1007/s11906-012-0246-y. [DOI] [PubMed] [Google Scholar]
- 18.Witteles RM, Kaplan EL, Roizen MF. Sensitivity of diagnostic and localization tests for pheochromocytoma in clinical practice. Arch Intern Med. 2000;160:2521–4. doi: 10.1001/archinte.160.16.2521. [DOI] [PubMed] [Google Scholar]
- 19.Jalil ND, Pattou FN, Combemale F, Chapuis Y, Henry JF, Peix JL, et al. Effectiveness and limits of preoperative imaging studies for the localisation of pheochromocytomas and paragangliomas: A review of 282 cases. French Association of Surgery (AFC), and the French Association of Endocrine Surgeons (AFCE) Eur J Surg. 1998;164:23–8. doi: 10.1080/110241598750004913. [DOI] [PubMed] [Google Scholar]
- 20.Pacak K, Eisenhofer G, Goldstein DS. Functional imaging of endocrine tumors: Role of positron emission tomography. Endocr Rev. 2004;25:568–80. doi: 10.1210/er.2003-0032. [DOI] [PubMed] [Google Scholar]
- 21.Timmers HJ, Taieb D, Pacak K. Current and future anatomical and functional imaging approaches to pheochromocytoma and paraganglioma. Horm Metab Res. 2012;44:367–72. doi: 10.1055/s-0031-1299712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plouin PF, Duclos JM, Soppelsa F, Boublil G, Chatellier G. Factors associated with perioperative morbidity and mortality in patients with pheochromocytoma: Analysis of 165 operations at a single center. J Clin Endocrinol Metab. 2001;86:1480–6. doi: 10.1210/jcem.86.4.7392. [DOI] [PubMed] [Google Scholar]
- 23.Lentschener C, Gaujoux S, Tesniere A, Dousset B. Point of controversy: Perioperative care of patients undergoing pheochromocytoma removal-time for a reappraisal? Eur J Endocrinol. 2011;165:365–73. doi: 10.1530/EJE-11-0162. [DOI] [PubMed] [Google Scholar]
- 24.Tauzin-Fin P, Sesay M, Gosse P, Ballanger P. Effects of perioperative alpha1 block on haemodynamic control during laparoscopic surgery for phaeochromocytoma. Br J Anaesth. 2004;92:512–7. doi: 10.1093/bja/aeh083. [DOI] [PubMed] [Google Scholar]
- 25.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–75. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 26.Kinney MA, Narr BJ, Warner MA. Perioperative management of pheochromocytoma. J Cardiothorac Vasc Anesth. 2002;16:359–69. doi: 10.1053/jcan.2002.124150. [DOI] [PubMed] [Google Scholar]
- 27.Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and paraganglioma: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–42. doi: 10.1210/jc.2014-1498. [DOI] [PubMed] [Google Scholar]
- 28.James M. The impact of changes in drug availability for hemodynamic management in pheochromocytoma: Prêt-à-porter or tailor-made? Can J Anaesth. 2015;62:1244–7. doi: 10.1007/s12630-015-0481-1. [DOI] [PubMed] [Google Scholar]
- 29.Shao Y, Chen R, Shen ZJ, Teng Y, Huang P, Rui WB, et al. Preoperative alpha blockade for normotensive pheochromocytoma: Is it necessary? J Hypertens. 2011;29:2429–32. doi: 10.1097/HJH.0b013e32834d24d9. [DOI] [PubMed] [Google Scholar]
- 30.Kocak S, Aydintug S, Canakci N. Alpha blockade in preoperative preparation of patients with pheochromocytomas. Int Surg. 2002;87:191–4. [PubMed] [Google Scholar]
- 31.Havlik RJ, Cahow CE, Kinder BK. Advances in the diagnosis and treatment of pheochromocytoma. Arch Surg. 1988;123:626–30. doi: 10.1001/archsurg.1988.01400290112020. [DOI] [PubMed] [Google Scholar]
- 32.Agrawal R, Mishra SK, Bhatia E, Mishra A, Chand G, Agarwal G, et al. Prospective study to compare peri-operative hemodynamic alterations following preparation for pheochromocytoma surgery by phenoxybenzamine or prazosin. World J Surg. 2014;38:716–23. doi: 10.1007/s00268-013-2325-x. [DOI] [PubMed] [Google Scholar]
- 33.Weingarten TN, Cata JP, O’Hara JF, Prybilla DJ, Pike TL, Thompson GB, et al. Comparison of two preoperative medical management strategies for laparoscopic resection of pheochromocytoma. Urology. 2010;76:508.e6–11. doi: 10.1016/j.urology.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 34.Desiniotis A, Kyprianou N. Advances in the design and synthesis of prazosin derivatives over the last ten years. Expert Opin Ther Targets. 2011;15:1405–18. doi: 10.1517/14728222.2011.641534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prys-Roberts C, Farndon JR. Efficacy and safety of doxazosin for perioperative management of patients with pheochromocytoma. World J Surg. 2002;26:1037–42. doi: 10.1007/s00268-002-6667-z. [DOI] [PubMed] [Google Scholar]
- 36.Miura Y, Yoshinaga K. Doxazosin: A newly developed, selective alpha 1-inhibitor in the management of patients with pheochromocytoma. Am Heart J. 1988;116(6 Pt 2):1785–9. doi: 10.1016/0002-8703(88)90230-x. [DOI] [PubMed] [Google Scholar]
- 37.Bongon J, Oliva R, Almelor L, Lantion-Ang FL. Terazosin as first line preoperative blockade in Filipino patients diagnosed with pheochromocytoma. J ASEAN Fed Endocr Soc. 2015;30:35. [Google Scholar]
- 38.Prys-Roberts C. Phaeochromocytoma – Recent progress in its management. Br J Anaesth. 2000;85:44–57. doi: 10.1093/bja/85.1.44. [DOI] [PubMed] [Google Scholar]
- 39.Sibal L, Jovanovic A, Agarwal SC, Peaston RT, James RA, Lennard TW, et al. Phaeochromocytomas presenting as acute crises after beta blockade therapy. Clin Endocrinol. 2006;65:186–90. doi: 10.1111/j.1365-2265.2006.02571.x. [DOI] [PubMed] [Google Scholar]
- 40.Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069–79. doi: 10.1210/jc.2007-1720. [DOI] [PubMed] [Google Scholar]
- 41.Solanki KK, Bomanji J, Moyes J, Mather SJ, Trainer PJ, Britton KE. A pharmacological guide to medicines which interfere with the biodistribution of radiolabelled meta-iodobenzylguanidine (MIBG) Nucl Med Commun. 1992;13:513–21. doi: 10.1097/00006231-199207000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Ulchaker JC, Goldfarb DA, Bravo EL, Novick AC. Successful outcomes in pheochromocytoma surgery in the modern era. J Urol. 1999;161:764–7. [PubMed] [Google Scholar]
- 43.Lebuffe G, Dosseh ED, Tek G, Tytgat H, Moreno S, Tavernier B, et al. The effect of calcium channel blockers on outcome following the surgical treatment of phaeochromocytomas and paragangliomas. Anaesthesia. 2005;60:439–44. doi: 10.1111/j.1365-2044.2005.04156.x. [DOI] [PubMed] [Google Scholar]
- 44.Sprung J, O’Hara JF, Jr, Gill IS, Abdelmalak B, Sarnaik A, Bravo EL. Anesthetic aspects of laparoscopic and open adrenalectomy for pheochromocytoma. Urology. 2000;55:339–43. doi: 10.1016/s0090-4295(99)00466-5. [DOI] [PubMed] [Google Scholar]
- 45.Kohl BA, Schwartz S. How to manage perioperative endocrine insufficiency. Anesthesiol Clin. 2010;28:139–55. doi: 10.1016/j.anclin.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Witteles RM, Kaplan EL, Roizen MF. Safe and cost-effective preoperative preparation of patients with pheochromocytoma. Anesth Analg. 2000;91:302–4. doi: 10.1097/00000539-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Hariskov S, Schumann R. Intraoperative management of patients with incidental catecholamine producing tumors: A literature review and analysis. J Anaesthesiol Clin Pharmacol. 2013;29:41–6. doi: 10.4103/0970-9185.105793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinney MA, Warner ME, vanHeerden JA, Horlocker TT, Young WF, Jr, Schroeder DR, et al. Perianesthetic risks and outcomes of pheochromocytoma and paraganglioma resection. Anesth Analg. 2000;91:1118–23. doi: 10.1097/00000539-200011000-00013. [DOI] [PubMed] [Google Scholar]
- 49.Kizer JR, Koniaris LS, Edelman JD, St John Sutton MG. Pheochromocytoma crisis, cardiomyopathy, and hemodynamic collapse. Chest. 2000;118:1221–3. doi: 10.1378/chest.118.4.1221. [DOI] [PubMed] [Google Scholar]
- 50.Mallat J, Pironkov A, Destandau MS, Tavernier B. Systolic pressure variation (Deltadown) can guide fluid therapy during pheochromocytoma surgery. Can J Anaesth. 2003;50:998–1003. doi: 10.1007/BF03018362. [DOI] [PubMed] [Google Scholar]
- 51.Matsuda Y, Kawate H, Shimada S, Matsuzaki C, Nagata H, Adachi M, et al. Perioperative sequential monitoring of hemodynamic parameters in patients with pheochromocytoma using the Non-Invasive Cardiac System (NICaS) Endocr J. 2014;61:571–5. doi: 10.1507/endocrj.ej13-0471. [DOI] [PubMed] [Google Scholar]
- 52.Tatsugami K, Eto M, Hamaguchi M, Yokomizo A, Harano M, Naito S. What affects the results of a laparoscopic adrenalectomy for pheochromocytoma. Evaluation with respect to intraoperative blood pressure and state of tumor? J Endourol. 2009;23:101–5. doi: 10.1089/end.2008.0279. [DOI] [PubMed] [Google Scholar]
- 53.Namekawa T, Utsumi T, Kawamura K, Kamiya N, Imamoto T, Takiguchi T, et al. Clinical predictors of prolonged postresection hypotension after laparoscopic adrenalectomy for pheochromocytoma. Surgery. 2016;159:763–70. doi: 10.1016/j.surg.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 54.Wang W, Li P, Wang Y, Wang Y, Ma Z, Wang G, et al. Effectiveness and safety of laparoscopic adrenalectomy of large pheochromocytoma: A prospective, nonrandomized, controlled study. Am J Surg. 2015;210:230–5. doi: 10.1016/j.amjsurg.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Hattori S, Miyajima A, Hirasawa Y, Kikuchi E, Kurihara I, Miyashita K, et al. Surgical outcome of laparoscopic surgery, including laparoendoscopic single-site surgery, for retroperitoneal paraganglioma compared with adrenal pheochromocytoma. J Endourol. 2014;28:686–92. doi: 10.1089/end.2013.0706. [DOI] [PubMed] [Google Scholar]
- 56.Scholten A, Vriens MR, Cromheecke GJ, Borel Rinkes IH, Valk GD. Hemodynamic instability during resection of pheochromocytoma in MEN versus non-MEN patients. Eur J Endocrinol. 2011;165:91–6. doi: 10.1530/EJE-11-0148. [DOI] [PubMed] [Google Scholar]
- 57.Bénay CE, Tahiri M, Lee L, Theodosopoulos E, Madani A, Feldman LS, et al. Selective strategy for intensive monitoring after pheochromocytoma resection. Surgery. 2016;159:275–82. doi: 10.1016/j.surg.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 58.Inabnet WB, Pitre J, Bernard D, Chapuis Y. Comparison of the hemodynamic parameters of open and laparoscopic adrenalectomy for pheochromocytoma. World J Surg. 2000;24:574–8. doi: 10.1007/s002689910094. [DOI] [PubMed] [Google Scholar]


