Abstract
Introduction:
The artificial urinary sphincter (AUS) is the mainstay of surgical treatment for male stress urinary incontinence. Although urethral erosions are a known complication, their temporal distribution and optimal management have not been well characterized. We seek to evaluate the timing, etiologies, and management of urethral erosions in primary AUS implantations.
Materials and Methods:
1802 male patients underwent AUS procedure at Mayo Clinic (Rochester) from 1983 to 2011, including 1082 primary placements. Of primary placements, 63 had a urethral erosion of their device requiring explanation and were included in our analysis. All cases of urethral erosion were confirmed at the time of explantation through cystoscopy and direct visualization. At our institution, explantation is typically performed without primary urethral repair.
Results:
There were 63 cases (5.8%) of urethral erosions of primary AUS devices during the study time frame. The median age at AUS implantation was 74 years (interquartile range [IQR] 68–77 years) and median time to explantation was 21 months (IQR 5–59 months). The temporal trend of AUS erosions demonstrates a peak in the 1st year, with a gradual tapering of cases thereafter, persisting beyond 10 years. Three of 36 (8.3%) patients with follow-up developed a urethral stricture. Overall, 32/63 patients (51%) underwent salvage AUS reimplantation at a median of 7.1 months (IQR 3.1–12.9 months).
Conclusions:
Urethral erosions tend to occur early (within 1–2 years), with gradual tapering over time. However, continued vigilance is needed after AUS placement to decrease late erosions. These data can be used for counseling and to help guide follow-up care of patients with AUS.
INTRODUCTION
Since its introduction in 1972, the artificial urinary sphincter (AUS) has been the gold standard treatment for the surgical management of severe male stress urinary incontinence.[1,2] While numerous studies demonstrate excellent long-term AUS success rates,[2,3,4] the development of a urethral cuff erosion in some patients is common to all reports. In fact, a recent pooled analysis demonstrated urethral cuff erosion in 8.5% of patients (range: 3.3%–27.8%).[2]
Despite the commonality of cuff erosions to the vast majority of AUS reports, data regarding the timing and etiology of these events are scarce. As AUS erosions requiring explantation is a known complication of the procedure, it is important for patient counseling and clinical decision-making to improve our understanding of these events. From the available literature, it appears that most of these events happen within the first 2 years after implantation[5] though late events have been reported.[3]
With regard to the management of urethral cuff erosion, there are little data to guide management, aside from recommending removal of all three components. Traditionally, the entire device is removed and a large bore catheter placed to facilitate urethral healing.[6,7,8] More recently, ventral urethroplasty at the time of explantation, in an attempt to decrease stricture formation, has been reported.[9]
We evaluated our experience with AUS urethral erosions to define trends in the temporal distribution of the events, potential underlying causes, describe our management technique and patient outcomes.
MATERIALS AND METHODS
After obtaining the Institutional Review Board approval, we identified 1802 male patients undergoing AUS implantation at a single institution from 1983 to 2011. Of these, 1082 (60%) were primary implantations. Among the primary implantations, 63 cases (5.8%) of urethral erosion were identified and comprised the study cohort. Exclusion criterion including patients with a neurogenic bladder, younger than 18 years of age or did not consent to database inclusion was applied before data acquisition.
All implanted devices were American Medical Systems 800 (American Medical Systems, Inc., Minnetonka, MN, USA) and placed through a perineal approach. All urethral cuffs were placed around the bulbar urethra, with cuff size and location (i.e., transcorporal) at the discretion of the treating surgeon. Of note, during primary AUS placement, we place the cuff around the bulbospongiosus muscle. All cuffs were 4.0 or greater. No 3.5 cuffs were utilized. AUS erosion was defined as perforation of the urethral cuff into the urethral lumen as verified in all cases either through preoperative cystoscopy [Figure 1a] with or without retrograde urethrogram, with intraoperative confirmation of the urethral defect during urethral irrigation at the time of explantation [Figure 1b]. At our institution, AUS urethral cuff erosion, regardless of the degree of erosion or comorbidities, is managed with removal of the urethral cuff and an indwelling urethral catheter for 4–6 weeks with no concomitant urethral reconstruction at the time of explantation. Patients are then evaluated with a pericatheter retrograde urethrogram at 6 weeks (before catheter removal).
Figure 1.
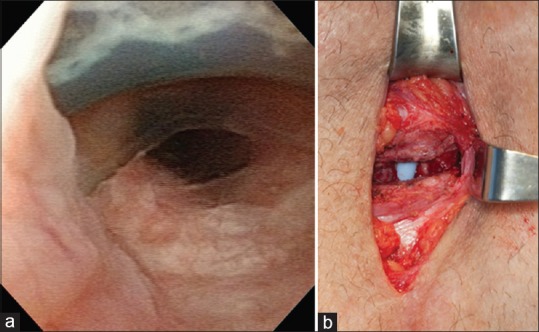
(a) Cystoscopic appearance of a dorsal 180° urethral artificial urinary sphincter cuff erosion. (b) Urethral defect secondary to cuff erosion as seen at the time of device explantation
Patient charts were retrospectively reviewed to evaluate patient age, history of prior pelvic radiation therapy, hypertension, diabetes mellitus, coronary artery disease, and body mass index (BMI). Likewise, operative reports were evaluated for details of the primary AUS implantation procedure and AUS explantation procedure.
Continuous variables are presented as mean (standard deviation) if they were normally distributed and as median (interquartile range [IQR]) if not normally distributed. Categorical variables are reported as number and percentage. The device erosion rate was calculated as the time from AUS implantation to AUS explantation for urethral erosion using the Kaplan–Meier method. All analyses were performed using JMP (version 10.0; SAS Institute Inc., Cary, NC, USA).
RESULTS
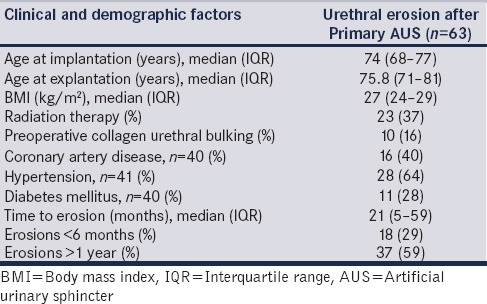
Of the 1082 patients undergoing primary AUS implantation, 63 patients (5.8%) subsequently underwent device explantation for urethral erosion. The clinical and demographic features of the 63 patients who underwent explantation in our study cohort are shown in Table 1. The median age at implantation was 74 years (IQR 68–77 years), and the median age at explant was 75.8 years (IQR 71–81 years). The median patient BMI at the time of AUS implantation was 27 kg/m2 (IQR 24–29).
Table 1.
Clinical and demographic factors of patients undergoing device explantation for artificial urinary sphincter cuff erosion
The median follow-up among the 1082 patients undergoing primary implantation was 4.1 years (IQR 0.8–7.7 years). The median time to erosion was 21 months (IQR 5–59 months). Overall, 18 of the 63 device erosions (26%) occurred within 6 months of AUS placement. Conversely, 37 device erosions (59%) occurred >1 year following placement. A failure curve evaluating the time from AUS placement to AUS erosion event is shown in Figure 2. While the majority of erosion events occurred relatively early (<2 years), urethral erosions continued to present even 10 years after AUS placement. Traumatic catheterization was one of the etiologies for late erosions among these patients (n = 10) with a median time to explantation of 74 months (IQR 43–108 months), and was a cause of 26% of erosions occurring >1 year from primary AUS implantation. None of these catheterizations were performed at our institution and were often performed by non-urologist providers at outside institutions.
Figure 2.
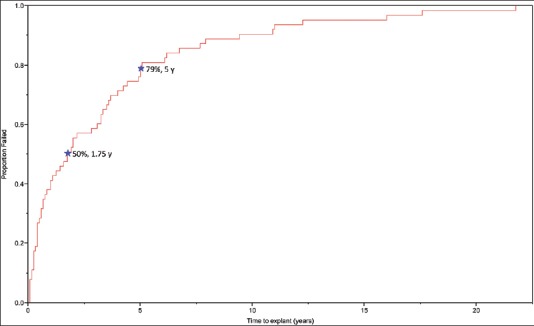
Time to artificial urinary sphincter explantation secondary to urethral cuff erosions
Following device explantation, all patients were managed with an indwelling catheter for 4–6 weeks. No urethral repair was performed at the time of explantation in 58 of 63 patients while 4 had a urethroplasty and 1 had urethral ligation with suprapubic tube placement. A pericatheter retrograde urethrogram or cystoscopy after device explantation was available for 36 of 58 patients managed with a catheter alone. None of them had a prior history of urethral stricture disease. Three of these 36 (8.3%) developed a urethral stricture and only one required subsequent management (primary bulbar urethroplasty). The remaining 33 did not develop a urethral stricture, and 22 of these patients underwent salvage AUS placement. Overall, 32 of the 63 patients (51%) subsequently underwent salvage AUS reimplantation at our institution, at a median of 7.1 months (IQR 3.1–12.9 months) after device explantation. Nine of the 32 salvage devices required subsequent explantation at a median of 5 months (IQR 4–42 months). The remaining 31 patients did not undergo salvage AUS reimplantation, either due to personal choice or due to other factors (poor urethral tissues, decrease in dexterity/cognition, etc.).
DISCUSSION
In this large series of men undergoing AUS device explantation for urethral erosion, we found that while the majority of events happen early (within 2 years), late events persist even beyond 10 years. Importantly, we found that a high rate (16%) of our AUS erosions was secondary to traumatic catheterization, which is an area for improving care and preventing patient morbidity. In addition, the management of AUS urethral erosions without urethroplasty appears to have a low de novo stricture rate (8.3%) and facilitates an acceptable rate of salvage AUS reimplantations (51%). Our overall rate of cuff erosion, 5.8% (63/1082), is consistent with the recently reported pooled analysis (8.5%, range 3.3%–27.8%)[2] though direct comparisons are difficult, given disparate patient population and variable follow-up between the series.
Our data suggest an early peak of explanations within 1–2 years of implantation, with a decreasing, but persistent rate over time. We hypothesize three major mechanisms for cuff erosion. The first consideration would be urethral injury or shallow dissection at the time of AUS implantation leading to early erosion. It would be expected that if this was the mechanism patients would present in the first few months after surgery. A second consideration would be poor urethral tissues secondary to comorbidities which lead to tissue atrophy or necrosis and eventual erosion. The last category would be sporadic and potentially preventable events, such as catheterization.
Traumatic and inappropriate catheterizations are an important consideration given the significant morbidity associated with cuff erosion. That is, a urethral cuff erosion necessitates a repeat operation for device explantation and impacts the patients future continence status as roughly 50% of patients in our series underwent salvage AUS implantation after an erosion. In addition, those who do undergo salvage reimplantation have a higher rate of repeat infection/erosion than primary implantations.[7,10] In our series, 26% of urethral erosions after 1 year were possibly preventable, being related to traumatic and/or inappropriate catheterization techniques at outside institutions. Similar to our results, this has been identified in the literature as a preventable cause of urethral erosion of AUS and stresses the need of patients and health-care professionals to be aware of these devices.[11,12] Several strategies have been proposed to help decrease the rate of traumatic catheterizations including: Patient education, medical alert bracelets or necklaces, and informing patients with an AUS to contact the urologist who placed the device to discuss catheter placement before a catheterization attempt.[11]
The goal of these patient instructions is that if catheterization is warranted, the AUS device should be completely deactivated and a small-caliber catheter (we typically use a 12 French catheter) should be used.[11,12] However, we discovered in our series that in some cases, when the patient informed the caregiver of their AUS and a urologist was involved, the cuff was inappropriately deactivated and/or the patient experienced prolonged catheterization with large-sized catheters. This may indicate a lack of understanding of the AUS, how it functions, and the serious ramifications of improper catheter use, considering that 26% of our erosions were preventable which represents major morbidity to the patient and an area for improvement in patient care.
For erosions in this series, the urethra was managed with a urinary catheter only and no urethroplasty at the time of AUS explantation in 92% of cases. The de novo stricture rate was 8.3%. Our results differ from that presented in a recent small series of patients treated with catheter-only (85%, n = 13) or ventral urethroplasty (38%, n = 13) at the time of device explantation.[9] In this comparative series, 54% of patients with a urethroplasty underwent AUS reimplantation, which is similar to the 51% we observed in this series with catheter-only management. Another recent series demonstrated an increased risk of postexplantation stricture rate (38.5% vs. 5%) for catheter management of erosions with >50% urethral circumference involvement compared to those with <50% involvement. The overall stricture rate reported in the study was 17%.[13] These differences may be secondary to disparate patient populations, differences in the degree of urethral injury, surgical selection bias, or follow-up.
Limitations of our study, including its nonrandomized, retrospective design should be noted. As a tertiary care center, some patients may have had additional care with their local providers which could impact our results. We attempt to capture this by yearly mailed follow-up surveys through the Mayo Clinic AUS registry. However, this mailing does not evaluate for interval stricture development. The traumatic catheterizations were all done at outside institutions, and further details of these events are very limited. Furthermore, while we present a large cohort for the clinical scenario evaluated, given the rarity of this entity, it is still rather limited in its scope. As such, further studies, ideally in a multi-institutional setting, are needed to better define the optimal management of AUS cuff erosions.
CONCLUSIONS
Urethral erosions tend to occur early (with 1–2 years) after implantation, with gradual tapering over time. Continued vigilance even beyond the first 2 years is needed after AUS placement (both from the patient and providers) in an effort to decrease late erosions, especially those secondary to traumatic catheterization. Health-care professionals and urologists must be better informed with the AUS and ramifications of improper catheterization and work in a collaborative fashion to avoid these events. These data can be used for counseling and to help guide follow-up care of patients with AUS.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Scott FB, Bradley WE, Timm GW. Treatment of urinary incontinence by an implantable prosthetic urinary sphincter. J Urol. 1974;112:75–80. doi: 10.1016/s0022-5347(17)59647-0. [DOI] [PubMed] [Google Scholar]
- 2.Van der Aa F, Drake MJ, Kasyan GR, Petrolekas A, Cornu JN Young Academic Urologists Functional Urology Group. The artificial urinary sphincter after a quarter of a century: A critical systematic review of its use in male non-neurogenic incontinence. Eur Urol. 2013;63:681–9. doi: 10.1016/j.eururo.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Léon P, Chartier-Kastler E, Rouprêt M, Ambrogi V, Mozer P, Phé V. Long-term functional outcomes after artificial urinary sphincter implantation in men with stress urinary incontinence. BJU Int. 2015;115:951–7. doi: 10.1111/bju.12848. [DOI] [PubMed] [Google Scholar]
- 4.Linder BJ, Rivera ME, Ziegelmann MJ, Elliott DS. Long-term outcomes following artificial urinary sphincter placement: An analysis of 1082 cases at Mayo Clinic. Urology. 2015;86:602–7. doi: 10.1016/j.urology.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Linder BJ, Viers BR, Ziegelmann MJ, Rivera ME, Rangel LJ, Elliott DS. Artificial urinary sphincter mechanical failures-is it better to replace the entire device or just the malfunctioning component? J Urol. 2016;195:1523–8. doi: 10.1016/j.juro.2015.10.084. [DOI] [PubMed] [Google Scholar]
- 6.Flynn BJ, Webster GD. Evaluation and surgical management of intrinsic sphincter deficiency after radical prostatectomy. Rev Urol. 2004;6:180–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Linder BJ, de Cogain M, Elliott DS. Long-term device outcomes of artificial urinary sphincter reimplantation following prior explantation for erosion or infection. J Urol. 2014;191:734–8. doi: 10.1016/j.juro.2013.08.089. [DOI] [PubMed] [Google Scholar]
- 8.Kowalczyk JJ, Nelson R, Mulcahy JJ. Successful reinsertion of the artificial urinary sphincter after removal for erosion or infection. Urology. 1996;48:906–8. doi: 10.1016/s0090-4295(96)00245-2. [DOI] [PubMed] [Google Scholar]
- 9.Rozanski AT, Tausch TJ, Ramirez D, Simhan J, Scott JF, Morey AF. Immediate urethral repair during explantation prevents stricture formation after artificial urinary sphincter cuff erosion. J Urol. 2014;192:442–6. doi: 10.1016/j.juro.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Brant WO, Erickson BA, Elliott SP, Powell C, Alsikafi N, McClung C, et al. Risk factors for erosion of artificial urinary sphincters: A multicenter prospective study. Urology. 2014;84:934–8. doi: 10.1016/j.urology.2014.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandhu JS. Artificial urinary sphincter: The workhorse for treatment of male stress urinary incontinence. Eur Urol. 2013;63:690–1. doi: 10.1016/j.eururo.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Anusionwu II, Wright EJ. Indications for revision of artificial urinary sphincter and modifiable risk factors for device-related morbidity. Neurourol Urodyn. 2013;32:63–5. doi: 10.1002/nau.22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chertack N, Chaparala H, Angermeier KW, Montague DK, Wood HM. Foley or fix: A comparative analysis of reparative procedures at the time of explantation of artificial urinary sphincter for cuff Erosion. Urology. 2016;90:173–8. doi: 10.1016/j.urology.2015.11.040. [DOI] [PubMed] [Google Scholar]


