Abstract
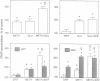
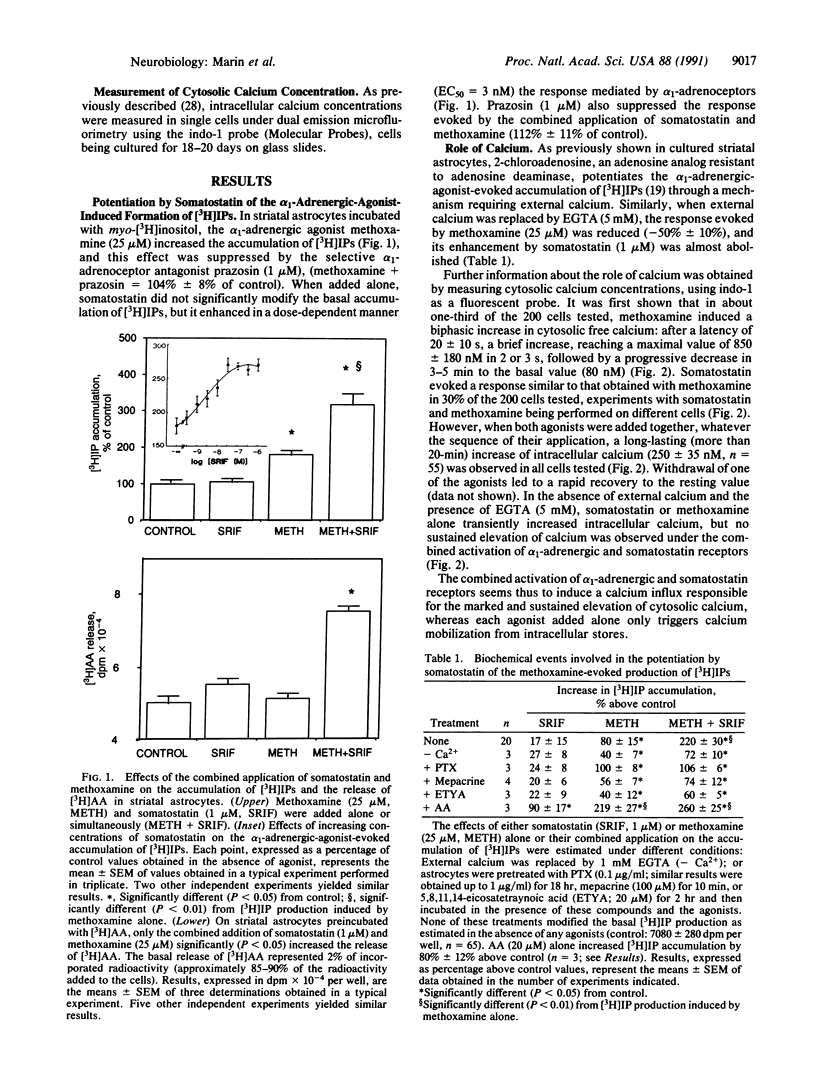
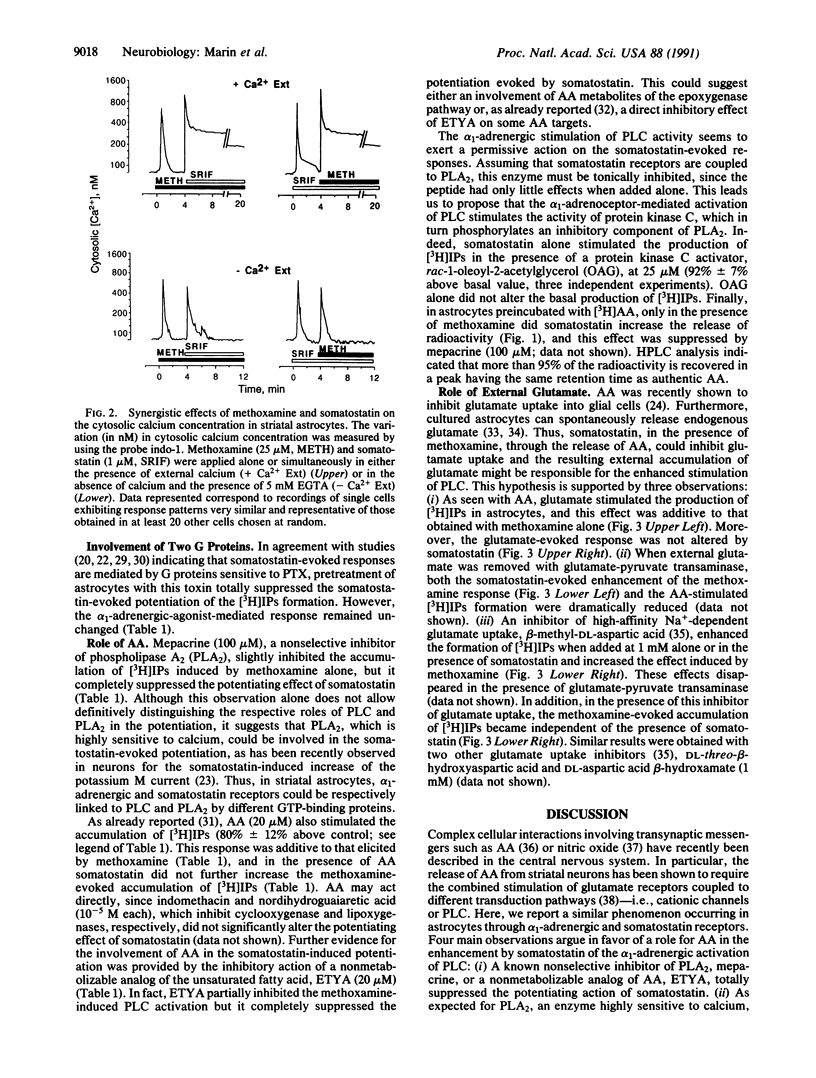
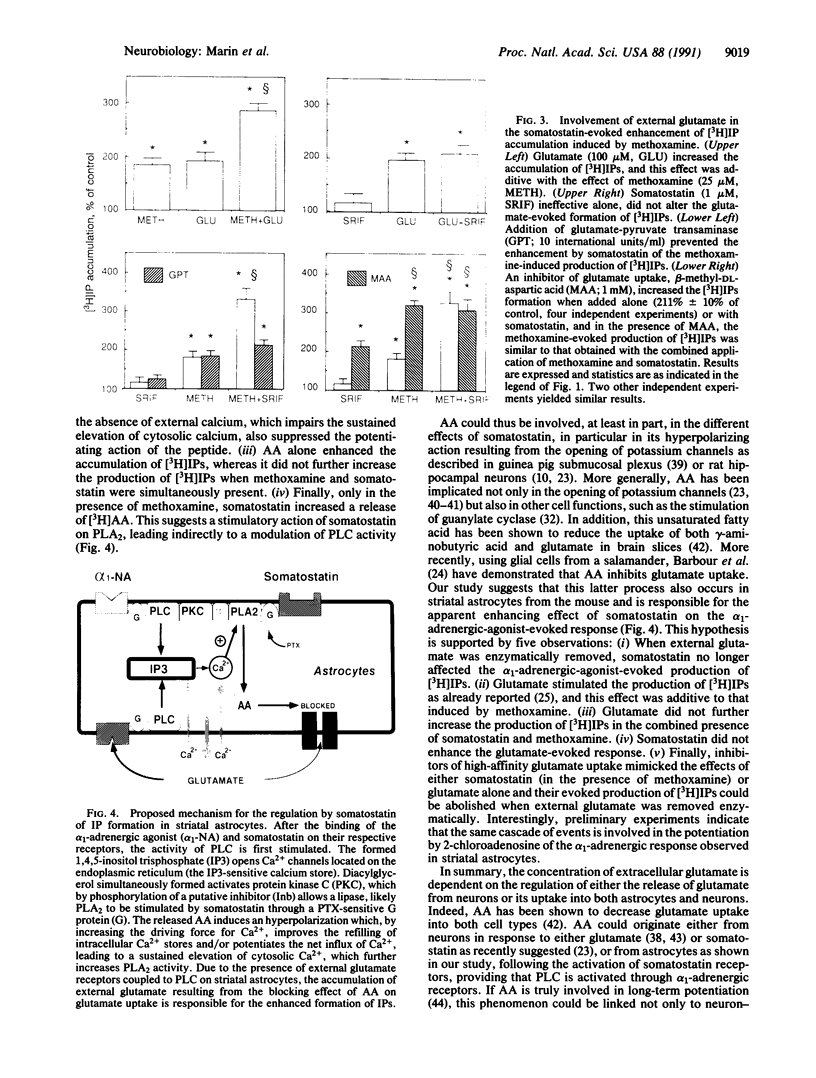
As previously shown with adenosine, somatostatin, which is ineffective alone, enhanced the alpha 1-adrenergic-agonist-stimulated production of inositol phosphates in cultured striatal astrocytes. This effect was suppressed in cells pretreated with pertussis toxin. It required external calcium and was selectively antagonized by both mepacrine, an inhibitor of phospholipase A2, and 5,8,11,14-eicosatetraynoic acid, a nonmetabolizable analog of arachidonic acid. In addition, a long-lasting elevation of cytosolic calcium and a release of arachidonic acid were observed only under the combined stimulation of somatostatin and alpha 1-adrenergic receptors. Arachidonic acid could in turn inhibit glutamate uptake into astrocytes, and the resulting external accumulation of glutamate could account for the somatostatin-evoked amplification of the alpha 1-adrenergic-agonist-stimulated hydrolysis of inositol-phospholipids. The effect of somatostatin was indeed reproduced by glutamate or glutamate uptake inhibitors and suppressed by enzymatic removal of external glutamate. Thus, astrocytes may contribute to long-term plasticity events in glutamatergic synapses through regulation of external glutamate levels.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Schultz G., Jakobs K. H. Adenylate cyclase inhibition and GTPase stimulation by somatostatin in S49 lymphoma cyc- variants are prevented by islet-activating protein. FEBS Lett. 1983 Jul 11;158(1):169–173. doi: 10.1016/0014-5793(83)80701-7. [DOI] [PubMed] [Google Scholar]
- Barbour B., Szatkowski M., Ingledew N., Attwell D. Arachidonic acid induces a prolonged inhibition of glutamate uptake into glial cells. Nature. 1989 Dec 21;342(6252):918–920. doi: 10.1038/342918a0. [DOI] [PubMed] [Google Scholar]
- Chan P. H., Kerlan R., Fishman R. A. Reductions of gamma-aminobutyric acid and glutamate uptake and (Na+ + K+)-ATPase activity in brain slices and synaptosomes by arachidonic acid. J Neurochem. 1983 Feb;40(2):309–316. doi: 10.1111/j.1471-4159.1983.tb11284.x. [DOI] [PubMed] [Google Scholar]
- Chneiweiss H., Glowinski J., Prémont J. Modulation by monoamines of somatostatin-sensitive adenylate cyclase on neuronal and glial cells from the mouse brain in primary cultures. J Neurochem. 1985 Jun;44(6):1825–1831. doi: 10.1111/j.1471-4159.1985.tb07175.x. [DOI] [PubMed] [Google Scholar]
- Delumeau Jean C., Tencé Martine, Marin Philippe, Cordier Jocelyne, Glowinski Jacques, Prémont Joël. Synergistic Regulation of Cytosolic Ca2+ Concentration by Adenosine and alpha1-Adrenergic Agonists in Mouse Striatal Astrocytes. Eur J Neurosci. 1991 Jun;3(6):539–550. doi: 10.1111/j.1460-9568.1991.tb00841.x. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Prestwich S. A. Pertussis toxin reverses adenosine inhibition of neuronal glutamate release. Nature. 1985 Jul 11;316(6024):148–150. doi: 10.1038/316148a0. [DOI] [PubMed] [Google Scholar]
- Dumuis A., Pin J. P., Oomagari K., Sebben M., Bockaert J. Arachidonic acid released from striatal neurons by joint stimulation of ionotropic and metabotropic quisqualate receptors. Nature. 1990 Sep 13;347(6289):182–184. doi: 10.1038/347182a0. [DOI] [PubMed] [Google Scholar]
- Dumuis A., Sebben M., Haynes L., Pin J. P., Bockaert J. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nature. 1988 Nov 3;336(6194):68–70. doi: 10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- Ebersolt C., Premont J., Prochiantz A., Perez M., Bockaert J. Inhibition of brain adenylate cyclase by A1 adenosine receptors: pharmacological characteristics and locations. Brain Res. 1983 May 9;267(1):123–129. doi: 10.1016/0006-8993(83)91045-4. [DOI] [PubMed] [Google Scholar]
- Eng L. F. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol. 1985 Jun;8(4-6):203–214. doi: 10.1016/s0165-5728(85)80063-1. [DOI] [PubMed] [Google Scholar]
- Epelbaum J. Somatostatin in the central nervous system: physiology and pathological modifications. Prog Neurobiol. 1986;27(1):63–100. doi: 10.1016/0301-0082(86)90012-2. [DOI] [PubMed] [Google Scholar]
- Gally J. A., Montague P. R., Reeke G. N., Jr, Edelman G. M. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc Natl Acad Sci U S A. 1990 May;87(9):3547–3551. doi: 10.1073/pnas.87.9.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzer R., Brash A. R., Hardman J. G. Activation of soluble guanylate cyclase by arachidonic acid and 15-lipoxygenase products. Biochim Biophys Acta. 1986 May 29;886(3):383–389. doi: 10.1016/0167-4889(86)90173-4. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990 Jul;13(7):244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Gross R. A., Macdonald R. L., Ryan-Jastrow T. 2-Chloroadenosine reduces the N calcium current of cultured mouse sensory neurones in a pertussis toxin-sensitive manner. J Physiol. 1989 Apr;411:585–595. doi: 10.1113/jphysiol.1989.sp017592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Nakajima S., Nakajima Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J Physiol. 1988 Dec;407:177–198. doi: 10.1113/jphysiol.1988.sp017409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg H. K., Goderie S. K., Higman S., Pang S., Waniewski R. A. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990 May;10(5):1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg H. K., Pang S., Treble D. H. Excitatory amino acid-stimulated uptake of 22Na+ in primary astrocyte cultures. J Neurosci. 1989 Apr;9(4):1141–1149. doi: 10.1523/JNEUROSCI.09-04-01141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch B. D., Blalock J. B., Schonbrunn A. Characterization of the cyclic AMP-independent actions of somatostatin in GH cells. I. An increase in potassium conductance is responsible for both the hyperpolarization and the decrease in intracellular free calcium produced by somatostatin. J Biol Chem. 1988 Jan 5;263(1):216–225. [PubMed] [Google Scholar]
- Lewis D. L., Weight F. F., Luini A. A guanine nucleotide-binding protein mediates the inhibition of voltage-dependent calcium current by somatostatin in a pituitary cell line. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9035–9039. doi: 10.1073/pnas.83.23.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D. J., Sheu F. S., Murakami K., Routtenberg A. Enhancement of long-term potentiation by cis-unsaturated fatty acid: relation to protein kinase C and phospholipase A2. J Neurosci. 1987 Nov;7(11):3783–3792. doi: 10.1523/JNEUROSCI.07-11-03783.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini A., Lewis D., Guild S., Schofield G., Weight F. Somatostatin, an inhibitor of ACTH secretion, decreases cytosolic free calcium and voltage-dependent calcium current in a pituitary cell line. J Neurosci. 1986 Nov;6(11):3128–3132. doi: 10.1523/JNEUROSCI.06-11-03128.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy N., Woolkalis M., Thermos K., Carlson K., Manning D., Reisine T. Pertussis toxin modifies the characteristics of both the inhibitory GTP binding proteins and the somatostatin receptor in anterior pituitary tumor cells. J Pharmacol Exp Ther. 1988 Aug;246(2):779–785. [PubMed] [Google Scholar]
- Mancillas J. R., Siggins G. R., Bloom F. E. Somatostatin selectively enhances acetylcholine-induced excitations in rat hippocampus and cortex. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7518–7521. doi: 10.1073/pnas.83.19.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara S., North R. A., Surprenant A. Somatostatin increases an inwardly rectifying potassium conductance in guinea-pig submucous plexus neurones. J Physiol. 1987 Sep;390:335–355. doi: 10.1113/jphysiol.1987.sp016704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. D., Madamba S. G., Joëls M., Siggins G. R. Somatostatin augments the M-current in hippocampal neurons. Science. 1988 Jan 15;239(4837):278–280. doi: 10.1126/science.2892268. [DOI] [PubMed] [Google Scholar]
- Murphy S., Welk G. Arachidonic acid evokes inositol phospholipid hydrolysis in astrocytes. FEBS Lett. 1989 Oct 23;257(1):68–70. doi: 10.1016/0014-5793(89)81788-0. [DOI] [PubMed] [Google Scholar]
- Pace C. S., Tarvin J. T. Somatostatin: mechanism of action in pancreatic islet beta-cells. Diabetes. 1981 Oct;30(10):836–842. doi: 10.2337/diab.30.10.836. [DOI] [PubMed] [Google Scholar]
- Pares-Herbuté N., Bonet A., Peraldi S., Pin J. P., Gabrion J., Astier H., Tapia-Arancibia L. The presence of non-neuronal cells influences somatostatin release from cultured cerebral cortical cells. Brain Res. 1988 May 1;468(1):89–97. doi: 10.1016/0165-3806(88)90011-9. [DOI] [PubMed] [Google Scholar]
- Pearce B., Albrecht J., Morrow C., Murphy S. Astrocyte glutamate receptor activation promotes inositol phospholipid turnover and calcium flux. Neurosci Lett. 1986 Dec 23;72(3):335–340. doi: 10.1016/0304-3940(86)90537-9. [DOI] [PubMed] [Google Scholar]
- Pennefather P. S., Heisler S., MacDonald J. F. A potassium conductance contributes to the action of somatostatin-14 to suppress ACTH secretion. Brain Res. 1988 Mar 22;444(2):346–350. doi: 10.1016/0006-8993(88)90944-4. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Shapiro E., Feinmark S. J., Schwartz J. H. Metabolites of arachidonic acid in the nervous system of Aplysia: possible mediators of synaptic modulation. J Neurosci. 1987 Nov;7(11):3675–3686. doi: 10.1523/JNEUROSCI.07-11-03675.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor W. R., Dunwiddie T. V. Adenosine inhibits calcium spikes in hippocampal pyramidal neurons in vitro. Neurosci Lett. 1983 Feb 21;35(2):197–201. doi: 10.1016/0304-3940(83)90550-5. [DOI] [PubMed] [Google Scholar]
- Reisine T., Zhang Y. L., Sekura R. Pertussis toxin treatment blocks the inhibition of somatostatin and increases the stimulation by forskolin of cyclic AMP accumulation and adrenocorticotropin secretion from mouse anterior pituitary tumor cells. J Pharmacol Exp Ther. 1985 Jan;232(1):275–282. [PubMed] [Google Scholar]
- Schweitzer P., Madamba S., Siggins G. R. Arachidonic acid metabolites as mediators of somatostatin-induced increase of neuronal M-current. Nature. 1990 Aug 2;346(6283):464–467. doi: 10.1038/346464a0. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Adenosine as a neuromodulator. Annu Rev Neurosci. 1985;8:103–124. doi: 10.1146/annurev.ne.08.030185.000535. [DOI] [PubMed] [Google Scholar]
- Tatsumi H., Costa M., Schimerlik M., North R. A. Potassium conductance increased by noradrenaline, opioids, somatostatin, and G-proteins: whole-cell recording from guinea pig submucous neurons. J Neurosci. 1990 May;10(5):1675–1682. doi: 10.1523/JNEUROSCI.10-05-01675.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Adenosine-activated potassium conductance in cultured striatal neurons. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4857–4861. doi: 10.1073/pnas.82.14.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher P., McKenzie J., Dufy B. Arachidonic acid affects membrane ionic conductances of GH3 pituitary cells. Am J Physiol. 1989 Aug;257(2 Pt 1):E203–E211. doi: 10.1152/ajpendo.1989.257.2.E203. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier C., Brazeau P., Guillemin R. Effects of somatostatin on the secretion of thyrotropin and prolactin. Endocrinology. 1974 Oct;95(4):968–977. doi: 10.1210/endo-95-4-968. [DOI] [PubMed] [Google Scholar]
- Wang H. L., Bogen C., Reisine T., Dichter M. Somatostatin-14 and somatostatin-28 induce opposite effects on potassium currents in rat neocortical neurons. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9616–9620. doi: 10.1073/pnas.86.23.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. H., Errington M. L., Lynch M. A., Bliss T. V. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989 Oct 26;341(6244):739–742. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Shibuya N., Ogata E. Hyperpolarization of the membrane potential caused by somatostatin in dissociated human pituitary adenoma cells that secrete growth hormone. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6198–6202. doi: 10.1073/pnas.83.16.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Etr M., Cordier J., Glowinski J., Premont J. A neuroglial cooperativity is required for the potentiation by 2-chloroadenosine of the muscarinic-sensitive phospholipase C in the striatum. J Neurosci. 1989 May;9(5):1473–1480. doi: 10.1523/JNEUROSCI.09-05-01473.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]