Abstract
Objective
To investigate whether the effects of nerve growth factor (NGF) inhibition with tanezumab on rats with medial meniscal tear (MMT) effectively model rapidly progressive osteoarthritis (RPOA) observed in clinical trials.
Methods
Male Lewis rats underwent MMT surgery and were treated weekly with tanezumab (0.1, 1 or 10 mg/kg), isotype control or vehicle for 7, 14 or 28 days. Gait deficiency was measured to assess weight-bearing on the operated limb. Joint damage was assessed via histopathology. A second arm, delayed onset of treatment (starting 3–8 weeks after MMT surgery) was used to control for analgesia early in the disease process. A third arm, mid-tibial amputation, evaluated the dependency of the model on weight-bearing.
Results
Gait deficiency in untreated rats was present 3–7 days after MMT surgery, with a return to normal weight-bearing by days 14–28. Prophylactic treatment with tanezumab prevented gait deficiency and resulted in more severe cartilage damage. When onset of treatment with tanezumab was delayed to 3–8 weeks after MMT surgery, there was no increase in cartilage damage. Mid-tibial amputation completely prevented cartilage damage in untreated MMT rats.
Conclusions
These data suggest that analgesia due to NGF inhibition during the acute injury phase is responsible for increased voluntary weight-bearing and subsequent cartilage damage in the rat MMT model. This model failed to replicate the hypotrophic bone response observed in tanezumab-treated patients with RPOA.
Keywords: Knee Osteoarthritis, Osteoarthritis, Arthritis
Introduction
Knee osteoarthritis (OA) is a condition characterised by pain, inflammation and functional disability.1 OA pain is complex and involves both inflammatory and neuropathic components mediated through persistent tissue injury and release of inflammatory mediators.2 Pain treatment for OA is problematic because many standard therapies provide minimal pain relief and do not address underlying mechanisms driving disease pathophysiology.3
The neurotrophin, nerve growth factor (NGF), is considered a key modulator of pain perception in several chronic pain conditions, including OA.4–7 Tanezumab, a humanised monoclonal antibody, binds NGF and prevents interaction with its receptors (high-affinity transmembrane tyrosine kinase receptor (TrkA) and the low-affinity NGF receptor [p75]).8 Tanezumab provided significant improvement in pain, physical function and patients' global assessments in a number of chronic pain conditions.9–16
Investigator reports of adverse events initially described as osteonecrosis leading to total joint replacement during the conduct of phase III clinical OA studies led the US Food and Drug Administration to put trials of all NGF inhibitors on partial clinical hold.17–19 Blinded adjudication of the results showed that there was no increase in osteonecrosis, nor frequency of total joint replacement with tanezumab monotherapy. Tanezumab treatment was however associated with increased incidence of rapidly progressive osteoarthritis (RPOA). A retrospective analysis of the data suggested ways to mitigate this risk, and based on these data the clinical hold was lifted to allow further trials to test these risk mitigation approaches.17–20
The increased frequency of RPOA was unexpected, as no issues with bone or joints were seen in non-clinical studies of anti-NGF therapy using large multiples of the clinical dose.21 Also, no evidence of abnormal bone or joint phenotypes exists in humans with TrkA or p75 null mutations other than that observed in congenital pain insensitivity mutations.5 Last, in an experimental fracture model, anti-NGF therapy was shown to ameliorate fracture pain without impacting bone healing.22
Meniscal injury and acute meniscectomy are known to increase the risk of knee OA.23 Up to 80% of patients with knee OA, as well as a high percentage of age-matched controls, have evidence of meniscal injury at the time of diagnosis yet abnormal load-bearing and joint instability stemming from meniscal injury resulting in substantial OA lesions may take years.23 Attempts to model this in animals have produced varied results.24 25 The medial meniscal tear (MMT)-induced joint damage model in rats has many features attractive for an animal model. These animals show joint instability and tibial cartilage damage in as little as 7–14 days after surgery.25 MMT-induced joint damage lesions are highly reproducible and include articular cartilage proteoglycan loss, chondrocyte degeneration and loss of matrix. Although erosion of cartilage is a feature of this model, rarely does it progress to ulceration within 14 or 28 days. Subchondral bone sclerosis and osteophyte formation, which are compensatory responses to altered mechanical loading and joint instability are present in this model.24 26–28
The objective of this study was to characterise the impact of NGF inhibition with tanezumab on voluntary weight-bearing and subsequent articular cartilage damage in MMT rats to see if this model would be useful for investigating potential mechanisms underlying the clinical findings of increased RPOA in patients treated with tanezumab.
Methods
Animals and tanezumab administration
For animal care and use, see online supplementary text S1.
annrheumdis-2015-208913supp_text.pdf (162.3KB, pdf)
All animals underwent MMT or sham surgery on study day 0. In MMT surgery rats, the medial meniscus in the right hind limb was cut through the full thickness to simulate a complete tear.24 25 In sham surgery animals, the knee was opened and the medial meniscus was touched only (not cut). Sham surgery rats received vehicle, and animals that underwent MMT surgery were treated with either isotype control (a non-specific antibody of the same type (immunoglobulin G) and class (2a) as tanezumab), vehicle (10 mM trehalose buffer) or tanezumab (0.1, 1 or 10 mg/kg) subcutaneously once a week for 7, 14 or 28 days.
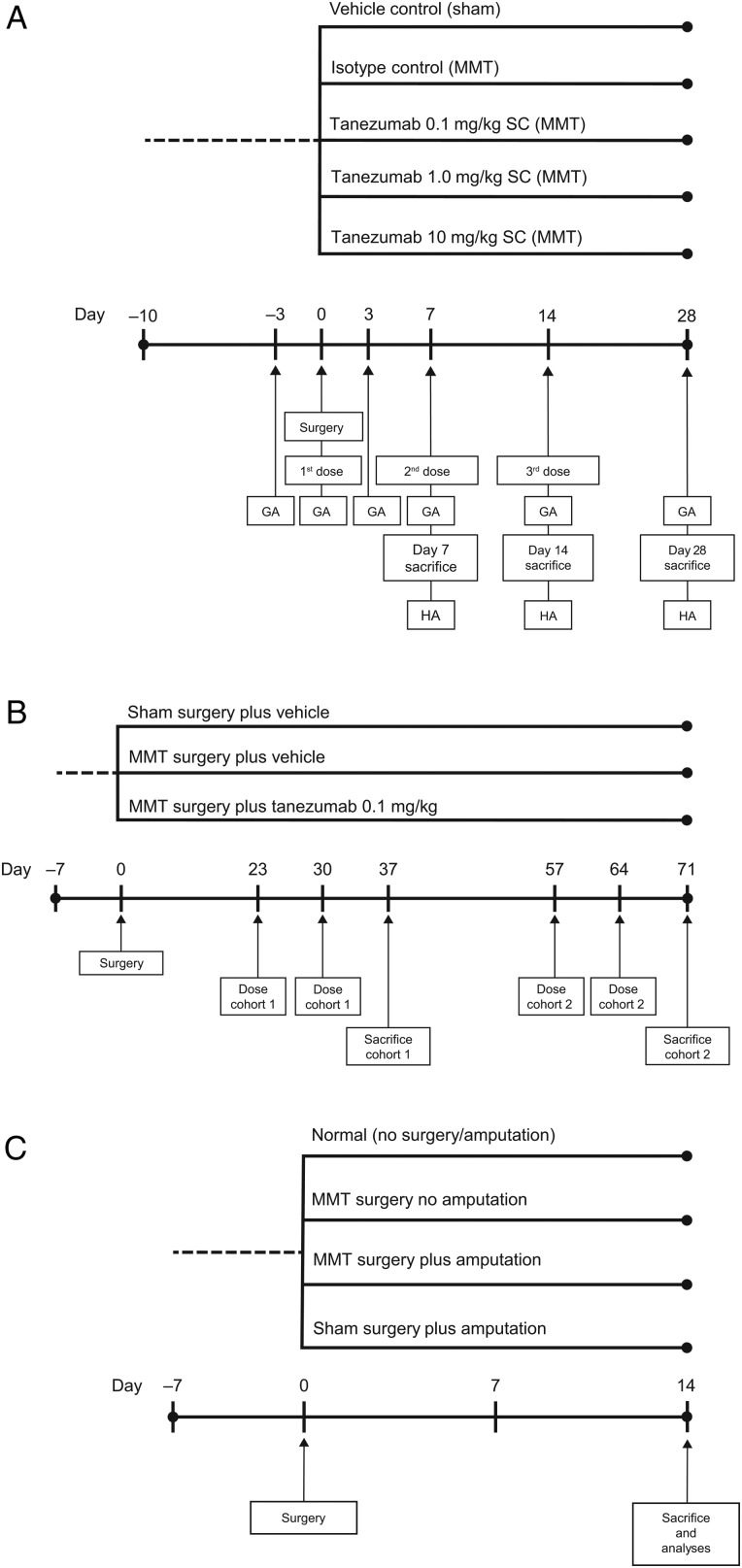
In the first arm of the study, treatment began on the day of surgery (day 0, figure 1A).29 To examine the impact of analgesia on weight-bearing and lesion severity, a second arm evaluated delaying the onset of tanezumab treatment (0.1 mg/kg weekly) until gait deficiency was no longer evident (day 23); half the animals started receiving treatment on day 23, while the other half were delayed another month (day 57) before treatment was initiated (figure 1B). In both arms of this study, anti-drug antibodies in plasma were measured, as well as concentration of parent compound in serum to evaluate the potential for clearance of tanezumab via immunologic response.
Figure 1.
Study designs for (A) the initial arm (28-day study), (B) the second arm (delayed-treatment onset study) and (C) the weight-bearing (amputation) study. GA, gait analysis; HA, histopathology analysis; MMT, medial meniscal tear; SC, subcutaneous.
To understand the role of weight-bearing in the progression of knee joint damage in the rat MMT model, a third arm of the study evaluated the impact of mid-tibial amputation on MMT lesion progression, eliminating weight-bearing as a variable. The knees were collected 14 days after MMT for micro-CT (µCT) imaging, followed by histopathology (figure 1C). In the first two study arms, the impact of NGF inhibition on subchondral bone, osteophyte size and growth plate thickness was evaluated in MMT rats to assess secondary effects on the overall joint.
Gait deficiency
Difference in dynamic weight-bearing between the ipsilateral (MMT operated; right side) limb and contralateral (control; left side) limb is an established approach to measuring gait in rat and mouse models of OA.30 31 Voluntary weight-bearing by non-amputated MMT rats was assessed on days −3, 3, 7, 14 and 28 in the first arm of the study and additional days 37, 42, 56, 63 and 71 in the delayed-treatment study to confirm that there was no pain relapse in any animals (see online supplementary text S2).
Histopathology and quantitative image analysis
Microscopy-based semiquantitative scoring of cartilage and bone changes is an established endpoint for describing response to compounds in MMT rats. Cohorts of animals were sacrificed 7, 14 or 28 days after surgery or 14 days after treatment onset (day 23 or 57). Knees were collected at necropsy and evaluated via light microscopy by a veterinary pathologist as described (see online supplementary text S3).25
Radiography and µCT imaging
Knees were X-rayed with a MX20 specimen scanner (Faxitron Bioptics, Tucson, Arizona, USA) using recommended settings; exposure 12–18 s at 31−35 kV. Radiographs were used to assess gross anatomy of the region of interest (ROI) evaluated by µCT and to inspect bone samples for presence of abnormalities.
µCT was conducted on the right tibial epiphysis and metaphysis using a MicroCT 100 system (Scanco Medical, Bassersdorf, Switzerland) with the following parameters: 800 slices, 10 µm resolution, total scanned area of 8.0 mm2 and source energy of 70 kVp, 115 µA at 8 W to capture the entire proximal tibia section. Cortical and cancellous bone of the entire epiphysis were scanned and analysed in an ROI on 100 consecutive slices with 1.0 mm thickness that best represented the central segment of the epiphysis. Parameters included tissue volume (TV), bone volume (BV), BV/TV ratio and bone mineral density (BMD). Cortical and cancellous bone of the proximal metaphysis were scanned and analysed in an ROI on 100 consecutive slices 1 mm below the growth plate with a thickness of 1.0 mm that best represented the central metaphysis segment. Parameters included TV, BV, BV/TV and BMD. Cancellous bone compartment of the metaphysis was analysed 1 mm below the growth plate and extending 3 mm distally to include only the secondary spongiosa. Cancellous bone was evaluated in an ROI drawn on 100 consecutive slices with a thickness of 1.0 mm that best represented the central segment of the tibia.32 Cancellous bone parameters included TV, BV, BV/TV, trabecular number, trabecular thickness, trabecular separation and BMD.
Pharmacokinetics and anti-drug antibody
To evaluate the potential for clearance of compound via immunologic response, we measured anti-drug antibodies in plasma as well as concentration of tanezumab in serum pharmacokinetic (PK) samples. Blood samples were collected on days 3, 7, 14 and 21. An additional PK sample was taken 4 days prior to termination. In the delayed-treatment study, blood samples for PK and anti-drug antibodies were collected on days 16, 30, 57 and 64. PK plasma concentrations of tanezumab were measured using a validated ELISA at ICON Development Solutions (Whitesboro, New York, USA). The lower limit of quantitation in Lewis rat plasma was 50 ng/mL. Concentrations below the limit of quantitation were treated as 0 ng/mL in the PK calculations. Anti-drug antibodies concentrations in serum were measured using a validated ELISA at ICON Development Solutions. Final assessment of induction of an anti-drug antibodies response was based on comparison of predose and postdose results for each animal.
Statistical analyses
Body weight change, weight-bearing (gait) deficiency at each time point and histopathology outcomes were analysed. Vehicle and sham surgery groups were compared with the isotype group using a two-sided t test (with a Welch correction for unequal variances if necessary) or Mann-Whitney U test. Each tanezumab-treated group was compared with the isotype group using a two-sided Dunnett or Dunn multiple comparisons test. Significance for all tests was set at p≤0.05. Gait deficiency was assessed by Dunnett and t-tests.
Results
Tanezumab treatment influences weight-bearing and subsequent cartilage damage in the rat MMT model
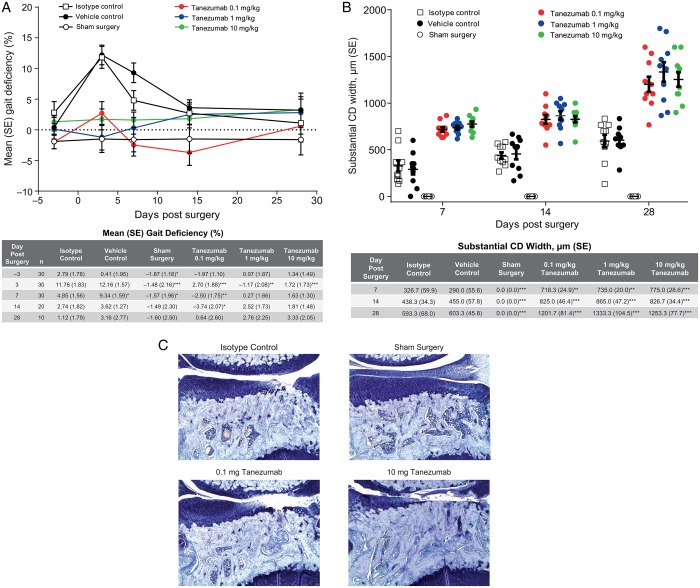
Although MMT rats with intact hind limbs appeared to ambulate normally after surgery, they experienced a measurable weight-bearing (gait) deficiency on the operated limb. Gait deficiency in MMT rats administered vehicle or isotype control peaked 3 days after surgery (11.8% and 12.2% decrease, respectively), continued through day 7 and resolved by days 14–28 (figure 2A). In contrast, tanezumab-treated MMT rats exhibited no gait deficiency at any point, indicating that tanezumab-treated MMT rats were bearing full weight on the operated limbs throughout the study (figure 2). Tanezumab-treated rats exhibited more severe total tibial cartilage substantial degeneration width than either vehicle or isotype controls on days 7, 14 and 28 (figure 2B). No significant differences in body weight gain were observed over the course of the study. Focal areas of alopecia along the mouth/muzzle began developing on day 14 and were seen in most animals in the 1 mg/kg tanezumab group by day 28. Gait analysis was also performed as part of the delayed-treatment study (see online supplementary table S1).
Figure 2.
Weight-bearing corresponds with severity of cartilage damage in MMT rats. (A) Gait deficiency in tanezumab-treated MMT rats compared with control treatment and sham surgery rats. Data expressed as per cent decrease from mean of right and left (SE). Starting on day 7, 10 animals per treatment group were removed for microscopic evaluation. (B) Substantial tibial cartilage degeneration width, as determined by histopathology on days 7, 14 and 28 (n=10 rats/group). (C) Representative photomicrographs (50× magnification) from the isotype and sham surgery controls and tanezumab treatment groups on day 28. Days reflect days after MMT surgery. *p<0.05 versus isotype control; **p<0.01 versus isotype control; ***p<0.001 versus isotype control. CD, cartilage degeneration; MMT, medial meniscal tear.
annrheumdis-2015-208913supp_tables.pdf (87.5KB, pdf)
There was evidence of a robust, compensatory (hypertrophic) response to the partially destabilised joint. Namely, a time-dependent increase in subchondral bone sclerosis was observed in untreated MMT rats (table 1), which was exacerbated by tanezumab (statistically significant greater subchondral sclerosis compared with isotype controls on days 7, 14 and 28). Larger osteophyte size was also observed in tanezumab-treated MMT rats compared with isotype controls on days 7 (tanezumab 10 mg/kg, p<0.05), 14 (tanezumab 1 mg/kg, p<0.01) and 28 (tanezumab 0.1, 1 and 10 mg/kg, p<0.001 for all; table 1), indicating a time–dose relationship with increasing effect. No osteophytes were observed in sham surgery animals. Last, a significant focal increase in growth plate thickness was also observed in tanezumab-treated MMT rats compared with isotype control-treated rats (1 and 10 mg/kg on days 14 and 28, p≤0.05, table 1).
Table 1.
Histopathology summary, n=10/group; mean (SE)
| Parameter/day | Isotype control | Vehicle control | Sham surgery | Tanezumab 0.1 mg/kg/week |
Tanezumab 1 mg/kg/week |
Tanezumab 10 mg/kg/week |
|---|---|---|---|---|---|---|
| Growth plate difference (medial-lateral), µm | ||||||
| 7 | 41.33 (5.43) | 33.33 (2.22) | 5.33 (2.95)*** | 50.67 (10.29) | 60.00 (8.94) | 70.67 (11.60) |
| 14 | 68.00 (15.83) | 50.67 (7.38) | 46.67 (5.71) | 74.67 (8.93) | 164.00 (26.15)** | 141.33 (22.59)* |
| 28 | 33.33 (4.10) | 32.00 (4.07) | 16.00 (5.55)* | 65.33 (10.60) | 185.33 (50.73)*** | 161.33 (76.24)** |
| Medial collateral ligament, µm | ||||||
| 7 | 865.33 (26.70) | 893.33 (13.91) | 870.67 (35.84) | 1068.00 (33.46)** | 1041.33 (48.42)* | 1128.00 (60.65)*** |
| 14 | 637.33 (26.28) | 633.33 (31.13) | 641.33 (28.63) | 664.00 (24.49) | 689.33 (31.74) | 688.00 (29.02) |
| 28 | 476.00 (16.03) | 466.67 (15.78) | 472.00 (20.09) | 530.67 (17.87) | 570.67 (22.82)** | 545.33 (15.71)* |
| Bone damage score† | ||||||
| 7 | 2.00 (0.15) | 2.00 (0.00) | 0.00 (0.00)*** | 2.40 (0.16) | 2.20 (0.13) | 2.20 (0.13) |
| 14 | 2.80 (0.25) | 2.60 (0.16) | 0.00 (0.00)*** | 3.10 (0.31) | 2.60 (0.16) | 2.70 (0.30) |
| 28 | 3.00 (0.26) | 3.40 (0.16) | 0.00 (0.00)*** | 4.60 (0.16)** | 4.40 (0.34)** | 4.20 (0.29)* |
| Bone sclerosis score‡ | ||||||
| 7 | 1.30 (0.15) | 1.20 (0.13) | 0.20 (0.13)*** | 2.30 (0.15)** | 2.10 (0.10)* | 2.70 (0.26)*** |
| 14 | 2.20 (0.20) | 2.50 (0.17) | 0.10 (0.10)*** | 3.50 (0.17)** | 3.70 (0.15)*** | 3.80 (0.13)*** |
| 28 | 2.80 (0.25) | 3.70 (0.26)* | 0.20 (0.13)*** | 4.60 (0.16)*** | 4.80 (0.13)*** | 4.70 (0.15)*** |
| Tibial osteophyte size, µm | ||||||
| 7 | 119.33 (26.79) | 121.33 (26.88) | 0 (0.00)*** | 167.33 (21.79) | 150.33 (29.65) | 223.67 (14.59)* |
| 14 | 309.67 (10.90) | 299.67 (16.03) | 0 (0.00)*** | 382.67 (27.93) | 476.67 (43.62)** | 417.67 (41.30) |
| 28 | 581.67 (34.20) | 601.67 (27.38) | 0 (0.00)*** | 1106.67 (49.20)*** | 1068.33 (75.30)*** | 1093.33 (50.63)*** |
*p<0.05 versus isotype control; **p<0.01 versus isotype control; ***p<0.001 versus isotype control.
†Damage to calcified cartilage and subchondral bone was scored on a 6-point numerical scale (0=no changes to 5=increased basophilia; marked to severe fragmentation of calcified cartilage, mesenchymal change in marrow involves up to three-fourths of the total area and articular cartilage has collapsed into the epiphysis to a depth of >250 µm from tidemark).
‡Medial tibial subchondral/epiphysial bone sclerosis was scored on a 6-point numerical scale (0=no changes to 5=76%–100% increase in subchondral or epiphysial trabecular bone thickness in medial versus lateral; very little marrow space remains in medial tibia).
Delaying onset of tanezumab treatment protects against cartilage damage in MMT rats
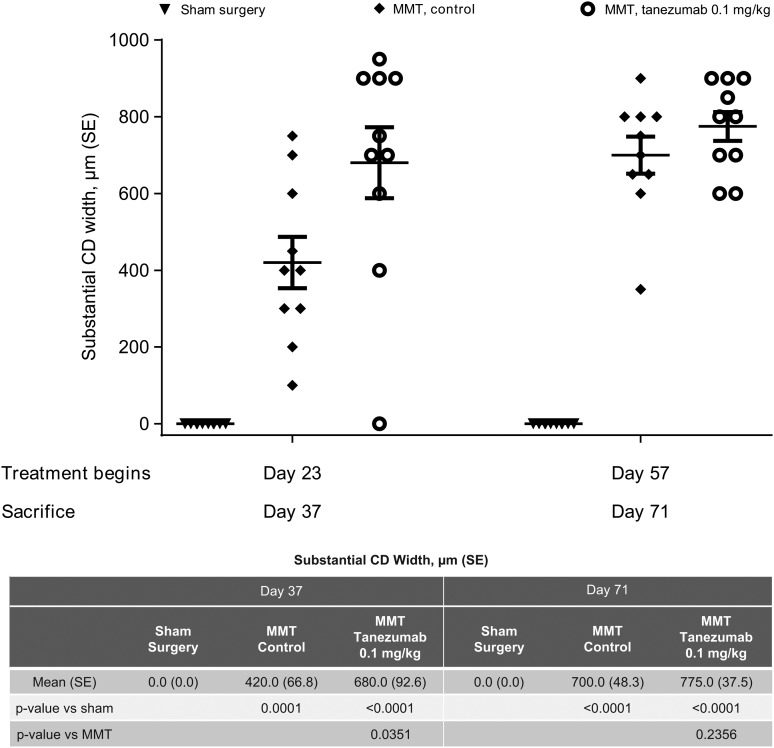
Gait deficiency began shortly after MMT surgery and appeared to resolve by days 14–28. To confirm whether changes in weight-bearing during this early postoperative period was the principal driver of increased joint damage in the tanezumab-treated MMT rats, we delayed the onset of treatment from day 0 to day 23 (cohort 1) or day 57 (cohort 2). The second cohort in particular initiated treatment well past the time that gait abnormalities normalised. Tanezumab-treated MMT rats had significantly greater cartilage degeneration width than MMT controls when treatment began on day 23 (p=0.035, figure 3). However, delaying treatment until weight-bearing fully returned to normal (ie, day 57 but not day 23) resulted in complete protection from worsening of joint damage when compared with control treatments (figure 3).
Figure 3.
Delaying onset of treatment from day 0 until after gait deficiency returns to normal (day 57 but not day 23) resulted in no difference in cartilage damage between treated and untreated MMT rats sacrificed 14 days after treatment. Data points include mean substantial tibial cartilage degeneration width measurements (tanezumab 0.1 mg/kg/week for 14 days; n=10 rats/group). Days reflect days after MMT surgery. CD, cartilage degeneration; MMT, medial meniscal tear.
Limb amputation protects against joint damage in untreated MMT rats
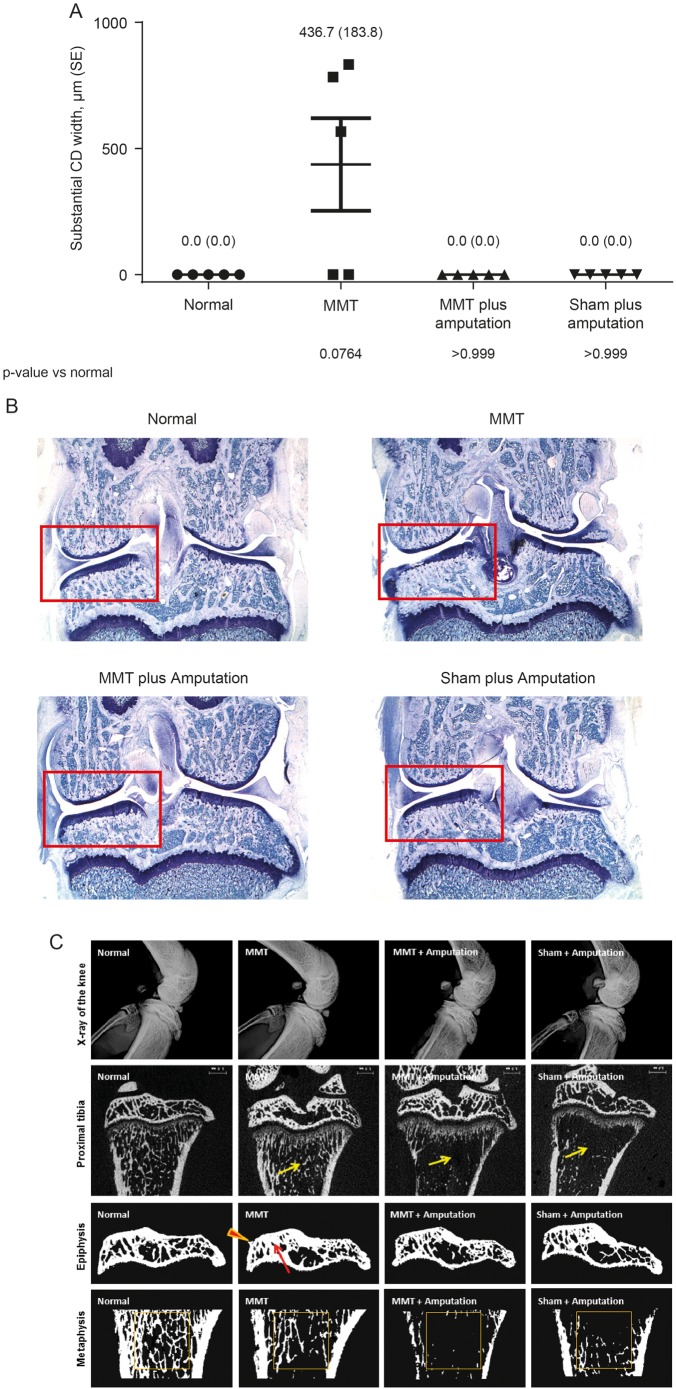
As reported,26 as well as in this study (figure 4) signs of tibial articular cartilage damage appear in normal weight-bearing MMT rats in as early as 7 days after surgery. In this study, those changes were prevented by tibial amputation (figure 4). MMT rats with tibial amputations are not placing weight on their operated limbs, as evidenced by less cortical and cancellous bone in amputated rats than unamputated rats, regardless of whether they had MMT surgery performed (table 2 and figure 4C).
Figure 4.
The rat MMT model is dependent on dynamic weight-bearing for cartilage damage. Rats underwent either MMT or sham surgery±amputation of the lower tibia. (A) Tibial cartilage substantial degeneration width (SE), as determined by histopathology at day 14 after MMT surgery was observed in MMT rats (no amputation). (B) Representative photomicrographs from each group (16× magnification). (C) Top row depicts X-ray images of the right knee. Second row shows representative two-dimensional μCT images of the proximal tibia (arrows indicate less cancellous bone at metaphysis). Third row shows the image of tibial epiphysis (arrow indicates thicker subchondral bone at medial tibial plateau and arrowhead points at osteophyte). Bottom row shows proximal tibial metaphysis (square indicates areas of secondary spongiosa where cancellous bone has been evaluated). MMT, medial meniscal tear; μCT, micro-CT.
Table 2.
Bone parameters evaluated ex vivo by µ-CT at proximal tibial epiphysis and metaphysis
| Parameter | Normal rats | MMT surgery | MMT plus amputation | Sham plus amputation |
|---|---|---|---|---|
| Proximal tibial epiphysis (cortical and cancellous bone) | ||||
| TV, mm3 | 1.65±0.11 | 1.87±0.14 | 1.83±0.07 | 1.67±0.11 |
| BV, mm3 | 0.76±0.10 | 0.74±0.09 | 0.67±0.04†,‡ | 0.64±0.05†,‡ |
| BV/TV, ratio | 0.46±0.03 | 0.43±0.02 | 0.37±0.03††,‡ | 0.38±0.02††,‡ |
| BMD, g/cm2 | 985.14±16.44 | 990.85±14.89 | 983.37±14.97 | 1005.05±11.05 |
| Proximal tibial metaphysis (cortical and cancellous bone) | ||||
| TV, mm3 | 2.81±0.05 | 2.88±0.04 | 2.87±0.05 | 2.80±0.14 |
| BV, mm3 | 0.73±0.06 | 0.57±0.06††† | 0.24±0.07†††,‡‡‡ | 0.26±0.05†††,‡‡‡ |
| BV/TV, ratio | 0.26±0.02 | 0.20±0.02††† | 0.08±0.02†††,‡‡‡ | 0.09±0.02†††,‡‡‡ |
| BMD, g/cm2 | 929.74±32.06 | 922.53±57.59 | 848.15±40.35 | 943.72±68.42 |
| Proximal tibial metaphysis (cancellous bone only) | ||||
| TV, mm3 | 0.93±0.01 | 0.95±0.01 | 0.94±0.02 | 0.96±0.00 |
| BV, mm3 | 0.15±0.02 | 0.07±0.01††† | 0.01±0.01†††,‡‡‡ | 0.03±0.02†††,‡‡‡ |
| BV/TV, ratio | 0.16±0.02 | 0.08±0.01††† | 0.01±0.01†††,‡‡‡ | 0.03±0.02†††,‡‡‡ |
| TbN, 1/mm | 2.82±0.35 | 1.65±0.26††† | 0.32±0.19†††,‡‡‡ | 0.63±0.32†††,‡‡‡ |
| TbT, ìm | 0.056±0.002 | 0.048±0.005† | 0.039±0.007†††,‡‡‡ | 0.041±0.010†,‡ |
| TbS, ìm | 0.30±0.04 | 0.57±0.09††† | 4.94±4.64†††,‡‡‡ | 2.01±1.23†††,‡‡‡ |
| BMD, g/cm2 | 918.43±19.86 | 923.92±37.10 | 864.96±41.79 | 921.60±45.66 |
†p<0.05 versus normal; ‡p<0.05 versus MMT; ††p<0.01 versus normal; ‡‡p<0.01 versus MMT; †††p<0.001 versus normal and ‡‡‡p<0.001 versus MMT.
BMD, bone mineral density; BV, bone volume; MMT, medial meniscal tear; TbN, trabecular number; TbS, trabecular separation; TbT, trabecular thickness; TV, tissue volume; μCT, micro-CT.
PK and anti-drug antibody levels
For PK and anti-drug antibodies results, see online supplementary text S4.
Discussion
The serious joint adverse events observed in placebo-controlled clinical trials of anti-NGF antibodies have been adjudicated as either normal or RPOA, the latter characterised by atrophic bone with subchondral insufficiency and/or occasional foci of secondary osteonecrosis.33 This clinical finding was unexpected, since extensive non-clinical safety studies failed to find adverse events of bone or joint. The current study was undertaken to see if a commonly used and relevant arthritis model, the rat MMT, would be useful for further understanding the finding.
Treating MMT rats with tanezumab did not recapitulate the atrophic bone destruction associated with RPOA in clinical trials. Although anti-NGF treatment early in the MMT disease process influenced weight-bearing and drove additional cartilage damage, secondary changes in subchondral bone were instead hypertrophic (subchondral bone thickening, with osteophytes present). There were however notable findings related to weight-bearing and its impact on joint damage, highlighting that this model is ideal for evaluating the relationship between joint instability, changes in weight/load-bearing and health of articular cartilage damage.24 27 Cartilage damage and subsequent effects on bony tissues (ie, subchondral bone sclerosis and osteophyte formation) appear to be exquisitely sensitive to weight-bearing early in the rat MMT disease process (ie, 0–14 days after meniscal tear). In contrast, treatment in the chronic phase after gait deficiency had already normalised, similar to the clinical trial experience, had no adverse effects on the joint.
Mid-tibial amputation protected against MMT-induced joint damage, further suggesting that dynamic weight-bearing is the key driver for joint damage in this model. For instance, there was more severe decreased bone volume in the proximal tibial metaphysis of amputated rats versus normal rats. The idea that weight-bearing drives joint damage in the MMT rat is also supported by observations that MMT rats given intra-articular injections of irritating substances leading to joint swelling and pain actually result in decreased cartilage damage and bone sclerosis scores, largely because the rats are unwilling to bear weight on the joint (A. Bendele, unpublished observations).30
In this study, MMT rats exhibited gait deficiency on the operated limb shortly after surgery, which returned to baseline by days 14–28. Transection of the meniscus leads to this gait abnormality, not general surgery trauma, as animals undergoing sham surgery exhibited no change in weight-bearing at any point. Treatment with tanezumab at ≥0.1 mg/kg resulted in MMT rats behaving similarly to sham surgery rats, since they did not exhibit altered weight-bearing. These results, combined with other work investigating the role of NGF in a meniscal transection rat model of OA, suggest that analgesia can significantly influence voluntary weight-bearing.
Results suggest that at least two separable phases of pain behaviours occur in the rat MMT model: (1) an early phase, in which gait abnormality is present and dynamic weight-bearing significantly alters the future trajectory of joint damage, and (2) a later phase, in which there is no detectable gait abnormality and tissue destruction is no longer sensitive to NGF inhibition. From this, we propose that increased joint damage seen in tanezumab-treated MMT rats was due to an increase in voluntary weight-bearing early in the disease process and not due to direct toxic effect of tanezumab on cartilage or bone. To support this, an additional arm of the study was conducted delaying onset of tanezumab treatment from day 0 to day 23 or 57 after MMT surgery when gait deficiency was no longer present. Overall, the results presented here support the conclusion that analgesia may exacerbate joint damage when it is present during the early phase. This is likely due to mechanical overloading shortly after meniscal injury. The relevance of this model to RPOA reported in human clinical trials should be considered. Specifically, results from this study may help guide future clinical trial design by suggesting exclusion criteria (ie, patients deemed sensitive to mechanical overloading/radiographic evidence of bone or joint insufficiency).
Acknowledgments
The authors acknowledge the excellent technical assistance of Phil Bendele at Bolder BioPATH for the tibial amputation model, as well as ICON laboratories for the determination of PK and anti-drug antibodies parameters. The authors also acknowledge Drs Larry Whiteley, Nasir Khan and Lloyd Dethloff (Pfizer) for their guidance and critical review of this work. Medical writing support was provided by Joseph Oleynek at Engage Scientific Solutions and was funded by Eli Lilly & Co. and Pfizer.
Footnotes
Contributors: TPL, KEG, TRC, DLS and MAZ designed the study. BCO, AMB, SIH and CMB generated data for the study. LEG performed the statistical analyses. All authors were involved in data interpretation and writing and reviewing the manuscript.
Funding: This study was funded by Pfizer, Inc. and Eli Lilly & Co.
Competing interests: KEG, SIH, CMB, TRC and MAZ are employees of Pfizer and own stock and/or stock options in Pfizer. TPL and DLS were employees of Pfizer at the time of these studies and own stock and/or stock options in Pfizer. LEG is a contracted resource of and owns stock in Pfizer.
Ethics approval: Oversight by local institutional animal care and use committees.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dillon CF, Rasch EK, Gu Q, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol 2006;33:2271–9. [PubMed] [Google Scholar]
- 2.Kidd BL, Langford RM, Wodehouse T. Arthritis and pain. Current approaches in the treatment of arthritic pain. Arthritis Res Ther 2007;9:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hochman JR, French MR, Bermingham SL, et al. The nerve of osteoarthritis pain. Arthritis Care Res (Hoboken) 2010;62:1019–23. 10.1002/acr.20142 [DOI] [PubMed] [Google Scholar]
- 4.Hefti FF, Rosenthal A, Walicke PA, et al. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci 2006;27:85–91. 10.1016/j.tips.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Mantyh PW, Koltzenburg M, Mendell LM, et al. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. Anesthesiology 2011;115:189–204. 10.1097/ALN.0b013e31821b1ac5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh DA, McWilliams DF, Turley MJ, et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 2010;49:1852–61. 10.1093/rheumatology/keq188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson JJ, Allen SJ, Dawbarn D. Targeting nerve growth factor in pain: what is the therapeutic potential? BioDrugs 2008;22:349–59. 10.2165/0063030-200822060-00002 [DOI] [PubMed] [Google Scholar]
- 8.Abdiche YN, Malashock DS, Pons J. Probing the binding mechanism and affinity of tanezumab, a recombinant humanized anti-NGF monoclonal antibody, using a repertoire of biosensors. Protein Sci 2008;17:1326–35. 10.1110/ps.035402.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown MT, Murphy FT, Radin DM, et al. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain 2012;13:790–8. 10.1016/j.jpain.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 10.Brown MT, Murphy FT, Radin DM, et al. Tanezumab reduces osteoarthritic hip pain: results of a randomized, double-blind, placebo-controlled phase III trial. Arthritis Rheum 2013;65:1795–803. 10.1002/art.37950 [DOI] [PubMed] [Google Scholar]
- 11.Ekman E, Gimbel J, Bello A, et al. Efficacy and Safety of Intravenous Tanezumab in Osteoarthritis Hip and Knee Pain: comparison to Placebo and Naproxen in Two Phase III Studies (NCT00830063 & NCT00863304). J Pain 2011;12:55 http://www.ampainsoc.org/abstract/view/4761/ [Google Scholar]
- 12.Evans RJ, Moldwin RM, Cossons N, et al. Proof of concept trial of tanezumab for the treatment of symptoms associated with interstitial cystitis. J Urol 2011;185:1716–21. 10.1016/j.juro.2010.12.088 [DOI] [PubMed] [Google Scholar]
- 13.Katz N, Borenstein DG, Birbara C, et al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 2011;152:2248–58. 10.1016/j.pain.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 14.Kivitz AJ, Gimbel JS, Bramson C, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain 2013;154:1009–21. 10.1016/j.pain.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 15.Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med 2010;363:1521–31. 10.1056/NEJMoa0901510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spierings EL, Fidelholtz J, Wolfram G, et al. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain 2013;154:1603–12. 10.1016/j.pain.2013.04.035 [DOI] [PubMed] [Google Scholar]
- 17.Food and Drug Administration Center for Drug Evaluation and Research. Background Materials. Meeting of the Arthritis Advisory Committee (AAC). http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisDrugsAdvisoryCommittee/UCM295202.pdf (accessed 10 Apr 2012).
- 18.Food and Drug Administration Center for Drug Evaluation and Research. Background Materials Addendum. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisDrugsAdvisoryCommittee/UCM295203.pdf (accessed 10 Apr 2012). [Google Scholar]
- 19.Pfizer Inc. Arthritis Advisory Committee Briefing Document. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisDrugsAdvisoryCommittee/UCM295205.pdf (accessed 10 Apr 2012).
- 20.Schnitzer TJ, Ekman EF, Spierings EL, et al. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis 2015;74:1202–11. 10.1136/annrheumdis-2013-204905 [DOI] [PubMed] [Google Scholar]
- 21.Zorbas M, Hurst S, Shelton D, et al. A multiple-dose toxicity study of tanezumab in cynomolgus monkeys. Regul Toxicol Pharmacol 2011;59:334–42. 10.1016/j.yrtph.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 22.Koewler NJ, Freeman KT, Buus RJ, et al. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J Bone Miner Res 2007;22:1732–42. 10.1359/jbmr.070711 [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharyya T, Gale D, Dewire P, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am 2003;85-A:4–9. [DOI] [PubMed] [Google Scholar]
- 24.Bendele AM. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact 2001;1:363–76. [PubMed] [Google Scholar]
- 25.Gerwin N, Bendele AM, Glasson S, et al. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage 2010;18(Suppl 3):S24–34. 10.1016/j.joca.2010.05.030 [DOI] [PubMed] [Google Scholar]
- 26.Bendele AM. Animal models of osteoarthritis in an era of molecular biology. J Musculoskelet Neuronal Interact 2002;2:501–3. [PubMed] [Google Scholar]
- 27.Bove SE, Laemont KD, Brooker RM, et al. Surgically induced osteoarthritis in the rat results in the development of both osteoarthritis-like joint pain and secondary hyperalgesia. Osteoarthr Cartil 2006;14:1041–8. 10.1016/j.joca.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 28.Janusz MJ, Bendele AM, Brown KK, et al. Induction of osteoarthritis in the rat by surgical tear of the meniscus: inhibition of joint damage by a matrix metalloproteinase inhibitor. Osteoarthr Cartil 2002;10: 785–91. 10.1053/joca.2002.0823 [DOI] [PubMed] [Google Scholar]
- 29.Shelton DL, Zeller J, Ho WH, et al. Nerve growth factor mediates hyperalgesia and cachexia in auto-immune arthritis. Pain 2005;116:8–16. 10.1016/j.pain.2005.03.039 [DOI] [PubMed] [Google Scholar]
- 30.Ashraf S, Mapp PI, Burston J, et al. Augmented pain behavioural responses to intra-articular injection of nerve growth factor in two animal models of osteoarthritis. Ann Rheum Dis 2014;73:1710–18. 10.1136/annrheumdis-2013-203416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulet B, de Souza R, Knights CB, et al. Modifications of gait as predictors of natural osteoarthritis progression in STR/Ort mice. Ann Rheum Dis 2014;66:1832–42. 10.1002/art.38616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanson NA, Bagi CM. Alternative approach to assessment of bone quality using micro-computed tomography. Bone 2004;35:326–33. 10.1016/j.bone.2004.02.019 [DOI] [PubMed] [Google Scholar]
- 33.Hochberg MC. Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage 2015;23(Suppl 1):S18–21. 10.1016/j.joca.2014.10.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2015-208913supp_text.pdf (162.3KB, pdf)
annrheumdis-2015-208913supp_tables.pdf (87.5KB, pdf)