Abstract
Objective
To assess the safety and efficacy of ixekizumab, a monoclonal antibody that inhibits interleukin-17A, in a double-blind phase III trial enrolling patients with active psoriatic arthritis (PsA).
Methods
Patients naive to biologic therapy with active PsA were randomised to subcutaneous injections of placebo (N=106), adalimumab 40 mg once every 2 weeks (active reference; N=101), ixekizumab 80 mg once every 2 weeks (IXEQ2W) (N=103), or ixekizumab 80 mg once every 4 weeks (IXEQ4W) (N=107). Both ixekizumab regimens included a 160-mg starting dose. The primary objective was to assess the superiority of IXEQ2W or IXEQ4W versus placebo as measured by the proportion of patients achieving an American College of Rheumatology 20 (ACR20) response at week 24.
Results
Significantly more patients treated with ixekizumab achieved an ACR20 response with IXEQ2W (62.1%) or IXEQ4W (57.9%) than placebo (30.2%) (p≤0.001; non-responder imputation method). Disease activity and functional disability were significantly improved with both ixekizumab doses versus placebo at weeks 12 and 24, and there was significantly less progression of structural damage at week 24 (p≤0.01). Clearance of plaque psoriasis was greater with ixekizumab than placebo (p≤0.001). Efficacy results with adalimumab, the active reference arm, showed significant improvements versus placebo. Treatment-emergent adverse events were more frequent with ixekizumab (65.7–66.4%) and adalimumab (64.4%) than placebo (47.2%) (p<0.05).
Conclusions
In biologic-naive patients with active PsA, ixekizumab treatment resulted in improvements in disease activity and physical function, as well as in the inhibition of structural damage progression. Overall, adverse events were more frequent in all active groups compared with placebo.
Trial registration number
NCT01695239; EudraCT2011-002326-49; Results.
Keywords: DMARDs (biologic), Psoriatic Arthritis, Treatment, Spondyloarthritis
Introduction
Psoriatic arthritis (PsA) is a chronic, systemic, immune-mediated, inflammatory arthritis commonly associated with plaque psoriasis, joint damage, dactylitis, enthesitis and axial involvement.1 2 PsA can be progressive and destructive, resulting in physical deformities, impaired function, decreased quality of life and increased mortality.3 4 The cytokine interleukin (IL)-17A promotes joint inflammation and damage by triggering the activation and trafficking of immune cells, inducing proinflammatory cytokines and chemokines, acting as a chemoattractant to neutrophils and monocytes, and stimulating release of matrix metalloproteases and receptor activator of nuclear factor kappa-B ligand, which contribute to cartilage and bone destruction, respectively.5 Increased numbers of IL-17A-producing cells are present in the peripheral blood, synovial tissue and fluid, and skin plaques of patients with PsA;6–11 the concentration of IL-17A-producing cells correlates with disease activity.10 Based on these findings, specific inhibition of IL-17A represents an emerging targeted approach to PsA management.12 13
Ixekizumab, a recombinant, high-affinity, humanised, immunoglobulin G4κ monoclonal antibody selectively binds and neutralises IL-17A. The safety and efficacy of ixekizumab in patients with active PsA not previously treated with biologic agents are under investigation in a phase III study (SPIRIT-P1). Here we report the results from the 24-week, placebo-controlled and active-controlled, double-blind period of this study.
Methods
Study design and patient population
The SPIRIT-P1 study (NCT01695239, EudraCT 2011-002326-49) is a 3-year, phase III, randomised, double-blind, placebo-controlled and active-controlled clinical trial comparing two regimens of ixekizumab and an active reference arm adalimumab (Humira; AbbVie) at the approved dose and regimen to treatment with placebo in patients not previously treated with biologic agents for plaque psoriasis or PsA. The double blind period of the study occurred in the first 24 weeks. Enrolled patients fulfilled the Classification Criteria for Psoriatic Arthritis (CASPAR);14 had ≥3 of 68 tender joint count and ≥3 of 66 swollen joint count; and had either ≥1 PsA-related hand or foot joint erosion on centrally read X-rays or C reactive protein >6 mg/L. Further discussion of study design and patient population is included in the online supplementary material.
annrheumdis-2016-209709supp1.pdf (627.1KB, pdf)
Treatment and randomisation
Randomisation was performed centrally via an interactive voice response system based on a computer-generated randomisation code, with stratification by country and by prior/current/no use of conventional (non-biologic) disease-modifying antirheumatic drugs (cDMARDs). Patients were randomised at a 1:1:1:1 ratio to one of four treatment groups: ixekizumab 80 mg every 2 weeks (IXEQ2W), ixekizumab 80 mg every 4 weeks (IXEQ4W), adalimumab 40 mg Q2W, or placebo, all administered via subcutaneous injection (see online supplementary figure S1). Patients randomised to IXEQ4W or IXEQ2W were administered a starting dose of 160 mg given as two injections at week 0. Because the different randomised treatments used distinct schedules and distinguishable prefilled syringes, a double-dummy design with Q2W dosing was employed to conceal treatment allocation (see online supplementary figure S2).
Patients in all treatment groups with an inadequate response at week 16, distinguished by predefined tender and swollen joint count criteria (Inadequate Responders), were required to add or modify concomitant medications. The investigators, study personnel and patients were blinded to the Inadequate Response criteria. The Inadequate Response criteria are being applied in another ongoing and currently blinded study (SPIRIT-P2) in bDMARD-experienced patients with active PsA and thus, must remain blinded. Inadequate Responders remained on their originally assigned dose of ixekizumab or, if receiving adalimumab or placebo, were randomised again to IXEQ2W or IXEQ4W in a 1:1 ratio (see online supplementary figure S1). Inadequate Responders from the adalimumab treatment group received 8 weeks of placebo as a washout therapy prior to initiating ixekizumab treatment at week 24.
Patients already on stable doses of allowed cDMARDs, oral corticosteroids, opiates and/or non-steroidal anti-inflammatory drugs/cyclo-oxygenase-2 inhibitors continued these on study. Further discussion of treatment, concomitant medications and randomisation is provided in the online supplementary material.
Study assessments
On scheduled study visits (weeks 1, 2, 4, 8, 12, 16, 20 and 24) during the double-blind treatment period, patients underwent assessments for safety and efficacy end points and were supplied with study medication if scheduled. Radiographic images of the hands and feet taken at the screening visit, week 16 and week 24 were assessed centrally by two expert readers who were blinded to treatment allocation, time point and patient data. The primary efficacy end point was the proportion of patients achieving an American College of Rheumatology (ACR) 20 response at week 24 versus placebo. Definitions of the ACR20, ACR50 and ACR70 responses used in the study are provided in the online supplementary material.15
Secondary end points included the proportion of patients achieving ACR50 or ACR70 responses; the proportion of patients achieving a 75%, 90% or 100% improvement in Psoriasis Area and Severity Index score (PASI 75, PASI 90 and PASI 100, respectively);16 17 percentage of psoriasis-affected body surface area (BSA); change from baseline in the Health Assessment Questionnaire-Disability Index (HAQ-DI);18 19 change from baseline in the van der Heijde modified total Sharp score (mTSS, a measure of structural disease progression based on radiographic assessment of bone erosions and joint space narrowing in the hands and feet);20 and change from baseline in 28-joint Disease Activity Score using C reactive protein (DAS28-CRP) versus placebo.21 Additional secondary end points for patients affected at baseline were enthesitis (inflammation of tendon and ligament insertions, as assessed by the presence or absence of tenderness at the six sites of the Leeds Enthesitis Index (LEI)),22 dactylitis (swelling of the whole digit, with assessment of the number of all 20 digits that are affected, according to the Leeds Dactylitis Index-Basic (LDI-B)),23 24 the Itch Numeric Rating Scale (itch NRS)25 and a modified version of the Nail Psoriasis Severity Index (NAPSI),26 which assessed fingernails only.
Safety evaluations included adverse event (AE) reporting, vital signs, physical exam findings, concomitant medications, ECG, and drug immunogenicity, haematology and laboratory evaluations. AEs of special interest included cytopenias, liver function test changes/enzyme elevations, infections, injection site reactions, hypersensitivity, cerebrocardiovascular events, malignancies, Pneumocystis pneumonia/interstitial lung disease, depression and Crohn's disease/ulcerative colitis.
Statistical analyses
Efficacy analyses were conducted on the intent-to-treat population (all randomised patients). Primary analyses of categorical variables were based on a logistic regression analysis with treatment, geographical region and baseline cDMARD experience in the model. Missing data were imputed using a non-responder imputation method, in which patients who were Inadequate Responders, or who discontinued treatment before week 24, were defined as non-responders. The primary analyses for all continuous variables were based on mixed-effects models for repeated measures with treatment, geographical region, baseline score, baseline cDMARD experience, visit and the interaction of treatment-by-visit in the model. To control the overall type I error rate at a two-sided α level of 0.05, a multiplicity-controlled analysis was used for the primary end point and the six predetermined secondary end points. If the week 24 ACR20 primary efficacy analysis was significant for one or both ixekizumab doses, the secondary analyses were considered in the following sequence: week 24 HAQ-DI, week 24 mTSS, week 12 ACR20, week 12 PASI 75, week 12 LEI and week 12 itch NRS. All other secondary end points were assessed at a significance level of p<0.05 with no adjustment for multiplicity. Safety analyses were conducted on the safety population (all patients who took at least one dose of study medication). Fisher's exact test was used for categorical safety data. Continuous safety variables used analysis of covariance (ANCOVA) with treatment and baseline value in the model. Details of additional statistical methods are provided in the online supplementary material.
The adalimumab 40 mg Q2W treatment arm served as active reference for comparison with placebo. The study was not powered to test equivalence or non-inferiority of ixekizumab versus adalimumab.
Results
Patient population
Of 719 patients screened, 417 were randomised (see online supplementary figure S3). The mean age was 49.5 years, 46.0% were male, 85.3% were cDMARD-experienced, 64% were currently using cDMARDs and 54.2% reported current methotrexate use. For those taking methotrexate at baseline, the average methotrexate dose was 15.8±5.04 mg/week (mean±SD). Overall, 69.5% had psoriasis involving ≥3% of BSA, 58% had enthesitis and 37.6% had dactylitis at baseline (table 1).
Table 1.
Baseline characteristics of the patients according to treatment group
| Placebo | IXEQ4W | IXEQ2W | Adalimumab 40 mg Q2W* | Total | |
|---|---|---|---|---|---|
| N=106 | N=107 | N=103 | N=101 | N=417 | |
| Age (years), mean (SD) | 50.6 (12.3) | 49.1 (10.1) | 49.8 (12.6) | 48.6 (12.4) | 49.5 (11.9) |
| Male, n (%) | 48 (45.3) | 45 (42.1) | 48 (46.6) | 51 (50.5) | 192 (46.0) |
| Weight (kg), mean (SD) | 83.8 (19.6) | 85.5 (23.0) | 81.6 (17.5) | 91.6 (21.9)† | 85.6 (20.9) |
| BMI (kg/m2), mean (SD) | 29.2 (6.3) | 30.2 (8.4) | 28.6 (6.6) | 32.1 (11.4)‡ | 30 (8.5) |
| Race, n (%) | |||||
| White | 99 (93.4) | 102 (95.3) | 96 (93.2) | 95 (94.1) | 392 (94.0) |
| Asian | 5 (4.7) | 2 (1.9) | 5 (4.9) | 3 (3.0) | 15 (3.6) |
| American Indian or Alaska native | 2 (1.9) | 2 (1.9) | 2 (1.9) | 3 (3.0) | 9 (2.2) |
| Other | 0 | 1 (0.9) | 0 | 0 | 1 (0.2) |
| Time since psoriatic arthritis diagnosis (years), mean (SD) | 6.3 (6.9) | 6.2 (6.4) | 7.2 (8.0) | 6.9 (7.5) | 6.7 (7.2) |
| Time since psoriasis diagnosis (years), mean (SD) | 16.0 (13.8) | 16.5 (13.8) | 17.0 (14.0) | 15.7 (12.7) | 16.3 (13.5) |
| Background cDMARD therapy, n (%) | |||||
| Naïve | 13 (12.3) | 17 (15.9) | 17 (16.5) | 14 (13.9) | 61 (14.6) |
| Past use | 24 (22.6) | 22 (20.6) | 23 (22.3) | 20 (19.8) | 89 (21.3) |
| Current use | 69 (65.1) | 68 (63.6) | 63 (61.2) | 67 (66.3) | 267 (64.0) |
| Methotrexate current use, n (%) | 59 (55.7) | 57 (53.3) | 53 (51.5) | 57 (56.4) | 226 (54.2) |
| Patients with specific disease characteristics, n (%) | |||||
| Current psoriasis§ | 102 (96.2) | 100 (93.5) | 95 (92.2) | 97 (96.0) | 394 (94.5) |
| Psoriasis BSA ≥3%¶ | 67 (67.7) | 73 (73.0) | 59 (64.8) | 68 (72.3) | 267 (69.5) |
| Fingernail psoriasis§ | 74 (69.8) | 70 (65.4) | 74 (71.8) | 71 (70.3) | 289 (69.3) |
| Dactylitis§ | 39 (36.8) | 54 (50.5) | 41 (39.8) | 23 (22.8)‡ | 157 (37.6) |
| Enthesitis§ | 57 (53.8) | 70 (65.4) | 59 (57.3) | 56 (55.4) | 242 (58.0) |
| Baseline disease and quality of life scores, mean (SD) | |||||
| Tender joint count (68 joints) | 19.2 (13.0) | 20.5 (13.7) | 21.5 (14.1) | 19.3 (13.0) | 20.1 (13.4) |
| Swollen joint count (66 joints) | 10.6 (7.3) | 11.4 (8.2) | 12.1 (7.2) | 9.9 (6.5) | 11.0 (7.4) |
| HAQ-DI | 1.2 (0.60) | 1.2 (0.54) | 1.2 (0.57) | 1.1 (0.59) | 1.2 (0.58) |
| Patient-reported pain VAS 0–100 | 58.5 (23.0) | 60.1 (19.4) | 58.4 (21.7) | 58.7 (19.7) | 58.9 (20.9) |
| Patient-assessed global disease activity VAS 0–100 | 61.1 (22.7) | 62.7 (19.1) | 62.5 (19.9) | 59.1 (19.1) | 61.4 (20.2) |
| Physician-assessed global disease activity VAS 0–100 | 55.9 (19.3) | 57.6 (18.7) | 58.5 (19.0) | 55.4 (18.7) | 56.9 (18.9) |
| CRP (mg/L) | 15.1 (23.6) | 12.8 (16.4) | 15.1 (25.9) | 13.2 (19.1) | 14.1 (21.5) |
| mTSS | 17.6 (28.6) | 19.2 (32.7) | 15.2 (28.9) | 15.9 (27.4) | 17.0 (29.4) |
| DAS28-CRP | 4.9 (1.0) | 5.0 (1.0) | 5.0 (1.1) | 4.9 (1.0) | 4.9 (1.0) |
| LEI** | 2.9 (1.7) | 2.7 (1.6) | 3.1 (1.8) | 3.0 (1.6) | 2.9 (1.7) |
| LDI-B†† | 46.2 (65.5) | 58.1 (96.7) | 40.6 (54.6) | 93.9 (111.9)‡ | 55.8 (83.6) |
| LDI-B‡‡ | 62.7 (69.3) | 73.0 (103.4) | 64.0 (56.6) | 119.9 (113.5)‡ | 75.9 (89.4) |
| % Psoriasis BSA involved¶ | 14.4 (20.2) | 15.1 (16.3) | 12.0 (15.6) | 14.8 (19.2) | 14.1 (17.9) |
| PASI total score¶ | 6.2 (7.5) | 6.9 (6.6) | 6.0 (7.0) | 5.5 (6.5) | 6.1 (6.9) |
| NAPSI§§ | 19.8 (17.2) | 21.3 (18.9) | 25.0 (21.2) | 20.9 (17.5) | 21.8 (18.8) |
| SF-36 PCS | 34.0 (8.3) | 32.4 (10.1) | 34.2 (8.7) | 33.9 (8.8) | 33.6 (9.0) |
*The adalimumab 40 mg Q2W treatment arm served as active reference for comparison with placebo. The study was not powered to test equivalence or non-inferiority of ixekizumab versus adalimumab.
†p≤0.01 vs placebo.
‡p<0.05 vs placebo.
§Presence or absence, as qualitatively assessed by the investigator.
¶Evaluated in patients with psoriasis, as qualitatively assessed by the investigator, at baseline.
**Evaluated in patients with enthesitis, as qualitatively assessed by the investigator, at baseline.
††Evaluated in patients with dactylitis, as qualitatively assessed by the investigator, at baseline.
‡‡Evaluated in patients with baseline LDI-B score >0; post hoc analysis.
§§Evaluated in patients with fingernail psoriasis, as qualitatively assessed by the investigator, at baseline.
BMI, body mass index; BSA, body surface area; cDMARD, conventional disease-modifying antirheumatic drug; CRP, C reactive protein; DAS28-CRP, 28-joint Disease Activity Score using C reactive protein; HAQ-DI, Health Assessment Questionnaire-Disability Index; IXEQ2W, 80 mg ixekizumab once every 2 weeks; IXEQ4W, 80 mg ixekizumab once every 4 weeks; LDI-B, Leeds Dactylitis Index-Basic; LEI, Leeds Enthesitis Index; mTSS, van der Heijde modified total Sharp score; NAPSI, Nail Psoriasis Severity Index; PASI, Psoriasis Area and Severity Index; Q2W, every 2 weeks; SF-36 PCS, Short Form (36 Items) Health Survey Physical Component Score; VAS, visual analogue scale.
Of the 382 patients completing the 24-week double-blind period, 57 were Inadequate Responders (11 IXEQ4W, 10 IXEQ2W, 9 adalimumab, 27 placebo) and received rescue medication from week 16 through week 24. A numerically greater percentage of patients in the IXEQ4W (90.7%), IXEQ2W (94.2%) and adalimumab (96%) groups than in the placebo group (85.8%) completed the 24 weeks.
Efficacy results
Table 2 summarises the outcomes of efficacy end points at 12 weeks and 24 weeks. The primary efficacy end point of ACR20 response at week 24 was met with both IXEQ4W (57.9%) and IXEQ2W (62.1%); response rates in both ixekizumab groups were significantly greater than in the placebo group (30.2%) (p≤0.001) (figure 1A). The adalimumab group (active reference) also had a significantly greater ACR20 response at week 24 (57.4%) compared with placebo (p≤0.001). Despite the adalimumab group having a statistically higher baseline weight and body mass index (BMI) compared with the placebo group (table 1), a Cochran-Armitage trend test within the adalimumab group demonstrated no effect of weight or BMI on the ACR20 response at week 24 (data not shown). The ACR20 response at 12 weeks, tested as a secondary end point, was also significantly greater in patients randomised to IXEQ4W (57%) and IXEQ2W (60.2%) versus placebo (31.1%) (p≤0.001). ACR20 response rates for both ixekizumab groups and the adalimumab group were significantly greater than the placebo group as early as week 1 (p≤0.01), and the differences were maintained throughout the 24-week period (p≤0.01). The treatment responses on ixekizumab were significantly greater than those on placebo as early as week 4 for ACR50 and week 8 for ACR70 and persisted through week 24 (p<0.05) (figure 1B, C). Mean reductions in each component of the ACR core set are presented in online supplementary figure S4 and table S1.
Table 2.
Comparison of efficacy during the 24 weeks of placebo-controlled therapy
| Placebo | IXEQ4W | IXEQ2W | Adalimumab 40 mg Q2W* | |||||
|---|---|---|---|---|---|---|---|---|
| 12 weeks | 24 weeks | 12 weeks | 24 weeks | 12 weeks | 24 weeks | 12 weeks | 24 weeks | |
| Responder rate: | ||||||||
| N=106 | N=107 | N=103 | N=101 | |||||
| ACR20, % | 31.1 | 30.2 | 57.0† | 57.9† | 60.2† | 62.1† | 51.5‡ | 57.4† |
| ACR50, % | 4.7 | 15.1 | 33.6† | 40.2† | 39.8† | 46.6† | 29.7† | 38.6† |
| ACR70, % | 0 | 5.7 | 15.0 | 23.4† | 16.5 | 34.0† | 17.8 | 25.7† |
| N=92 | N=100 | N=90 | N=89 | |||||
| HAQ-DI MCID, %§ | 29.3 | 26.1 | 49.0‡ | 49.0† | 64.4† | 57.8† | 49.4‡ | 49.4† |
| N=28 | N=39 | N=26 | N=18 | |||||
| LDI-B (0), %¶,** | 53.6 | 25.0 | 74.4 | 79.5† | 69.2 | 76.9† | 61.1 | 77.8† |
| N=57 | N=68 | N=57 | N=54 | |||||
| LEI (0), %¶,†† | 28.1 | 19.3 | 27.9 | 42.6‡ | 47.4‡‡ | 38.6§§ | 35.2 | 33.3 |
| N=67 | N=73 | N=59 | N=68 | |||||
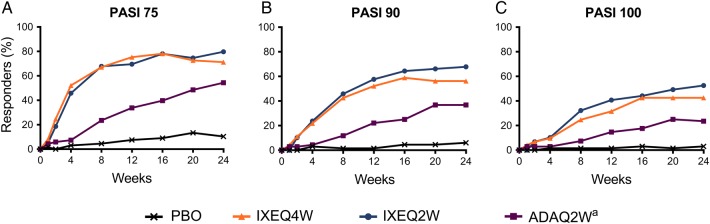
| PASI 75, %¶¶ | 7.5 | 10.4 | 75.3† | 71.2† | 69.5† | 79.7† | 33.8† | 54.4† |
| PASI 90, %¶¶ | 1.5 | 6.0 | 52.1† | 56.2† | 57.6† | 67.8† | 22.1‡ | 36.8† |
| PASI 100, %¶¶ | 1.5 | 3.0 | 31.5† | 42.5† | 40.7† | 52.5† | 14.7§§ | 23.5‡ |
| N=41 | N=52 | N=41 | N=37 | |||||
| sPGA (0, 1), %*** | 7.3 | 17.1 | 75.0† | 65.4† | 80.5† | 73.2† | 45.9† | 62.2† |
| sPGA (0), %*** | 2.4 | 2.4 | 30.8‡ | 38.5‡ | 36.6‡ | 39.0‡ | 10.8 | 18.9‡‡ |
| N=74 | N=70 | N=74 | N=71 | |||||
| NAPSI (0), %††† | 8.1 | 18.9 | 20.0‡‡ | 25.7 | 27.0‡ | 36.5§§ | 19.7‡‡ | 39.4‡ |
| LS mean change from baseline (SE): | ||||||||
| N=106 | N=107 | N=103 | N=101 | |||||
| DAS28-CRP | −0.57 (0.11) | −0.84 (0.13) | −1.63 (0.11)† | −1.96 (0.12)† | −1.67 (0.11)† | −2.04 (0.12)† | −1.57 (0.12)† | −1.74 (0.12)† |
| N=106 | N=107 | N=103 | N=101 | |||||
| HAQ-DI | −0.13 (0.05) | −0.18 (0.05) | −0.37 (0.05)† | −0.44 (0.05)† | −0.47 (0.05)† | −0.50 (0.05)† | −0.35 (0.05)† | −0.37 (0.05)‡ |
| N=106 | N=107 | N=103 | N=101 | |||||
| SF-36 PCS | 2.3 (0.8) | 2.9 (1.0) | 5.8 (0.8)† | 7.5 (0.9)† | 7.6 (0.8)† | 8.2 (0.9)† | 5.7 (0.8)† | 6.8 (0.9)‡ |
| N=28 | N=39 | N=26 | N=18 | |||||
| LDI-B¶,** | −36.3 (10.3) | −33.7 (9.7) | −72.8 (8.8) † | −75.4 (8.1)† | −63.9 (10.6)‡‡ | −66.1 (9.8)‡ | −62.1 (11.9) | −76.0 (10.9)† |
| N=57 | N=70 | N=59 | N=56 | |||||
| LEI‡‡‡ | −0.8 (0.24) | −0.8 (0.26) | −0.9 (0.21) | −1.3 (0.21) | −1.5 (0.24)‡‡ | −1.4 (0.24) | −0.8 (0.24) | −0.9 (0.23) |
| N=102 | N=100 | N=95 | N=97 | |||||
| % BSA§§§ | −1.6 (1.2) | −2.7 (1.4) | −10.4 (1.2)† | −12.0 (1.3)† | −8.8 (1.2)† | −10.6 (1.4)† | −7.7 (1.2)† | −9.5 (1.4)† |
| N=74 | N=70 | N=74 | N=71 | |||||
| NAPSI††† | −1.1 (1.4) | −2.4 (1.7) | −8.4 (1.5)† | −14.0 (1.5)† | −7.7 (1.4)† | −15.5 (1.5)† | −6.8 (1.4)‡ | −10.7 (1.5)† |
*The adalimumab 40 mg Q2W treatment arm served as active reference for comparison with placebo. The study was not powered to test equivalence or non-inferiority of ixekizumab versus adalimumab.
†p≤0.001 vs placebo.
‡p≤0.01 vs placebo.
§Data reported for patients with a baseline HAQ-DI score ≥0.35. The MCID for HAQ-DI is an improvement from baseline ≥0.35.
¶Post hoc analysis.
**Data are reported for patients with dactylitis, as qualitatively assessed by the investigator, at baseline and baseline LDI-B score >0.
††Data are reported for patients with enthesitis, as qualitatively assessed by the investigator, at baseline and baseline LEI score >0.
‡‡p<0.05 vs placebo.
§§p≤0.025 vs placebo.
¶¶Data are reported for patients with baseline psoriatic lesion(s) involving ≥3% BSA.
***Data are reported for patients with sPGA ≥3 at baseline.
†††Data are reported for patients with fingernail psoriasis, as qualitatively assessed by the investigator, at baseline.
‡‡‡Data are reported for patients with enthesitis, as qualitatively assessed by the investigator, at baseline.
§§§Data are reported for patients with psoriasis, as qualitatively assessed by the investigator, at baseline.
ACR20/50/70, 20/50/70% American College of Rheumatology response; BSA, body surface area; DAS28-CRP, 28-joint Disease Activity Score using C reactive protein; HAQ-DI, Health Assessment Questionnaire-Disability Index; IXEQ2W, 80 mg ixekizumab once every 2 weeks; IXEQ4W, 80 mg ixekizumab once every 4 weeks; LDI-B, Leeds Dactylitis Index-Basic; LEI, Leeds Enthesitis Index; LS, least squares; MCID, minimal clinically important difference; NAPSI, Nail Psoriasis Severity Index; PASI 75/90/100, Psoriasis Area and Severity Index Improvement Response for 75/90/100%; Q2W, every 2 weeks; SF-36 PCS, Short Form (36 Items) Health Survey Physical Component Score; sPGA, static Physician Global Assessment of psoriasis.
Figure 1.
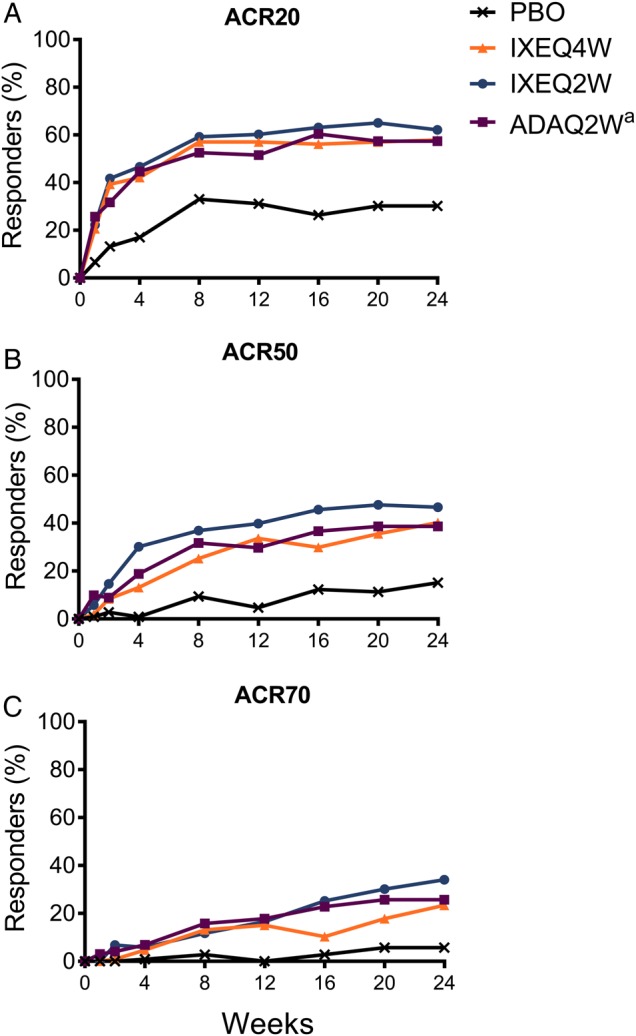
Time course of ACR responses. The percentages of patients achieving ACR20 (A), ACR50 (B) and ACR70 (C) are shown. Patients with inadequate responses to treatment at week 16 or missing data were analysed as non-response up to week 24. aActive reference arm for comparison with placebo. The study was not powered to test equivalence or non-inferiority of ixekizumab versus adalimumab. ACR20/50/70, 20/50/70% American College of Rheumatology response; ADAQ2W, 40 mg adalimumab once every 2 weeks; IXEQ2W, 80 mg ixekizumab once every 2 weeks; IXEQ4W, 80 mg ixekizumab once every 4 weeks; PBO, placebo; Q2W, once every 2 weeks.
Mean reductions in the level of disease activity observed with the DAS28-CRP at week 24 were −0.84 with placebo and −1.96, −2.04 and −1.74 in the IXEQ4W, IXEQ2W and adalimumab groups (all p≤0.001), respectively (table 2 and see online supplementary figure S5A). At week 24, improvements from baseline in physical function, measured by HAQ-DI, were significantly greater in patients receiving IXEQ4W (−0.44), IXEQ2W (−0.50) and adalimumab (−0.37) than in those receiving placebo (−0.18) (p≤0.01) (table 2 and see online supplementary figure S4F). For patients with HAQ-DI ≥0.35 at baseline, a greater percentage of patients achieved the minimal clinically important difference in HAQ-DI (improvement ≥0.35 from baseline)27 at week 24 in the IXEQ4W (49.0%), IXEQ2W (57.8%) and adalimumab (49.4%) groups compared with the placebo group (26.1%) (p≤0.001) (table 2). The improvement from baseline in Short Form (36 Items) Health Survey Physical Component Score was also significantly greater at week 24 for patients receiving IXEQ4W (7.5), IXEQ2W (8.2) and adalimumab (6.8) compared with those receiving placebo (2.9) (p≤0.01) (table 2).
Progression of structural damage, measured by changes from baseline in mTSS at week 24, was significantly less in the IXEQ4W (0.17), IXEQ2W (0.08) and adalimumab (0.10) groups than in the placebo group (0.49) (p≤0.01) (table 3). Significantly greater percentages of patients in the ixekizumab groups and adalimumab group at week 24 experienced no structural progression as defined by thresholds of ≤0.5 or ≤0.95 compared with the placebo group (table 3).
Table 3.
Effect on structural disease progression
| Placebo | IXEQ4W | IXEQ2W | Adalimumab 40 mg Q2W* | ||
|---|---|---|---|---|---|
| N=106 | N=107 | N=103 | N=101 | ||
| LS mean change from baseline mTSS (SE)† | Week 16 | 0.36 (0.07) | 0.13 (0.07)‡ | 0.06 (0.07)§ | 0.12 (0.08)‡ |
| Week 24 | 0.49 (0.09) | 0.17 (0.08)§ | 0.08 (0.08)¶ | 0.10 (0.09)¶ | |
| Percentage of patients with change in mTSS at week 24 | ≤0 | 72.0 | 83.0 | 83.5 | 91.6¶ |
| ≤0.5 | 77.4 | 89.0** | 94.8¶ | 95.8¶ | |
| ≤0.95 | 83.9 | 94.0‡ | 96.9§ | 95.8§ |
*The adalimumab 40 mg Q2W treatment arm served as active reference for comparison with placebo. The study was not powered to test equivalence or non-inferiority of ixekizumab versus adalimumab.
†Higher mTSS scores indicate more articular damage. The mean change from baseline at week 24 was assessed with a statistical significance threshold of p<0.025 in a hierarchical analysis; all other end points were assessed with a statistical significance threshold of p<0.05.
‡p≤0.025 vs placebo.
§p≤0.01 vs placebo.
¶p≤0.001 vs placebo.
**p<0.05 vs placebo.
IXEQ2W, 80 mg ixekizumab once every 2 weeks; IXEQ4W, 80 mg ixekizumab once every 4 weeks; LS, least squares; mTSS, van der Heijde modified total Sharp score; Q2W, every 2 weeks.
For patients with dactylitis and LDI-B >0 at baseline, significantly greater improvements in mean LDI-B scores at week 24 (post hoc analysis) were observed for the IXEQ4W, IXEQ2W and adalimumab groups compared with the placebo group (p≤0.01) (table 2 and see online supplementary figure S5B), and complete resolution of dactylitis symptoms (LDI-B=0) at week 24 (post hoc analysis) occurred at a greater rate in the IXEQ4W (80%), IXEQ2W (77%) and adalimumab (78%) groups than in the placebo group (25%) (p≤0.001) (table 2 and see online supplementary figure S6A). For patients with enthesitis at baseline, the reduction in LEI score at week 12 was greater only in the IXEQ2W group than in the placebo group (p=0.038); the change did not reach the significance threshold (p<0.025) based on the multiplicity-controlled analysis. At week 24, reductions in LEI score were numerically greater in both ixekizumab groups than in the placebo group (IXEQ4W, −1.3 p=0.151; IXEQ2W, −1.4 p=0.099; placebo, −0.8) but did not meet statistical significance (table 2 and see online supplementary figure S5C). Among patients with LEI>0 at baseline, complete resolution of enthesitis symptoms (LEI=0) at week 24 (post hoc analysis) occurred at a greater rate with IXEQ4W (43%) and IXEQ2W (39%) than with placebo (19%) (p<0.05) (table 2 and see online supplementary figure S6B).
Among patients with psoriasis at baseline affecting ≥3% BSA, a significantly greater percentage of patients achieved PASI 75 at week 12 for the IXEQ4W (75.3%), IXEQ2W (69.5%) and adalimumab (33.8%) groups compared with the placebo group (7.5%) (p<0.001) (table 2 and figure 2A). Significantly greater PASI responses compared with placebo were observed as early as week 4 for PASI 75 and PASI 90, and week 8 for PASI 100 (p≤0.01) (figure 2). For patients with nail involvement at baseline, mean changes from baseline in the NAPSI score at week 24 were significantly greater for the IXEQ4W (−14.0), IXEQ2W (−15.5) and adalimumab (−10.7) groups than for the placebo group (−2.4) (p≤0.001) (table 2). At week 24, a significantly greater percentage of patients achieved complete resolution of nail psoriasis (NAPSI=0) in the IXEQ2W (36.5%) and adalimumab (39.4%) groups compared with the placebo group (18.9%) (p<0.025).
Figure 2.
Time course of PASI responses. The percentages of patients achieving PASI 75 (A), PASI 90 (B) and PASI 100 (C) are shown. PASI was measured in patients with baseline psoriatic lesion(s) involving ≥3% body surface area. Patients with inadequate responses to treatment at week 16 or missing data were analysed as non-response up to week 24. aActive reference arm for comparison with placebo. The study was not powered to test equivalence or non-inferiority of ixekizumab versus adalimumab. PASI 75/90/100, Psoriasis Area and Severity Index Improvement Response for 75/90/100%; IXEQ2W, 80 mg ixekizumab once every 2 weeks; IXEQ4W, 80 mg ixekizumab once every 4 weeks; Q2W, once every 2 weeks.
Safety profile
A greater percentage of patients receiving ixekizumab (66%) and adalimumab (64%) reported at least one treatment-emergent AE compared with patients receiving placebo (47.2%). These were mostly mild or moderate, and the most common were injection site reaction, injection site erythema and nasopharyngitis (table 4).
Table 4.
Safety overview
| Placebo | IXEQ4W | IXEQ2W | Adalimumab 40 mg Q2W* | |
|---|---|---|---|---|
| (N=106) | (N=107) | (N=102) | (N=101) | |
| TEAE, n (%) | 50 (47.2) | 71 (66.4)† | 67 (65.7)† | 65 (64.4)‡ |
| Mild | 27 (25.5) | 43 (40.2)§ | 41 (40.2)§ | 39 (38.6) |
| Moderate | 21 (19.8) | 24 (22.4) | 21 (20.6) | 25 (24.8) |
| Severe | 2 (1.9) | 4 (3.7) | 5 (4.9) | 1 (1.0) |
| Most frequent TEAEs¶, n (%) | ||||
| Injection site reaction | 0 | 13 (12.1)** | 16 (15.7)** | 2 (2.0) |
| Injection site erythema | 0 | 7 (6.5)‡ | 13 (12.7)** | 2 (2.0) |
| Nasopharyngitis | 5 (4.7) | 7 (6.5) | 3 (2.9) | 7 (6.9) |
| Headache | 1 (0.9) | 4 (3.7) | 4 (3.9) | 3 (3.0) |
| Upper respiratory tract infection | 7 (6.6) | 5 (4.7) | 3 (2.9) | 5 (5.0) |
| ALT increased | 0 | 3 (2.8) | 4 (3.9) | 3 (3.0) |
| Diarrhoea | 3 (2.8) | 2 (1.9) | 5 (4.9) | 3 (3.0) |
| Muscle spasms | 1 (0.9) | 3 (2.8) | 4 (3.9) | 1 (1.0) |
| Bronchitis | 3 (2.8) | 3 (2.8) | 3 (2.9) | 4 (4.0) |
| AST increased | 0 | 2 (1.9) | 3 (2.9) | 2 (2.0) |
| Nausea | 2 (1.9) | 0 | 5 (4.9) | 4 (4.0) |
| Psoriatic arthropathy | 1 (0.9) | 3 (2.8) | 2 (2.0) | 3 (3.0) |
| Back pain | 0 | 2 (1.9) | 2 (2.0) | 3 (3.0) |
| Serious adverse events, n (%) | 2 (1.9) | 6 (5.6) | 3 (2.9) | 5 (5.0) |
| Serious infection, n (%) | 0 | 1 (0.9) | 2 (2.0) | 2 (2.0) |
| Discontinued due to AE, n (%) | 2 (1.9) | 2 (1.9) | 4 (3.9) | 2 (2.0) |
| AEs of special interest††, n (%) | 36 (34.0) | 52 (48.6)§ | 56 (54.9)† | 45 (44.6) |
| Infection | 27 (25.5) | 30 (28.0) | 24 (23.5) | 26 (25.7) |
| Any candida infection | 0 | 1 (0.9) | 1 (1.0) | 0 |
| Active or reactivated tuberculosis | 0 | 0 | 0 | 0 |
| Injection site reactions | 5 (4.7) | 26 (24.3)** | 27 (26.5)** | 6 (5.9) |
| Hepatic event | 7 (6.6) | 5 (4.7) | 9 (8.8) | 13 (12.9) |
| Allergic reaction/hypersensitivity | 3 (2.8) | 2 (1.9) | 5 (4.9) | 5 (5.0) |
| Cytopenia (all types) | 6 (5.7) | 1 (0.9) | 4 (3.9) | 4 (4.0) |
| Neutropenia | 0 | 0 | 1 (1.0) | 0 |
| Depression | 0 | 2 (1.9) | 1 (1.0) | 1 (1.0) |
| Cerebrocardiovascular event | 0 | 0 | 0 | 3 (3.0) |
| Malignancy | 1 (0.9) | 0 | 0 | 1 (1.0) |
*The adalimumab 40 mg Q2W treatment arm served as active reference for comparison with placebo. The study was not powered to test equivalence or non-inferiority of ixekizumab versus adalimumab.
†p≤0.01 vs placebo.
‡p≤0.025 vs placebo.
§p<0.05 vs placebo.
¶Adverse events are listed according to the preferred term in MedDRA, V.17.1, and are events that occurred in ≥2.0% of the patients in the combined ixekizumab group.
**p≤0.001 vs placebo.
††Reported as adverse events and coded using MedDRA, V.17.1. Groups of adverse events of special interest are shown; adverse events of special interest not reported in any group included pneumocystis pneumonia, Crohn's disease/ulcerative colitis and interstitial lung disease.
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IXEQ2W, 80 mg ixekizumab once every 2 weeks; IXEQ4W, 80 mg ixekizumab once every 4 weeks; MedDRA, Medical Dictionary for Regulatory Activities; Q2W, once every 2 weeks; TEAE, treatment-emergent adverse event.
Across all groups, 10 patients (2.4%) discontinued because of an AE. No deaths occurred. Injection site reactions were mostly mild in intensity and were more frequent in the ixekizumab groups than in the placebo group (see online supplementary table S2). No serious AEs (SAEs) of injection site reactions were reported, and four patients discontinued due to an AE of injection site reactions (two IXEQ2W, one adalimumab, one placebo). Reductions in neutrophils from normal levels, meeting the definition of National Cancer Institute Common Terminology Criteria for Adverse Events of grade 1 (lower limit of normal to 1500 cells/mm3) and grade 2 (<1500–1000 cells/mm3) neutropenia,28 occurred in 6.2% and 4.8%, respectively, of the patients treated with ixekizumab (data not shown). One patient had grade 2 neutropenia on more than one assessment; no infection onset occurred within 14 days of any report of grade 2 neutropenia. There were no instances of grade 3 (<1000–500 cells/mm3) or higher neutropenia, major cerebrocardiovascular events (cardiovascular death, myocardial infarction stroke, etc), interstitial lung disease/Pneumocystis pneumonia, Crohn's disease or ulcerative colitis in the ixekizumab-treated patients. Depression-related symptoms were reported in three patients in the ixekizumab groups; none were reported in the placebo group. One patient randomised to IXEQ2W discontinued from the study because of worsening of mild depression existing at baseline. No AEs of suicidal ideation or suicide attempt were reported. AEs of infection were similar in frequency between all treatment groups; the most commonly reported infections in the combined ixekizumab groups were nasopharyngitis, upper respiratory tract infection, bronchitis, conjunctivitis, oral herpes and pharyngitis (see online supplementary table S3). One patient treated with IXEQ2W experienced herpes zoster involving the eyelid, which was classified as a SAE. Four other SAEs of infection were gastroenteritis (IXEQ4W), oesophageal candidiasis (IXEQ2W), cellulitis (adalimumab) and mycoplasma pneumonia (adalimumab). All SAEs of infection resolved with treatment and did not lead to study discontinuation. One case of oral candidiasis (mild) was reported as an AE (IXEQ4W). There were no cases of invasive fungal disease or clinically active or reactivated tuberculosis. Mild or moderate hypersensitivity events, most commonly manifesting as rash or urticaria, were reported in seven patients in the ixekizumab groups; none were reported as serious. One patient treated with IXEQ4W discontinued the study due to rash. In the ixekizumab treatment groups, 11 patients had treatment-emergent anti-ixekizumab antibodies, and none had detectable neutralising antibodies; 72.7% (n=8/11) of these patients achieved an ACR20 response at week 24.
Discussion
In this phase III study, 80 mg of ixekizumab administered every 2 weeks or 4 weeks after a starting dose of 160 mg significantly improved signs and symptoms, including enthesitis and dactylitis, of PsA and patient-reported physical functioning, while also inhibiting bone destruction. Both ixekizumab dose regimens significantly reduced radiographic progression of joint damage when compared with placebo. A rapid therapeutic response was apparent as early as 1 week after beginning therapy. Moreover, nearly half of the patients with ≥3% affected BSA who were treated with ixekizumab achieved complete clearance of their psoriasis, a result consistent with earlier studies of ixekizumab in patients with moderate-to-severe psoriasis.29 30
A numerically greater proportion of patients receiving IXEQ2W achieved, for many end points, greater efficacy at week 24 compared with IXEQ4W. However, a formal comparison of the two ixekizumab treatments was not prespecified in the study design. Of note, the study was not intended to assess the effects of the 160 mg starting dose.
Patients treated with adalimumab, the active reference group, demonstrated significant efficacy compared with placebo across disease activity, functional outcome and radiographic endpoints. The inclusion of adalimumab, an active control, served as an internal reference arm to assess the relative efficacy of an approved and established treatment compared with placebo in the same study patient population as that for ixekizumab. The positive efficacy results for adalimumab further support the ability of SPIRIT-P1 to distinguish active treatments from placebo.
Within this manuscript, improvement in LDI-B is presented for two different baseline patient populations (see online supplementary table S4): (1) presence of dactylitis, based on a qualitative assessment conducted by the investigator (prespecified) or (2) presence of dactylitis, based on a qualitative assessment conducted by the investigator, and a baseline LDI-B score >0 (post hoc). Both assessments demonstrate a significant improvement in dactylitis with ixekizumab or adalimumab treatment versus placebo. However, the latter (post hoc) analysis uses a baseline population identified by applying a more objective measure (ie, based on a measurement of digit circumference and digit tenderness), which is intended to provide a clinically meaningful, yet more rigorous, estimation of the treatment effect.
The efficacy findings further highlight the role of IL-17A in the pathogenesis of PsA.12 13 31
The safety profile of ixekizumab was consistent with findings reported aligned with the safety profile in moderate-to-severe plaque psoriasis.29 30 Other than findings related to mild-to-moderate, transient injection site reactions, most AEs occurred at similar rates to those observed with placebo. IL-17A can regulate neutrophil homoeostasis via several mediators of neutrophil production, trafficking and function, such as granulocyte colony-stimulating factor, growth-related oncogene-α and IL-8. Furthermore, IL-17A is integrally involved in host mucosal defences against Candida albicans,32–34 which may contribute to the increased number of cases of candidiasis associated with IL-17A inhibition,12 13 30 including the two reported in the current study. Treatment-emergent neutropenia was of grade 1 or 2. Observed infections occurred at similar rates in active treatment and placebo groups. No obvious correlation between ixekizumab dosing frequency and AEs was observed in this study.
The 24-week placebo controlled study duration was based on existing data and ethical considerations to minimise the duration of exposure to placebo treatment. An extension period of up to 3 years will permit longer-term safety and efficacy evaluations. Given that the current study was restricted to patients who were naive to biologic therapy, the results cannot be generalised to treatment of patients with a history of failed therapy or loss of efficacy to therapy or intolerance to anti-tumor necrosis factor (TNF) agents. Evaluation of ixekizumab in patients with PsA with previous experience with biologic therapy is currently under investigation in the SPIRIT-P2 clinical trial (NCT02349295; EudraCT 2011-002328-42).
In conclusion, 24 weeks of treatment with ixekizumab improved the key clinical domains of PsA. The safety findings were consistent with the current understanding of IL-17A inhibition and the clinical profile observed in previous ixekizumab studies in patients with moderate-to-severe psoriasis. The present findings support the view that IL-17A is a key cytokine in the pathogenesis of PsA and an important therapeutic target.
Acknowledgments
The authors thank David S Shrom, Suvajit Samanta and Stephanie M Krupski (Eli Lilly and Company, Indianapolis, Indiana, USA), and David Hartree, Molly R Nixon, Millie S Hollandbeck and Jennifer N Bodie (ClinGenuity, Cincinnati, Ohio, USA) for medical writing support and assistance with preparation and submission of this paper.
Footnotes
Collaborators: The authors thank the SPIRIT-P1 study investigators (see online supplementary material for the full investigator list): N Barkham, L Bessette, R Blanco Alonso, EJ Box, M Brooks, R Burgos Vargas, V Chandran, R Dhar, A Fernandez Nebro, R Fleischmann, K Flint, J Forstot, F Galvan Villegas, F Garcia-Fructuoso, O Garmish, M Geneva-Popova, P Geusens, G Gladstein, H Goto, E Griep, R Harrell, A Hou, M Howell, A Kivitz, S Klein, A Kolczewska, M Korkosz, D Lopez Montilla, C Lue, Y Makino, JL Marenco de la Fuente, H Marzo-Ortega, P Mease, T Miyamura, E Mueller, L Myasoutova, F Navarro Sarabia, M Okada, A Ostor, K Papp, L Podrazilova, G Pulka, E-K Raussi, C Ritchlin, J Rosa, E Roussou, A Rychlewska-Hanczewska, K Sada, L Sedova, D Sikes, S Solomon, M Stack, M Stanislavchuk, K Suzuki, H Tahir, J Tälli, A Toncheva, A Turkiewicz, I Valter, S Vladeva, S Wolfe and N Zyablova.
Contributors: PJM, CTR, CLS, DKB and DDG were involved in the conception and design of the clinical study; PJM, RSC, CLS and CHL were involved in the acquisition of the data; and all authors were involved in the analysis and interpretation of the data. All authors were involved in the drafting and revising of the manuscript. C-YL was involved in the statistical analysis.
Funding: This study was funded and sponsored by Eli Lilly and Company.
Competing interests: PJM reports grants and personal fees from Eli Lilly & Company during the conduct of the study. PJM also reports, outside the submitted work, grants, personal fees, and non-financial support from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Crescendo, Genentech, Janssen, Pfizer and UCB Pharma; grants and personal fees from Merck and Novartis; and non-financial support from Corrona. DvdH reports personal fees from Eli Lilly & Company, during the conduct of the study. DvdH also reports personal fees from AbbVie, Amgen, AstraZeneca, Augurex, Bristol Myers Squibb, Celgene, Centocor, Chugai, Covagen, Daiichi, Galapagos, GlaxoSmithKline, Janssen, Merck, Novo Nordisk, Otsuka, Pfizer, Roche, Sanofi-Aventis, UCB Pharma and Vertex, outside the submitted work. DvdH is the director at Imaging Rheumatology BV. CTR reports personal fees from AbbVie, Amgen, Janssen, Novartis, UCB, Boerhinger Ingelheim, as well as grants from Amgen, outside the submitted work. MO reports grants and non-financial support from Eli Lilly & Company, during the conduct of this study. MO also reports non-financial support from Santen Pharmaceutical, Mitsubishi Tanabe Pharma, Pfizer and Abbott Japan, outside the submitted work. RSC and DKB were employees of Eli Lilly & Company, at the time this study was conducted. CLS, C-YL and CHL are employees of Eli Lilly & Company. DDG reports grants and personal fees from AbbVie, Amgen, Celgene, Janssen, Novartis, Pfizer and UCB, and personal fees from Eli Lilly & Company, outside the submitted work.
Ethics approval: The protocol was approved by each site's institutional review board or ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: N Barkham, L Bessette, R Blanco Alonso, EJ Box, M Brooks, R Burgos Vargas, V Chandran, R Dhar, A Fernandez Nebro, R Fleischmann, K Flint, J Forstot, F Galvan Villegas, F Garcia-Fructuoso, O Garmish, M Geneva-Popova, P Geusens, G Gladstein, H Goto, E Griep, R Harrell, A Hou, M Howell, A Kivitz, S Klein, A Kolczewska, M Korkosz, D Lopez Montilla, C Lue, Y Makino, JL Marenco de la Fuente, H Marzo-Ortega, T Miyamura, E Mueller, L Myasoutova, F Navarro Sarabia, A Ostor, K Papp, L Podrazilova, G Pulka, E-K Raussi, J Rosa, E Roussou, A Rychlewska-Hanczewska, K Sada, L Sedova, D Sikes, S Solomon, M Stack, M Stanislavchuk, K Suzuki, H Tahir, J Tälli, A Toncheva, A Turkiewicz, I Valter, S Vladeva, S Wolfe, and N Zyablova
References
- 1.Gladman DD. Psoriatic arthritis. Dermatol Ther 2009;22:40–55. 10.1111/j.1529-8019.2008.01215.x [DOI] [PubMed] [Google Scholar]
- 2.de Vlam K, Gottlieb AB, Mease PJ. Current concepts in psoriatic arthritis: pathogenesis and management. Acta Derm Venereol 2014;94:627–34. 10.2340/00015555-1833 [DOI] [PubMed] [Google Scholar]
- 3.Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64(Suppl 2):ii14–17. 10.1136/ard.2004.032482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung YY, Tam LS, Kun EW, et al. Psoriatic arthritis as a distinct disease entity. J Postgrad Med 2007;53:63–71. 10.4103/0022-3859.30334 [DOI] [PubMed] [Google Scholar]
- 5.Raychaudhuri SP. Role of IL-17 in psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol 2013;44:183–93. 10.1007/s12016-012-8307-1 [DOI] [PubMed] [Google Scholar]
- 6.Jandus C, Bioley G, Rivals JP, et al. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum 2008;58:2307–17. 10.1002/art.23655 [DOI] [PubMed] [Google Scholar]
- 7.Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol 2010;130:1373–83. 10.1038/jid.2009.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin AM, Rubin CJ, Khandpur R, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol 2011;187:490–500. 10.4049/jimmunol.1100123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noordenbos T, Yeremenko N, Gofita I, et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum 2012;64:99–109. 10.1002/art.33396 [DOI] [PubMed] [Google Scholar]
- 10.Menon B, Gullick NJ, Walter GJ, et al. Interleukin-17+CD8+T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. 2014;66:1272–81. 10.1002/art.38376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leijten EF, van Kempen TS, Boes M, et al. Brief report: enrichment of activated group 3 innate lymphoid cells in psoriatic arthritis synovial fluid. Arthritis Rheumatol 2015;67:2673–8. 10.1002/art.39261 [DOI] [PubMed] [Google Scholar]
- 12.McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137–46. 10.1016/S0140-6736(15)61134-5 [DOI] [PubMed] [Google Scholar]
- 13.Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med 2015;373:1329–39. 10.1056/NEJMoa1412679 [DOI] [PubMed] [Google Scholar]
- 14.Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. 10.1002/art.21972 [DOI] [PubMed] [Google Scholar]
- 15.Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. 10.1002/art.1780380602 [DOI] [PubMed] [Google Scholar]
- 16.Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis 2005;64(Suppl 2):ii65–8; discussion ii69–73 10.1136/ard.2004.031237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisman S, Pollack CR, Gottschalk RW. Psoriasis disease severity measures: comparing efficacy of treatments for severe psoriasis. J Dermatolog Treat 2003;14:158–65. 10.1080/09546630310013360 [DOI] [PubMed] [Google Scholar]
- 18.Fries JF, Spitz P, Kraines RG, et al. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. 10.1002/art.1780230202 [DOI] [PubMed] [Google Scholar]
- 19.Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol 1982;9: 789–93. [PubMed] [Google Scholar]
- 20.van der Heijde D, Sharp J, Wassenberg S, et al. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis 2005;64(Suppl 2):ii61–4. 10.1136/ard.2004.030809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells G, Becker JC, Teng J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 2009;68:954–60. 10.1136/ard.2007.084459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686–91. 10.1002/art.23568 [DOI] [PubMed] [Google Scholar]
- 23.Helliwell PS, Firth J, Ibrahim GH, et al. Development of an assessment tool for dactylitis in patients with psoriatic arthritis. J Rheumatol 2005;32:1745–50. [PubMed] [Google Scholar]
- 24.Healy PJ, Helliwell PS. Measuring dactylitis in clinical trials: which is the best instrument to use? J Rheumatol 2007;34:1302–6. [PubMed] [Google Scholar]
- 25.Kimball AB, Naegeli AN, Edson-Heredia E, et al. Psychometric properties of the itch numeric rating scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol 2016;175:157–62. 10.1111/bjd.14464 [DOI] [PubMed] [Google Scholar]
- 26.Rich P, Scher RK. Nail psoriasis severity index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol 2003;49:206–12. 10.1067/S0190-9622(03)00910-1 [DOI] [PubMed] [Google Scholar]
- 27.Mease PJ, Woolley JM, Bitman B, et al. Minimally important difference of Health Assessment Questionnaire in psoriatic arthritis: relating thresholds of improvement in functional ability to patient-rated importance and satisfaction. J Rheumatol 2011;38:2461–5. 10.3899/jrheum.110546 [DOI] [PubMed] [Google Scholar]
- 28.Common Terminology Criteria for Adverse Events (CTCAE), version 4.03 Bethesda, MD: National Cancer Institute, 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf (accessed 14 Jun 2010). [Google Scholar]
- 29.Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med 2012;366: 1190–9. 10.1056/NEJMoa1109997 [DOI] [PubMed] [Google Scholar]
- 30.Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015;386:541–51. 10.1016/S0140-6736(15)60125-8 [DOI] [PubMed] [Google Scholar]
- 31.Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 2014;370: 2295–306. 10.1056/NEJMoa1315231 [DOI] [PubMed] [Google Scholar]
- 32.Huang W, Na L, Fidel PL, et al. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 2004;190:624–31. 10.1086/422329 [DOI] [PubMed] [Google Scholar]
- 33.Chimenti MS, Ballanti E, Perricone C, et al. Immunomodulation in psoriatic arthritis: focus on cellular and molecular pathways. Autoimmun Rev 2013;12:599–606. 10.1016/j.autrev.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto T. Angiogenic and inflammatory properties of psoriatic arthritis. ISRN Dermatol 2013;2013:630620 10.1155/2013/630620 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2016-209709supp1.pdf (627.1KB, pdf)