Abstract
Background
Assembly of cytochrome c oxidase (COX, complex IV, cIV), the terminal component of the mitochondrial respiratory chain, is assisted by several factors, most of which are conserved from yeast to humans. However, some of them, including COA7, are found in humans but not in yeast. COA7 is a 231aa-long mitochondrial protein present in animals, containing five Sel1-like tetratricopeptide repeat sequences, which are likely to interact with partner proteins.
Methods
Whole exome sequencing was carried out on a 19 year old woman, affected by early onset, progressive severe ataxia and peripheral neuropathy, mild cognitive impairment and a cavitating leukodystrophy of the brain with spinal cord hypotrophy. Biochemical analysis of the mitochondrial respiratory chain revealed the presence of isolated deficiency of cytochrome c oxidase (COX) activity in skin fibroblasts and skeletal muscle. Mitochondrial localization studies were carried out in isolated mitochondria and mitoplasts from immortalized control human fibroblasts.
Results
We found compound heterozygous mutations in COA7: a paternal c.410A>G, p.Y137C, and a maternal c.287+1G>T variants. Lentiviral-mediated expression of recombinant wild-type COA7 cDNA in the patient fibroblasts led to the recovery of the defect in COX activity and restoration of normal COX amount. In mitochondrial localization experiments, COA7 behaved as the soluble matrix protein Citrate Synthase.
Conclusions
We report here the first patient carrying pathogenic mutations of COA7, causative of isolated COX deficiency and progressive neurological impairment. We also show that COA7 is a soluble protein localized to the matrix, rather than in the intermembrane space as previously suggested.
Keywords: Neurology, Molecular genetics, mitochondrial disease, COX assembly, mitochondrial respiratory chaixn
Introduction
Cytochrome c oxidase (COX) is the terminal component of the mitochondrial respiratory chain (MRC). COX transfers four electrons from cytochrome c to oxygen. The energy liberated by this redox reaction sustains the translocation of four protons from the matrix to the intermembrane space (IMS) and contributes to the formation of the mitochondrial electrochemical gradient. In humans, COX deficiency can be either isolated or combined to defects in other MRC complexes. Phenotypes may vary1 2 from multisystem disorders to isolated myopathies or encephalopathies.3 4 Isolated COX deficiency may be caused by mutations in mitochondrial DNA (mtDNA) or nuclear DNA genes encoding structural COX subunits, but in most cases is due to recessive mutations in nuclear genes encoding factors involved in COX biogenesis.5 COA7 is a putative COX assembly factor containing five Sel1-like repeat domains (Interpro IPR006597), is conserved in animals, but neither in plants nor in fungi, and has been reported to localise in the mitochondrial IMS.6 Here, we describe the first biallelic pathogenic COA7 mutations in a patient with isolated COX deficiency and leukoencephalopathy, and show that the protein is localised in the inner mitochondrial compartment, predominantly in the matrix.
Case report
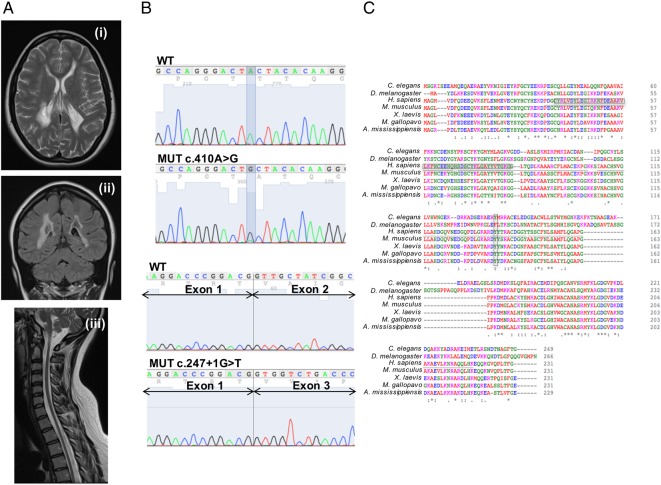
The proband is a 19-year-old woman, first child of healthy unrelated parents. Her family history was unremarkable. She was born at term after a normal pregnancy. The perinatal period was uneventful and her early development was referred to as normal, but after 1 year of age, psychomotor delay became evident. She started walking autonomously at 22 months, with poor balance and frequent falls. At 3 years of age, she developed a demyelinating sensorimotor neuropathy and a brain MRI disclosed supratentorial leukodystrophy. During her childhood, the clinical signs remained stable. At 10 years, her walking difficulties worsened, and limb weakness and tremor ensued. The neurological evaluation showed dysarthria, dysmetria, ataxic gait and hyporeflexia in the four limbs with muscle wasting. She was able to walk alone only for a few steps with an ataxic gait. Mild cognitive impairment was documented (IQ 75, WISC-R scale). Histological analysis of a muscle biopsy showed hypo/atrophy of fibres. The clinical evolution was slowly progressive. At her last follow-up examination, at 19 years of age, she was able to walk alone only with ankle-foot orthotic aids and had developed a marked dorsal-lumbar scoliosis. Other clinical signs were stable. Neurophysiological studies confirmed worsening of her mixed axonal demyelinating peripheral neuropathy. Brain and spinal cord MRI showed mild extension of signal abnormalities and extensive cavitations in the cerebral white matter; the cerebellum and brainstem were spared but the spinal cord was thin with no obvious focal lesions (figure 1A). Plasma lactate was 2.9 mM (n.v. <2.1).
Figure 1.
Clinical and genetic findings. (A) Transverse supratentorial (i), coronal (ii) and sagittal brain/spinal cord (iii) T2- fluid attenuated inversion recovery (FLAIR) MRI sequences. (B) Sanger sequence of the mutated regions in cDNA from mutant fibroblasts. (C) Clustal W diagram. Boxed areas correspond to mutations. Sel1-like conserved domains are underlined.
Methods
Whole exome sequencing (WES) was performed as described.7
Fibroblasts from skin biopsies were immortalised by lentiviral transduction using the pLOX-Ttag-iresTK vector (Tronolab, Addgene #12246). Primary and immortalised fibroblasts and HEK293T cells were grown in standard conditions. For mitochondrial localisation studies, mitochondria and mitoplasts were prepared and trypsin-digested as previously described.8 9 For membrane association studies, sonicated mitochondrial supernatants containing the soluble fractions were separated from the membrane-enriched pellets by ultracentrifugation. Membrane-containing pellets were treated with increasing ionic strength buffers, and for the final protein dissociation, they were suspended in equal volumes of isotonic buffer containing 0.2% SDS. These fractions were analysed by western-blot (WB) immunodetection, using several proteins as markers of distinct mitochondrial compartments.
The COA7 cDNA sequence was obtained from the IMAGE clone (ID: 4430419/IRATp970-0D0921D). PCR products were cloned into the lentiviral vector pWPXLd-ires-PuroR (derived from pWPXLd, Tronolab, Addgene #12258). Lentiviral particle production and transduction of target cells were performed as recommended (https://www.addgene.org/tools/protocols/plko/#E).
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and blue native gel electrophoresis (BNGE) were performed as described.10 Antibodies were purchased from Proteintech, Abcam and Sigma.
MRC and other enzymatic activities were measured as described.11 12
Results
Biallelic mutations in COA7 are present in the proband
This study was approved by the Ethical Committee of the ‘Carlo Besta’ Neurological Institute, Milan, Italy, in agreement with the Declaration of Helsinki. Informed consent was signed by the parents of the patient. We ruled out the presence of pathogenic mutations in mtDNA by Sanger sequencing. WES7 was then carried out. After standard filtering steps and assuming a recessive trait, we prioritised 13 genes with either one homozygous variant or two heterozygous variants. Of these 13 genes, the only one encoding a mitochondria-targeted protein was COA7,6 which carried a (paternal) heterozygous nucleotide transition c.410A>G (NM_023077), p.Y137C and a (maternal) heterozygous G>T transversion affecting the first intronic nucleotide of the exon 2/intron 2 junction (c.287+1G>T). The two variants were not reported in the ExAc database (March 2016). The presence of both mutations was validated by Sanger sequencing.
COA7 mutations are associated with absence of the COA7 protein
Amplification of the patient COA7 cDNA yielded two bands. The upper band, which had the same size as that amplified from control samples, showed the c.410A>G paternal missense mutation. The lower band was originated from the maternal allele containing the c.287+1G>T-splicing mutation, which produces the deletion of the whole 141bp-long exon 2 of COA7 (figure 1B). The missense mutation affects the highly conserved Tyr137, which is replaced by a non-conservative Cys residue, whereas the splice site mutation produces the in-frame deletion of the 47 amino acids encoded by exon 2. This deletion spans almost all of the first conserved Sel1-like domain and half of the second (figure 1C).
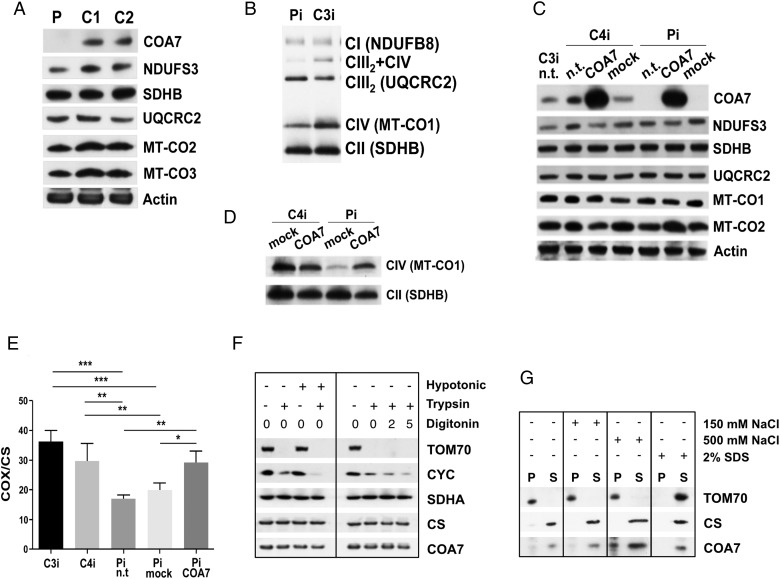
Next, total protein lysates extracted from patient-derived cells were analysed by WB using a specific anti-COA7 antibody. No COA7 cross-reacting material was immunodetectable, and the amount of two COX structural subunits (MT-CO2 and MT-CO3) was reduced in the mutant sample, relative to control cells, whereas that of subunits belonging to complex I and complex III was normal (figure 2A).
Figure 2.
Western blot (WB) immunodetection and enzymatic activities. (A) WB immunodetection of SDS-PAGE in primary fibroblasts. P, patient, C1 and C2, control cell lines. (B) WB immunodetection of BNGE in immortalised patient (Pi) and control (C3i) fibroblasts. Immunovisualised subunits are between brackets. (C) WB immunodetection of SDS-PAGE in immortalised patient fibroblasts (Pi) and a control (C4i). n.t., not transduced; mock, transduced with empty vector; COA7, transduced with COA7. (D) WB immunodetection of BNGE in COA7-transduced immortalised fibroblasts from patient (Pi) and a control (C4i). (E) Cytochrome c oxidase (COX)/citrate synthase (CS) activity in immortalised fibroblasts of two controls (C3i, C4i), and the patient (Pi). Vertical bars indicate SEM. Statistical analysis was by ANOVA (*p<0.05; **p<0.01; ***p<0.001). (F) WB immunodetection of SDS-PAGE of mitochondria and mitoplasts from HEK293T cells. (G) WB immunodetection of SDS-PAGE of soluble and membrane fractions from intact mitochondria. ANOVA, analysis of variance.
COX activity and assembly are recovered after expression of wild-type COA7
In patient-derived immortalised fibroblasts, the levels of fully assembled cIV and of the cIII2+cIV supercomplex were clearly lower relative to controls (figure 2B).
In order to test complementation, wild-type (wt) COA7 (COA7wt) was transduced in immortalised mutant fibroblasts. Robust overexpression of COA7wt was detected in both mutant and wt transduced cells, and, in mutant cells, this was associated with the recovery of normal amount of MT-CO2 subunit (figure 2C) and of both COX assembly (figure 2D) and activity (figure 2E).
COA7 is localised in the mitochondrial matrix
To further characterise COA7, we isolated mitochondria from HEK293T cells and performed WB analysis in organellar and suborganellar fractions. Trypsin digestion was carried out in both intact mitochondria or after stripping off the outer mitochondrial membrane (mitoplasts), by either hypotonic shock or digitonin treatment. The outer membrane protein TOM70 was completely digested by trypsin in mitochondria and mitoplasts; the IMS protein cytochrome c was protected in mitochondria but progressively digested in mitoplasts, whereas the inner membrane protein succinate dehydrogenase subunit A (SDHA) (part of cII) and the matrix protein citrate synthase were protected from digestion in both mitochondria and mitoplasts, as was COA7 (figure 2F). Next, mitochondria were disrupted by sonication, and the ionic strength was increased by adding NaCl to soluble versus membrane fractions. As shown in figure 2G, COA7 was predominantly immunodetected in the supernatant, similar to citrate synthase, although a small percentage was also detected in the pellet, before SDS treatment. Taken together, these results indicate that COA7 is predominantly localised in the mitochondrial matrix, although a small aliquot appears to be associated with the inner mitochondrial membrane.
Discussion
COA7 is involved in MRC biogenesis, as depletion by RNAi in cultured cells clearly affected COX assembly.6 Here, we present the first pathological mutations in COA7 in humans, associated with a severe, slowly progressive mitochondrial neurological disorder. We found biallelic mutations in one patient, according to the autosomal recessive mode of inheritance suggested by the patient's family tree. The COA7 mRNA skipping of exon 2 in the maternal allele and the drastic amino acid change in the paternal allele suggested pathogenicity. Indeed, WB immunodetection showed the virtual absence of the protein in cultured cells. Furthermore, the expression of COA7wt restored COX assembly and activity in mutant cells.
Our case was diagnosed as a mitochondrial encephalopathy and peripheral neuropathy. Although the onset was in infancy, the clinical course was slowly progressive, as the patient has reached adulthood in relatively stable conditions. This could be related to a partially dispensable role of COA7 in COX biogenesis.
Finally, our suborganellar fractionation studies, supported by in vitro import results, indicate that COA7 localises in the mitochondrial inner compartment, mostly in the matrix, rather than in the IMS, as previously proposed.6 We suggest that the proposed localisation to the IMS was in fact the consequence of a incorrect interpretation of results similar to ours, that is, protection of COA7 from proteinase K-treated mitoplasts, thus indicating its localisation within the inner mitochondrial compartment.6
Future work is warranted to establish the interactions and partners of COA7, its mechanisms of action and the steps of its intervention during the formation of COX.
Footnotes
Contributors: AM-L carried out most of the molecular biology and cell biology experiments as part of her PhD programme; AA and IM carried out the clinical follow up; AR carried out WES and part of the work on COA7 mitochondrial localisation; AJR provided bioinformatics management of the WES data; DG carried out the biochemical diagnosis and part of the molecular biology; EF-V and MZ organised the experimental set-up, collected the data and wrote the manuscript.
Funding: Supported by Telethon-Italy grant GGP15041 (to DG); Telethon-Italy Network of Genetic Biobank grant GTB12001J; ERC advanced grant ERC FP7-322424 (to MZ). MRC QQR grant MC_UP_1002/1.
Competing interests: None declared.
Ethics approval: Ethical Committee of the Fondazione Istituto Neurologico ‘Carlo Besta’, Milan, Italy.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Shoubridge EA. Cytochrome c oxidase deficiency. Am J Med Genet 2001;106:46–52. 10.1002/ajmg.1378 [DOI] [PubMed] [Google Scholar]
- 2.DiMauro S, Tanji K, Schon EA. The many clinical faces of cytochrome c oxidase deficiency. Adv Exp Med Biol 2012;748:341–57. 10.1007/978-1-4614-3573-0_14 [DOI] [PubMed] [Google Scholar]
- 3.Zeviani M, Carelli V. Mitochondrial disorders. Curr Opin Neurol 2007;20:564–71. [DOI] [PubMed] [Google Scholar]
- 4.Zeviani M, Di Donato S. Mitochondrial disorders. Brain 2004;127(Pt 10):2153–72. 10.1093/brain/awh259 [DOI] [PubMed] [Google Scholar]
- 5.Rak M, Benit P, Chretien D, Bouchereau J, Schiff M, El-Khoury R, Tzagoloff A, Rustin P. Mitochondrial cytochrome c oxidase deficiency. Clin Sci (Lond) 2016;130:393–407. 10.1042/CS20150707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozjak-Pavlovic V, Prell F, Thiede B, Gotz M, Wosiek D, Ott C, Rudel T. C1orf163/RESA1 is a novel mitochondrial intermembrane space protein connected to respiratory chain assembly. J Mol Biol 2014;426:908–20. 10.1016/j.jmb.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Legati A, Reyes A, Nasca A, Invernizzi F, Lamantea E, Tiranti V, Garavaglia B, Lamperti C, Ardissone A, Moroni I, Robinson A, Ghezzi D, Zeviani M. New genes and pathomechanisms in mitochondrial disorders unraveled by NGS technologies. Biochim Biophys Acta 2016;1857:1326–35. 10.1016/j.bbabio.2016.02.022 [DOI] [PubMed] [Google Scholar]
- 8.Gai X, Ghezzi D, Johnson MA, Biagosch CA, Shamseldin HE, Haack TB, Reyes A, Tsukikawa M, Sheldon CA, Srinivasan S, Gorza M, Kremer LS, Wieland T, Strom TM, Polyak E, Place E, Consugar M, Ostrovsky J, Vidoni S, Robinson AJ, Wong LJ, Sondheimer N, Salih MA, Al-Jishi E, Raab CP, Bean C, Furlan F, Parini R, Lamperti C, Mayr JA, Konstantopoulou V, Huemer M, Pierce EA, Meitinger T, Freisinger P, Sperl W, Prokisch H, Alkuraya FS, Falk MJ, Zeviani M. Mutations in FBXL4, encoding a mitochondrial protein, cause early-onset mitochondrial encephalomyopathy. Am J Hum Genet 2013;93:482–95. 10.1016/j.ajhg.2013.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reyes A, He J, Mao CC, Bailey LJ, Di Re M, Sembongi H, Kazak L, Dzionek K, Holmes JB, Cluett TJ, Harbour ME, Fearnley IM, Crouch RJ, Conti MA, Adelstein RS, Walker JE, Holt IJ. Actin and myosin contribute to mammalian mitochondrial DNA maintenance. Nucleic Acids Res 2011;39:5098–108. 10.1093/nar/gkr052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvaruso MA, Smeitink J, Nijtmans L. Electrophoresis techniques to investigate defects in oxidative phosphorylation. Methods 2008;46:281–7. 10.1016/j.ymeth.2008.09.023 [DOI] [PubMed] [Google Scholar]
- 11.Tiranti V, Munaro M, Sandona D, Lamantea E, Rimoldi M, DiDonato S, Bisson R, Zeviani M. Nuclear DNA origin of cytochrome c oxidase deficiency in Leigh's syndrome: genetic evidence based on patient's-derived rho degrees transformants. Hum Mol Genet 1995;4:2017–23. 10.1093/hmg/4.11.2017 [DOI] [PubMed] [Google Scholar]
- 12.Kirby DM, Thorburn DR, Turnbull DM, Taylor RW. Biochemical assays of respiratory chain complex activity. Methods Cell Biol 2007;80:93–119. 10.1016/S0091-679X(06)80004-X [DOI] [PubMed] [Google Scholar]