Abstract
Chronic kidney disease (CKD) is a global health problem, and novel therapies to treat CKD are urgently needed. Here, we show that inhibition of G0/G1 switch 2 (G0s2) ameliorates renal inflammation in a mouse model of CKD. Renal expression of chemokine (C-C motif) ligand 2 (Ccl2) was increased in response to p65 activation in the kidneys of wild-type 5/6 nephrectomy (5/6Nx) mice. Moreover, 5/6Nx Clk/Clk mice, which carry homozygous mutations in the gene encoding circadian locomotor output cycles kaput (CLOCK), did not exhibit aggravation of apoptosis or induction of F4/80-positive cells. The renal expression of G0s2 in wild-type 5/6Nx mice was important for the transactivation of Ccl2 by p65. These pathologies were ameliorated by G0s2 knockdown. Furthermore, a novel small-molecule inhibitor of G0s2 expression was identified by high-throughput chemical screening, and the inhibitor suppressed renal inflammation in 5/6Nx mice. These findings indicated that G0s2 inhibitors may have applications in the treatment of CKD.
Keywords: Chronic renal disease, Circadian clock, G0s2, Small compound inhibitor
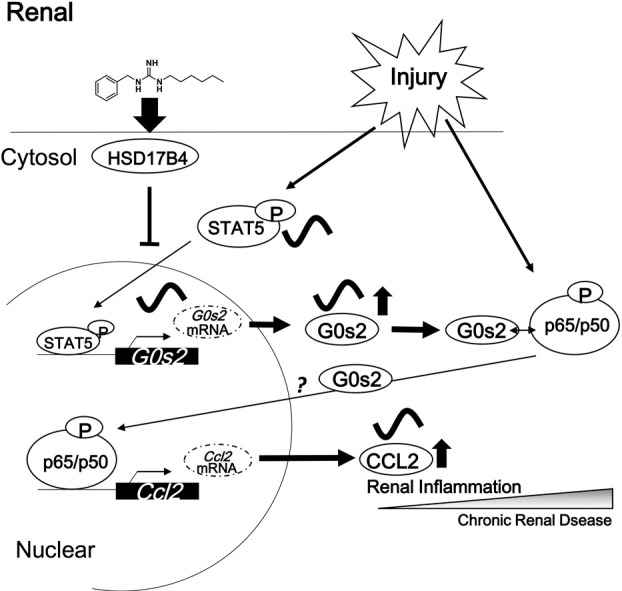
Graphical abstract
Highlights
-
•
Chronic renal inflammation in CKD was ameliorated by clock mutation.
-
•
G0s2 was important for the anti-inflammatory effects of clock mutation.
-
•
A novel small-molecule inhibitor of G0s2 ameliorated renal inflammation in CKD.
Matsunaga et al. found that G0/G1 switch 2 (G0s2) was important for renal inflammation and that a novel small-molecule inhibitor of G0s2 ameliorated renal dysfunction in chronic renal disease (CKD). G0s2 enhanced p65-mediated transcription. Moreover, renal inflammation was regulated by G0s2/Stat5 through the molecular clock mechanism. Finally, G0s2 knockdown or inhibition ameliorated renal dysfunction in CKD.
1. Introduction
Chronic kidney disease (CKD) is a global health problem (Barsoum, 2006, Hedayati et al., 2008, Murray, 2008, Enomoto et al., 2008, Krishnan and Kiernan, 2007). Therapeutic strategies for the treatment of CKD involve attenuation of progressive renal dysfunction, blood pressure control, reduction of proteinuria, and exclusion of uremic toxins (Ruggenenti et al., 1998, Ruggenenti et al., 1999, Niwa and Ise, 1994, Ruggenenti et al., 2012, Codreanu et al., 2005). Although inhibition of the renin-angiotensin-aldosterone pathway and elimination of uremic toxins confer renal protection, there are no adequate treatments available for alleviation of all the symptoms of CKD.
Most living organisms exhibit behavioral and physiological rhythms with a periodicity close to 24 h (Vitaterna et al., 1994, Kume et al., 1999, Jin et al., 1999, Preitner et al., 2002). Mammalian clock genes regulate biological functions in the central and/or peripheral tissues (Ueda et al., 2002, Siepka et al., 2007, Isojima et al., 2005). Molecular dissection of the circadian biological clock has revealed links between genetic mutations and/or alteration of clock genes and diseases, including cancer, metabolic syndrome, and diabetes, in humans and animal models (Filipski et al., 2005, Ohdo et al., 2011). In patients with CKD, the rhythms of sleep and serum hormone levels are dysregulated (Niemczyk et al., 2006). Moreover, many mediators of inflammation contribute the pathology of CKD (Yu et al., 2014), and the activities of these inflammatory mediators in various inflammatory disorders reveal the circadian characteristics of the diseased state (Hashiramoto et al., 2010, Gibbs et al., 2014). In a recent study, the core molecular clock protein circadian locomotor output cycles kaput (CLOCK) was shown to be important for nuclear factor-kappaB (NF-κB)-mediated transcription of various pro-inflammatory cytokines (Spengler et al., 2012, Narasimamurthy et al., 2012). Indeed, although it is difficult to discern causes from effects, these insights have suggested that there may be a relationship between the molecular clock and CKD pathology. Thus, elucidation of these relationships may facilitate the development of new therapies for treating CKD.
The use of high-throughput screening (HTS) techniques has long been employed by the pharmaceutical industry to increase discovery rates for new drugs that could be useful for disease treatment (Brey et al., 2011, Patel et al., 2012). Additionally, chemical screening has emerged as a powerful tool to investigate biological mechanisms and other processes (Hirota and Kay, 2009, Hirota et al., 2010, Isojima et al., 2005). Inflammation is a risk factor for various pathological conditions, such as renal disease, hepatitis, diabetes, and cardiovascular diseases (Meijer et al., 2015). Chronic inflammation is affected by activation of the NF-κB pathway, a prototypical pro-inflammatory signaling pathway; NF-κB induces the expression of various pro-inflammatory genes, including cytokines, chemokines, and adhesion molecules (Toby, 2009). Because NF-κB has various biological functions, targeting NF-κB in therapeutic strategies for inflammatory diseases is complicated (Toby, 2009). Therefore, identification of novel factors regulating inflammation in disorders such as CKD is urgently needed to facilitate the discovery of new therapeutic agents.
In this study, we aimed to identify the novel molecular mechanisms that mediate renal dysfunction in mice with CKD by examining the relationships between the circadian clock and CKD aggravation. Our data showed that the expression of the gene encoding G0/G1 switch 2 (G0s2), controlled by the molecular clock pathway (Turek et al., 2005), increased transcriptional activation of Ccl2 and that G0s2 knockdown or inhibition by a novel small-molecule inhibitor ameliorated renal inflammation in CKD. Thus, our data suggested that molecular clock-dependent changes in G0s2 expression aggravated renal inflammation in CKD mice.
2. Results
2.1. Renal CLOCK Expression Was Altered in Wild-Type 5/6Nx Mice
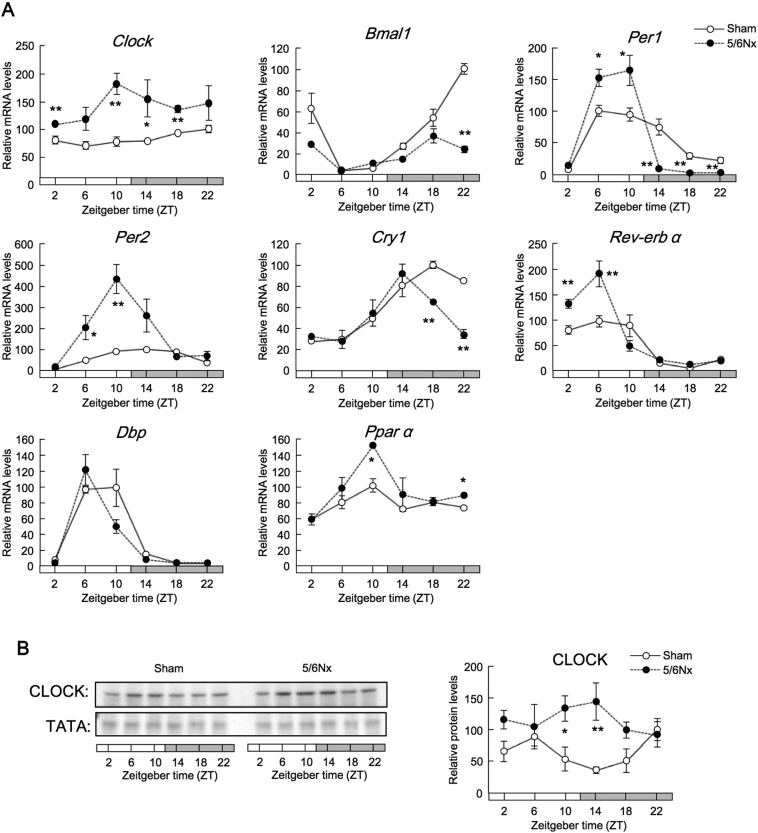
First, we sought to elucidate the association between the molecular clock and CKD pathology. We found that 24-h locomotor activities were altered in mice that underwent nephrectomy (hereafter referred to as 5/6Nx mice) at 7–9 weeks after the second operation (Fig. S1A). To evaluate the renal clock genes involved in various renal functions, we quantified the temporal expression profiles of renal clock genes in 5/6Nx mice. Renal Clock, Bmal1, Per1, Per2, Cry1, Rev-erbα, Dbp, and Pparα mRNA expression oscillated in wild-type sham-operated mice at 8 weeks after operation (Fig. 1A). The expression of clock genes containing cis-element E-boxes increased. Additionally, wild-type 5/6Nx mice exhibited increased expression of CLOCK protein (Fig. 1B), a critical regulator of renal function (Zuber et al., 2009, Nikolaeva et al., 2012). These results suggested that there may be a relationship between increased CLOCK expression and CKD pathology.
Fig. 1.
Renal CLOCK expression was increased in wild-type 5/6Nx mice.
(A) Temporal expression profiles of Clock, Bmal1, Per1, Per2, Cry1, Nr1d1 (Rev-erbα), Dbp, and Ppara mRNA in the kidneys of wild-type 5/6Nx and sham-operated mice. (B) Representative temporal CLOCK protein expression profiles. Values are the means ± SEMs for triplicate experiments (n = 3–6). All experiments were performed three times. *P < 0.05, **P < 0.01 vs. sham-operated mice at the same time point by Bonferroni/Dunn test.
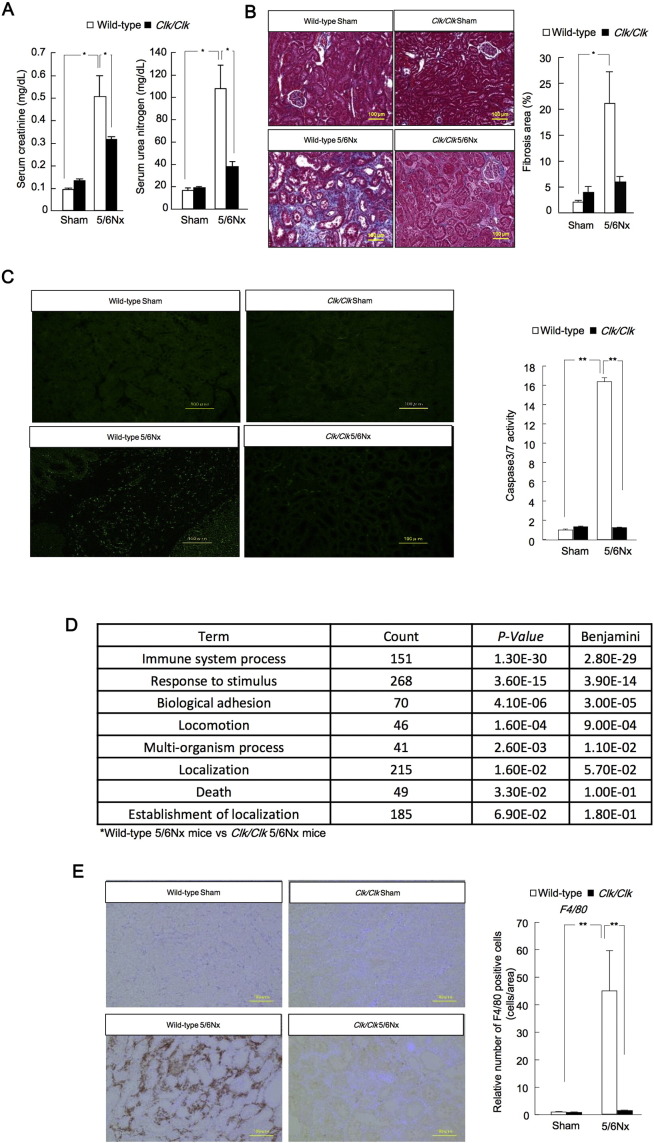
Thus, we further examined the role of CLOCK in 5/6Nx mice using Clock mutant mice (Clk/Clk), which exhibit more severe behavioral and molecular phenotypes than Clock-knockout mice (Debruyne et al., 2006). The Δ19 Clock mouse, which carries a deletion of exon 19 in the Clk locus, produces a protein that has been characterized as dominant negative by some researchers, but as functionally null by others (Gekakis et al., 1998). After the second operation at Zeitgeber time (ZT) 6, serum creatinine and serum urea nitrogen (SUN) levels were increased at 8 weeks in wild-type 5/6Nx mice, but decreased at 8 weeks in Clk/Clk 5/6Nx mice (Fig. 2A). The decrease in glomerular filtration rates (GFRs) in wild-type 5/6Nx mice at 8 weeks was ameliorated in Clk/Clk mutant mice (Fig. S1C). The region of renal fibrosis, indicated by blue staining of histological sections subjected to Masson's trichrome staining, decreased markedly in Clk/Clk 5/6Nx mice (Fig. 2B). The area of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells, as a marker of apoptosis, and the activity of caspase 3/7 were suppressed in Clk/Clk 5/6Nx mice compared with those in wild-type mice (Fig. 2C). Functional microarray analysis of renal genes in Clk/Clk 5/6Nx versus wild-type mice showed that the biological pathways related to the immune system were altered (Fig. 2D; NCBI accession no. GSE35135). Importantly, immune system function is correlated with inflammation and apoptosis (Sanz et al., 2008). The area of F4/80-positive cells was decreased in Clk/Clk 5/6Nx mice compared with that in wild-type 5/6Nx mice (Fig. 2E). These results suggested that renal inflammation in Clk/Clk 5/6Nx mice was less than that in WT 5/6Nx mice.
Fig. 2.
The progression of renal fibrosis was suppressed in Clk/Clk 5/6Nx mice.
(A) Serum creatinine and serum urea nitrogen (SUN) production in sham-operated and 5/6Nx wild-type or Clk/Clk mice. (B) Left: Masson's trichome staining of tissue fibrosis (blue). Right: quantitative analysis of interstitial fibrosis by light microscopy in 5/6Nx and sham-operated mouse kidney tissues 8 weeks after the second operation in wild-type or Clk/Clk mice. (C) Left: apoptotic cells are identified by TUNEL staining (green). Right: Caspase-3/7 activity in sham-operated and 5/6Nx wild-type or Clk/Clk mouse kidneys 8 weeks after the second operation. (D) Functional analysis of gene expression in sham-operated and 5/6Nx wild-type and Clk/Clk mice based on functional annotation clustering by the Database for Annotation, Visualization, and Integrated Discovery (DAVID). (E) Left: F4/80 immunostaining (brown). Right: F4/80 protein expression profiles in the kidneys of sham-operated and 5/6Nx wild-type or Clk/Clk mice at Zeitgeber time (ZT) 6 after the second operation. Quantitative analysis of F4/80 staining in tissues from sham-operated and 5/6Nx wild-type or Clk/Clk mice by light microscopy 8 weeks after the second operation. Values are the means ± SEMs for triplicate experiments (n = 3–6). *P < 0.05, **P < 0.01 by Bonferroni/Dunn test.
2.2. Transcriptional Control of Renal Ccl2 Expression by p65
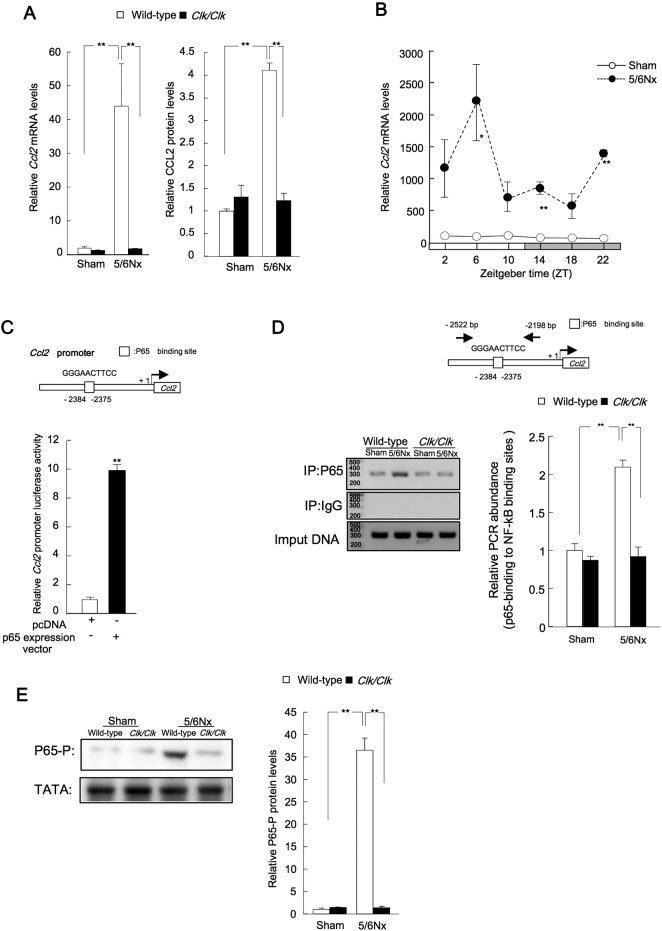
Increased tubular expression of chemokine (C-C motif) ligand 2 (Ccl2) is observed in human progressive renal disease, and interstitial inflammatory infiltrates express CC chemokine receptor 2 (CCR2) (Eardley et al., 2006). Thus, we next examined the expression of Ccl2 mRNA and protein in wild-type 5/6Nx mice (Fig. 3A). Renal Ccl2 transcript levels were increased in wild-type 5/6Nx mice, peaking at ZT6 and exhibiting a trough at ZT18 (Fig. 3B). We then investigated the consensus sequences within the promoter region of the Ccl2 gene. Cotransfection with mouse Ccl2 luciferase reporters and p65 expression constructs led to significant increases in transcriptional activity (Fig. 3C). In vivo binding of the p65 protein to the p65 binding site in the Ccl2 promoter at ZT6 was greater in lysates from 5/6Nx mice than in lysates from Clk/Clk 5/6Nx mice (Fig. 3D). The levels of phosphorylated p65 were decreased in Clk/Clk 5/6Nx mice compared with those in wild-type 5/6Nx mice (Fig. 3E). These results suggested that induction of Ccl2 by p65 in Clk/Clk 5/6Nx mice was lower than that in wild-type 5/6Nx mice.
Fig. 3.
Transcriptional control of renal Ccl2 expression by p65.
(A) Left: Ccl2 mRNA expression in the kidneys of sham-operated and 5/6Nx wild-type or Clk/Clk mice. Right: Ccl2 protein expression in the kidneys of sham-operated and 5/6Nx wild-type or Clk/Clk mice. (B) Temporal profiles of Ccl2 mRNA expression in sham-operated and 5/6Nx mice. (C) Effects of p65 transfection on mouse Ccl2-luciferase reporter activity in NIH3T3 cells. **P < 0.01 compared with the pcDNA3.1 group by Student's t-test. (D) Left: endogenous p65 protein binding to the Ccl2 promoter in sham-operated or 5/6Nx kidney cells. (E) Renal p65 phosphorylation (Ser-536) in sham-operated and 5/6Nx wild-type or Clk/Clk mice at ZT6. Values are the means ± SEMs for triplicate experiments (n = 3–6). **P < 0.01, *P < 0.05 vs. sham controls at the corresponding time points by Bonferroni/Dunn test.
2.3. G0s2 Functioned as a Transcriptional Co-activator in Response to p65 Activation
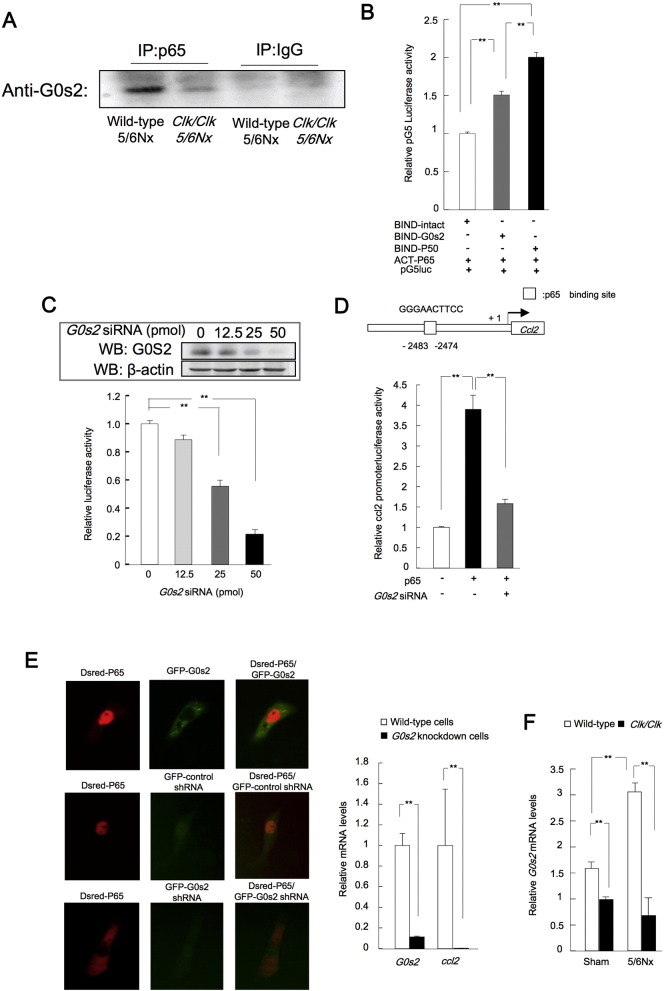
Next, we examined the mechanism responsible for reduced the binding of p65 to the p65 binding site in the ccl2 promoter in Clk/Clk 5/6Nx mice. In previous studies, the G0s2 has the relationship between pathophysiology of cancer, diabetes and inflammation (Welch et al., 2009, Zhang et al., 2014, Cristillo et al., 1997). And, cytosolic G0s2 is important for transactivation by p65 in inflammation. We demonstrated that the microarray analysis in hepatic knockdown of G0s2 in culture cells or mice induced the alteration of the cytokine and chemokine signaling genes expression (Table S1A, B). Thus, we examined this interaction in 5/6Nx or Clk/Clk 5/6Nx kidney cell lysates by immunoprecipitation. The binding of G0s2 was increased in wild-type 5/6Nx renal cell lysates compared with that in Clk/Clk 5/6Nx renal cell lysates (Fig. 4A). Next, to clarify the interactions between p65 and G0s2 proteins, we performed mammalian two-hybrid assays in Hepa1-6 cells (Fig. 4B). Co-transfection with ACT-p65 and BIND-G0s2 expression plasmids significantly increased the interactions of these proteins, suggesting that there may be an interaction of between p65 and G0s2 proteins. The transcriptional activity by of p65 was decreased by G0s2 knockdown (Fig. 4C). Additionally, p65-dependent Ccl2 promoter activity was suppressed by G0s2 knockdown (Fig. 4D). Next, to clarify the effects of G0s2 on function of p65 protein, we investigated the localization of p65 in G0s2-knockdown cells. In intact cells, G0s2 protein accumulated in the cytosol, and p65 protein accumulated in the nucleus (Fig. 4E). In contrast, nuclear accumulation of p65 protein and Ccl2 mRNA expression were suppressed in G0s2-knockdown cells (Fig. 4E). Moreover, the expression of G0s2 mRNA was suppressed in the kidneys of Clk/Clk sham-operated or 5/6Nx mice compared with that in wild-type mice (Fig. 4F). G0s2 may be involved in immunoregulation in autoimmune diseases, cancer, and metabolic syndrome (Nakamura et al., 2006; Yim et al., 2016, Yang et al., 2010, Zhang et al., 2014, Cristillo et al., 1997, Russell and Forsdyke, 1991). Thus, these results suggested that G0s2 expression may be important for the p65-dependent transcription of inflammation-related genes in wild-type 5/6Nx mice.
Fig. 4.
G0s2 interacts with p65 to regulate Ccl2 expression.
(A) Immunoprecipitation was performed using anti-p65 antibodies in renal cell lysates from 5/6Nx wild-type or Clk/Clk mice. G0s2 expression in the immunoprecipitated samples was then analyzed by Western blotting. (B) The G0s2 and p65 interaction was assessed in NIH3T3 cells using mammalian two-hybrid assays. Cells cotransfected with the BIND (GAL4)-p50 and ACT (VP16)-p65 expression vectors and pG5luc (GAL4 binding site promoter) luciferase reporter plasmid. (C) Upper panels show G0s2 expression in knockdown cells. (D) Relative p65 binding element (NRE)-luciferase activity in NIH3T3 cells cotransfected with G0s2 siRNA and p65 binding element (NRE)-luciferase plasmids. (D) Relative luciferase activity in NIH3T3 cells cotransfected with the p65 expression and Ccl2-promoter luciferase reporter plasmids and G0s2 siRNA. (E) G0s2 protein and p65 protein localization in Hepa1-6 cells. Left: cells at 48 h after GFP-G0s2 and Dsred-p65 expression vector transfection. Green: G0s2 protein; red: p65 protein. Center: the influence of G0s2 knockdown on p65 protein localization. G0s2 mRNA-GFP, green; p65 protein (red); colocalization (orange). Right bar graph: levels of G0s2 and Ccl2 mRNAs in G0s2-knockdown cells following transfection with the G0s2 mRNA-GFP expression vector. (F) Renal G0s2 expression in sham-operated and 5/6Nx wild-type or Clk/Clk mice at ZT6 after the second operation. Values are the means ± SEMs for triplicate experiments (n = 3–6). **P < 0.01, *P < 0.05 vs. controls at the corresponding time points by Bonferroni/Dunn test.
2.4. G0s2 Expression Was Stimulated by the Signal Transducer and Activator of Transcription 5 (Stat5) Pathway in Wild-type 5/6Nx Mice
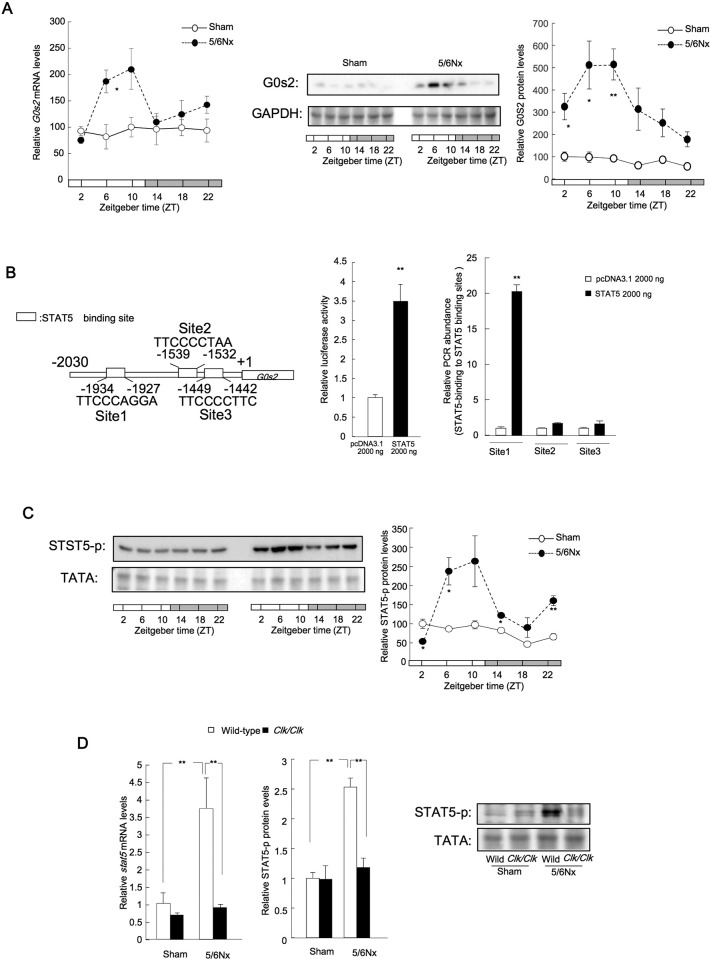
Our results described above showed that the temporal expression of G0s2 mRNA and protein peaked during the light phase in 5/6Nx mice (Fig. 5A). The circadian oscillation of G0s2 mRNA in sham mice was not detected. Moreover, CKD has been shown to induce significant changes in the plasma levels of a myriad biomolecules, including increased levels of serum retinol (Gibbs et al., 2014, Spengler et al., 2012, Narasimamurthy et al., 2012). Transcription of the G0s2 gene is controlled by the retinoic acid response element (Kitareewan et al., 2008), and G0s2 expression exhibits a 24-h rhythm in the livers of wild-type mice, in which G0s2 expression is elevated compared with that in other organs (Jiang et al., 2014). Thus, we next examined the influence of retinol production on G0s2 expression.
Fig. 5.
Stat5 expression and activation in 5/6Nx mouse kidneys.
(A) G0s2 temporal mRNA (left) and protein expression profiles. Center: Representative protein expression data. Right: temporal Stat5 protein phosphorylation in the kidneys of wild-type sham-operated and 5/6Nx mice. (B) Right: G0s2 promoter activity in response to Stat5 overexpression. **P < 0.01 compared with the pcDNA3.1 vector control group by Student's t-test. Left: Stat5 protein binding to the G0s2 promoter in Stat5-transfected NIH3T3 cells. **P < 0.01 compared with pcDNA3.1 in site1, site2, and site3 groups by Bonferroni/Dunn test. (C) Left: representative protein data. Right: temporal Stat5 phosphorylation (Tyr694/699) profiles in the kidneys of wild-type sham-operated and 5/6Nx mice. (D) Left: Stat5a mRNA expression in sham-operated and 5/6Nx wild-type or Clk/Clk mice. Right: Stat5 phosphorylation (Tyr694/699) in sham-operated and 5/6Nx wild-type or Clk/Clk mice. **P < 0.01, *P < 0.05 vs. controls at the corresponding time points by Bonferroni/Dunn test. Values are the means ± SEMs for triplicate experiments (n = 3–6).
Our results showed that the rhythmic expression of G0s2 mRNA was suppressed in livers from Clk/Clk mice (Fig. S3A). Moreover, transcription of the G0s2 gene was regulated by retinoic acid receptor α (Rarα) and retinoic acid in our mouse model (Fig. S3B–G). Serum retinol levels in wild-type mice were also markedly increased 8 weeks after the 5/6Nx operation (Fig. S1F). Notably, renal Rarα mRNA levels were also markedly decreased in wild-type 5/6Nx mice at 8 weeks after the 5/6Nx operation (Fig. S1G).
The G0s2 5′-flanking region (from − 2030 bp to − 1057 bp) was important for promoter activity (Fig. S3D). Moreover, this region appeared to contain a binding site for Stat5 (Fig. 5B), which has various roles in inflammation and immunity (Yu et al., 2009). Consistent with this, cotransfection with the mouse G0s2 luciferase reporter and Stat5 expression construct led to significantly increased transcriptional activity (Fig. 5B). Chromatin immunoprecipitation revealed that Stat5 may bind to Stat5 binding site 1 in the G0s2 5′-flanking region (Fig. 5B). The phosphorylation of Stat5 (Stat5-p) was markedly increased in the kidneys of wild-type 5/6Nx mice (Fig. 5C), and the expressions of Stat5 mRNA and levels of Stat5-p protein were lower in the kidneys of Clk/Clk 5/6Nx mice than in those of wild-type 5/6Nx mice (Fig. 5D).
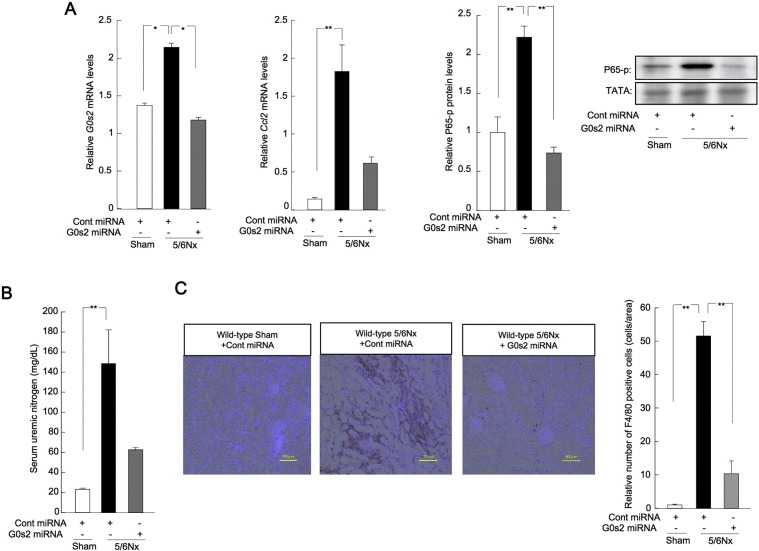
2.5. Treatment With G0s2 siRNA or a Novel Transcriptional Inhibitor of G0s2 Ameliorated Renal Dysfunction in Wild-type 5/6Nx Mice
Renal G0s2 expression may be important for renal inflammation mediated by p65 in mice with CKD. Thus, we carried out knockdown of G0s2 in the kidneys of 5/6Nx mice kidney (Fig. 6). Treatment of wild-type 5/6Nx mice with control or G0s2 miRNA expression plasmids resulted in decreased levels of G0s2 and Ccl2 mRNA in the kidneys (Fig. 6A). The phosphorylation of nuclear p65 protein was decreased in wild-type 5/6Nx mice treated with G0s2 miRNA (Fig. 6A), and SUN concentrations decreased in 5/6Nx mice treated with G0s2 miRNA (Fig. 6B). Finally, the F4/80-positive area and F4/80 protein levels were decreased in 5/6Nx mice treated with G0s2 miRNA (Fig. 6C). These results revealed that knockdown of G0s2 ameliorated renal dysfunction in our mouse model of CKD.
Fig. 6.
G0s2 knockdown ameliorated renal inflammation in CKD.
(A) Renal expression profiles for G0s2 and Ccl2 mRNA and p65 protein phosphorylation in wild-type sham-operated and 5/6Nx mice treated with control or G0s2 miRNA expression plasmids (50 μg/μL/animal, two times/week, i.v.) from 4 to 8 weeks after the second operation. *P < 0.05, **P < 0.01 by Bonferroni/Dunn test. (B) SUN levels in wild-type sham-operated and 5/6Nx mice treated with G0s2 or control miRNA expression plasmids (50 μg/μL/animal, two times/week, i.v.) from 4 to 8 weeks after the second operation. (C) Left: F4/80 immunostaining (brown) in wild-type sham-operated and 5/6Nx mice treated with G0s2 or control miRNA expression plasmids (50 μg/μL/animal, two times/week, i.v.) from 4 to 8 weeks after the second operation. Right: quantitative analysis of F4/80 staining by light microscopy. *P < 0.05, **P < 0.01 by Bonferroni/Dunn test. Values are the means ± SEMs for triplicate experiments (n = 3–6).
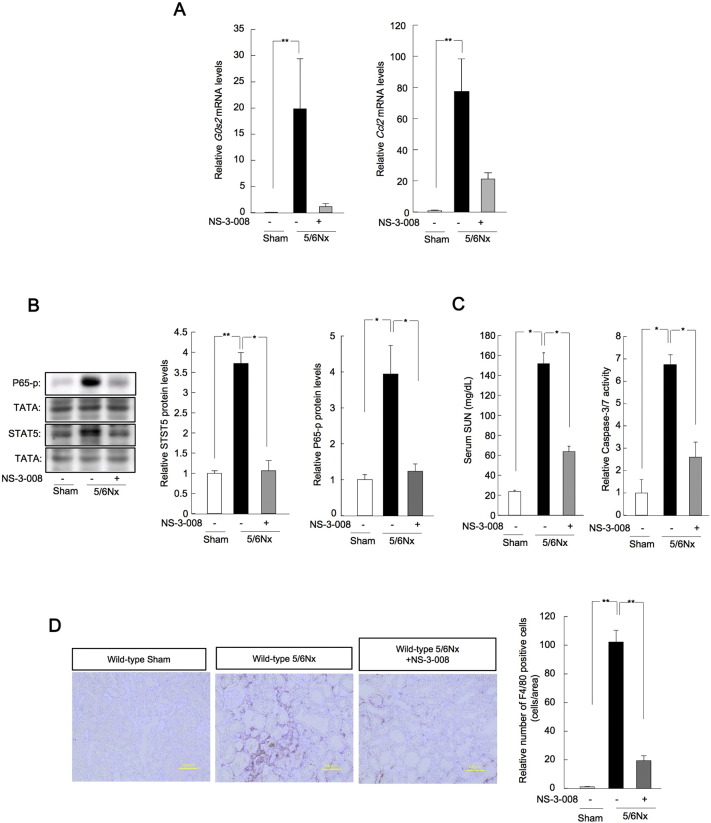
In addition, we carried out high-throughput screening of 9600 small compounds to identify novel transcriptional inhibitors of G0s2 (Figs. S4, S5). NS-3-008 inhibited the transcription of G0s2 with a half-maximal inhibitory concentration (IC50) of 2.25 μM (Fig. S6). Moreover, treatment of wild-type 5/6Nx mice with NS-3-008 (5 mg/kg, P.O.) resulted in decreased levels of G0s2 and Ccl2 mRNA in the kidneys (Fig. 7A). The phosphorylation of Stat5 and p65 protein was decreased in wild-type 5/6Nx mice treated with NS-3-008 (Fig. 7B), and SUN concentrations and renal caspase 3/7 activity decreased in 5/6Nx mice treated with NS-03-08 (Fig. 7). Finally, the F4/80-positive area and F4/80 protein levels were decreased in 5/6Nx mice treated with NS-3-008 (Fig. 7D). Interestingly, NS-3-008 bound to Hsd17b4 (Fig. S7A–D); knockdown of Hsd17b4 decreased G0s2 mRNA levels, whereas overexpression of Hsd17b4 induced G0s2 mRNA expression (Fig. S8A, 8B). The inhibitory effects of NS-3-008 were blocked by knockdown of Hsd17b4 or deletion of the Stat5 binding site in the G0s2 promoter (Fig. S8A, 8C). NS-3-008 decreased the nuclear phosphorylation of Stat5 (Fig. S8D). Thus, these results revealed that G0s2 inhibition by the novel compound NS-3-008 ameliorated renal dysfunction in our mouse model of CKD.
Fig. 7.
Treatment with the G0s2 inhibitor NS-3-008 ameliorated renal inflammation in CKD.
(A) Renal expression profiles of G0s2 and Ccl2 mRNA in wild-type sham-operated and 5/6Nx mice treated with NS-3-008 (5 mg/kg oral [PO]) from 4 to 8 weeks after the second operation. (B) Profiles of Stat5 phosphorylation (left) and p65 phosphorylation (right) in the kidneys of wild-type sham-operated and 5/6Nx mice treated with NS-3-008 (5 mg/kg, PO) 4 to 8 weeks after the second operation. (C) SUN (left) and renal caspase 3/7 activity (right) in wild-type sham-operated and 5/6Nx mice treated with NS-3-008 (5 mg/kg, PO) 4 to 8 weeks after the second operation. (D) Left: F4/80 immunostaining (brown) in wild-type sham-operated and 5/6Nx mice treated with NS-3-008 (5 mg/kg, PO) from 4 to 8 weeks after the second operation. Right: quantitative analysis of F4/80 staining by light microscopy. *P < 0.05, **P < 0.01 by Bonferroni/Dunn test. Values are the means ± SEMs for triplicate experiments (n = 3–6).
3. Discussion
In patients with CKD, the circadian rhythm and serum hormone levels are dysregulated (Niemczyk et al., 2006). Moreover, inflammation, which can be mediated by the molecular clock (Hashiramoto et al., 2010), is related to the pathology of CKD (Yu et al., 2014). Thus, in this study, we aimed to examine the relationships between circadian rhythms and CKD pathology. To this end, we investigated the role of G0s2 in CKD. Our data indicated that G0s2 may aggravate renal dysfunction in the context of CKD through mechanisms involving increased renal expression of the Clock gene. Subsequent induction of G0s2 expression by Stat5 in the kidney may further exacerbate renal dysfunction by inducing inflammation. These data, combined with our identification of a novel G0s2 inhibitor, suggest that G0s2 may be a promising therapeutic target in the treatment of CKD.
The molecular clock system is important for renal function in wild-type mice (Zuber et al., 2009, Nikolaeva et al., 2012). In this study, we found that the expression of renal clock genes was altered in kidneys from 5/6Nx mice, suggesting that increased expression of CLOCK, which is important for the core loop in the circadian clock system and is involved in inflammation, may affect the expression of other clock genes. Interestingly, we also found that mutations in the Clock gene ameliorated renal fibrosis, apoptosis, and inflammatory reactions in mice with CKD. Circadian proteins are known to regulate both blood pressure and kidney function (Curtis et al., 2007, Stow et al., 2012, Doi et al., 2010). In this study, we found that AT II levels in Clk/Clk mice were higher than those in wild-type mice (Fig. S1D). In addition, food intake in mice affects AT II levels, which are also highly dependent on salt intake and renal perfusion pressure. No significant changes were observed in the amount of food and water intake between wild-type and Clk/Clk mice in this study (Fig. S1E). In addition, angiotensin-converting enzyme (ACE) levels in the lungs at ZT8 were higher in intact Clk/Clk mice than in intact wild-type mice (Fig. S1E). In addition, there were no differences in mean blood pressure (MBP) increases between 5/6Nx wild-type mice and 5/6Nx Clk/Clk mice (Fig. S1E). This may have contributed to the clock-related pathology observed in our model. Renal fibrosis, apoptosis, and inflammation were ameliorated in Clk/Clk mice following the 5/6Nx procedure. These data provided insights into the mechanisms of renal inflammatory reactions induced by Clock, directing our further studies.
Ccl2 is expressed at sites of injury and inflammation to direct macrophage recruitment; it binds with CCR2 to promote macrophage adhesion and chemotaxis to disease sites. Increased tubular expression of CCL2 is present in human progressive renal disease, and interstitial inflammatory infiltrates express CCR2 (Eardley et al., 2006). Although Ccl2 expression was increased in wild-type 5/6Nx mice, this phenotype was not observed in Clk/Clk mice. Moreover, Ccl2 mRNA expression varied in a time-dependent manner in wild-type 5/6Nx mice and was regulated by p65. The expression of Ccl2 is activated by binding of transcription factors to NF-κB elements in the Ccl2 promoter (Deng et al., 2013). Although the CLOCK protein enhances transcription mediated by NF-κB, CLOCKΔ19 protein does not affect NF-κB-dependent transcription (Spengler et al., 2012), and the mechanism by which NF-κB regulates transcription in Clk/Clk mice remains unclear. In our study, we showed that G0s2, which is involved in the transition from G0 phase to G1 phase and is mediated by cyclosporine A (Cristillo et al., 1997), interacted with p65 protein in the kidneys of wild-type 5/6Nx mice. Nuclear accumulation of p65 protein was suppressed in G0s2-knockdown cells. Therefore, G0s2 protein may promote the nuclear localization of p65 protein. Moreover, knockdown of G0s2 blocked the transactivation of renal Ccl2 expression and the progression of renal dysfunction. Knockdown of G0s2 also reduced the number of F4/80-positive cells. These effects were also identified in a diethylnitrosamine (DEN) hepatitis mouse model (Fig. S2A–C). G0s2 mRNA expression was also decreased in the kidneys of Clk/Clk 5/6Nx mice, suggesting that G0s2 may be a novel activator of inflammatory reactions via p65 in 5/6Nx mice. Further analysis showed that repression of G0s2 expression in Clk/Clk 5/6Nx mice may be related to the lack of induction of Stat5, which has been shown to be directly regulated by the molecular clock in humans and rodents (Mavroudis et al., 2013). Thus, these data provided important insights into the regulation of inflammation and CKD by G0s2 and related pathways.
Chemical screening is a powerful tool to investigate various biological pathways (Pieper et al., 2010). Several recent studies have employed cell-based chemical screening strategies to identify small molecules with biological activities (Chen et al., 2012), providing evidence supporting the use of chemical screening approaches. In this study, we performed a high-throughput chemical screen of 9600 synthetic small molecules from a chemical library (Open Innovation Centre for Drug Discovery, The University of Tokyo) to identify transcriptional inhibitors of G0s2. Administration of NS-3-008 inhibited the expression of G0s2 in healthy mice liver. In contrast, administration of NS-3-008 did not affect the MBP in wild-type sham and 5/6Nx mice. We identified NS-3-008 as a novel G0s2 inhibitor and showed that administration of this inhibitor ameliorated renal dysfunction in wild-type 5/6Nx mice. In addition, the lipopolysaccharide (LPS)- or tumor necrosis factor alpha (TNFα)-dependent transactivation of G0s2 and Ccl2 was inhibited by administration of NS-3-008 (Fig. S10A–E). Moreover, NS-3-008 bound to Hsd17b4 to block G0s2 expression. Similar studies have shown that chromeceptin affects the transcription by Stats via binding to Hsd17b4 (Choi et al., 2006). Thus, our experiments identified a novel inhibitor of G0s2 and provided evidence for the mechanism of inhibition by this novel inhibitor, supporting the potential utility of G0s2 inhibition in the treatment of CKD.
In conclusion, our results showed that altered expression of molecular components of the clock signaling machinery was associated with the degree of CKD pathology. Environmental factors, such as light exposure, feeding habits, and working conditions, in human societies are complex; although these environmental conditions may also contribute to the observed effects, our data clearly indicated that inhibition of G0s2 function may have therapeutic benefits in the treatment of CKD.
4. Experimental Procedures
4.1. Mouse Experiments
Male ICR mice (Charles River Laboratory Japan, Inc.; Yokohama, Japan) were housed in a light-controlled room (lights on from ZT0 to ZT12) at 24 ± 1 °C and 60% ± 10% humidity, with food and water available ad libitum. Mice were synchronized to the light/dark cycle for 2 weeks before surgery. Clock mutant mice (C57BL/6J-ClockmlJt/J) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and backcrossed to wild-type Jcl: ICR mice for more than eight generations to enhance breeding and offspring care. Experiments were performed using wild-type ICR, homogeneous, and Clock-mutant mice. CKD was induced in 6-week-old mice by 5/6Nx two-stage surgery under isoflurane anesthesia (Baxter, Deerfield, IL, USA). During the first procedure, two-thirds of the left kidney was removed by cutting off both poles. Seven days later, the right kidney was completely removed. After the operations, mice were housed for 8 weeks to allow CKD development. Sham mice were subjected to laparotomy on the same days as the 5/6Nx mouse surgeries. For G0s2 inhibitor NS-3-008 treatment, mice were administered NS-3-008 (5 mg/kg body weight) or vehicle control in the drinking water from weeks 4 to 8 following the second 5/6Nx operation. All animals used in this study were treated in accordance with the guidelines stipulated by the Animal Care and Use Committee of Kyushu University.
4.2. Cell Culture
NIH3T3 cells (supplied by the Cell Resource Center for Biochemical Research, Tohoku University) were grown at 37 °C in a humidified environment (5% CO2, 95% air) in Dulbecco's modified Eagle's medium (Sigma-Aldrich; St. Louis, MO, USA) containing 10% fetal bovine serum (SAFC Biosciences; Kansas City, MO, USA), 0.1 μM insulin, 0.1 μM dexamethasone, and 2% penicillin/streptomycin.
4.3. Statistical Analysis
All data represent the mean ± standard error of the mean (SEM) of at least three individual experiments. Differences were assessed using Student's t or analysis of variance (ANOVA) with Bonferroni/Dunn post hoc testing as indicated. P > 0.05 was considered statistically significant.
Author Contributions
N. M. and S.O. participated in the design of the study, conducted the experiments, analyzed the data, and drafted the manuscript. E. I., K.K., M.W., N.S., T.Y., K.H., T.Y., Y.Y., M.M., T.O., K.H., A.T., H.K., H.I., K.T., K.I., H.I., Y.F., T.A., H.A., M.T., K.I., T.N., A.O. and S.K. conducted the experiments A.D. and K.Y. analyzed microarray data. All authors have read and approved the final manuscript.
Competing Financial Interests
The authors have no competing interests to declare.
Acknowledgements
This study was supported in part by Grants-in-Aid for Scientific Research (A; 25253038, 16H02636; S. Ohdo), for Scientific Research on Innovative Areas (25136716; S. Ohdo), and for Challenging Exploratory Research (25670079; S. Ohdo); The Uehara Memorial Foundation and Scientific Research (C; 24590196, 15K08098; N. Matsunaga); and a Grant-in-Aid for JSPS Fellows (25-4175; K. Hamamura) from the Japan Society for the Promotion of Science (JSPS) and the Fukuoka Foundation for Sound Health. This work was also supported by the Platform for Drug Discovery, Informatics, and Structural Life Science from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We would like to thank the Research Support Center, Graduate School of Medical Sciences, Kyushu University for technical support.
Footnotes
Additional methods and reagents are described in the supplementary methods available at http://www.ebiomedicine.com/. Supplementary data associated with this article can be found in the online version, at http://dx.doi.org/10.1016/j.ebiom.2016.10.008.
Appendix A. Supplementary Data
Supplementary Information
References
- Barsoum R.S. Chronic kidney disease in the developing world. N. Engl. J. Med. 2006;354:997–999. doi: 10.1056/NEJMp058318. [DOI] [PubMed] [Google Scholar]
- Brey D.M., Motlekar N.A., Diamond S.L., Mauck R.L., Garino J.P., Burdick J.A. High-throughput screening of a small molecule library for promoters and inhibitors of mesenchymal stem cell osteogenic differentiation. Biotechnol. Bioeng. 2011;108:163–174. doi: 10.1002/bit.22925. [DOI] [PubMed] [Google Scholar]
- Chen Z., Yoo S.H., Park Y.S., Kim K.H., Wei S., Buhr E., Ye Z.Y., Pan H.L., Takahashi J.S. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc. Natl. Acad. Sci. U. S. A. 2012;109:101–106. doi: 10.1073/pnas.1118034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Shimogawa H., Murakami K., Ramdas L., Zhang W., Qin J., Uesugi M. Chemical genetic identification of the IGF-linked pathway that is mediated by STAT6 and MFP2. Chem. Biol. 2006;13:241–249. doi: 10.1016/j.chembiol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Codreanu I., Perico N., Remuzzi G. Dual blockade of the renin-angiotensin system: the ultimate treatment for renal protection? J. Am. Soc. Nephrol. 2005;16:S34–S38. doi: 10.1681/asn.2004110966. [DOI] [PubMed] [Google Scholar]
- Cristillo A.D., Heximer S.P., Russell L., Forsdyke D.R. Cyclosporin A inhibits early mRNA expression of G0/G1 switch gene 2 (G0S2) in cultured human blood mononuclear cells. DNA Cell Biol. 1997;16:1449–1458. doi: 10.1089/dna.1997.16.1449. [DOI] [PubMed] [Google Scholar]
- Curtis A.M., Cheng Y., Kapoor S., Reilly D., Price T.S., Fitzgerald G.A. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3450–3455. doi: 10.1073/pnas.0611680104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne J.P., Noton E., Lambert C.M., Maywood E.S., Weaver D.R., Reppert S.M. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron. 2006;50:465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Deng X., Xu M., Yuan C., Yin L., Chen X., Zhou X., Li G., Fu Y., Feghali-Bostwick C.A., Pang L. Transcriptional regulation of increased CCL2 expression in pulmonary fibrosis involves nuclear factor-κB and activator protein-1. Int. J. Biochem. Cell Biol. 2013;45:1366–1376. doi: 10.1016/j.biocel.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Doi M., Takahashi Y., Komatsu R., Yamazaki F., Yamada H., Haraguchi S., Emoto N., Okuno Y., Tsujimoto G., Kanematsu A., Ogawa O., Todo T., Tsutsui K., van der Horst G.T., Okamura H. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat. Med. 2010;16:67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- Eardley K.S., Zehnder D., Quinkler M., Lepenies J., Bates R.L., Savage C.O., Howie A.J., Adu D., Cockwell P. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006;69:1189–1197. doi: 10.1038/sj.ki.5000212. [DOI] [PubMed] [Google Scholar]
- Enomoto M., Inoue Y., Namba K., Munezawa T., Matsuura M. Clinical characteristics of restless legs syndrome in end-stage renal failure and idiopathic RLS patients. Mov. Disord. 2008;23:811–816. doi: 10.1002/mds.21882. [DOI] [PubMed] [Google Scholar]
- Filipski E., Innominato P.F., Wu M., Li X.M., Iacobelli S., Xian L.J., Lévi F. Effects of light and food schedules on liver and tumor molecular clocks in mice. J. Nat. Cancer. Inst. 2005;97:507–517. doi: 10.1093/jnci/dji083. [DOI] [PubMed] [Google Scholar]
- Gekakis N., Staknis D., Nguyen H.B., Davis F.C., Wilsbacher L.D., King D.P., Takahashi J.S., Weitz C.J. Role of CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Gibbs J., Ince L., Matthews L., Mei J., Bell T., Yang N., Saer B., Begley N., Poolman T., Pariollaud M., Farrow S., DeMayo F., Hussell T., Worthen G.S., Ray D., Loudon A. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat. Med. 2014;20:919–926. doi: 10.1038/nm.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiramoto A., Yamane T., Tsumiyama K., Yoshida K., Komai K., Yamada H., Yamazaki F., Doi M., Okamura H., Shiozawa S. Mammalian clock gene cryptochrome regulates arthritis via proinflammatory cytokine TNF-alpha. J. Immunol. 2010;184:1560–1565. doi: 10.4049/jimmunol.0903284. [DOI] [PubMed] [Google Scholar]
- Hedayati S.S., Bosworth H.B., Briley L.P., Sloane R.J., Pieper C.F., Kimmel P.L., Szczech L.A. Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int. 2008;74:930–936. doi: 10.1038/ki.2008.311. [DOI] [PubMed] [Google Scholar]
- Hirota T., Kay S.A. High-throughput screening and chemical biology: new approaches for understanding circadian clock mechanisms. Chem. Biol. 2009;16:921–927. doi: 10.1016/j.chembiol.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T., Lee J.W., Lewis W.G., Zhang E.E., Breton G., Liu X., Garcia M., Peters E.C., Etchegaray J.P., Traver D., Schultz P.G., Kay S.A. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIalpha as a clock regulatory kinase. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isojima Y., Nakajima M., Ukai H., Fujishima H., Yamada R.G., Masumoto K.H., Kiuchi R., Ishii T., Onda H., Tanigawa A., Ohshima S., Fujiwara H., Mima T., Katada Y., Deguchi H., Suemura M., Miyake T., Miyatake K., Kawase I., Zhao H., Tomiyama Y., Saeki Y., Nojima H. Isolation and expression profiling of genes upregulated in the peripheral blood cells of systemic lupus erythematosus patients. DNA Res. 2005;12:429–439. doi: 10.1093/dnares/dsi020. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Cen W., Xing S., Chen J., Xu H., Wen A., Zhu L., Tang G., Li M., Jiang A., Li X. Tissue expression pattern and polymorphism of G0S2 gene in porcine. Gene. 2014;539:173–179. doi: 10.1016/j.gene.2014.01.058. [DOI] [PubMed] [Google Scholar]
- Jin X., Shearman L.P., Weaver D.R., Zylka M.J., de Vries G.J., Reppert S.M. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- Kitareewan S., Blumen S., Sekula D., Bissonnette R.P., Lamph W.W., Cui Q., Gallagher R., Dmitrovsky E. G0S2 is an all-trans-retinoic acid target gene. Int. J. Oncol. 2008;33:397–404. [PMC free article] [PubMed] [Google Scholar]
- Krishnan A.V., Kiernan M.C. Uremic neuropathy: clinical features and new pathophysiological insights. Muscle Nerve. 2007;35:273–290. doi: 10.1002/mus.20713. [DOI] [PubMed] [Google Scholar]
- Kume K., Zylka M.J., Sriram S., Shearman L.P., Weaver D.R., Jin X., Maywood E.S., Hastings M.H., Reppert S.M. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- Mavroudis P.D., Scheff J.D., Calvano S.E., Androulakis I.P. Systems biology of circadian-immune interactions. J. Innate. Immun. 2013;5:153–162. doi: 10.1159/000342427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer K., Vonk R.J., Priebe M.G., Roelofsen H. Cell-based screening assay for anti-inflammatory activity of bioactive compounds. Food Chem. 2015;166:158–164. doi: 10.1016/j.foodchem.2014.06.053. [DOI] [PubMed] [Google Scholar]
- Murray A.M. Cognitive impairment in the aging dialysis and chronic kidney disease populations: an occult burden. Adv. Chronic Kidney Dis. 2008;15:123–132. doi: 10.1053/j.ackd.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N., Shimaoka Y., Tougan T., Onda H., Okuzaki D., Zhao H., Fujimori A., Yabuta N., Nagamori I., Tanigawa A., Sato J., Oda T., Hayashida K., Suzuki R., Yukioka M., Nojima H., Ochi T. Isolation and expression profiling of genes upregulated in bone marrow-derived mononuclear cells of rheumatoid arthritis patients. DNA Res. 2006;13:169–183. doi: 10.1093/dnares/dsl006. [DOI] [PubMed] [Google Scholar]
- Narasimamurthy R., Hatori M., Nayak S.K., Liu F., Panda S., Verma I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. U. S. A. 2012;109:12662–12667. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemczyk S. Circadian profile of the prolactine concentration in the patients with end-stage renal failure. Pol. Arch. Med. Wewn. 2006;116:1137–1143. [PubMed] [Google Scholar]
- Nikolaeva S., Pradervand S., Centeno G., Zavadova V., Tokonami N., Maillard M., Bonny O., Firsov D. The circadian clock modulates renal sodium handling. J. Am. Soc. Nephrol. 2012;23:1019–1026. doi: 10.1681/ASN.2011080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa T., Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J. Lab. Clin. Med. 1994;124:96–104. [PubMed] [Google Scholar]
- Ohdo S., Koyanagi S., Matsunaga N., Hamdan A. Molecular basis of chronopharmaceutics. J. Pharm. Sci. 2011;100:3560–3576. doi: 10.1002/jps.22656. [DOI] [PubMed] [Google Scholar]
- Patel D.A., Patel A.C., Nolan W.C., Zhang Y., Holtzman M.J. High throughput screening for small molecule enhancers of the interferon signaling pathway to drive next-generation antiviral drug discovery. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper A.A., Xie S., Capota E., Estill S.J., Zhong J., Long J.M., Becker G.L., Huntington P., Goldman S.E., Shen C.H., Capota M., Britt J.K., Kotti T., Ure K., Brat D.J., Williams N.S., MacMillan K.S., Naidoo J., Melito L., Hsieh J., De Brabander J., Ready J.M., McKnight S.L. Discovery of a proneurogenic, neuroprotective chemical. Cell. 2010;142:39–51. doi: 10.1016/j.cell.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner N., Damiola F., Lopez-Molina L., Zakany J., Duboule D., Albrecht U., Schibler U. The orphan nuclear receptor REV-ERB alpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P., Perna A., Gherardi G., Gaspari F., Benini R., Remuzzi G. Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril efficacy in nephropathy. Lancet. 1998;352:1252–1256. doi: 10.1016/s0140-6736(98)04433-x. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P., Perna A., Gherardi G., Garini G., Zoccali C., Salvadori M., Scolari F., Schena F.P., Remuzzi G. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354:359–364. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P., Cravedi P., Remuzzi G. Mechanisms and treatment of CKD. J. Am. Soc. Nephrol. 2012;23:1917–1928. doi: 10.1681/ASN.2012040390. [DOI] [PubMed] [Google Scholar]
- Russell L., Forsdyke D.R. A human putative lymphocyte G0/G1 switch gene containing a CpG-rich island encodes a small basic protein with the potential to be phosphorylated. DNA Cell Biol. 1991;10:581–591. doi: 10.1089/dna.1991.10.581. [DOI] [PubMed] [Google Scholar]
- Sanz A.B., Santamaría B., Ruiz-Ortega M., Egido J., Ortiz A. Mechanisms of renal apoptosis in health and disease. J. Am. Soc. Nephrol. 2008;19:1634–1642. doi: 10.1681/ASN.2007121336. [DOI] [PubMed] [Google Scholar]
- Siepka S.M., Yoo S.H., Park J., Song W., Kumar V., Hu Y., Lee C., Takahashi J.S. Circadian mutant overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129:1011–1023. doi: 10.1016/j.cell.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler M.L., Kuropatwinski K.K., Comas M., Gasparian A.V., Fedtsova N., Gleiberman A.S., Gitlin I.I., Artemicheva N.M., Deluca K.A., Gudkov A.V., Antoch M.P. Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E2457–E2465. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow L.R., Richards J., Cheng K.Y., Lynch I.J., Jeffers L.A., Greenlee M.M., Cain B.D., Wingo C.S., Gumz M.L. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension. 2012;59:1151–1156. doi: 10.1161/HYPERTENSIONAHA.112.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toby L. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek F.W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., Laposky A., Losee-Olson S., Easton A., Jensen D.R., Eckel R.H., Takahashi J.S., Bass J. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H.R., Chen W., Adachi A., Wakamatsu H., Hayashi S., Takasugi T., Nagano M., Nakahama K., Suzuki Y., Sugano S., Iino M., Shigeyoshi Y., Hashimoto S. A transcription factor response element for gene expression during circadian night. Nature. 2002;418:534–539. doi: 10.1038/nature00906. [DOI] [PubMed] [Google Scholar]
- Vitaterna M.H., King D.P., Chang A.M., Kornhauser J.M., Lowrey P.L., McDonald J.D., Dove W.F., Pinto L.H., Turek F.W., Takahashi J.S. Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch C., Santra M.K., El-Assaad W., Zhu X., Huber W.E., Keys R.A., Teodoro J.G., Green M.R. Identification of a protein, G0S2, that lacks Bcl-2 homology domains and interacts with and antagonizes Bcl-2. Cancer Res. 2009;69:6782–6789. doi: 10.1158/0008-5472.CAN-09-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Lu X., Lombès M., Rha G.B., Chi Y.I., Guerin T.M., Smart E.J., Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim C.Y., Sekula D.J., Hever-Jardine M.P., Liu X., Warzecha J.M., Tam J., Freemantle S.J., Dmitrovsky E., Spinella M.J. G0S2 suppresses oncogenic transformation by repressing a MYC-regulated transcriptional program. Cancer Res. 2016;76:1204–1213. doi: 10.1158/0008-5472.CAN-15-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Zheng Y., Nettleton J.A., Alexander D., Coresh J., Boerwinkle E. Serum metabolomic profiling and incident CKD among African Americans. Clin. J. Am. Soc. Nephrol. 2014;9:1410–1417. doi: 10.2215/CJN.11971113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Xie X., Heckmann B.L., Saarinen A.M., Czyzyk T.A., Liu J. Targeted disruption of G0/G1 switch gene 2 enhances adipose lipolysis, alters hepatic energy balance, and alleviates high-fat diet-induced liver steatosis. Diabetes. 2014;63:934–946. doi: 10.2337/db13-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber A.M., Centeno G., Pradervand S., Nikolaeva S., Maquelin L., Cardinaux L., Bonny O., Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16523–16528. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information