Abstract
Objective
A potential mechanism by which obesity could promote hypertension and kidney diseases is through accumulation of adipose tissue in the renal sinus (RS). The aim of the study was to quantify RS and abdominal adipose tissue volumes and to evaluate serum kidney injury molecule (sKIM)-1 and fibroblast growth factor (FGF)-21 association with different adipose tissue compartments.
Methods
The cross-sectional study included 280 and follow-up study-40 asymptomatic participants; aged 38.30 ± 4.10. For all study participants computed tomography examination was performed, sKIM-1 and FGF-21 levels were measured.
Results
The results indicated asymmetrical deposition of adipose tissue into the RS even after corresponding kidney volume adjustment. The cross-sectional and the follow-up studies showed that sKIM-1 level was positively associated with RS adipose tissue volume increase for both genders. FGF-21 was positively associated with RS and retroperitoneal adipose tissue amount.
Conclusions
Regardless of gender adipose tissue in RS accumulates asymmetrically–the left RS accumulates a significantly higher amount of adipose tissue. Thus, primarily RS adipose tissue effects should be assessed on the left kidney. Accumulation of adipose tissue in the RS is related with the visceral adipose amount, KIM-1 and FGF-21 concentration increase in the blood serum.
Keywords: Asymmetry, Renal sinus, KIM-1, FGF-21, Obesity, Visceral adipose tissue
Highlights
-
•
Renal sinus adipose tissue accumulation is asymmetric
-
•
KIM (kidney injury molecule)-1 level is positively associated with renal sinus adipose tissue amount
-
•
FGF (fibroblast growth factor)-21 level is associated with renal sinus and retroperitoneal adipose tissue amount
Obesity is a common contributing factor to the development of chronic kidney disease, hypertension, and predispose to the calcium oxalate and urate stones. The distribution of adipose tissue between different depots seems to be more important than the total adipose tissue mass for the risk of developing obesity-associated diseases. This research is dedicated to the renal sinus adipose tissue. The article reveals that renal sinus adipose tissue deposition is asymmetric–the left compared to the right renal sinus accumulates significantly higher adipose tissue amount. Additionally, there is association between renal sinus adipose tissue increase and proximal tubule epithelial cells damage.
1. Introduction
In humans and most animal models, development of obesity also leads to ectopic adipose tissue storage (Bjorndal et al., 2011). Normal renal sinus (RS) contains a small amount of adipose tissue that lines other structures within it. By excess deposition of perirenal adipose tissue in the RS, compression of various renal structures may occur, especially the inner medulla that, unlike the entire kidney, is not protected by the fibrous capsule (Lamacchia et al., 2011). It has been previously shown that RS adipose tissue may have an independent association with renal functions (Chughtai et al., 2010, Foster et al., 2011b). However, RS adipose tissue research is limited to only a couple of recently published studies. Foster et al. (Foster et al., 2011b) showed that RS adipose tissue is associated with an increased risk of hypertension and chronic renal disease. Similarly, Chughtai et al. (Chughtai et al., 2010) indicated that RS adipose tissue volume was associated with the number of prescribed antihypertensive medications and stage II hypertension.
Overall, obesity can lead to renal functional disorders and induce hypertension through a variety of mechanisms: (Hall, 1997, Hall et al., 1999, Hall et al., 2002, Hall et al., 2010, Hall et al., 2015, Hall et al., 2014). Activation of sympathetic nervous system; Physical–mechanical compression; Renin–angiotensin–aldosterone system. It has been hypothesized that RS adipose tissue effect is local and mostly occurs through the physical–mechanical compression (Montani et al., 2004, Hall et al., 2014). However, unfortunately exact mechanisms how RS adipose tissue could influence kidney functions are not described. Additionally, published RS adipose tissue research has some significant disadvantages. Since the object segmentation and 3D reconstruction of organs is labor-intensive and time-consuming, often chosen strategy is computed tomography (CT) or magnetic resonance (MR) single-scan measurement at a specific anatomic level. In this way, the manual data processing is reduced significantly. However, as the RS adipose tissue is a small and diffuse object, this also results in loss of the measurement quality. Since RS adipose tissue compartment is relatively small object, then in our study, strategy to segment the entire RS adipose tissue and analyze it's full volumes, was used, thus more accurately characterizing RS adipose tissue and it's role.
In our study we analyze asymptomatic, middle age participants, therefore, it was important to find an earlier diagnostic biomarkers of kidney injury. It is useful to keep in mind that creatinine level may not raise until more than half of the kidney function has been lost due to renal reserve. Kidney injury factor (KIM)-1 serves as an earlier diagnostic biomarker of kidney injury when compared to any of the conventional diagnostic markers, e.g., serum creatinine and cystatin C, increased proteinuria. It has been approved by the US Food and Drug Administration as an acute kidney injury biomarker for preclinical drug development (Dieterle et al., 2010). KIM-1 expression and secretion was observed in the kidney epithelial cells in vitro (Ichimura et al., 2004), in animal models and in patients with various kidney diseases (Lim and Meigs, 2013). Experiments in rodents and studies in humans have shown that kidney ischemia or hypoxia promotes KIM-1 concentrations increase in the urine and blood serum. Chronic KIM-1 expression can promote further tubulointestinal inflammation and hypoxia, further inducing KIM-1 expression that culminate in chronic kidney disease (Humphreys et al., 2013). The ectodomain of KIM-1 is shed into the lumen, and serves as a urinary biomarker of kidney injury (Bonventre, 2009, Kuwata et al., 2015, Jin et al., 2013). Recent study showed that KIM-1 may be released into the circulation and identified KIM-1 as a blood biomarker that specifically reflects injury to the proximal tubule of the kidney, the primary site of injury for ischemia and most nephrotoxicants (Sabbisetti et al., 2014, Bonventre et al., 2010). The proposed mechanisms for the KIM-1 reabsorption are: (1) After kidney proximal tubule injury when tubular cell polarity is lost and KIM-1 may be released directly into the interstitium; (2) Renal microvascular endothelial cells are detached from the basement membrane that facilitate KIM-1 reabsorption into the circulation (Sabbisetti et al., 2014) (Hall et al., 2014).
Recently, fibroblast growth factor (FGF)-21 was introduced as a renal function marker. FGF-21 levels were higher in the patients with both chronic and acute renal dysfunctions. Additionally, FGF-21 levels gradually increased with the development or renal diseases from the early to late stage (Zhang et al., 2015). FGF-21 serve as renal function marker because renal elimination is a major route by which physiological FGF-21 serum levels are maintained (Stein et al., 2009). Respectively, if renal function is impaired, serum FGF-21 level rise.
In general, there is limited information about the mechanism how RS adipose tissue induce renal disease and hypertension. There is no information about RS adipose tissue and early diagnostic biomarkers of kidney injury. Additionally, all previously conducted researches were based on RS adipose tissue single slice measurements. Thereby, the aim of the study was to quantify RS and abdominal adipose tissue volumes and to evaluate sKIM-1 (as primary kidneys damage marker) and FGF-21 association with different adipose tissue compartments in the observational study with a cross-sectional design and a prospective one-year naturalistic follow-up study. We hypothesized that RS adipose tissue would be independently associated with sKIM-1 and FGF-21 levels after accounting for abdominal adipose tissue segments (retroperitoneal (RP), intraperitoneal (IP) and subcutaneous (SC)) as a measure of abdominal obesity.
2. Methods
2.1. Participants and Study Design
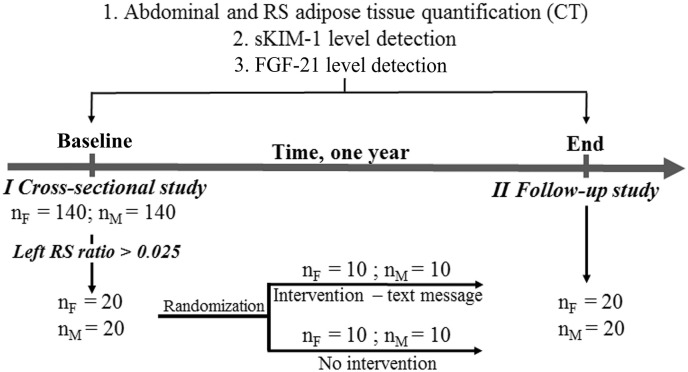
An observational study with a cross-sectional design and a prospective one-year naturalistic follow-up study. The period of recruitment was from January 2011 till January 2014. The sample size was calculated according to proportions (n = 1.962 × 4 p(1 − p)/d2; where: p – expected population proportion; d - the desired width of the confidence interval (CI)). In our case: an estimate of the prevalence of adipose tissue accumulation in the RS is 0.99 (99%). The width of the 95% CI is 0.1. Estimated minimal sample size was 153 subjects.
The cross-sectional study participants were selected from family physician's practices of a Health care center in Riga. The exclusion criteria for the participants selection were a recorded diagnosis of chronic kidney diseases (nephrolithiasis; renal cysts; nephroptosis; an extra kidney; renal lipomatosis), cardiovascular diseases, thyroid diseases, diabetes or malignancy; use of tobacco and alcohol (male > 140 g ethanol per week; female > 70 g ethanol per week); anamnesis of arterial hypertension, pregnancy and lactation, taking any regular medications; body mass index (BMI) < 18 kg/m2 and BMI > 35 kg/m2. The inclusion criteria were BMI = 18–35 kg/m2, age 30–45 years. In the cross-sectional study 280 participants were recruited (144/136, F/M) aged 37.30 ± 4.10 years, ethnicity – Caucasians.
Participants (n = 40; 20/20, F/M) for the prospective one-year naturalistic follow-up study were recruited from the cross-sectional study group. The inclusion criteria for this study were left RS ratio > 0.025 (see Section 2.3). The participants were randomized into two groups: participants (10/10, F/M) randomized for the intervention received daily text messages for one year. Text messages included practical recommendations on how to balance caloric intake and physical activity to achieve and maintain a healthy body weight. The recommendations were prepared by the general practitioner according to the diet and lifestyle recommendations (Eckel et al., 2014). Other randomized 10 males and 10 females did not receive any intervention for one year. The study design is shown in the Fig. 1.
Fig. 1.
Design of the cross-sectional and the prospective one-year naturalistic follow-up study.
Experimental procedures were approved by the Ethical Committee of the Institute of Experimental and Clinical Medicine, University of Latvia. All procedures performed in this study were in accordance with the ethical standards of the institutional and international research committee and with the 1964 Helsinki declaration. Informed consent was obtained from all study participants.
2.2. Anthropometric Parameters
BMI was calculated as the ratio of weight to height squared. Normal weight was defined as BMI 18.0–24.9 kg/m2; overweight – as BMI 25.0–29.9 kg/m2 and obesity – as BMI ≥ 30 kg/m2. Waist circumference (WC) was measured using a tape rule, and the point of measurement was the anterior superior iliac spine (Kovesdy et al., 2010). Blood pressure (systolic blood pressure (SBP) and diastolic blood pressure (DBP)) measurements were performed by professional medical assistant.
2.3. Abdominal and RS Adipose Tissue Quantification
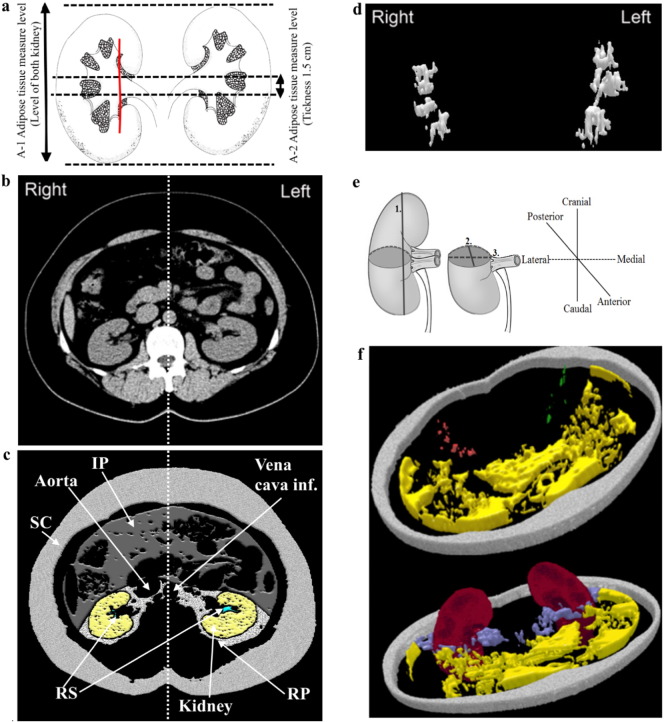
Native abdominal (~ Th10 to ~ L4) CT scans were captured using spiral (128-slice configuration) CT SOMATOM Definition AS/AS (Siemens AG, Forchheim, Germany) with automated tube current modulation (CareDose4D, Siemens) during inspiratory breath-hold (slice thickness 5 mm). CT scans were post-processed using the 3D Doctor software (Able Software Corp., Lexington, MA, USA). According to the techniques and anatomical landmarks published previously (Chughtai et al., 2010), abdominal adipose tissue was manually segmented into SC, RP, and IP compartments (Fig. 2. c). Two abdominal adipose tissue measurement series were performed. First, total abdominal adipose tissue volume was measured at the level of both kidneys (n = 100) (Fig. 2a, A-1) for all compartments (RPfull, IPfull, SCfull). Second, CT measurements of abdominal adipose tissue was performed in three axial slices (RPthree-slices, IPthree-slices, SCthree-slices) (Fig. 2a, A-2) positioned at the level of the right renal hilum. Next, total volumetric RS adipose tissue segmentation from both kidneys were performed. According to the Foster et al. (Foster et al., 2011a, Foster et al., 2011b) protocol, the boundary of RS was defined as a straight line between both dimples at the edge of the RS opening (Fig. 2a, A-1). Since RS adipose tissue compartment is relatively small object, strategy to segment the entire RS adipose tissue in the RS and analyze its full volumes was used, thus more accurately characterizing RS adipose tissue (Fig. 2d and f). Additionally, total volumetric kidney segmentation was performed (we excluded major branches of the renal artery and vein as well as any visible branches of the renal collecting system) (Chughtai et al., 2010). According to Glodny et al. (Glodny et al., 2009) a kidney length (pole to pole) in the coronal plane and according to Breau et al. (Breau et al., 2013) sagittal and lateral diameter in the axial plane were measured (Fig. 2e).
Fig. 2.
Abdominal computed tomography scans demonstrating renal sinus and abdominal adipose tissue compartment measurements. a: The levels of abdominal adipose tissue measurements. A-1 – the level where total abdominal adipose tissue measurements were performed (RPfull, IPfull, SCfull). A-2 – the level where abdominal adipose tissue measurements in three axial slices were performed (RPthree-slices, IPthree-slices, SCthree-slices). The red line represents the boundary of RS; b: Example of single abdominal CT slice selected for RS and abdominal adipose tissue compartment measurements; c: Visualization of RS and abdominal adipose tissue compartments as well as kidney using 3D Doctor software; d: Volumetric right and left RS adipose tissue compartment visualization. e: Measure of the kidney length (pole to pole) (1.) in the sagittal plane and sagittal (2.) as well as lateral diameter (3.) in the axial plane; f: 3D reconstruction of RS, IP, RP, SC and kidneys. RS adipose tissue and kidneys were segmented fully; RP, IP and SC adipose tissue at the renal hilum's level. Subcutaneous adipose tissue (SC) (gray); Intraperitoneal (IP) adipose tissue (yellow); RP adipose tissue (purple); Right RS adipose tissue (red); Left RS adipose tissue (green).
Overall, for adipose tissue compartments and kidney volume detection, first, region of interest was manually outlined with the mouse by using a tool called “Polygon” on each section. Second, segmentation of adipose tissue and kidney volumes was performed based on Hounsfield units (HU). Adipose tissue was identified using an attenuation range of (− 195)–(− 45) HU (Foster et al., 2011a, Foster et al., 2011b), for kidneys - (+ 20)–(+ 40) HU (Hofer, 2010). Third, we used 3D Rendering Full Complex Surface command and constructed 3D models (Fig. 2d and f). Different adipose tissue compartment's volumes (cm3) were measured by volume and surface rendering technique according to which the 3D Doctor software calculates segmental volume of each respective adipose tissue compartment slice as the product the slice area and the inter-slice distance (slice thickness). The volume of the entire adipose tissue compartment was then calculated as a sum of segmental volumes as indicated by the Cavalieri's principle (Szczepaniak et al., 2013): the volume of the abdominal or the RS adipose tissue = Σ (volumes of all respective adipose tissue slices).
In order to exclude the kidney size effect on RS adipose tissue volume, ratio of RS adipose tissue volume to corresponding total kidney size was calculated using the arithmetical mean for each participant:
The participants for the prospective one-year naturalistic follow-up study were recruited from the cross-sectional study group according to 75th percentile of the left RS ratio (75th percentile = 0.025). The left RS ratio was chosen because the left RS accumulates significantly higher amount of adipose tissue compared to the right RS (see Section 3.4).
2.4. Analytical Methods
sKIM-1 level was measured by Human Kidney Toxicity Magnetic Bead Panel 1, HKTX1MAG-38K (Millipore, Billerica, MA, USA), interleukin (IL)-6 and tumor necrosis factor alpha (TNFα) were measured by HADCYMAG-61 (Millipore, Billerica, MA, USA) on a Luminex 200 analyzer (Austin, TX, USA). FGF-21 was measured by ELISA kits (#EZHFGF21-19K; Millipore, Billerica, MA, USA) on an Infinite®M200 analyzer (Tecan Trading AG, Switzerland). The tests were performed in accordance with the manufacturer's instructions. Serum creatinine, cystatin C, glucose, triglycerides (TG), high density lipoprotein cholesterol (HDLC) and low density lipoprotein cholesterol (LDLC) tests were performed in the clinical laboratory ‘E.Gulbja laboratorija’, Riga, Latvia. The estimated glomerular filtration rate (eGFREPI cyst&crea) was determined using cystatin C and creatinine concentrations and Chronic Kidney Disease Epidemiology equation (Inker et al., 2012).
Venous blood samples for blood tests were collected without anticoagulant and were allowed to coagulate for 25–30 min at the room temperature. After that, samples were centrifuged at 4 °C for 10 min at 1600 × g. All specimens were immediately aliquoted, frozen, and stored at − 80 °C until use (for serum sKIM-1 and FGF-21 analysis; standard blood tests were performed using fresh serum samples). Serum samples were obtained after an overnight fast.
2.5. Statistical analysis
For the cross-sectional study the fatty kidney group was defined as the presence of high left RS ratio based on gender specific 75th percentiles (Control group < 75th percentiles). For the prospective one-year naturalistic follow-up study we divided all (n = 40) participants in the three groups according to the significant (> 5%) visceral adipose tissue (IP adipose tissue + RP adipose tissue) volume change after follow-up period: Group 1 (control) – no significant visceral adipose tissue volume change; Group 2 - significant visceral adipose tissue volume decrease; Group 3 - significant visceral adipose tissue volume increase.
Data were analyzed using SPSS22.0. After testing normality (Kolmogorov-Smirnov test) unpaired data with normal distribution (presented as mean ± SD) were analyzed using unpaired Student t-test. Mann-Whitney U test was used as nonparametric method for unpaired data (data with skewed distribution presented as median and interquartile range). p < 0.05 was considered significant. Paired data (left vs. right RS ratio, left vs. right kidney and follow up study's data (baseline vs. end)) were compared using Wilcoxon rank-sum test. Square root transformation (sqrt) was used for positive skew data, when Pearson and age and gender adjusted partial Pearson correlations as well as multiple linear regression analysis were performed. Pearson correlation was used to detect association between different abdominal and RS adipose tissue compartments. Associations between sKIM-1 or FGF-21 and different adipose tissue compartments were assessed by an age and gender adjusted stepwise-method multiple linear regression analysis (results presented as standardized regression coefficient (β); 95% CI; R2% and p value). Collinearity was assessed using variance inflation factor (VIFs) and tolerance (VIF > 10.0 and tolerance < 0.2 indicating model instability). Binary logistic regression was used to calculate age and gender adjusted p values. All adipose tissue compartments were measured separately by two readers for inter-reader variation and then repeated by the first reader for intra-reader variation. Bland-Altman plots were utilized to assess potential systematic biases within the intra-reader and inter-reader repeated measurements.
3. Results
The 3.1, 3.2, 3.3, 3.4, 3.5 summarize the cross-sectional study results.
3.1. General Characteristics of Participants
RS adipose tissue volume ranged from 0.07 cm3 to 11.23 cm3. Individuals with fatty kidney had a higher BMI, higher level of serum KIM-1, FGF-21 and TG levels, as well as significantly lower LDLC level (Table 1). However, WC and BMI adjusted binary logistic regression shows that serum TG level is positively associated with IP adipose tissue segment volume (effect size 56%, p < 0.01). In general males had significantly greater (p < 0.05) WC and higher visceral adipose tissue (RP and IP) accumulation. However, amount of SC adipose tissue is significantly higher in females. These results are in agreement with those reported previously. Concerning to the inflammatory cytokine levels there are no significant difference between control and fatty kidney group (Table 1). Participant's eGFREPI cyst&crea was > 60 ml/min/1.73 m2 (Table 1).
Table 1.
General characteristics of cross-sectional study participants and abdominal adipose tissue measurements (ntotal = 280).
| Measurements | Control (no fatty kidney) n = 199 (F/M; 96/103) | Fatty kidney n = 81 (F/M; 44/37) | Age and gender adjusted p |
|---|---|---|---|
| Anthropometric parameters | |||
| Age, yearsa | 38.00 (34.00, 40.25) | 38.00 (35.00, 40.00) | 0.116 |
| BMI, kg/m2 | 27.02 (23.98, 30.89) | 30.86 (26.86, 33.35) | 0.005 |
| WC, cm | 92.00 (82.00, 101.00) | 100.00 (89.00, 105.00) | 0.322 |
| SBP, mm Hg | 120.00 (110.00, 125.00) | 120.00 (115.00, 129.00) | 0.136 |
| DBP, mm Hg | 75.00 (70.00, 85.00) | 80.00 (75.00, 85.00) | 0.110 |
| Adipose tissue measurements | |||
| RP, cm3 | 27.56 (13.33, 54.86) | 52.37 (31.89, 84.53) | 0.175 |
| IP, cm3 | 89.81 (40.94, 168.23) | 171.61 (92.50, 253.49) | 0.204 |
| SC, cm3 | 175.41 (111.55, 309.66) | 249.26 (144.34, 340.68) | 0.731 |
| RSRight, cm3 | 0.39 (0.10, 0.95) | 2.72 (1.83, 4.89) | < 0.001 |
| Right RSRatio | 0.0026 (0.0007, 0.0061) | 0.0165 (0.0102, 0.0288) | < 0.001 |
| RSLeft, cm3 | 1.42 (0.57, 2.56) | 5.20 (4.28, 7.47) | < 0.001 |
| Left RS Ratio | 0.0084 (0.0035, 0.0148) | 0.0329 (0.0258, 0.0464) | < 0.001 |
| Kidney biochemical parameters | |||
| sKIM-1, pg/ml | 110.10 (52.21, 235.14) | 178.26 (115.25, 304.26) | < 0.05 |
| FGF21, pg/ml | 106.92 (66.59, 189.40) | 204.26 (76.89, 404.26) | < 0.05 |
| eGFREPI cyst&crea, ml/min/1.73 m2 | 144.25 (108.37, 121.66) | 110.39 (105.25, 119.04) | 0.202 |
| Cystatin C, mg/lb | 0.69 (0.61, 0.77) | 0.74 (0.63, 0.81) | 0.200 |
| Creatinine, μmol/lb | 0.76 (0.68, 0.87) | 0.80 (0.74, 0.96) | 0.251 |
| Glucose and lipid profile | |||
| Glucose, mmol/l | 5.00 (4.77, 5.35) | 5.10 (4.80, 5.37) | 0.171 |
| TG, mmol/l | 1.06 (0.78, 1.51) | 1.47 (0.88, 1.94) | < 0.05 |
| HDLC, mmol/l | 1.52 (1.29, 1.77) | 1.43 (1.09, 1.72) | 0.190 |
| LDLC, mmol/l | 3.27 (2.79, 3.95) | 2.93 (2.44, 3.78) | 0.017 |
| Inflammatory cytokine levels | |||
| IL-6, pg/ml | 1.41 (1.09, 1.77) | 1.21 (0.89, 1.52) | 0.275 |
| TNFα, pg/ml | 2.32 (1.69, 3.74) | 2.41 (1.94, 3.44) | 0.343 |
Data are presented as median (25th, 75 percentile) (age and gender adjusted p values calculated by binary logistic regression). BMI - body mass index; WC - Waist circumference; SBP - systolic blood pressure; DBP - diastolic blood pressure; SC – subcutaneous adipose tissue; IP – intraperitoneal adipose tissue; RP – retroperitoneal adipose tissue; RS – renal sinus adipose tissue; RSRatio - ratio of RS adipose tissue volume to corresponding total kidney size; sKIM-1 – serum kidney injury molecule-1; FGF-21 – fibroblast growth factor – 21; eGFREPI cyst&crea – estimated glomerular filtration rate; TG – triglycerides; HDLC – high density lipoprotein cholesterol; LDLC - low density lipoprotein cholesterol; IL-6 – interleukin 6; TNFα - tumor necrosis factor alpha. The level of significance was set as p < 0.05.
Data show the gender adjusted p value.
Serum cystatin and creatinine were used to estimate GFR.
3.2. Abdominal Adipose Tissue Measurements
Abdominal adipose tissue compartments measured in full volumetric measurement along kidney (Fig. 2a, A-1) and three-slices (Fig. 2a, A-2) at the level of renal hilum, showed strong Pearson correlation coefficients for both males (RPfull and RPthree-slices, r = 0.93; IPfull and IPthree-slices, r = 0.91; SCfull and SCthree-slices, r = 0.83; p < 0.001) and females (RPfull and RPthree-slices, r = 0.95; IPfull and IPthree-slices, r = 0.98; SCfull and SCthree-slices, r = 0.98; p < 0.001) participants. The results suggest, that three slice abdominal adipose tissue measurements at the level of the right renal hilum (Fig. 2a, A-1) completely represent IP, RP and SC adipose tissue relative quantity at the kidney region (Fig. 2f). Additionally, since RS is connected with a perinephric space only at the level of a renal hilum then for further data analysis three sliced abdominal adipose tissue measurements are used. Three- slice abdominal adipose tissue compartment volumes are given in Table 1.
3.3. Kidney Size Measurements
Table 2 shows that females kidney (left and right) volumes are significantly (p < 0.001) lower than males. For both genders no significant differences between the right and left kidney volume are found. However, for both males and females right kidney shows significant higher sagittal diameter and significant lower lateral diameter compared to the left kidney (Table 2). Consequently, the left kidney is slightly laterally flattened which is compensated with a sagittal diameter increase.
Table 2.
A kidney length (pole to pole) in the coronal plane, sagittal and lateral diameter in the axial plane, and a kidney volume.
| Side | Male | p, R vs. L | Female | p, R vs. L | p, M vs. F | |
|---|---|---|---|---|---|---|
| Kidney lenght, cm | Right | 10.48 ± 1.29 | 0.452 | 10.50 ± 1.65 | 0.321 | 0.921 |
| Left | 10.89 ± 1.56 | 10.56 ± 1.29 | 0.845 | |||
| Sagittal diameter, cm | Right | 5.52 ± 0.68 | < 0.001 | 5.51 ± 0.55 | < 0.001 | 0.973 |
| Left | 5.15 ± 0.52 | 5.03 ± 0.53 | 0.174 | |||
| Lateral diameter, cm | Right | 5.24 ± 0.67 | < 0.001 | 4.77 ± 0.61 | 0.002 | < 0.001 |
| Left | 5.59 ± 0.67 | 4.92 ± 0.55 | < 0.001 | |||
| Kidney volume, cm3 | Right | 174.96 ± 25.96 | 0.268 | 151.19 (137.70, 169.88) | 0.175 | < 0.001 |
| Left | 177.51 ± 27.94 | 151.34 (134.61, 167.59) | < 0.001 |
Median (25th, 75 percentile) for skewed data; Mean with standard deviation (± SD) for normally distributed data. The left (L) and the right (R) kidney is compared by using paired Student's t test (normally distributed data) and Wilcoxon rank-sum test (skewed data); Male (M) and female (F) groups are compared by using Mann-Whitney U test. The level of significance was set as p < 0.05.
3.4. Measurements of RS Adipose Tissue
Anatomically, RS adipose tissue can be described as diffuse structure which in a “drop-like” manner is situated between other structures in the RS (Fig. 2d and f). RS adipose tissue volumes in the left and the right kidney were 2.7 (1.49 to 4.62) cm3 and 1.07 (0.46 to 2.45) cm3, respectively, in males and 1.39 (0.57 to 2.99) cm3 and 0.39 (0.10 to 0.95) cm3, respectively, in females participants.RS adipose tissue volume shows no significant (p > 0.05) correlation corresponding to respective (right or left) kidney volume for neither gender. However, to be able to effectively compare females and males RS adipose tissue, as well as to exclude the possibility that in some cases kidney volume could affect RS adipose tissue volume, further cross-sectional study data analysis and interpretation is done by using relative ratio - RS adipose tissue volume corresponding to kidneys (right or left) volume.
All participants (n = 280) regardless of gender, accumulate significantly more (p < 0.001) adipose tissue in the left RS (Fig. 3). Additionally, the same asymmetric distribution pattern of adipose tissue we find for participants with high normal blood pressure (Supplemental material Table S1.) and patients with bilateral nephrolithiasis (Supplemental material Table S2.). Moreover, both participants with the high normal blood pressure in comparison to the participants with the optimal blood pressure and patients with bilateral nephrolithiasis in comparison to the asymptomatic participants show statistically higher adipose tissue accumulation both in the left and the right RS (Supplemental material Tables S1 and S2.).
Fig. 3.
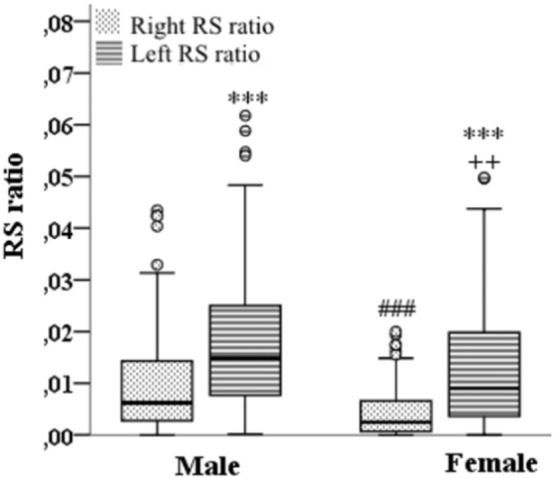
Gender and topography (right and left kidney) effects on the renal sinus adipose tissue accumulation. ***, ### p < 0.001; ++ p = 0.006, °labels potential extremes. Right and left kidney compared by using Wilcoxon rank-sum test; Male and female groups compared by using Mann-Whitney test. Symbols used in the graph: *Right RS ratio vs. Left RS ratio (for male and female group, respectively); #Female right RS ratio vs. Male right RS ratio; + Female left RS ratio vs. Male left RS ratio. RSRatio - ratio of RS adipose tissue volume to corresponding total kidney size.
Age and gender adjusted correlations of RS ratio with adiposity measures and continuous covariates are presented in Table 3.
Table 3.
Age and gender adjusted partial Pearson correlations (r) with sqrt-transformed left or right RS ratio.
| sqrt right RS ratio |
sqrt left RS ratio |
|||
|---|---|---|---|---|
| r | p | r | p | |
| Anthropometric parameters | ||||
| Agea | 0.30 | < 0.001 | 0.32 | < 0.001 |
| BMI, kg/m2 | 0.37 | < 0.001 | 0.36 | < 0.001 |
| WC, cm | 0.40 | < 0.001 | 0.36 | < 0.001 |
| SBP, mm Hg | 0.12 | 0.213 | 0.13 | 0.104 |
| DBP, mm Hg | 0.17 | 0.088 | 0.15 | 0.114 |
| Adipose tissue measurements | ||||
| sqrt RP,cm3 | 0.60 | < 0.001 | 0.52 | < 0.001 |
| sqrt IP,cm3 | 0.50 | < 0.001 | 0.42 | < 0.001 |
| sqrt SC, cm3 | 0.40 | < 0.001 | 0.35 | < 0.001 |
| Kidney biochemical parameters | ||||
| eGFREPI cyst&crea, ml/min/1.73 m2 | − 0.10 | 0.451 | − 0.17 | 0.330 |
| Cystatin C, mg/l | 0.12 | 0.221 | 0.13 | 0.193 |
| Creatinine, μmol/l | 0.02 | 0.856 | 0.04 | 0.703 |
| Sqrt sKIM-1, pg/ml | 0.49 | 0.040 | 0.56 | 0.023 |
| Sqrt FGF21, pg/ml | 0.57 | 0.001 | 0.64 | < 0.001 |
| Glucose and lipid profile | ||||
| Glucose, mmol/l | 0.03 | 0.897 | 0.06 | 0.717 |
| Sqrt TG, mmol/l | 0.25 | 0.009 | 0.21 | 0.033 |
| Sqrt HDLC, mmol/l | − 0.04 | 0.688 | − 0.05 | 0.626 |
| Sqrt LDLC, mmol/l | − 0.22 | 0.024 | − 0.20 | 0.038 |
| Inflammatory cytokine levels | ||||
| Sqrt IL-6, pg/ml | − 0.25 | 0.107 | − 0.05 | 0.734 |
| Sqrt TNFα, pg/ml | − 0.10 | 0.541 | − 0.12 | 0.450 |
sqrt – square root-transformation; RS – renal sinus; SC – subcutaneous adipose tissue; IP – intraperitoneal adipose tissue; RP – retroperitoneal adipose tissue; RSRatio - ratio of RS adipose tissue volume to corresponding total kidney size; BMI – body mass index; WC – waist circumference; SBP - systolic blood pressure; DBP – diastolic blood pressure; eGFREPI cyst&crea – estimated glomerular filtration rate; sKIM-1 – serum kidney injury molecule-1; FGF-21 – fibroblast growth factor – 21; TG – triglycerides; HDLC – high density lipoprotein cholesterol; LDLC - low density lipoprotein cholesterol; IL-6 – interleukin 6; TNFα - tumor necrosis factor alpha. The level of significance was set as p < 0.05.
Data show the gender adjusted partial Pearson correlation.
RS ratio was correlated with all of the adipose tissue and anthropometric measurements covariates examined (p < 0.001) except SBP and DBP (p > 0.05), with the strongest correlations observed for other RP adipose tissue measures. Only sKIM-1 and FGF-21 was correlated with RS ratio according to the kidney biochemical parameters (p < 0.05). Additionally, RS ratio increase was associated with an increased TG level and decreased LDLH level. Concerning to the inflammatory cytokine levels neither left RS ratio nor right RS ratio showed significant association with inflammatory cytokine levels. It is possible that smaller adipose tissue compartments, like the RS adipose tissue one, could be devoid of systemic inflammatory effects.
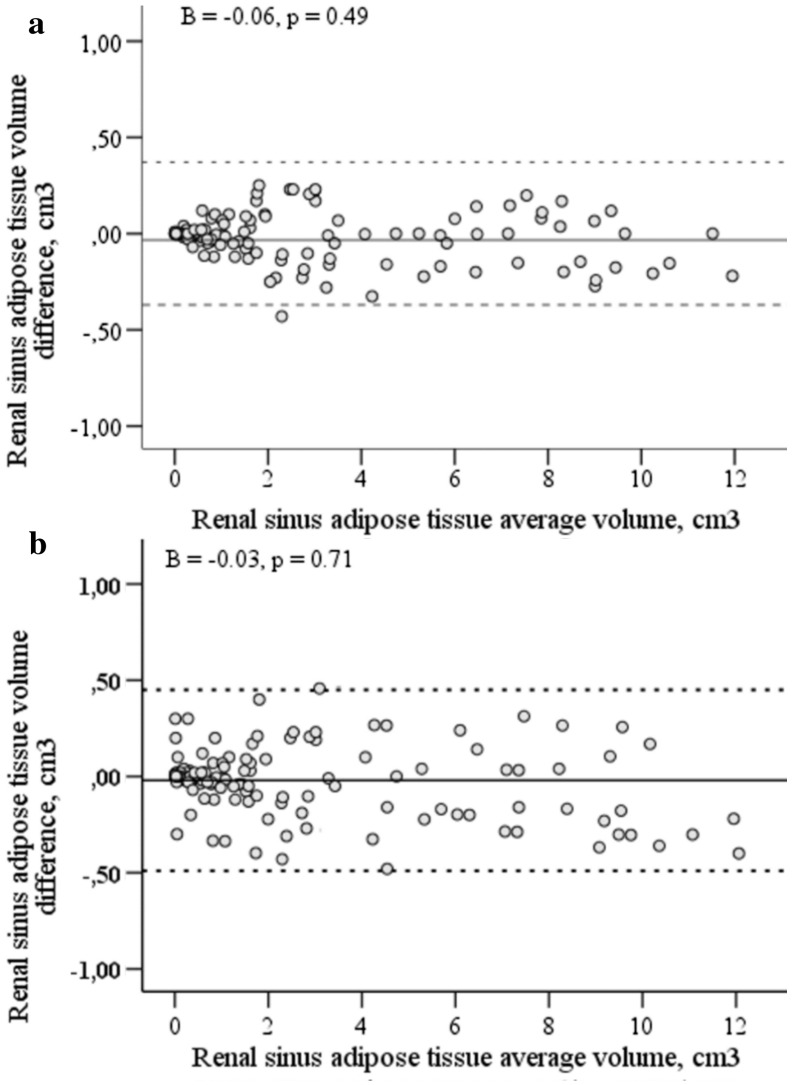
Intra-(Fig. 4a) and inter-(Fig. 4b) reader reproducibility of RS adipose tissue measurement are shown in the Bland-Altman plots. Overall, the mean difference between the repeated measurements was 0.001 cm2 with upper and lower confidence limits of 0.442 cm2 and − 0.430 cm2, respectively, suggesting minimal systematic bias between the intra-reader measurements. For the inter-reader measurements the mean difference between repeated measures was 0.005 cm2, with upper and lower confidence limits of 0.481 cm2 and − 0.497 cm2. Linear multiple regression analysis shows that there is no proportional bias in intra-(p = 0.49) or inter-reader (p = 0.71) measurements.
Fig. 4.
Bland and Altman plots of the intra-reader (a) and the inter-reader (b) renal sinus adipose tissue volume measurements. The average of the repeated measurements is presented on the x-axis and the difference between two measurements is presented on y-axis. The middle continuous line represents the mean difference between the repeated measures, upper and lower dotted lines represent upper and lower confidence limits for the mean difference, respectively (n = 150 (M/F, 75/75)). The level of significance was set as p < 0.05.
3.5. RS Adipose Tissue Association With Serum KIM-1 and FGF-21 Level
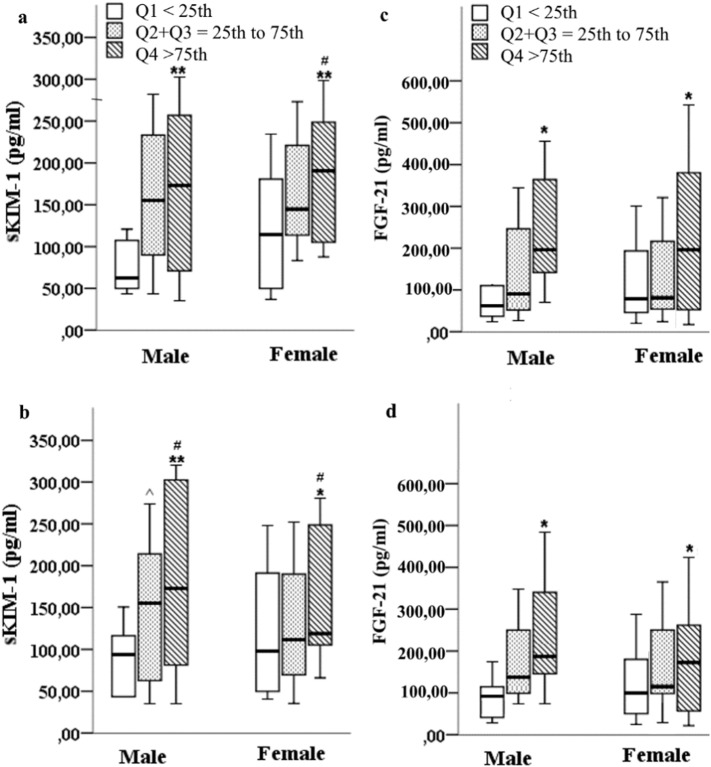
sKIM-1 and FGF-21 levels were significantly higher for the fatty kidney group compared to the control group (Table 1). After grouping of study participants into three groups according to left (Fig. 5a and c) or right (Fig. 5b and d) RS ratio quartiles sKIM-1 and FGF-21 levels were significantly (p < 0.05) higher in the Q4 (> 75th) quartile group for both males and females.
Fig. 5.
Serum KIM-1 and FGF-21 levels according to left (a and c) or right (b and d) RS ratio quartiles. Male (M) and female (F) were divided into 3 groups based on left or right RS ratio quartiles: < 25th quartile Q1 (F/M; 34/38); 25th–75th inter quartile range Q2 + Q3 (F/M; 62/60); > 75th quartile Q4 (F/M; 44/42). *Q1 vs. Q4; #Q2 + Q3 vs. Q4; ^Q1 vs. Q2 + Q3. *, #, ^p < 0.05; ⁎⁎p < 0.01; Mann-Whitney U test.
We also performed an age and gender adjusted stepwise multivariable linear regression analysis using sKIM-1 or FGF-21 level as a dependent variable and all abdominal adipose tissue compartments as well as BMI and WC as predictors. The significant association between sKIM-1 level and right RS ratio was lost (β = 0.06; p = 0.750) but there remained a persistent association between the left RS ratio and sKIM-1 level for both males (β = 0.43; 95% CI (0.19 to 0.65); R2 = 29%; p = 0.009) and females (β = 0.38; 95% CI (0.15 to 0.47); R2 = 26%; p = 0.023). Significant association between sKIM-1 level and abdominal adipose tissue segments (IP, RP and SC), BMI and WC were not found. Significant positive association between FGF-21 level and left RS ratio + RP adipose tissue was found for both males (β = 0.56; 95% CI (0.29 to 0.72); R2 = 30%; p = 0.009) and females (β = 0.61; 95% CI (0.24 to 0.77); R2 = 34%; p = 0.023). Additionally, eGFREPI cyst&crea negatively predicts FGF-21 concentration in multiple regression analyses (β = − 0.31; 95% CI (0.11 to 0.54); R2 = 34%; p = 0.023) for both genders (FGF-21 level as a dependent variable and eGFREPI cyst&crea as well as cystatin C and creatinine as predictors). There was no collinearity between independent variables: VIF < 5 and tolerance > 0.2.
3.6. Follow-up Study Results
There was no statistically significant benefit of text messaging intervention in obese participants. Table 4 represents general characteristics, abdominal adipose tissue measurements, FGF-21 and sKIM-1 levels for males and females at the baseline and the end of the follow-up study (age and gender adjusted p values were not significant (p > 0.05) – data not shown in the Table 4).
Table 4.
General characteristics of follow-up study participants and abdominal adipose tissue measurements (ntotal = 40).
| Measurements | Intervention (text message) |
No intervention |
||
|---|---|---|---|---|
| Baseline | End | Baseline | End | |
| n = 20 | n = 20 | n = 20 | n = 20 | |
| BMI, kg/m2 | 35.68 ± 4.31 | 33.24 ± 3.51 | 35.86 ± 6.88 | 34.77 ± 7.37 |
| WC, cm | 107.52 ± 11.37 | 103.30 ± 9.14 | 106.71 ± 12.58 | 102.04 ± 13.82 |
| RP, cm3 | 76.50 ± 35.02 | 74.78 ± 34.89 | 75.01 ± 30.15 | 74.51 ± 28.69 |
| IP, cm3 | 245.94 ± 75.90 | 238.44 ± 74.66 | 244.45 ± 69.91 | 231.06 ± 77.76 |
| SC, cm3 | 300.05 ± 132.99 | 280.56 ± 112.77 | 333.42 ± 174.61 | 275.88 ± 120.27 |
| Left RS, cm3 | 3.56 (2.96, 6.95) | 3.33 (2.78, 6.37) | 3.50 (1.25, 4.09) | 3.65 (1.03, 4.01) |
| Right RS, cm3 | 3.07 (1.00, 4.56) | 2.83 (1.24, 4.65) | 3.33 (0.39, 2.62) | 2.40 (0.37, 3.11) |
| sKIM-1, pg/ml | 150.37 (69.39, 290.96) | 137.61 (47.67, 238.83) | 159.54 (43.41, 197.10) | 147.91 (56.26, 184.13) |
| FGF-21, pg/ml | 198.41 (160.32, 475.06) | 177.25 (132.14, 412.11) | 209.52 (176.12, 499.27) | 180.33 (143.17, 398.19) |
Median (25th, 75 percentile) for skewed data; Mean with standard deviation (± SD) for normally distributed data. BMI - body mass index; WC - waist circumference; SC – subcutaneous adipose tissue; IP – intraperitoneal adipose tissue; RP – retroperitoneal adipose tissue; Left RS- left renal sinus adipose tissue; Right RS – right renal sinus adipose tissue; sKIM-1 – serum kidney injury molecule-1; FGF-21 - fibroblast growth factor-21.
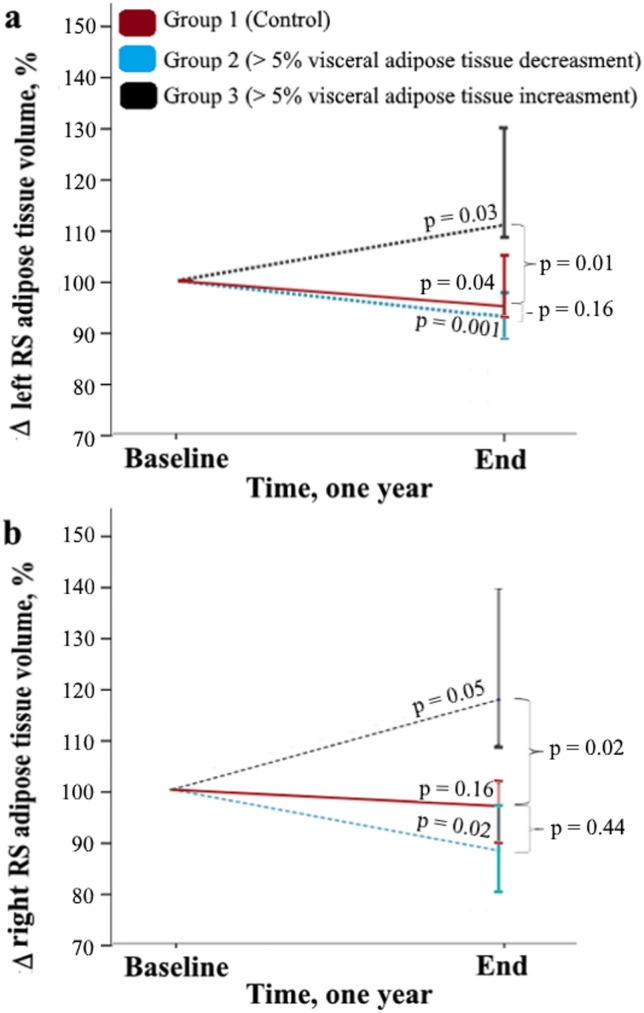
Main results of the one year follow-up study show that, compared to the control group, significant (> 5%) increase in total amount of visceral adipose tissue also induce significant (p < 0.05) increase in amount of adipose tissue in the left (Fig. 6a) or the right (Fig. 6b) RS. However, when significant (> 5%) decrease in total amount of visceral adipose tissue is observed, there is not significant (p > 0.05) decrease in amount of adipose tissue in the left (Fig. 6a) or the right (Fig. 6b) RS. In general the right and the left RS adipose tissue are positively associated with visceral adipose tissue amount (Table 5).
Fig. 6.
Left (a) and right (b) renal sinus adipose tissue volumes changes (%) in the one year follow-up period, based on the visceral adipose tissue significant (> 5%) increase or decrease. The mean value of the time point “Baseline” was arbitrarily set at 100%. p Value shown above the black, blue and red lines represent difference between “Baseline” and “End” (Wilcoxon rank-sum test). p Value which are indicated under the brace represent difference between control group (Mann Whitney U test). The level of significance was set as p < 0.05.
Table 5.
Age and gender adjusted partial Person correlations (r) between the left and the right renal sinus adipose tissue and visceral adipose tissue in the one year follow-up study (ntotal = 40).
| Visceral adipose tissue Baseline, cm3 | Visceral adipose tissue End, cm3 | ΔVisceral adipose tissue, % | |
|---|---|---|---|
| Right RS Baseline, cm3 | 0.82⁎⁎⁎ | ||
| Left RS Baseline, cm3 | 0.79⁎⁎⁎ | ||
| Right RS End, cm3 | 0.76⁎⁎⁎ | ||
| Left RS End, cm3 | 0.78⁎⁎⁎ | ||
| ΔRight RS, % | 0.65⁎⁎⁎ | ||
| ΔLeft RS, % | 0.71⁎⁎⁎ |
Δ – percentage change from baseline; Left RS - left renal sinus adipose tissue; Right RS – right renal sinus adipose tissue.
p < 0.001.
The aim of the follow-up study was to confirm the cross-sectional study data: whether sKIM-1 and FGF-21 levels are associated with the change of RS adipose tissue or abdominal adipose tissue amount. Therefore, we calculated percentage change from baseline for all the adipose tissue segments (ΔSC, ΔIP, ΔRP, Δleft RS and Δright RS adipose tissue) as well as for sKIM-1 and FGF-21 level (ΔsKIM-1 and ΔFGF-21). RS adipose tissue volume shows no significant (p > 0.05) correlation with the corresponding (right or left) kidney volume for neither gender in the cross-sectional study, therefore, RS adipose tissue volumes were taken for analysis. ΔsKIM-1 shows significant positive Spearman correlation with the Δleft and the Δright RS adipose tissue. Accordingly, both the cross-sectional and the one year follow-up study indicates positive association between sKIM-1 level and increase in amount of adipose tissue in the renal sinus. The abdominal adipose tissue segments (ΔSC, ΔIP, ΔRP) did not show significant association with change in ΔsKIM-1 serum level (Table 6). ΔFGF-21shows significant positive Spearman correlation with the Δleft RS and RP adipose tissue amount. These results also confirms cross-sectional study results.
Table 6.
Spearman correlations between percentage change from baseline for the all adipose tissue segments (ΔSC, ΔIP, ΔRP, Δleft RS and Δright RS adipose tissue), sKIM-1 (ΔsKIM-1) and FGF-21 (ΔFGF-21) level.
| ΔsKIM-1, % | ΔFGF-21, % | ||
|---|---|---|---|
| ΔRP,% | r | 0.19 | 0.61 |
| p | 0.609 | 0.002 | |
| ΔIP,% | r | 0.21 | 0.14 |
| p | 0.510 | 0.110 | |
| ΔSC, % | r | 0.16 | 0.14 |
| p | 0.667 | 0.091 | |
| ΔLeft RS,% | r | 0.69 | 0.47 |
| p | 0.031 | 0.044 | |
| ΔRight RS, % | r | 0.61 | 0.36 |
| p | 0.039 | 0.051 |
Δ – percentage change from baseline; SC – subcutaneous adipose tissue; IP – intraperitoneal adipose tissue; RP – retroperitoneal adipose tissue; Left RS - left renal sinus adipose tissue; Right RS – right renal sinus adipose tissue; sKIM-1 – serum kidney injury molecule-1; FGF-21 – fibroblast growth factor-21. The level of significance was set as p < 0.05.
4. Discussion
To the best of our knowledge findings reported in this study add some aspects to the function of RS adipose tissue. First, results indicate asymmetrical deposition of adipose tissue into the RS even after corresponding kidney volume adjustment in both males and females. Second, the cross-sectional and the follow-up studies shows that sKIM-1 level is positively associated with RS adipose tissue volume increase for both genders. Third, FGF-21 is associated with RS and RP adipose tissue amount and shows inverse association with GFR. Fourth, follow up study shows that accumulation of adipose tissue in the RS is related with the visceral adipose tissue volume increase. Additionally, significant visceral adipose tissue volume increase is related with significant increase of adipose tissue amount in the RS. However, significant visceral adipose tissue volume decrease does not show significant decrease of adipose tissue amount in the RS. From this we can draw a hypothesis that the RS adipose tissue accumulation happens faster than elimination.
Interestingly, that the left RS accumulate significantly more adipose tissue than the right RS for both genders. Moreover, we did not find association between kidney size and RS adipose tissue volume. To the best of our knowledge, this anatomical phenomenon previously has not been described. One of the reason could be, that since the object segmentation and 3D reconstruction of RS adipose tissue compartment is labor-intensive and time-consuming, often chosen strategy is CT or MR single slice cross-sectional area measurement at a specific anatomic level (Foster et al., 2011a, Chughtai et al., 2010, Wagner et al., 2012) or RS adipose tissue volume detection only in one kidney (Foster et al., 2011a, Foster et al., 2011b). This way data processing time is significantly optimized, however, this also results in loss of measurement quality. Based on the results can be said that the RS adipose tissue compartments are a diffuse object that have “drop-like” form and are located between major and minor renal calices. Therefore, using only one scan could lead to inaccurate result that does not represent total amount of adipose tissue in the RS. Also fact that normal humans have two kidneys, therefore, two RS, must be taken into account when making measurements. Since humans are not bilaterally symmetrical (especially when talking about abdominal organs) and kidneys have both functional as well as anatomical asymmetry described, then it is important to analyze both RS.
We speculate that, first, asymmetrical RS adipose tissue accumulation could be result of anatomical differences between left and right renal vein. The right renal vein receives blood only from the right kidney, but the left renal vein receives left gonadal and adrenal veins in addition to the vein coming from the left kidney (Anjamrooz et al., 2012). There is no difference in diameter between the left and the right renal vein (Satyapal et al., 1995) but possibly this asymmetry predispose the left RS for higher RS adipose tissue deposition. Second, there is wide variation in calyceal orientation (Miller et al., 2013) According to the Kaye and Reinke results (Kaye and Reinke, 1984), the calyceal angles in the right and the left kidney differs.
The concept of anatomical, physiological, and functional asymmetry between kidneys has been a matter of interest for researchers. Clinicians recommend that functional asymmetry of the kidneys should be investigated preoperatively to determine which kidney should be donated and transplanted (Salehipour et al., 2008). Although, we did not find information about asymmetric RS deposition, considerable differences in renal blood flow have been reported between the right and the left kidneys in some previous investigations. Van Onna and co-authors showed that asymmetry of renal blood flow was present in up to 51% of hypertensive patients. Moreover, mean left renal blood flow was on average significantly lower than the right mean renal blood flow (van Onna et al., 2002). Similar, Caralps et al. reported that in 10 out of 11 asymmetry cases the left kidney was more affected than the right one (Caralps et al., 1974). Van Onna and co-authors showed that there is no association between kidney volume and asymmetry of renal blood flow in their study subjects (van Onna et al., 2002). We hypothesized that significant higher RS adipose tissue deposition in the left kidney possibly could be inborn structural phenomenon that might cause asymmetrical renal blood flow.
Results of this study show that sKIM-1 level is positively associated with the RS adipose tissue volume increase for both males and females. In normal kidney proximal tubules KIM-1 are expressed at a very low level (Ichimura et al., 2004). However, its expression is significantly increased in the post-ischemic kidney (Ichimura et al., 1998). Jin and co-authors in their study detected sKIM-1 level (126.2 ± 53.5 pg/ml) for healthy middle age control subjects (Jin et al., 2013). Our study showed comparable average sKIM-1 concentrations for females and males in RS ratio quartile Q1 (63.56 (41.67 to 98.11) pg/ml and 79.20 (41.67 to 111.55) pg/ml, respectively). However, significant sKIM-1 level increase in the RS ratio quartile Q4 was observed. We propose that one of the initial mechanisms for obesity-induced renal damage and hypertension could be linked to proximal convoluted tubule epithelial cell damage caused by RS adipose tissue produced compression of intrarenal collecting system, blood and lymph vessel. As the left RS accumulate significantly more adipose tissues than the right RS, possibly, early changes could be observed directly in the left kidney. Together these findings indicate that there is some structural and functional asymmetry between deposition of adipose tissue in the right and the left RS.
In agreement with other studies (Stein et al., 2009, Zhang et al., 2015, Lee et al., 2015) serum levels of the FGF-21 depend on renal functions because renal elimination is a major route by which physiological FGF-21 serum levels are maintained. Like other researches (Stein et al., 2009, Lee et al., 2015) we found that GFR is significantly and inversely associated with circulating FGF-21 level. Furthermore, the cross-sectional and follow-up study confirms that FGF-21 level was positively associated with the RS and RP adipose tissue amount. We hypothesized that this association could be due to brown adipose tissue accumulation, respectively, RS and RP adipose tissues has different morphological profile compared with the IP and SC adipose tissues. Brown adipose tissue presence in the perirenal adipose tissue compartment was confirmed with biopsies from healthy kidney donors (Svensson et al., 2014) and with a positron emission tomography/CT investigations (Nedergaard et al., 2007). Ouellet et al. confirmed brown adipose tissue presence in perirenal fat compartment in a large cohort of patients (n = 4842) (Ouellet et al., 2011). Another research on mice suggested that perirenal adipose tissue compartment contains a mixture of brown and white adipose tissue (Fisher et al., 2012). Accumulation of brown adipose tissue in RS could induce compression of various renal structures described before (Montani et al., 2004, Foster et al., 2011b, Chughtai et al., 2010). However, effect of brown adipose tissue on serum lipoprotein level could be favorable. This is consistent with our data because fatty kidney group showed significantly lower LDLC level. Bartelt et al. identified brown adipose tissue as a major organ involved in plasma very light density lipoprotein triglycerides clearance in mice. Cold exposure activated brown adipose tissue significantly lowered level of TG. However, HDLC level slightly increased, which is probably explained by an increase of TG-rich lipoproteins derived HDLC precursors. For further studies we have to detect serum level of uncoupling protein-1 – a marker of brown adipose tissue.
Our study has limitations. First, we analyzed only serum KIM-1. There are studies that reflect serum KIM-1 as blood marker for early kidney injury detection as mentioned before, however, detection of urine KIM-1 could give more precise results. Second, relatively small number of individuals were included in the follow-up study. Strengths of our study are that volumetric analysis of various adipose tissue segments was carried out. Amount of adipose tissue in the RS was standardized by using corresponding kidney, thus excluding possibility that size of the kidney influence amount of adipose tissue in the RS. Moreover, our results show that it is important to measure the left and the right RS adipose tissue volumes because of significant asymmetrical RS adipose tissue deposition. The research group was selected so that both genders and all BMI groups were evenly represented.
Regardless of gender adipose tissue in RS accumulates asymmetrically – the left RS accumulates a significantly higher amount of adipose tissue. Thus, primarily RS adipose tissue effects should be assessed on the left kidney. Accumulation of adipose tissue in the RS is related with the visceral adipose amount and KIM-1 concentration increase in the blood serum. Additionally, FGF-21 level is positively associated with the RS and RP adipose tissue amount.
Funding
Research relating to this abstract were funded by the European Social Fund within the project “Innovative technologies for acquisition and processing of biomedical images” No.2013/0009/1DP/1.1.1.2.0/13/APIA/VIAA/014, European Social Fund within the project “Support for Doctoral Studies at University of Latvia”, and by the grant No. 2014.10-4/VPP-5/16 BIOMEDICINE of the framework of the Latvian National Program. These funding sources had no role in the study design; collection, analysis and interpretation of data; writing of the manuscript; and decision to submit the manuscript for publication.
Author Contributions
Research idea and study design: G. Krievina and P. Tretjakovs; Data acquisition: G. Krievina, I. Skuja, V. Silina, D. Krievina, and L. Keisa; Data collection/analysis: G. Krievina, I. Skuja, and V. Silina; Data interpretation: G. Krievina, P. Tretjakovs, D. Krievina, and G. Bahs; Manuscript writing: G. Krievina, P. Tretjakovs, I. Skuja, V. Silina, D. Krievina, L. Keisa, and G. Bahs
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.10.020.
Appendix A. Supplementary data
Supplementary tables
References
- Anjamrooz S.H., Azari H., Abedinzadeh M. Abnormal patterns of the renal veins. Anat. Cell. Biol. 2012;45:57–61. doi: 10.5115/acb.2012.45.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorndal B., Burri L., Staalesen V., Skorve J., Berge R.K. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J. Obes. 2011;2011:490650. doi: 10.1155/2011/490650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre J.V. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol. Dial. Transplant. 2009;24:3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- Bonventre J.V., Vaidya V.S., Schmouder R., Feig P., Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nat. Biotechnol. 2010;28:436–440. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breau R.H., Clark E., Bruner B., Cervini P., Atwell T., Knoll G., Leibovich B.C. A simple method to estimate renal volume from computed tomography. Can. Urol. Assoc. J. 2013;7:189–192. doi: 10.5489/cuaj.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caralps A., Rius J.M., Vilardell M., Magrina N., Sarrias J., Brulles A. Asymmetrical interlobar nephrosclerosis. Lancet. 1974;1:534–536. doi: 10.1016/s0140-6736(74)92716-0. [DOI] [PubMed] [Google Scholar]
- Chughtai H.L., Morgan T.M., Rocco M., Stacey B., Brinkley T.E., Ding J., Nicklas B., Hamilton C., Hundley W.G. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension. 2010;56:901–906. doi: 10.1161/HYPERTENSIONAHA.110.157370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterle F., Sistare F., GOODSAID F., Papaluca M., Ozer J.S., Webb C.P., Baer W., Senagore A., Schipper M.J., Vonderscher J., Sultana S., Gerhold D.L., Phillips J.A., Maurer G., Carl K., Laurie D., Harpur E., Sonee M., Ennulat D., Holder D., Andrews-Cleavenger D., Gu Y.Z., Thompson K.L., Goering P.L., Vidal J.M., Abadie E., Maciulaitis R., Jacobson-Kram D., Defelice A.F., Hausner E.A., Blank M., Thompson A., Harlow P., Throckmorton D., Xiao S., Xu N., Taylor W., Vamvakas S., Flamion B., Lima B.S., Kasper P., Pasanen M., Prasad K., Troth S., Bounous D., Robinson-Gravatt D., Betton G., Davis M.A., Akunda J., Mcduffie J.E., Suter L., Obert L., Guffroy M., Pinches M., Jayadev S., Blomme E.A., Beushausen S.A., Barlow V.G., Collins N., Waring J., Honor D., Snook S., Lee J., Rossi P., Walker E., Mattes W. Renal biomarker qualification submission: a dialog between the FDA-EMEA and predictive safety testing consortium. Nat. Biotechnol. 2010;28:455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- Eckel R.H., Jakicic J.M., Ard J.D., De Jesus J.M., Houston Miller N., Hubbard V.S., Lee I.M., Lichtenstein A.H., Loria C.M., Millen B.E., Nonas C.A., Sacks F.M., Smith J.S.C., Svetkey L.P., Wadden T.A., Yanovski S.Z. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J. Am. Coll. Cardiol. 2014;63 doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F., Wu J., Kharitonenkov A., Flier J.S., Maratos-Flier E., Spiegelman B.M. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M.C., Hwang S.J., Porter S.A., Massaro J.M., Hoffmann U., Fox C.S. Development and reproducibility of a computed tomography-based measurement of renal sinus fat. BMC Nephrol. 2011;12:52. doi: 10.1186/1471-2369-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M.C., Hwang S.J., Porter S.A., Massaro J.M., Hoffmann U., Fox C.S. Fatty kidney, hypertension, and chronic kidney disease: the Framingham heart study. Hypertension. 2011;58:784–790. doi: 10.1161/HYPERTENSIONAHA.111.175315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glodny B., Unterholzner V., Taferner B., Hofmann K.J., Rehder P., Strasak A., Petersen J. Normal kidney size and its influencing factors - a 64-slice MDCT study of 1.040 asymptomatic patients. BMC Urol. 2009;9:19. doi: 10.1186/1471-2490-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.E. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am. J. Hypertens. 1997;10:49S–55S. [PubMed] [Google Scholar]
- Hall J.E., Brands M.W., Henegar J.R. Angiotensin II and long-term arterial pressure regulation: the overriding dominance of the kidney. J. Am. Soc. Nephrol. 1999;10(Suppl 12):S258–S265. [PubMed] [Google Scholar]
- Hall J.E., Crook E.D., Jones D.W., Wofford M.R., Dubbert P.M. Mechanisms of obesity-associated cardiovascular and renal disease. Am. J. Med. Sci. 2002;324:127–137. doi: 10.1097/00000441-200209000-00003. [DOI] [PubMed] [Google Scholar]
- Hall J.E., Da Silva A.A., Do Carmo J.M., Dubinion J., Hamza S., Munusamy S., Smith G., Stec D.E. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J. Biol. Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M.E., Do Carmo J.M., Da Silva A.A., Juncos L.A., Wang Z., Hall J.E. Obesity, hypertension, and chronic kidney disease. Int. J. Nephrol. Renov. Dis. 2014;7:75–88. doi: 10.2147/IJNRD.S39739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.E., Do Carmo J.M., Da Silva A.A., Wang Z., Hall M.E. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ. Res. 2015;116:991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer M. Thieme; 2010. CT Teaching Manual: A Systematic Approach to CT Reading. [Google Scholar]
- Humphreys B.D., Xu F., Sabbisetti V., Grgic I., Naini S.M., Wang N., Chen G., Xiao S., Patel D., Henderson J.M., Ichimura T., Mou S., Soeung S., Mcmahon A.P., Kuchroo V.K., Bonventre J.V. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J. Clin. Invest. 2013;123:4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura T., Bonventre J.V., Bailly V., Wei H., Hession C.A., Cate R.L., Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- Ichimura T., Hung C.C., Yang S.A., Stevens J.L., Bonventre J.V. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Ren. Physiol. 2004;286:F552–F563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- Inker L.A., Schmid C.H., Tighiouart H., Eckfeldt J.H., Feldman H.I., Greene T., Kusek J.W., Manzi J., Van Lente F., Zhang Y.L., Coresh J., Levey A.S., Investigators C.-E. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z.K., Tian P.X., Wang X.Z., Xue W.J., Ding X.M., Zheng J., Ding C.G., Mao T.C., Duan W.L., Xi M. Kidney injury molecule-1 and osteopontin: new markers for prediction of early kidney transplant rejection. Mol. Immunol. 2013;54:457–464. doi: 10.1016/j.molimm.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Kaye K.W., Reinke D.B. Detailed caliceal anatomy for endourology. J. Urol. 1984;132:1085–1088. doi: 10.1016/s0022-5347(17)50042-7. [DOI] [PubMed] [Google Scholar]
- Kovesdy C.P., Czira M.E., Rudas A., Ujszaszi A., Rosivall L., Novak M., Kalantar-Zadeh K., Molnar M.Z., Mucsi I. Body mass index, waist circumference and mortality in kidney transplant recipients. Am. J. Transplant. 2010;10:2644–2651. doi: 10.1111/j.1600-6143.2010.03330.x. [DOI] [PubMed] [Google Scholar]
- Kuwata K., Nakamura I., Ide M., Sato H., Nishikawa S., Tanaka M. Comparison of changes in urinary and blood levels of biomarkers associated. J. Toxicol. Pathol. 2015;28:151–164. doi: 10.1293/tox.2014-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamacchia O., Nicastro V., Camarchio D., Valente U., Grisorio R., Gesualdo L., Cignarelli M. Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol. Dial. Transplant. 2011;26:892–898. doi: 10.1093/ndt/gfq522. [DOI] [PubMed] [Google Scholar]
- Lee C.H., Hui E.Y., Woo Y.C., Yeung C.Y., Chow W.S., Yuen M.M., Fong C.H., Xu A., Lam K.S. Circulating fibroblast growth factor 21 levels predict progressive kidney disease in subjects with type 2 diabetes and normoalbuminuria. J. Clin. Endocrinol. Metab. 2015;100:1368–1375. doi: 10.1210/jc.2014-3465. [DOI] [PubMed] [Google Scholar]
- Lim S., Meigs J.B. Ectopic fat and cardiometabolic and vascular risk. Int. J. Cardiol. 2013;169:166–176. doi: 10.1016/j.ijcard.2013.08.077. [DOI] [PubMed] [Google Scholar]
- Miller J., Durack J.C., Sorensen M.D., Wang J.H., Stoller M.L. Renal calyceal anatomy characterization with 3-dimensional in vivo computerized tomography imaging. J. Urol. 2013;189:562–567. doi: 10.1016/j.juro.2012.09.040. [DOI] [PubMed] [Google Scholar]
- Montani J.P., Carroll J.F., Dwyer T.M., Antic V., Yang Z., Dulloo A.G. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int. J. Obes. Relat. Metab. Disord. 2004;28(Suppl 4):S58–S65. doi: 10.1038/sj.ijo.0802858. [DOI] [PubMed] [Google Scholar]
- Nedergaard J., Bengtsson T., Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Ouellet V., Routhier-Labadie A., Bellemare W., Lakhal-Chaieb L., Turcotte E., Carpentier A.C., Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J. Clin. Endocrinol. Metab. 2011;96:192–199. doi: 10.1210/jc.2010-0989. [DOI] [PubMed] [Google Scholar]
- Sabbisetti V.S., Waikar S.S., Antoine D.J., Smiles A., Wang C., Ravisankar A., Ito K., Sharma S., Ramadesikan S., Lee M., Briskin R., De Jager P.L., Ngo T.T., Radlinski M., Dear J.W., Park K.B., Betensky R., Krolewski A.S., Bonventre J.V. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J. Am. Soc. Nephrol. 2014;25:2177–2186. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehipour M., Bahador A., Jalaeian H., Salahi H., Nikeghbalian S., Khajehee F., Malek-Hosseini S.A. Comparison of right and left grafts in renal transplantation. Saudi J. Kidney Dis. Transpl. 2008;19:222–226. [PubMed] [Google Scholar]
- Satyapal K.S., Rambiritcj V., Pillai G. Morphometric analysis of the renal veins. Anat. Rec. 1995;241:268–272. doi: 10.1002/ar.1092410213. [DOI] [PubMed] [Google Scholar]
- Stein S., Bachmann A., Lössner U., Kratzsch J., Blüher M., Stumvoll M., Fasshauer M. Serum levels of the adipokine FGF21 depend on renal function. Diabetes Care. 2009;32:126–128. doi: 10.2337/dc08-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson P.A., Lindberg K., Hoffmann J.M., Taube M., Pereira M.J., Mohsen-Kanson T., Hafner A.L., Rizell M., Palming J., Dani C., Svensson M.K. Characterization of brown adipose tissue in the human perirenal depot. Obesity (Silver Spring) 2014;22:1830–1837. doi: 10.1002/oby.20765. [DOI] [PubMed] [Google Scholar]
- Szczepaniak E.W., Malliaras K., Nelson M.D., Szczepaniak L.S. Measurement of pancreatic volume by abdominal MRI: a validation study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Onna M., Houben A.J.H.M., Kroon A.A., Wierema T.K.A., Koster D., Van Engelshoven J.M.A., De Leeuw P.W. Asymmetry of renal blood flow in patients with moderate to severe hypertension. Hypertension. 2002;41:108–113. doi: 10.1161/01.hyp.0000050928.96979.a5. [DOI] [PubMed] [Google Scholar]
- Wagner R., Machann J., Lehmann R., Rittig K., Schick F., Lenhart J., Artunc F., Linder K., Claussen C.D., Schleicher E., Fritsche A., Haring H.U., Weyrich P. Exercise-induced albuminuria is associated with perivascular renal sinus fat in individuals at increased risk of type 2 diabetes. Diabetologia. 2012;55:2054–2058. doi: 10.1007/s00125-012-2551-z. [DOI] [PubMed] [Google Scholar]
- Zhang F., Yu L., Lin X., Cheng P., He L., Li X., Lu X., Tan Y., Yang H., Cai L., Zhang C. Minireview: roles of fibroblast growth factors 19 and 21 in metabolic regulation and chronic diseases. Mol. Endocrinol. 2015;29:1400–1413. doi: 10.1210/me.2015-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables