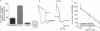Abstract
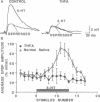
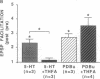
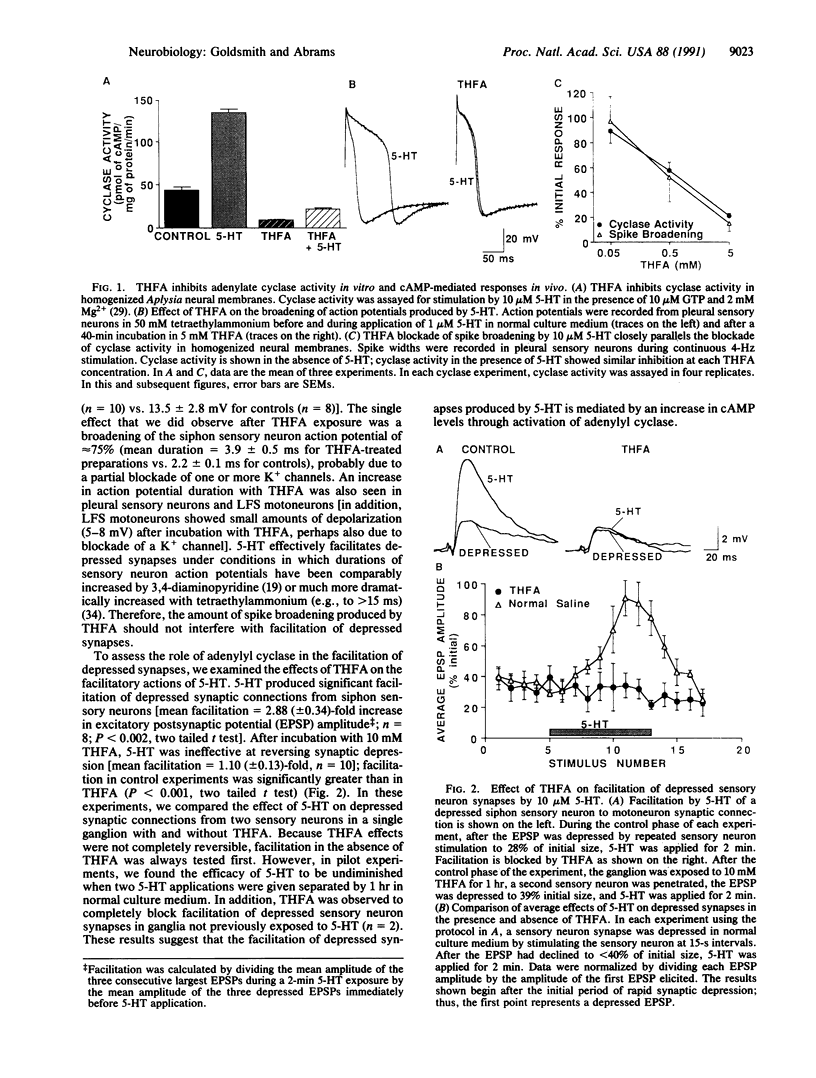
Facilitation of the monosynaptic connection between siphon sensory neurons and gill and siphon motor neuron contributes to sensitization and dishabituation of the gill and siphon withdrawal reflex in Aplysia. The facilitatory transmitter serotonin (5-HT) initiates two mechanisms that act in parallel to increase transmitter release from siphon sensory neurons. 5-HT acts, at least partly through cAMP, to broaden the presynaptic action potential. 5-HT also initiates a second process that facilitates depressed sensory neuron synapses by a mechanism independent of changes in action potential duration. Recent experiments indicated that either of two protein kinases, cAMP-dependent protein kinase A and protein kinase C, are capable of effectively activating this second facilitatory mechanism, restoring synaptic transmission in depressed synapses. We have used the adenylyl cyclase inhibitor SQ 22,536 [9-(tetrahydro-2-furyl)adenine or THFA] to explore the contribution of cAMP to the reversal of synaptic depression. THFA effectively inhibited both adenylyl cyclase activity in vitro and known cyclase-mediated effects in intact sensory neurons. THFA also completely blocked facilitation of depressed synapses by 5-HT. These results suggest that adenylyl cyclase plays a critical role in the reversal of synaptic depression that contributes to dishabituation in this system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams T. W., Castellucci V. F., Camardo J. S., Kandel E. R., Lloyd P. E. Two endogenous neuropeptides modulate the gill and siphon withdrawal reflex in Aplysia by presynaptic facilitation involving cAMP-dependent closure of a serotonin-sensitive potassium channel. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7956–7960. doi: 10.1073/pnas.81.24.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter D. A., Byrne J. H. Serotonergic modulation of two potassium currents in the pleural sensory neurons of Aplysia. J Neurophysiol. 1989 Sep;62(3):665–679. doi: 10.1152/jn.1989.62.3.665. [DOI] [PubMed] [Google Scholar]
- Belardetti F., Biondi C., Brunelli M., Fabri M., Trevisani A. Heterosynaptic facilitation and behavioral sensitization are inhibited by lowering endogenous cAMP in Aplysia. Brain Res. 1983 Dec 12;288(1-2):95–104. doi: 10.1016/0006-8993(83)90084-7. [DOI] [PubMed] [Google Scholar]
- Bernier L., Castellucci V. F., Kandel E. R., Schwartz J. H. Facilitatory transmitter causes a selective and prolonged increase in adenosine 3':5'-monophosphate in sensory neurons mediating the gill and siphon withdrawal reflex in Aplysia. J Neurosci. 1982 Dec;2(12):1682–1691. doi: 10.1523/JNEUROSCI.02-12-01682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H., Spira M. E., Kandel E. R., Siegelbaum S. A. Facilitatory and inhibitory transmitters modulate calcium influx during action potentials in aplysia sensory neurons. Neuron. 1990 Oct;5(4):487–499. doi: 10.1016/0896-6273(90)90088-w. [DOI] [PubMed] [Google Scholar]
- Braha O., Dale N., Hochner B., Klein M., Abrams T. W., Kandel E. R. Second messengers involved in the two processes of presynaptic facilitation that contribute to sensitization and dishabituation in Aplysia sensory neurons. Proc Natl Acad Sci U S A. 1990 Mar;87(5):2040–2044. doi: 10.1073/pnas.87.5.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli M., Castellucci V., Kandel E. R. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976 Dec 10;194(4270):1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Davies N. W., Lux H. D., Morad M. Site and mechanism of activation of proton-induced sodium current in chick dorsal root ganglion neurones. J Physiol. 1988 Jun;400:159–187. doi: 10.1113/jphysiol.1988.sp017116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D., Atwood H. L. Adenylate cyclase system is essential for long-term facilitation at the crayfish neuromuscular junction. J Neurosci. 1989 Dec;9(12):4246–4252. doi: 10.1523/JNEUROSCI.09-12-04246.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc B., Castellucci V. F. Receptive fields and properties of a new cluster of mechanoreceptor neurons innervating the mantle region and the branchial cavity of the marine mollusk Aplysia californica. J Exp Biol. 1991 Mar;156:315–334. doi: 10.1242/jeb.156.1.315. [DOI] [PubMed] [Google Scholar]
- Edmonds B., Klein M., Dale N., Kandel E. R. Contributions of two types of calcium channels to synaptic transmission and plasticity. Science. 1990 Nov 23;250(4984):1142–1147. doi: 10.1126/science.2174573. [DOI] [PubMed] [Google Scholar]
- Eppler C. M., Bayley H., Greenberg S. M., Schwartz J. H. Structural studies on a family of cAMP-binding proteins in the nervous system of Aplysia. J Cell Biol. 1986 Jan;102(1):320–331. doi: 10.1083/jcb.102.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost W. N., Clark G. A., Kandel E. R. Parallel processing of short-term memory for sensitization in Aplysia. J Neurobiol. 1988 Jun;19(4):297–334. doi: 10.1002/neu.480190402. [DOI] [PubMed] [Google Scholar]
- Gingrich K. J., Byrne J. H. Simulation of synaptic depression, posttetanic potentiation, and presynaptic facilitation of synaptic potentials from sensory neurons mediating gill-withdrawal reflex in Aplysia. J Neurophysiol. 1985 Mar;53(3):652–669. doi: 10.1152/jn.1985.53.3.652. [DOI] [PubMed] [Google Scholar]
- Glanzman D. L., Mackey S. L., Hawkins R. D., Dyke A. M., Lloyd P. E., Kandel E. R. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci. 1989 Dec;9(12):4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. M., Bernier L., Schwartz J. H. Distribution of cAMP and cAMP-dependent protein kinases in Aplysia sensory neurons. J Neurosci. 1987 Jan;7(1):291–301. doi: 10.1523/JNEUROSCI.07-01-00291.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. N., Asaad M. M., Phillips M. B., Goldenberg H. J., Antonaccio M. J. Inhibition of adenylate cyclase in human blood platelets by 9-substituted adenine derivatives. J Cyclic Nucleotide Res. 1979;5(2):125–134. [PubMed] [Google Scholar]
- Hochner B., Klein M., Schacher S., Kandel E. R. Additional component in the cellular mechanism of presynaptic facilitation contributes to behavioral dishabituation in Aplysia. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8794–8798. doi: 10.1073/pnas.83.22.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- JASPER H., SHARPLESS S. Habituation of the arousal reaction. Brain. 1956 Dec;79(4):655–680. doi: 10.1093/brain/79.4.655. [DOI] [PubMed] [Google Scholar]
- Klein M., Camardo J., Kandel E. R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Mechanism of calcium current modulation underlying presynaptic facilitation and behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6912–6916. doi: 10.1073/pnas.77.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Shapiro E., Kandel E. R. Synaptic plasticity and the modulation of the Ca2+ current. J Exp Biol. 1980 Dec;89:117–157. doi: 10.1242/jeb.89.1.117. [DOI] [PubMed] [Google Scholar]
- Kruger K. E., Sossin W. S., Sacktor T. C., Bergold P. J., Beushausen S., Schwartz J. H. Cloning and characterization of Ca(2+)-dependent and Ca(2+)-independent PKCs expressed in Aplysia sensory cells. J Neurosci. 1991 Aug;11(8):2303–2313. doi: 10.1523/JNEUROSCI.11-08-02303.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S. L., Kandel E. R., Hawkins R. D. Identified serotonergic neurons LCB1 and RCB1 in the cerebral ganglia of Aplysia produce presynaptic facilitation of siphon sensory neurons. J Neurosci. 1989 Dec;9(12):4227–4235. doi: 10.1523/JNEUROSCI.09-12-04227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Ocorr K. A., Byrne J. H. Membrane responses and changes in cAMP levels in Aplysia sensory neurons produced by serotonin, tryptamine, FMRFamide and small cardioactive peptideB (SCPB). Neurosci Lett. 1985 Apr 9;55(2):113–118. doi: 10.1016/0304-3940(85)90004-7. [DOI] [PubMed] [Google Scholar]
- Rankin C. H., Carew T. J. Dishabituation and sensitization emerge as separate processes during development in Aplysia. J Neurosci. 1988 Jan;8(1):197–211. doi: 10.1523/JNEUROSCI.08-01-00197.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor T. C., Schwartz J. H. Sensitizing stimuli cause translocation of protein kinase C in Aplysia sensory neurons. Proc Natl Acad Sci U S A. 1990 Mar;87(5):2036–2039. doi: 10.1073/pnas.87.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S., Castellucci V. F., Kandel E. R. cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science. 1988 Jun 17;240(4859):1667–1669. doi: 10.1126/science.2454509. [DOI] [PubMed] [Google Scholar]
- Schacher S., Montarolo P., Kandel E. R. Selective short- and long-term effects of serotonin, small cardioactive peptide, and tetanic stimulation on sensorimotor synapses of Aplysia in culture. J Neurosci. 1990 Oct;10(10):3286–3294. doi: 10.1523/JNEUROSCI.10-10-03286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz K. P., Byrne J. H. Intracellular injection of cAMP induces a long-term reduction of neuronal K+ currents. Science. 1988 Jun 17;240(4859):1664–1666. doi: 10.1126/science.2837826. [DOI] [PubMed] [Google Scholar]
- Shuster M. J., Camardo J. S., Siegelbaum S. A., Kandel E. R. Cyclic AMP-dependent protein kinase closes the serotonin-sensitive K+ channels of Aplysia sensory neurones in cell-free membrane patches. 1985 Jan 31-Feb 6Nature. 313(6001):392–395. doi: 10.1038/313392a0. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Sweatt J. D., Kandel E. R. Persistent and transcriptionally-dependent increase in protein phosphorylation in long-term facilitation of Aplysia sensory neurons. Nature. 1989 May 4;339(6219):51–54. doi: 10.1038/339051a0. [DOI] [PubMed] [Google Scholar]
- Thompson R. F., Spencer W. A. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966 Jan;73(1):16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Yovell Y., Kandel E. R., Dudai Y., Abrams T. W. Biochemical correlates of short-term sensitization in Aplysia: temporal analysis of adenylate cyclase stimulation in a perfused-membrane preparation. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9285–9289. doi: 10.1073/pnas.84.24.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]