Abstract
Frontal fibrosing alopecia (FFA) is a primary lymphocytic cicatricial alopecia characterized by a progressive band-like recession of the frontotemporal hairline and frequent loss of the eyebrows. It predominantly affects postmenopausal women. Coexistence of FFA and vitiligo is rarely reported in the literature. We retrospectively studied 20 cases diagnosed with FFA in a 14-month period in our Department. Among them, there were 2 cases, a 72-year-old woman and a 48-year-old man, who developed FFA on preexisting vitiligo of the forehead. Anatomical colocalization of the two dermatoses supports the notion that a causal link may exist and their association may not be coincidental. We suggest that interrelated immunologic events and pathologic processes may underlie both these skin conditions.
Key Words: Alopecia, Vitiligo, Frontal fibrosing alopecia, Coexistence
Established Facts
Frontal fibrosing alopecia (FFA) is a non-uncommon lymphocyte-mediated cicatricial alopecia.
Immunologic and hormonal factors have been implicated in its pathogenesis.
Novel Insights
A considerable prevalence of vitiligo was observed in our series of patients diagnosed with FFA.
Potential immunologic mechanisms interrelating these two dermatoses are presented, suggesting that this association may be more than coincidental.
Introduction
Frontal fibrosing alopecia (FFA) is a primary lymphocytic cicatricial alopecia characterized by a progressive symmetric recession of the frontotemporal hairline, frequent loss of the eyebrows, and, less often, alopecia of the axillae, the pubic area, or the limbs [1]. It is almost exclusively seen in postmenopausal women [1,2]. Its occurrence in men is rare, with only 25 cases reported to date [3,4,5,6,7,8,9,10,11,12,13,14].
The etiopathogenesis of FFA remains obscure, yet a key element seems to be the destruction of the epithelial hair follicle stem cells located in the bulge region by a lymphocytic infiltrate [15]. In addition, the localization at the frontal hairline, the predominance of postmenopausal women, the increased incidence of early menopause [11], and the clinical improvement with anti-androgen drugs [11,16,17,18] suggest that hormonal influences may also play an important role. The true nature of FFA is yet debated. It is not clear if it represents a distinct type of lymphocyte-mediated cicatricial alopecia or a variant of lichen planopilaris (LPP) with selective topography [19,20].
In the literature, there are rare reports of vitiligo coexisting with FFA. Herein, we present two additional cases of FFA that developed on preexisting vitiligo of the forehead, and we discuss possible causal immunologic associations.
Case Presentation
We conducted a retrospective study of all histologically proven FFA cases diagnosed at the Hair Clinic of the 2nd Academic Department of Dermatology and Venereology, “Attikon” General University Hospital, Athens, Greece, from January 1, 2015 to February 29, 2016. During the period studied, 20 cases of FFA were diagnosed. Of these, 19 (95%) were females with a mean age of 58 years (range 23-78) and 1 (5%) was male, aged 48 years. Associated vitiligo was present in 2 (10%) of the 20 patients included.
Case 1
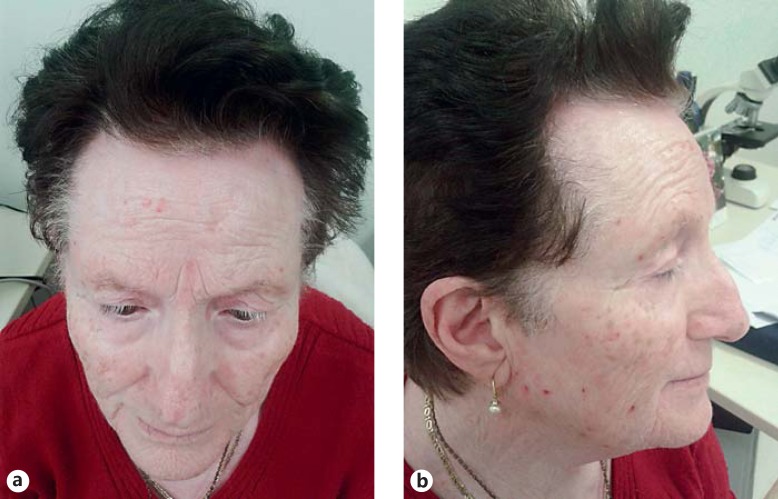
A 72-year-old Caucasian presented with a 5-year history of progressive hair loss on the frontal scalp following a stressful event, accompanied by pruritus (Fig. 1a, b). Clinical examination revealed also thinning of the eyebrows, almost complete alopecia of the limbs and the pubic area, and partial loss of the axillary hair, dating back 10 years. Dermoscopic examination of the frontal hairline and eyebrows showed loss of follicular openings, mild perifollicular erythema, white scar-like depigmentation, and red dots (Fig. 2a, b). Dermoscopy-guided 3-mm punch biopsy of the scalp confirmed the diagnosis of FFA. The patient had been diagnosed with vitiligo at the age of 8 years, and at the time of the examination, she presented with generalized vitiligo. She mentioned that her mother, grandmother (father's side), brother, and sister also had vitiligo and that her daughter suffered from multiple sclerosis. No family history of FFA was given. Autoimmune thyroid disease and histologically confirmed morphea were also present in the patient, the latter of 1-year duration.
Fig. 1.
a, b Coexistence of vitiligo of the face with FFA of the frontal hairline and the eyebrows in a 72-year-old woman.
Fig. 2.
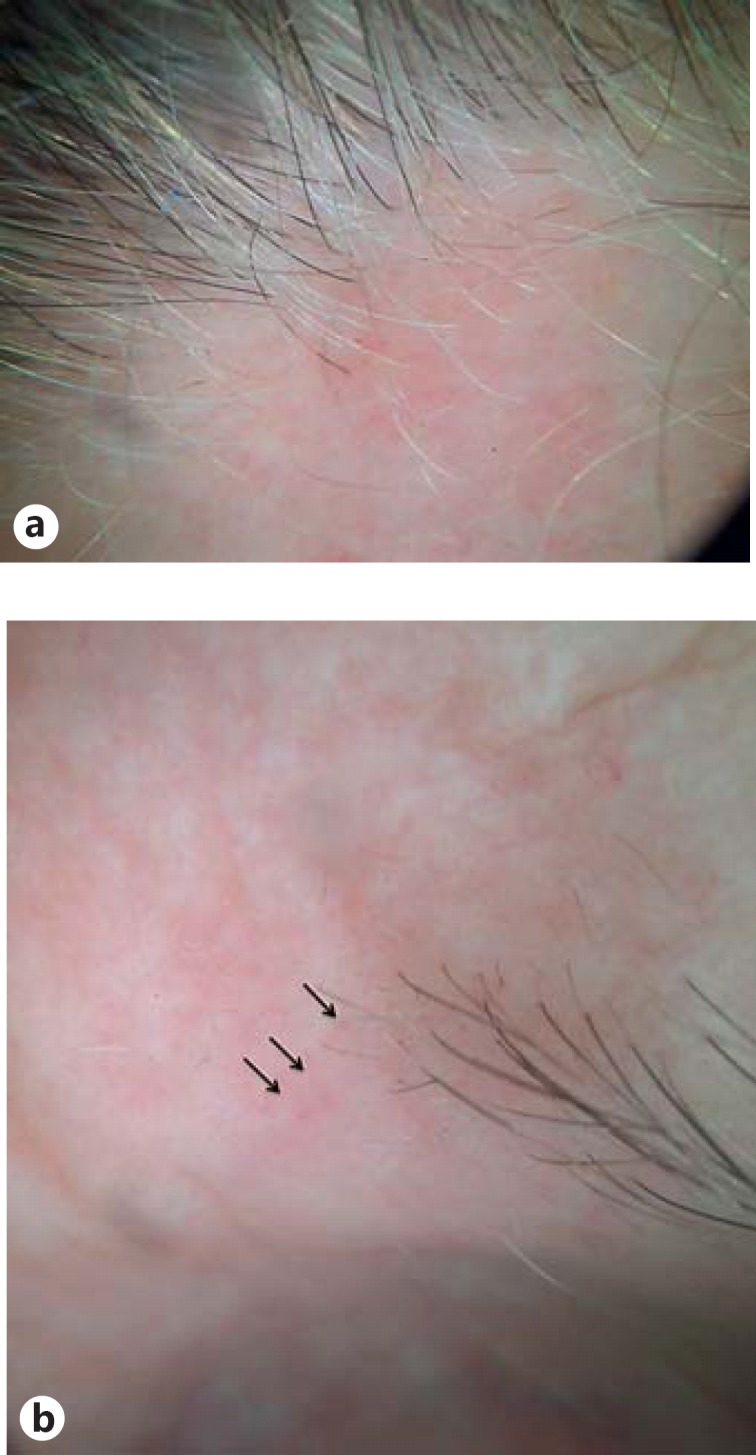
a Dermoscopy of the frontal hairline showing lonely hairs and perifollicular erythema on a whitish background. b Typical red dots on an alopecic area of the left eyebrow.
Case 2
A 48-year-old Caucasian male was referred to us with an asymptomatic recession of the frontotemporal hairline dating back 1 year. The patient had been diagnosed with vitiligo 7 years before. He had a history of severe head injury following a car accident. There was no history of FFA or autoimmune disease in his family. No patches of vitiligo were observed elsewhere. On clinical examination, a white, band-like patch on the temporal and forehead areas was present, extending below the lower margin of cicatricial skin, clinically consistent with vitiligo. Dermoscopy of the hairline showed loss of follicular orifices, lonely hair, and mild perifollicular erythema and scaling. Histological findings from the frontal hairline were consistent with FFA.
Discussion
Association of FFA with vitiligo is scarcely described in the literature, with only 8 cases reported so far, to the best of our knowledge [11,13,21,22]. In our series, we found 2 cases of FFA coexisting with vitiligo among 20 cases included in the study. In addition, the anatomical colocalization of these two dermatoses in both of our cases may suggest that a true association may exist, and vitiligo and FFA may share common pathogenic pathways.
Lichen planus (LP) has been previously associated with vitiligo [23,24,25,26,27,28,29,30,31]. Furthermore, some authorities consider FFA as a variant of LPP [19,20]. There is evidence that most lymphocytic clones involved in both LP and vitiligo are of CD8+ cytotoxic origin [32,33,34]. In LP, the cytotoxic lymphocytes, alleged to target yet unknown antigen(s), conjugate to the basal layer keratinocytes and induce apoptosis of the latter. It is known that melanocytes and keratinocytes form functional units; therefore, apoptosis of keratinocytes can result in decreased melanocytic survival and apoptosis, as occurs in vitiligo. The aforementioned findings could imply that the keratinocytes of the outer root sheath, being continuous with the epidermal keratinocytes, can also express the adhesion molecules necessary for attachment of the cytotoxic lymphocytes to the affected follicles [21]. In this context, vitiligo and LPP/FFA may develop simultaneously. In addition, vitiligo could alter the exposure of dermoepidermal junction antigens identified by effector T cells of FFA, or alternatively, vitiligo may nonspecifically inactivate suppressive mechanisms on the effector cells responsible for FFA [30].
The Koebner phenomenon, which LP is well known to exhibit, could also explain the anatomical colocalization of these two dermatoses, as observed in our two cases (especially case 2, who showed features of vitiligo solely on the frontal area). Additionally, there are several reports describing lesions of LP that developed mainly over sun-exposed vitiliginous skin [25,26,29,30,31]. Extrapolating this to FFA, actinic damage in vitiliginous skin may trigger initiation of FFA on vitiligo-affected skin. However, the literature also contains cases of associated LP and vitiligo that are not readily accommodated by this theory [24,28,35,36].
FFA is extremely uncommon in men. Therefore, case 2 is interesting not only because it involves a male patient, but also because, to the best of our knowledge, it is the first description of coexistence of vitiligo and FFA in a male. Another interesting point is the occurrence of FFA on the sole vitiliginous patch of the patient.
In conclusion, we report 2 cases of FFA coexisting with vitiligo in a series of 20 FFA patients studied. The anatomical colocalization supports the notion that the association is not coincidental. Both diseases have a common pathogenic background and we assume that this association may be explained by co-stimulation of immunological mechanisms, or alternatively, inactivation of nonspecific suppressor mechanisms. Koebner phenomenon related to subclinical photodamage inducing FFA preferentially over vitiliginous skin could also be considered. At present, there is insufficient evidence to resolve the uncertainties, but the possible immunologic association between FFA and vitiligo seems to merit further study.
Statement of Ethics
The two patients presented in this paper gave their consent for the above presentation.
Disclosure Statement
There is no conflict of interest concerning this paper.
References
- 1.Kossard S. Postmenopausal frontal fibrosing alopecia. Scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770–774. [PubMed] [Google Scholar]
- 2.Moreno-Ramírez D, Ferrandiz L, Camacho FM. Diagnostic and therapeutic assessment of frontal fibrosing alopecia. Actas Dermosifiliogr. 2007;98:594–602. [PubMed] [Google Scholar]
- 3.Stockmeier M, Kunte C, Sander CA, Wolff H. Kossard frontal fibrosing alopecia in a man. Hautarzt. 2002;53:409–411. doi: 10.1007/s001050100273. [DOI] [PubMed] [Google Scholar]
- 4.Kossard S, Shiell RC. Frontal fibrosing alopecia developing after hair transplantation for androgenetic alopecia. Int J Dermatol. 2005;44:321–323. doi: 10.1111/j.1365-4632.2004.02251.x. [DOI] [PubMed] [Google Scholar]
- 5.Nusbaum BP, Nusbaum AG. Frontal fibrosing alopecia in a man: results of follicular unit test grafting. Dermatol Surg. 2010;36:959–962. doi: 10.1111/j.1524-4725.2010.01580.x. [DOI] [PubMed] [Google Scholar]
- 6.Ramaswamy P, Mendese G, Goldberg LJ. Scarring alopecia of the sideburns: a unique presentation of frontal fibrosing alopecia in men. Arch Dermatol. 2012;148:1095–1096. doi: 10.1001/archdermatol.2012.848. [DOI] [PubMed] [Google Scholar]
- 7.Chen W, Kigitsidou E, Prucha H, Ring J, Andres C. Male frontal fibrosing alopecia with generalised hair loss. Australas J Dermatol. 2014;55:e37–e39. doi: 10.1111/ajd.12005. [DOI] [PubMed] [Google Scholar]
- 8.Dlova NC, Goh CL. Frontal fibrosing alopecia in an African man. Int J Dermatol. 2015;54:81–83. doi: 10.1111/j.1365-4632.2012.05821.x. [DOI] [PubMed] [Google Scholar]
- 9.Khan S, Fenton DA, Stefanato CM. Frontal fibrosing alopecia and lupus overlap in a man: guilt by association? Int J Trichology. 2013;5:217–219. doi: 10.4103/0974-7753.130420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296–1300. doi: 10.1111/j.1365-2133.2010.09965.x. [DOI] [PubMed] [Google Scholar]
- 11.Vañó-Galván S, Molina-Ruiz AM, Serrano-Falcón C, Arias-Santiago S, Rodrigues-Barata AR, Garnacho-Saucedo G, Martorell-Calatayud A, Fernández-Crehuet P, Grimalt R, Aranegui B, Grillo E, Diaz-Ley B, Salido R, Pérez-Gala S, Serrano S, Moreno JC, Jaén P, Camacho FM. Frontal fibrosing alopecia: a multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670–678. doi: 10.1016/j.jaad.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Dlova NC, Jordaan HF, Skenjane A, Khoza N, Tosti A. Frontal fibrosing alopecia: a clinical review of 20 black patients from South Africa. Br J Dermatol. 2013;169:939–941. doi: 10.1111/bjd.12424. [DOI] [PubMed] [Google Scholar]
- 13.Banka N, Mubki T, Bunagan MJ, McElwee K, Shapiro J. Frontal fibrosing alopecia: a retrospective clinical review of 62 patients with treatment outcome and long-term follow-up. Int J Dermatol. 2014;53:1324–1330. doi: 10.1111/ijd.12479. [DOI] [PubMed] [Google Scholar]
- 14.AlGaadi S, Miteva M, Tosti A. Frontal fibrosing alopecia in a male presenting with sideburn loss. Int J Trichology. 2015;7:72–73. doi: 10.4103/0974-7753.160115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaisse V, Matard B, Assouly P, Jouannique C, Reygagne P. Postmenopausal frontal fibrosing alopecia: 20 cases. Ann Dermatol Venereol. 2003;130:607–610. [PubMed] [Google Scholar]
- 16.Tosti A, Piraccini BM, Iorizzo M, Misciali C. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55–60. doi: 10.1016/j.jaad.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Georgala S, Katoulis AC, Befon A, Danopoulou I, Georgala C. Treatment of postmenopausal frontal fibrosing alopecia with oral dutasteride. J Am Acad Dermatol. 2009;61:157–158. doi: 10.1016/j.jaad.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Ladizinski B, Bazakas A, Selim MA, Olsen EA. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749–755. doi: 10.1016/j.jaad.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 19.Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59–66. doi: 10.1016/s0190-9622(97)70326-8. [DOI] [PubMed] [Google Scholar]
- 20.Poblet E, Jiménez F, Pascual A, Piqué E. Frontal fibrosing alopecia versus lichen planopilaris: a clinicopathological study. Int J Dermatol. 2006;45:375–380. doi: 10.1111/j.1365-4632.2006.02507.x. [DOI] [PubMed] [Google Scholar]
- 21.Miteva M, Aber C, Torres F, Tosti A. Frontal fibrosing alopecia occurring on scalp vitiligo: report of four cases. Br J Dermatol. 2011;165:445–447. doi: 10.1111/j.1365-2133.2011.10382.x. [DOI] [PubMed] [Google Scholar]
- 22.Tan KT, Messenger AG. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160:75–79. doi: 10.1111/j.1365-2133.2008.08861.x. [DOI] [PubMed] [Google Scholar]
- 23.Gül U, Soylu S, Demiriz M. Colocalization of lichen planus and vitiligo associated with selective IgA deficiency. Skinmed. 2007;6:202–203. doi: 10.1111/j.1540-9740.2007.06386.x. [DOI] [PubMed] [Google Scholar]
- 24.Göktay F, Mansur AT, Aydingöz IE. Colocalization of vitiligo and lichen planus on scrotal skin: a finding contrary to the actinic damage theory. Dermatology. 2006;212:390–392. doi: 10.1159/000092295. [DOI] [PubMed] [Google Scholar]
- 25.Ujiie H, Sawamura D, Shimizu H. Development of lichen planus and psoriasis on lesions of vitiligo vulgaris. Clin Exp Dermatol. 2006;31:375–377. doi: 10.1111/j.1365-2230.2006.02066.x. [DOI] [PubMed] [Google Scholar]
- 26.Baghestani S, Moosavi A, Eftekhari T. Familial colocalization of lichen planus and vitiligo on sun exposed areas. Ann Dermatol. 2013;25:223–225. doi: 10.5021/ad.2013.25.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golchai J, Ramezanpour A. Report of a new case with four skin diseases. Dermatol Online J. 2003;9:15. [PubMed] [Google Scholar]
- 28.Porter SR, Scully C, Eveson JW. Coexistence of lichen planus and vitiligo is coincidental. Clin Exp Dermatol. 1994;19:366. doi: 10.1111/j.1365-2230.1994.tb01218.x. [DOI] [PubMed] [Google Scholar]
- 29.Veitch D, Kravvas G, Hughes S, Bunker C. A rare colocalization of lichen planus and vitiligo. Case Rep Dermatol Med. 2015;2015:840193. doi: 10.1155/2015/840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anstey A, Marks R. Colocalization of lichen planus and vitiligo. Br J Dermatol. 1993;128:103–104. doi: 10.1111/j.1365-2133.1993.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 31.Sardana K, Sharma RC, Koranne RV, Mahajan S. An interesting case of colocalization of segmental lichen planus and vitiligo in a 14-year-old boy. Int J Dermatol. 2002;41:508–509. doi: 10.1046/j.1365-4362.2002.01552_2.x. [DOI] [PubMed] [Google Scholar]
- 32.Piguet V, Breathnach SM, Le Cleach L. Lichen planus and lichenoid disorders. In: Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D, editors. Rook's Textbook of Dermatology. 9th edition. West Sussex: Wiley-Blackwell; pp. 37.1–37.20. [Google Scholar]
- 33.Glassman SJ. Vitiligo, reactive oxygen species and T-cells. Clin Sci (Lond) 2011;120:99–120. doi: 10.1042/CS20090603. [DOI] [PubMed] [Google Scholar]
- 34.Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28:100–108. doi: 10.1016/j.clindermatol.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Wayte J, Wilkinson JD. Unilateral lichen planus, sparing vitiliginous skin. Br J Dermatol. 1995;133:817–818. doi: 10.1111/j.1365-2133.1995.tb02768.x. [DOI] [PubMed] [Google Scholar]
- 36.Baran R, Ortonne JP, Perrin C. Vitiligo associated with a lichen planus border. Dermatology. 1997;194:199. doi: 10.1159/000246170. [DOI] [PubMed] [Google Scholar]