In 1955, Christian de Duve discovered a novel organelle packaged with hydrolytic enzymes for which he coined the terminology ‘lysosome’ (De Duve et al., 1955). Two decades later, he received the Nobel prize for this work and more than six decades later, our knowledge of the lysosome as an organelle for end-point degradation has expanded enormously to one that plays a central role in cellular metabolism, implicated not only in rare lysosomal storage diseases but also in common and complex diseases such as Parkinson's disease, dementia, and cancer (Coutinho & Alves, 2016). In this issue of EBioMedicine, Jian, Tian, Hettinghouse, et al. (http://dx.doi.org/10.1016/j.ebiom.2016.10.010) presented novel findings on progranulin (PGRN), a growth factor with anti-inflammation properties, that also functions as a co-chaperone with the heat shock protein HSP70 disaggregation system. During stress, PGRN-HSP70 prevents the aggregation of lysosomal glucocerebrosidase (GCase) and lysosomal integral membrane protein LIMP2 in the cytoplasm, and facilitates their trafficking to the lysosome. These findings have implications in Gaucher disease (GD), an autosomal recessively inherited and most prevalent lysosomal storage disease, and demonstrate the myriad of important cellular physiological functions of the lysosome, in addition to serving as the cell's ‘waste bin’.
GD results from deficient GCase (GBA1, EC3.2.1.45). Three clinical forms of GD have been described: Type 1, non-neuropathic, Type 2, acute neuropathic, and Type 3, sub-acute neuropathic (Beutler & Grabowski, 2001). GD may also result from deficient saposin C (Tamaro et al., 2012) or LIMP2 (Reczek et al., 2007), the former is an activator protein of GCase (Tamaro et al., 2012) and the latter is the receptor protein for targeting GCase to the lysosome (Reczek et al., 2007). In a previous study (Jian et al., 2016), these investigators reported that serum PGRN level in GD patients is significantly lower than that in healthy controls, and four SNPs in the GRN gene that encodes PGRN may contribute to this low level. In the present study, they noted that when PGRN KO (knock-out) mice and wild type (WT) mice were challenged using ovalbumin injections and nasal sprays to induce lung inflammation, the PGRN KO mice developed a Gaucher-like phenotype. While the level of GCase activity in the KO mice cells was unaffected, GCase was found aggregated with LIMP2 in the cytoplasm co-localized with the autophagy marker LC3. They demonstrated that this defect is specific to GCase-LIMP2 since the localization of the lysosomal protein marker LAMP2 (lysosomal associated membrane protein 2) was unaffected. In addition, LAMP2 and LIMP2 as well as GCase trafficking to the lysosomes in the WT mice were also unaffected.
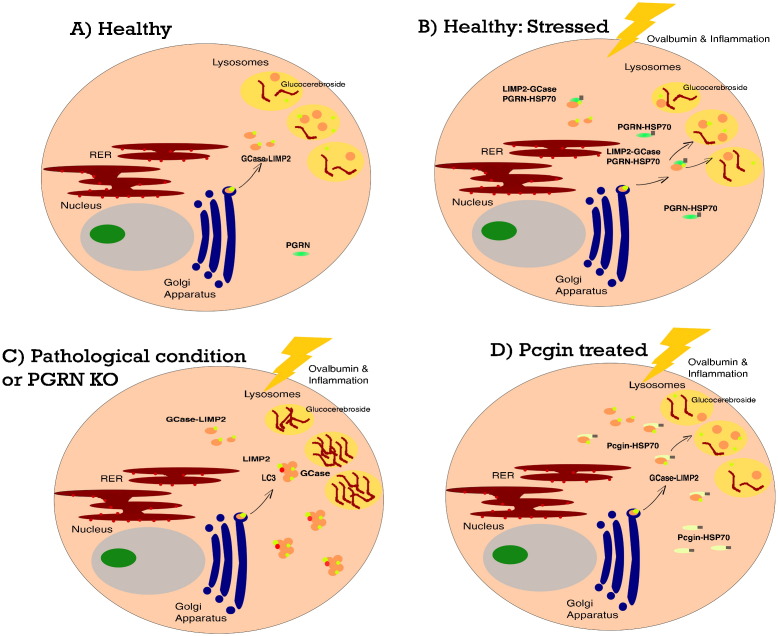
Immunoprecipitation studies followed by MS and other analyses of WT and PGRN KO mouse cells showed that an additional protein, the heat shock protein HSP70, was involved. It was postulated that during stress induced by ovalbumin or certain pathological conditions, PGRN functions as a co-chaperone of HSP70 via the HSP70 disaggregation system (Nillegoda & Bukau, 2015) that prevents GCase-LIMP2 aggregation in the cytoplasm and facilitates their lysosomal localization. In PGRN KO cells, this intricate stress-response is perturbed, and GCase-LIMP2 ends up aggregated in the cytoplasm. Consequently, glucocerebroside substrate accumulates in the lysosome, resulting in cytotoxicity and expression of Gaucher-like phenotype (Fig. 1). To prove their point, the authors used siRNA to specifically suppress HSP70 in lipid-stimulated stressed cells and noted a reduction of lysosomal GCase detection and simultaneously GCase aggregation in the cytoplasm.
Fig. 1.
Interaction of PGRN as a co-chaperone with HSP70, GCase, and LIMP2. (A) In healthy cells, GCase binds to its receptor protein LIMP1 for lysosomal trafficking and localization. (B) When ovalbumin is used to induce stress, PGRN serves as a co-chaperone with HSP70 in the HSP70 disaggregation system that prevents GCase-LIMP1 aggregation in the cytoplasm and facilitates their trafficking/localization to the lysosome. (C) In stressed PGRN KO cells, PGRN deficiency results in GCase-LIMP1 aggregation in the cytoplasm, GCase deficiency in the lysosome, excessive glucocerebroside substrate accumulation, and cytotoxicity. (D) Pcgin treatment of PGRN KO cells and Gaucher Types 1 and 2 fibroblasts results in the delivery of GCase to the lysosome, restoration of lysosomal GCase activity, and degradation of glucocerebroside. PGRN, progranulin; HSP, heat shock protein; GCase, glucocerebrosidase; LIMP2, lysosomal integral membrane protein 2; KO, knock-out; Pcgin, progranulin C-terminus for GCase interaction.
Because PGRN is a growth factor that has oncogenic activities, this limits its application for potential treatment of GD. To this end, the authors performed expression studies of PGRN cDNA deletion mutants and a 98 amino acid of C-terminal PGRN, termed ‘Pcgin’ (PGRN C-terminus for GCase Interaction) was identified that retains binding capacity to GCase and HSP70 without oncogenic activity. Pcgin treatment led to significant reduction in glucocerebroside storage and increase of GCase localization and activity in Type 1 and Type 2 GD cultured skin fibroblasts, as well as in ovalbumin challenged PGRN-KO mice.
As stated, future investigation with additional GD models are warranted. As a macromolecule, however, Pcgin may not be effective for treatment of Types 2 and 3 GD as it may be excluded by the blood brain-barrier. Future direction should include expression of Pcgin as a fusion with the eleven amino acid protein transduction domain of the HIV-Tat protein or other cell penetrating peptides for potential permeation to reach the brain (Gramlich et al., 2016, Campbell et al., 2014). If successful, similar expression studies of PGRN are warranted since its deficiency has been associated with neurodegenerative diseases including Parkinson's disease, Alzheimer's disease, multiple sclerosis, and amyotrophic lateral sclerosis (Petkau & Leavitt, 2014). As GD is clinically heterogeneous and patients with identical genotype may have variable clinical phenotype (Beutler & Grabowski, 2001), it will also be interesting to compare the level of PGRN among these patients.
Disclosure
The authors declared no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.10.018.
Appendix A. Supplementary data
Supplementary materials.
References
- Beutler E., Grabowski G.A. In: Gaucher disease, in The Metabolic and Molecular Bases of Inherited Disease. Scriver C.R., Beaudet A.L., Sly W.S., editors. McGraw-Hill; New York: 2001. pp. 3635–3668. [Google Scholar]
- Campbell, T.N., Jack, A.T. & Choy, F.Y.M. ‘Gaucher disease and associated dementia’, in: Diet and Nutrition in Dementia and Cognitive Decline, Chapter 7, pp. 62–75, Eds. Martin, C. and Preedy, V., Elsevier-Academic Press, London, ISBN: 978-0-12-407824-6 (2014).
- Coutinho M.F., Alves S. From rare to common and back again: 60 years of lysosomal dysfunction. Mol. Genet. Metab. 2016;117:53–65. doi: 10.1016/j.ymgme.2015.08.008. [DOI] [PubMed] [Google Scholar]
- De Duve C. Tissue fractionation studies. 6. Intracellular distribution pattern of enzyme in rat-liver tissue. Biochem. J. 1955;60:604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramlich P.A. A peptide-linked recombinant glucocerebrosidase for targeted neuronal delivery: design, production, and assessment. J. Biotechnol. 2016;221:1–12. doi: 10.1016/j.jbiotec.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian J. Association between progranulin and Gaucher's disease. EBioMedicine. 2016 doi: 10.1016/j.ebiom.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian, J., Tian, Q.Y., Hettinghouse, A. et al. Progranulin recruits HSP70 to β-glucocerebrosidase and is therapeutic against Gaucher disease. EBioMedicine. http://dx.doi.org/10.1016/j.ebiom.2016.10.010 [DOI] [PMC free article] [PubMed]
- Nillegoda N.B., Bukau B. Metazoan Hsp70-based protein disaggregases: emergence and mechanisms. Frontiers in molecular sciences. 2015;2:57. doi: 10.3389/fmolb.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkau T.L., Leavitt B.R. Progranulin in neurodegenerative disease. Trends Neurosci. 2014;37:388–398. doi: 10.1016/j.tins.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Reczek D. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131:770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Tamaro R.J. The role of saposin C in Gaucher disease. Mol. Genet. Metab. 2012;106:257–263. doi: 10.1016/j.ymgme.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.