Abstract
Individual differences in the performance profiles of neuropsychologically-impaired patients are pervasive yet there is still no resolution on the best way to model and account for the variation in their behavioural impairments and the associated neural correlates. To date, researchers have generally taken one of three different approaches: a single-case study methodology in which each case is considered separately; a case-series design in which all individual patients from a small coherent group are examined and directly compared; or, group studies, in which a sample of cases are investigated as one group with the assumption that they are drawn from a homogenous category and that performance differences are of no interest. In recent research, we have developed a complementary alternative through the use of principal component analysis (PCA) of individual data from large patient cohorts. This data-driven approach not only generates a single unified model for the group as a whole (expressed in terms of the emergent principal components) but is also able to capture the individual differences between patients (in terms of their relative positions along the principal behavioural axes). We demonstrate the use of this approach by considering speech fluency, phonology and semantics in aphasia diagnosis and classification, as well as their unique neural correlates. PCA of the behavioural data from 31 patients with chronic post-stroke aphasia resulted in four statistically-independent behavioural components reflecting phonological, semantic, executive–cognitive and fluency abilities. Even after accounting for lesion volume, entering the four behavioural components simultaneously into a voxel-based correlational methodology (VBCM) analysis revealed that speech fluency (speech quanta) was uniquely correlated with left motor cortex and underlying white matter (including the anterior section of the arcuate fasciculus and the frontal aslant tract), phonological skills with regions in the superior temporal gyrus and pars opercularis, and semantics with the anterior temporal stem.
Keywords: Individual differences, Principal component analysis, Post-stroke aphasia, Symptom–lesion mapping
1. Introduction
For both clinical practice and basic research, it is important to understand the patterns and neural bases of impaired performance observed in both individual and groups of neuropsychological patients. Since the beginning of behavioural neurology and neuropsychology, contrastive impairments across patients have been used to make important inferences about the underlying cognitive computations and the neural structures that support them. In addition, stable models of variable patient performance are critical in accurate neurological differential diagnosis, clinical management and treatment.
The generation of stable, reliable models of normal and impaired function rely on the ability to understand the nature and sources of individual differences across patients. This has always been a key challenge for the field (Shallice, 1988), leading to numerous debates and discussions (Caramazza and McCloskey, 1988, Lambon Ralph et al., 2011, Schwartz and Dell, 2011, Shallice, 1988), and arguably still is. The kernel of the problem relates to the fact that there are multiple sources that underlie variable neuropsychological results. Previous formal considerations of the issue have set out a series of potential factors (Lambon Ralph et al., 2002, Shallice, 1988) but, for brevity, we will note three types here: (A) the type of difference that neuropsychological dissociations and differential diagnosis are based on, namely stable performance differences that arise when a task is supported by one or more computations underpinned by neuroanatomically-separated regions; (B) differences that relate, linearly or nonlinearly, to the severity of damage to the underlying neurocomputational component(s) – which can provide critical information about the nature and characteristics of the core neurocognitive computations, as well as the degree of recovery/deterioration in a patient's disease or disorder; and (C) sources of individual differences of no interest including random variations, measurement noise, and so forth.
Over the years, researchers have adopted different approaches to this issue. The most classical and common approach (also utilised by experimental psychology and functional neuroimaging) is to recruit a coherent sample of patients and, through the power of central tendency, average the behavioural results or lesion distributions in order to remove noise and other individual variations of no interest. As noted previously (Shallice, 1988), this method relies on having or defining a ‘coherent’ group and, at worst, can suffer from two types of statistical error: (a) failing to uncover meaningful differences within the sample of patients which is lost through the averaging process; and (b) failing to detect or understand how systematic variation in patient/lesion severity changes task performance (which otherwise is falsely considered to reflect a behavioural dissociation). An alternative approach, single-case study, possesses the opposite set of advantages and disadvantages. Of course, whilst single-case study avoids averaging away important behavioural dissociations, it means that it is hard/impossible to place each patient within a broader context or to generate a coherent, overarching cognitive and neuroanatomical model of the disorder. From a basic science perspective, this approach could lead to the logical absurdity of there being as many theories as there are patients; whilst, from a clinical perspective, it reduces our ability to generalise clinical knowledge from one patient to another – ultimately making it impossible to successfully diagnose, manage and treat the patient's impairments. The single-case study makes it impossible to map the relationship between severity of damage and level of performance (which, like any mathematical function, requires more than one datum). Over the past decade or so, multiple researchers have adopted a hybrid of these two approaches in the form of case-series studies (Hodges et al., 1992, Lambon Ralph et al., 2001, Schwartz et al., 2006, Woollams et al., 2007). These investigations recruit a sample of patients whom are all studied in detail at the individual level (i.e., often to the same precision as single-case studies), which can be used: to assess the consistency of performance, i.e., the coherence, in the sampled cases (Lambon Ralph et al., 2001, Schwartz et al., 2006, Woollams et al., 2007); to highlight patterns/mechanisms/lesion correlates which generalise reliably across cases; to identify meaningful, consistent patterns of individual differences (rather than random noise: Woollams et al., 2007); as well as to relate impairment severity to task performance (leading to mathematical/computational models of the severity-based functions: Lambon Ralph et al., 2001, Schwartz et al., 2006, Woollams et al., 2007). Furthermore, ‘comparative’ case-series can be used to strengthen important behavioural dissociations by demonstrating that, despite variations in severity, (i) the two case-series are systematically different in the expected way, yet (ii) are internally coherent (Jefferies and Lambon Ralph, 2006, Lambon Ralph et al., 2007).
Whilst the case-series methodology clearly has a number of strengths which benefit basic science and clinical practice, like group studies, the approach still relies heavily both on the ability to recruit reasonably coherent patient groups and also to know, a priori, what the relevant groupings of patient are. Indeed, we note here that one of the most common uses and development platforms of the case-series methodology were made through investigations of semantic dementia (Hodges et al., 1992, Lambon Ralph et al., 2001, Woollams et al., 2007), which is a highly consistent group of patients and thus makes this methodology particularly successful and powerful. It is in this context, therefore, that we consider a related approach – the use of principal component analysis (PCA) as a data-driven method which uses the patterns of individual differences in order both to reveal the statistically-reliable distinctions within a patient dataset and also to place individual cases, relative to each other, in the resultant multi-dimensional model (Butler et al., 2014, Lambon Ralph et al., 2002, Lambon Ralph et al., 2003). In effect, the PCA method spans group and individual levels of analysis because the emergent set of principal components provide a coherent, generalisable set of factors or model of the underlying systems whilst, unlike group studies, variations in individual patient performance can be placed systematically along one or more of the principal axes. If a group is relatively homogenous then PCA only generates one principal component along which all patients can be mapped. If, however, there are systematic differences within the cohort, then one or more statistically-independent (orthogonal) factors emerge. In contrast unsystematic sources of variation, such as measurement error and other random fluctuations are left as unexplained variance (or loaded onto many additional components with little explanatory power, i.e., with eigenvalues less than 1). In addition, in more recent work, we have demonstrated that the PCA factors can also be used to explore behaviour–brain correlates in a new fashion; rather than traditional lesion-overlapping methods which rely on coherent group studies (Caramazza and McCloskey, 1988, Shallice, 1988): or correlating raw test scores with brain voxel status (as per lesion–symptom mapping: Bates et al., 2003), we have found that clear results emerge when the behavioural principal components are related to the patients' lesion distributions (Butler et al., 2014, Chase, 2014).
In this study, therefore, we demonstrate and use the PCA approach in order to explore the nature of post-stroke aphasia. Aphasia is an interesting test case/problem for multiple reasons. Whilst it is possible to distinguish between patients with and without acquired language impairments, within the aphasic group there are multiple sources of individual differences. Traditionally, category-based classification schemes have been developed in order to assist with different diagnosis of aphasic subgroups, clinical management and interventions, as well as for basic science studies that have tried to relate aphasic profiles to underlying lesion distributions. Whilst these category-based systems may provide a useful ‘broad brushstroke’ summary for individual patients, there are multiple limitations which follow from the fact that the underlying distribution of patient data reflects continuous variations along multiple dimensions rather than coherent, mutually-exclusive categories. Instead, there are both very fuzzy boundaries between ‘categories’ and considerable variation of profile within each ‘category’. If this conceptualisation of post-stroke aphasia is correct then we might be able to use PCA in order to define the core underlying dimensions and the relative positions of each individual patient along these axes. Secondly, in turn, this multi-dimensional behavioural description can then be compared directly to the variation in the patients' lesion distributions.
Before considering the PCA findings in this study, we first briefly consider some of the key clinical characteristics of chronic post-stroke aphasia. Speech production, phonological and semantic impairments are common following left hemisphere stroke and the nature of these deficits forms the principal behavioural divisions in most aphasia classification systems. Typically, patients are first divided into fluent (relatively effortless speech output) or non-fluent types (effortful, slow or reduced complexity and length) and then additionally by the status of their phonological and semantic skills (Goodglass, 1993, Goodglass and Kaplan, 1983, Kertesz, 1982). Despite their prominence in aphasia diagnostics and importance for understanding intact language function, the unique neural correlates of speech dys-/fluency, semantic and phonological deficits remain unclear. Furthermore, when these have been considered, they have been investigated separately and thus the inter-relationship between them is unclear (e.g., is speech fluency, in part, a reflection of patients' phonological or semantic skills?).
Classically, damage to left frontal areas was associated with poor fluency (Broca, 1861); however, studies using high-resolution Magnetic Resonance Imaging (MRI) techniques have found that left frontal lesions do not always result in Broca's aphasia (Basso et al., 1985, Willmes and Poeck, 1993) and production deficits can occur following lesions outside of the frontal lobe, including white matter tracts and the anterior insula (aINS) (Bates et al., 2003, Blank et al., 2002, Damasio, 1992, Dronkers, 1996, Mohr et al., 1978, Wise et al., 1999). Functional neuroimaging studies have identified a broader neural network during connected-speech production. For example, by comparing propositional with automatic speech (e.g., counting), a positron emission tomography (PET) imaging study found extensive left hemisphere activation including the frontal lobe, pre-supplementary motor area (pre-SMA), angular gyrus (AG), fusiform gyrus (FG), and perisylvian areas (Blank et al., 2002). A large-scale left hemisphere speech production network was also identified in a study that measured fluency, complexity and variety of post-stroke aphasic speech (Borovsky, Saygin, Bates, & Dronkers, 2007). These critical sub-components could not be separated when they were related to the patients' neural damage, which hints at the multi-faceted nature of speech fluency. Specifically, considerable anatomical overlap was found across the measures: the number of speech tokens (T) correlated with aINS, pre-motor cortex (PMC); mean length of utterance (MLU) with aINS, PMC and anterior superior temporal gyrus (aSTG); and type–token ratio (TTR) with AG, posterior middle temporal gyrus (pMTG) and aSTG. This outcome probably reflects the inter-correlations between both the behavioural measures and lesion distributions (middle cerebral artery – MCA lesions tend to sample the same subset of brain regions) (Phan, Donnan, Wright, & Reutens, 2005), and because severity-related variance is shared across individual measurements. In addition, it is likely that fluency itself is a multi-faceted aphasic feature (Basilakos et al., 2014), which may benefit from decomposition into unique elements before the relationship with lesions can be satisfactorily explored. Plus, to identify the neural correlates of the full range of aphasic features, it is critical to consider fluency simultaneously alongside phonological and semantic skills.
To achieve this aim, the current study utilised the PCA methodology (Butler et al., 2014, Chase, 2014) to reveal, for the first time, the unique neural correlates of speech fluency, semantic and phonological abilities in chronic post-stroke aphasia. This extends previous findings by taking into account speech fluency measures as part of the wider behavioural profile (phonological and semantic deficits), which is a key dimension on which aphasia patients are typically classified. Specifically, two approaches for tackling the analytical challenges associated with brain–behaviour mapping in chronic aphasia are to derive statistically-independent behavioural factors/dimension from a rotated PCA, and lesion volume as a covariate for anatomical severity. Previous studies using PCA on neuropsychological data have also shown that it offers various benefits. First, there is additional statistical reliability by combining results from multiple tests (Lambon Ralph et al., 2002, Lambon Ralph et al., 2003). Secondly, by maintaining orthogonal components, it is possible to avoid problems of collinearity when undertaking lesion–symptom correlations. Thirdly, the use of varimax rotation promotes a clear cognitive interpretation of each of the principal components. Finally, when the rotated components are combined with lesion–symptom mapping, it is possible to establish the neural correlates of unique components of post-stroke aphasic behaviour (Butler et al., 2014, Mirman et al., 2015a, Mirman et al., 2015b).
In summary, this study investigated the multi-dimensionality of post-stroke aphasic deficits and fluency classification using this novel PCA-lesion mapping approach (Butler et al., 2014). The investigation was conducted in three stages: (i) confirmation of the basic results from a previous focussed-investigation of post-stroke fluency (Borovsky et al., 2007) – which represents a more “standard” approach to brain–behaviour correlation; (ii) decomposition of speech production measures using rotated PCA and identification of the neural correlates; and then, most importantly, (iii) simultaneous decomposition of a large behavioural dataset that included measures of speech production, phonology, semantics and executive abilities using an omnibus PCA, and subsequent identification of their unique neural correlates.
2. Methods
2.1. Participants
Thirty one chronic stroke patients (either ischaemic or haemorrhagic) were recruited (the same patients as reported by Butler et al., 2014), who had impairment in producing and/or understanding spoken language and their aphasia was classified using the Boston Diagnostic Aphasia Examination (BDAE). No restrictions were placed according to aphasia type or severity (spanning global to minimal aphasia). All patients were at least 12 months post-stroke at the time of scanning and assessment, were native English speakers with normal or corrected-to-normal hearing and vision (see Table 1 for a summary of demographic details). Our selection criteria excluded participants if they had any contraindications for scanning, were pre-morbidly left handed, had more than one stroke or had any other significant neurological conditions. Informed consent was obtained from all participants prior to participation under approval from the local ethics committee. Structural imaging data from a healthy age and education matched control group (eight female, 11 male) were used to determine the lesion outline in the patients using the Seghier, Ramlackhansingh, Crinion, Leff, and Price (2008) lesion identification toolbox.
Table 1.
Participant background information. Cases are ordered according to BNT score.
| Initials | Age (years) | Gender | Years of education | Time post-stroke (months) | Lesion volume (voxels) | BDAE classification | |
|---|---|---|---|---|---|---|---|
| 1 | DBb | 66 | M | 12 | 59 | 42,687 | Wernicke |
| 2 | ES | 69 | M | 11 | 39 | 28,146 | Global |
| 3 | ESb | 68 | M | 11 | 142 | 33,193 | Global |
| 4 | KW | 81 | M | 10 | 24 | 11,393 | Broca |
| 5 | BS | 59 | M | 11 | 103 | 27,242 | Broca |
| 6 | KL | 55 | M | 13 | 31 | 14,625 | Mixed non-fluent |
| 7 | LM | 63 | M | 11 | 13 | 14,990 | Global |
| 8 | DB | 60 | M | 12 | 44 | 31,644 | Wernicke |
| 9 | PE | 73 | F | 16 | 22 | 6959 | Wernicke/conduction |
| 10 | KS | 59 | M | 12 | 12 | 5822 | Transcortical sensory aphasia |
| 11 | KK | 48 | M | 12 | 33 | 20,043 | Broca |
| 12 | WM | 77 | M | 11 | 66 | 33,282 | Mixed non-fluent |
| 13 | GL | 47 | M | 12 | 18 | 26,319 | Broca |
| 14 | DCS | 45 | F | 12 | 12 | 5273 | Broca |
| 15 | JSa | 73 | M | 11 | 190 | 45,875 | Mixed non-fluent |
| 16 | JSc | 78 | M | 12 | 76 | 18,459 | Broca |
| 17 | JA | 65 | M | 11 | 128 | 26,097 | Mixed non-fluent |
| 18 | JJ | 84 | M | 12 | 25 | 8951 | Anomia |
| 19 | JM | 62 | M | 11 | 110 | 15,492 | Anomia |
| 20 | JSb | 72 | M | 11 | 23 | 1481 | Anomia |
| 21 | ER | 64 | F | 14 | 181 | 26,480 | Mixed non-fluent |
| 22 | HN | 81 | M | 10 | 56 | 25,963 | Anomia |
| 23 | BH | 64 | M | 11 | 26 | 8795 | Mixed non-fluent |
| 24 | EB | 61 | M | 17 | 12 | 4806 | Anomia |
| 25 | DM | 49 | M | 17 | 42 | 11,915 | Broca |
| 26 | DS | 72 | M | 11 | 106 | 11,446 | Transcortical motor aphasia |
| 27 | AG | 55 | M | 11 | 131 | 21,270 | Broca |
| 28 | LH | 65 | M | 11 | 81 | 10,073 | Anomia |
| 29 | JMf | 70 | F | 11 | 84 | 8921 | Anomia |
| 30 | AL | 49 | F | 12 | 69 | 9767 | Anomia |
| 31 | TJ | 60 | M | 12 | 23 | 19,975 | Anomia |
2.2. Neuropsychological assessments and analysis
In order to assess speech production deficits, participants were asked to undertake a picture description task (‘Cookie theft’ from the Boston Diagnostic Aphasia Examination) (Goodglass & Kaplan, 1983), which was audio recorded. Participants were instructed to “tell me everything you see going on in this picture”. In addition to the fluency measures, we utilised an extensive battery of neuropsychological tests to assess participants' language and cognitive abilities (described in Butler et al., 2014), enabling us to understand how speech production measures relate to the patients' input and output phonological, semantic and general executive abilities. These included subtests from the Psycholinguistic Assessments of Language Processing in Aphasia (PALPA) battery (Kay, Lesser, & Coltheart, 1992): auditory discrimination using non-word (PALPA 1) and word minimal pairs (PALPA 2); and immediate and delayed repetition of non-words (PALPA 8) and words (PALPA 9). Tests from the 64-item Cambridge Semantic Battery (Bozeat, Lambon Ralph, Patterson, Garrard, & Hodges, 2000) were included: spoken and written versions of the word-to-picture matching task; Camel and Cactus Test (CCT – picture); and the picture naming test. To increase the sensitivity to mild naming and semantic deficits we used the Boston Naming Test (BNT) (Kaplan, Goodglass, & Weintraub, 1983) and a written 96-trial synonym judgement test (Jefferies, Patterson, Jones, & Lambon Ralph, 2009). The spoken sentence comprehension task from the Comprehensive Aphasia Test (CAT) (Swinburn, Baker, & Howard, 2005) was used to assess sentential receptive skills. The additional cognitive tests included forward and backward digit span (Wechsler, 1987), the Brixton Spatial Rule Anticipation Task (Burgess & Shallice, 1997), and Raven's Coloured Progressive Matrices (Raven, 1962). All scores were converted into percentage. Assessments were conducted with participants over several testing sessions, with the pace and number determined by the participant.
The ‘Cookie theft’ description was recorded and then transcribed. The coding procedure followed the method used by Borovsky et al. (2007). Each utterance was marked and bound morphemes, repetitions, false starts, retraces, unintelligible material and interruptions were coded separately. Repeated and retraced utterances were excluded from analysis and only correct full utterances were coded. When the boundary of an utterance was unclear, or quite lengthy, transcribers applied the rule from Lee (1974) that only one “and” conjunction per sentence was allowed when the “and” connected two independent clauses. From these transcriptions, we extracted the number of word T and types, TTR, number of morphemes and MLU in morphemes. In order to control for the length of response given by each participant, we also computed words-per-minute (WPM). We considered these measures as indices of speech fluency as they are among the simplest to quantify and mirror those commonly used in other studies and clinical practice. There are a number of additional measures that could be used to elaborate the model of fluency, including syntactical features (i.e., see Thompson et al., 2012). These, however, require a more open-ended speech sample and are relatively time-consuming to code. We believe that between the four measures used here, we are capturing the amount of speech output within a confined context. All scores were converted into percentage, where the max score across participants was used.
2.3. Principal component analysis
This analysis was split into two parts. First, only the speech production measures were entered into a PCA with varimax rotation (SPSS 16.0). Factors with an eigenvalue exceeding 1.0 were extracted and then rotated. Following orthogonal rotation, the loadings of each test allowed a clear behavioural interpretation of each factor. Individual participants' scores on each extracted factor were then used as behavioural covariates in the neuroimaging analysis. A second PCA was performed on a larger dataset which combined the background neuropsychological data with speech production measures. This allowed us to determine how the speech production factors related to the three previously-extracted (Butler et al., 2014) factors relating to phonology, semantic and executive abilities.
2.4. Acquisition of neuroimaging data
High-resolution structural T1-weighted MRI scans were acquired on a 3.0 T Philips Achieva scanner (Philips Healthcare, Best, The Netherlands) using an eight-element SENSE head coil. A T1-weighted inversion recovery sequence with 3D acquisition was employed, with the following parameters: TR (repetition time) = 9.0 msec, TE (echo time) = 3.93 msec, flip angle = 8°, 150 contiguous slices, slice thickness = 1 mm, acquired voxel size 1.0 × 1.0 × 1.0 mm3, matrix size 256 × 256, field of view (FOV) = 256 mm, TI (inversion time) = 1150 msec, SENSE acceleration factor 2.5, total scan acquisition time = 575 sec.
2.5. Analysis of neuroimaging data
Structural MRI scans were pre-processed with Statistical Parametric Mapping software (SPM8: Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/). The images were normalised into standard Montreal Neurological Institute (MNI) space using a modified unified segmentation–normalisation procedure optimised for focal lesioned brains (Seghier et al., 2008). Data from all participants with stroke aphasia and all healthy controls were entered into the segmentation–normalisation. This procedure combines segmentation, bias correction and spatial normalisation through the inversion of a single unified model (see Ashburner & Friston, 2005 for more details). In brief, the unified model combines tissue class (with an additional tissue class for abnormal voxels), intensity bias and non-linear warping into the same probabilistic models that are assumed to generate subject-specific images. Images were then smoothed with an 8 mm full-width-half-maximum (FWHM) Gaussian kernel and used in the lesion analyses described below. The lesion of each patient was automatically identified using an outlier detection algorithm, compared to healthy controls, based on fuzzy clustering. The default parameters were used apart from the lesion definition ‘U-threshold’, which was set to .5 to create a binary lesion image. We modified the U-threshold from .3 to .5 after comparing the results obtained from a sample of patients to what would be nominated as lesioned tissue by an expert neurologist. The images generated for each patient were individually checked and visually inspected with respect to the original scan, and were used to create the lesion overlap map in Fig. 1 and the individual lesion outlines in Fig. 3. We selected the Seghier et al. (2008) method as it is objective and efficient for a large sample of patients (Wilke, de Haan, Juenger, & Karnath, 2011), in comparison to a labour intensive hand-traced lesion mask. The method has been shown to have a Dice overlap >.64 with manual segmentation of the lesion and >.7 with a simulated ‘real’ lesion (where real lesions are superimposed onto healthy brains; Seghier et al., 2008). We should note here, explicitly, that although commonly referred to as an automated ‘lesion’ segmentation method, the technique detects areas of unexpected tissue class – and, thus, identifies missing grey and white matter but also areas of augmented cerebral spinal fluid (CSF) space.
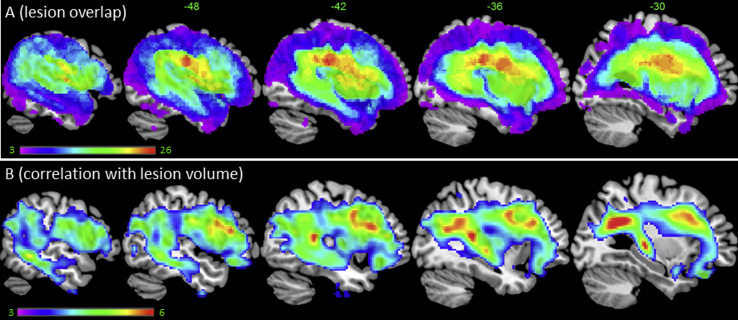
Fig. 1.
(A) Lesion overlap map across the 31 patients (threshold 1–26). (B) VBCM between regional integrity and lesion volume (t-scale 3–6).
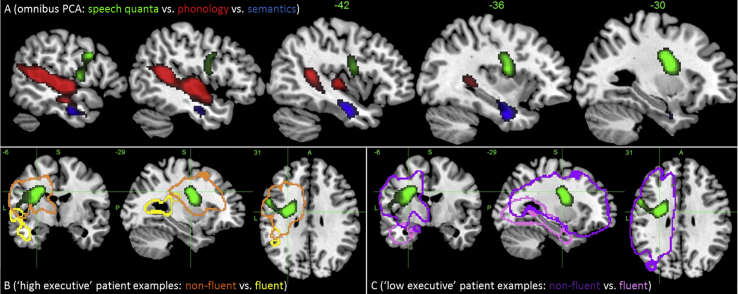
Fig. 3.
(A) VBCM analysis showing significant and unique neural correlates to phonological (red), semantic (blue) and speech quanta (green) performance, including lesion volume covariate. Image thresholded at p < .005, cluster corrected at FWE of p < .01 [image scale (t) 3–5]. Illustrative exemplar cases are shown in panels B and C. Panel B (patients with good executive scores) – patient EB (yellow) with good fluency versus KW (orange) with poor fluency. Panel C (patients with poor executive skills) – patient KS (pink) with good fluency versus ESb (purple) with poor fluency.
We used the T1-weighted images with continuous signal intensity values across the whole brain and correlated these with individual measures or PCA factor scores using a voxel-based correlational methodology (VBCM: Tyler, Marslen-Wilson, & Stamatakis, 2005), a variant of voxel-lesion symptom mapping (VSLM: Bates et al., 2003). VBCM does not require a binary classification of the intact/lesioned brain to be marked, as in the case of VSLM, as both the behaviour and tissue concentration measures are treated as continuous variables (conducted in SPM8). Three analyses were conducted and all reported anatomical regions were located on the left hemisphere. First, each fluency measure was analysed separately, in an attempt to confirm the basic results from Borovsky et al. (2007). Secondly, the factor scores from the first speech production PCA were simultaneously entered to investigate how variation in tissue concentration corresponded to the unique components of speech dys-/fluency. Finally, the participants' factor scores from the omnibus PCA of the entire neuropsychological test battery and speech measures were entered into a VBCM analysis. In order to ensure that the results were not merely attributable to lesion size, each participants' lesion volume was calculated from the lesion identified by the automated lesion identification method (Seghier et al., 2008) and this was entered as a covariate in each VBCM. Hence, all analyses were performed with and without a correction for lesion volume. All anatomical labels were based on the Harvard–Oxford atlas in MNI space.
3. Results
3.1. Neuropsychological and lesion profiles
Table 2 summarises the participants' scores on the speech production measures and a large neuropsychological battery (Butler et al., 2014) and is ordered according to patients' scores on the BNT. A lesion overlap map for stroke aphasic participants is provided in Fig. 1A, and primarily covers the left hemisphere area supplied by the MCA (Phan et al., 2005). The maximum number of participants who had a lesion in any one voxel was 26 (−45, −23, 27; ventral portion of the postcentral gyrus).
Table 2.
Participant scores on the behavioural assessment battery and speech production measures.
| ID | NW rep I | NW rep d | W rep I | W rep d | Picture naming | Boston naming | NW min pairs | W min pairs | SWPM | WWPM | CAT–sentence | Synonym | CCT | Brixton | Ravens | Digit F | Digit B | WPM | TTR | MLU | T |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBb | 0 | 0 | 37.5 | 0 | 0 | 0 | 22.22 | 52.78 | 57.81 | 31.25 | 12.5 | 48.96 | 53.13 | 38.18 | 30.56 | 25 | 0 | 63.33 | 53.91 | 60.74 | 23.89 |
| ES | 0 | 0 | 0 | 0 | 4.69 | 0 | 48.61 | 54.17 | 78.13 | 90.63 | 25 | 72.92 | 73.44 | 40 | 66.67 | 0 | 0 | 15.12 | 94.09 | 35.19 | 24.63 |
| ESb | 0 | 0 | 0 | 0 | 0 | 0 | 54.17 | 50 | 87.5 | 60.94 | 34.38 | 52.08 | 43.75 | 23.64 | 38.89 | 0 | 0 | 0 | 0 | 0 | 0 |
| KW | 0 | 0 | 3.75 | 0 | 1.56 | 0 | 75 | 65.28 | 95.31 | 92.19 | 84.38 | 82.29 | 89.06 | 50.91 | 80.56 | 50 | 42.86 | 2 | 86.25 | 12.35 | 2.99 |
| BS | 3.33 | 0 | 5 | 1.25 | 3.13 | 1.67 | 65.28 | 75 | 92.19 | 100 | 31.25 | 78.13 | 84.38 | 38.18 | 91.67 | 0 | 0 | 52.61 | 63.45 | 48.15 | 21.65 |
| KL | 0 | 0 | 6.25 | 0 | 4.69 | 1.67 | 75 | 77.78 | 92.19 | 98.44 | 28.13 | 68.75 | 78.13 | 61.82 | 88.89 | 0 | 0 | 11.87 | 44.72 | 21.16 | 13.44 |
| LM | 13.33 | 3.33 | 27.5 | 0 | 1.56 | 1.67 | 43.06 | 54.17 | 67.19 | 53.13 | 28.13 | 57.29 | 68.75 | 32.73 | 61.11 | 0 | 0 | 14.51 | 46 | 18.52 | 7.47 |
| DB | 70 | 30 | 85 | 83.75 | 7.81 | 8.33 | 87.5 | 58.33 | 64.06 | 76.56 | 31.25 | 59.38 | 82.81 | 40 | 86.11 | 37.5 | 14.29 | 17.42 | 38.33 | 65.36 | 89.58 |
| PE | 13.33 | 3.33 | 45 | 41.25 | 20.31 | 11.67 | 77.78 | 86.11 | 96.88 | 100 | 50 | 79.17 | 84.38 | 41.82 | 80.56 | 25 | 28.57 | 49.1 | 60.62 | 93.7 | 100 |
| KS | 73.33 | 80 | 93.75 | 95 | 31.25 | 13.33 | 94.44 | 95.83 | 71.88 | 67.19 | 84.38 | 84.38 | 68.75 | 52.73 | 86.11 | 100 | 57.14 | 70.53 | 86.6 | 90.53 | 60.47 |
| KK | 33.33 | 3.33 | 56.25 | 26.25 | 42.19 | 15 | 72.22 | 95.83 | 93.75 | 95.31 | 46.88 | 81.25 | 84.38 | 76.36 | 100 | 0 | 0 | 14.75 | 62.21 | 50 | 45.54 |
| WM | 36.67 | 30 | 55 | 41.25 | 39.06 | 25 | 47.22 | 63.89 | 92.19 | 75 | 50 | 61.46 | 51.56 | 43.64 | 61.11 | 37.5 | 28.57 | 5.44 | 65.71 | 22.22 | 10.45 |
| GL | 93.33 | 63.33 | 100 | 81.25 | 68.75 | 31.67 | 98.61 | 97.22 | 96.88 | 95.31 | 65.63 | 75 | 73.44 | 58.18 | 91.67 | 37.5 | 28.57 | 9.24 | 55.86 | 29.97 | 52.26 |
| DCS | 40 | 56.67 | 72.5 | 68.75 | 67.19 | 43.33 | 97.22 | 97.22 | 100 | 98.44 | 93.75 | 91.67 | 95.31 | 81.82 | 100 | 62.5 | 57.14 | 14.83 | 92.74 | 70.37 | 23.14 |
| JSa | 30 | 3.33 | 75 | 65 | 62.5 | 46.67 | 75 | 77.78 | 92.19 | 98.44 | 59.38 | 81.25 | 76.56 | 67.27 | 83.33 | 50 | 28.57 | 30.84 | 74.41 | 49.38 | 25.38 |
| JSc | 36.67 | 63.33 | 90 | 91.25 | 71.88 | 53.33 | 75 | 86.11 | 98.44 | 98.44 | 75 | 76.04 | 82.81 | 43.64 | 77.78 | 62.5 | 42.86 | 23.9 | 90.36 | 61.11 | 20.9 |
| JA | 36.67 | 40 | 85 | 78.75 | 79.69 | 63.33 | 90.28 | 95.83 | 100 | 98.44 | 78.13 | 63.54 | 87.5 | 61.82 | 80.56 | 37.5 | 0 | 46.26 | 74.41 | 60.74 | 25.38 |
| JJ | 36.67 | 23.33 | 82.5 | 73.75 | 85.94 | 63.33 | 51.39 | 80.56 | 98.44 | 98.44 | 56.25 | 93.75 | 53.13 | 43.64 | 41.67 | 62.5 | 42.86 | 41.98 | 85.19 | 82.72 | 40.31 |
| JM | 83.33 | 83.33 | 100 | 98.75 | 81.25 | 63.33 | 93.06 | 95.83 | 100 | 100 | 100 | 82.29 | 81.25 | 76.36 | 94.44 | 50 | 57.14 | 79.16 | 77.63 | 84.77 | 59.72 |
| JSb | 63.33 | 36.67 | 86.25 | 81.25 | 75 | 63.33 | 76.39 | 88.89 | 93.75 | 90.63 | 84.38 | 75 | 78.13 | 60 | 86.11 | 62.5 | 28.57 | 41.18 | 92 | 47.62 | 26.13 |
| ER | 53.33 | 36.67 | 70 | 81.25 | 71.88 | 65 | 81.94 | 88.89 | 95.31 | 93.75 | 56.25 | 84.38 | 90.63 | 41.82 | 38.89 | 25 | 0 | 46.49 | 82.14 | 95.06 | 47.03 |
| HN | 36.67 | 23.33 | 83.75 | 80 | 65.63 | 65 | 77.78 | 76.39 | 93.75 | 93.75 | 37.5 | 85.42 | 85.94 | 25.45 | 75 | 50 | 42.86 | 25.4 | 91.27 | 91.36 | 47.03 |
| BH | 86.67 | 80 | 100 | 96.25 | 95.31 | 66.67 | 93.06 | 94.44 | 98.44 | 93.75 | 78.13 | 83.33 | 73.44 | 67.27 | 66.67 | 62.5 | 57.14 | 36.77 | 78.68 | 60.49 | 28.37 |
| EB | 83.33 | 53.33 | 100 | 100 | 81.25 | 66.67 | 94.44 | 98.61 | 98.44 | 100 | 71.88 | 94.79 | 90.63 | 80 | 100 | 75 | 57.14 | 78.87 | 67.16 | 98.52 | 93.31 |
| DM | 60 | 10 | 73.75 | 68.75 | 75 | 71.67 | 80.56 | 93.06 | 98.44 | 98.44 | 56.25 | 95.83 | 98.44 | 50.91 | 91.67 | 37.5 | 0 | 23.63 | 84.74 | 50.79 | 28.37 |
| DS | 56.67 | 33.33 | 88.75 | 91.25 | 84.38 | 73.33 | 79.17 | 77.78 | 100 | 100 | 87.5 | 93.75 | 89.06 | 72.73 | 72.22 | 50 | 28.57 | 34.53 | 100 | 100 | 17.17 |
| AG | 73.33 | 83.33 | 77.5 | 87.5 | 87.5 | 78.33 | 100 | 98.61 | 100 | 100 | 87.5 | 89.58 | 75 | 56.36 | 75 | 100 | 100 | 13.06 | 80.5 | 54.81 | 22.4 |
| LH | 56.67 | 50 | 82.5 | 88.75 | 81.25 | 78.33 | 95.83 | 97.22 | 96.88 | 100 | 90.63 | 92.71 | 87.5 | 76.36 | 88.89 | 87.5 | 57.14 | 66.11 | 74.33 | 52.91 | 61.21 |
| JMf | 93.33 | 66.67 | 96.25 | 98.75 | 96.88 | 80 | 90.28 | 95.83 | 100 | 100 | 71.88 | 91.67 | 93.75 | 50.91 | 83.33 | 62.5 | 57.14 | 50.53 | 69 | 73.15 | 48.52 |
| AL | 90 | 90 | 100 | 98.75 | 93.75 | 88.33 | 91.67 | 100 | 100 | 100 | 84.38 | 93.75 | 79.69 | 60 | 91.67 | 87.5 | 85.71 | 100 | 86.25 | 87.65 | 44.79 |
| TJ | 93.33 | 83.33 | 98.75 | 92.5 | 95.31 | 95 | 87.5 | 98.61 | 98.44 | 100 | 68.75 | 88.54 | 70.31 | 52.73 | 50 | 75 | 28.57 | 38.28 | 76.67 | 81.48 | 38.07 |
Abbreviations: W – word; NW – non-word; rep I – immediate repetition; rep D – delayed repetition; S/WWPM – spoken/written word–picture matching; Digit F – forward digit span; Digit B – backward digit span.
CAT column refers to spoken sentence comprehension subtest.
3.2. Confirmation of previous basic findings on speech fluency alone
A bivariate correlation analysis on the speech production measures showed a high degree of inter-correlation. The token measure was correlated with MLU and WPM (r = .633, p < .001 and r = .513, p < .001 respectively). The MLU measure was correlated with TTR and WPM (r = .505, p = .004 and r = .674, p < .001 respectively). No other correlations reached significance. As noted by Borovsky et al. (2007), it is not unexpected to find inter-correlations between these measures. Indeed, the shared variance could reflect common factors underlying speech, including semantics, phonology and general aphasia severity. As highlighted in the Introduction, these high inter-correlations provide a challenge when attempting to relate the behavioural measures to unique aspects of the patients' lesions (see below).
The first VBCM analysis examined the neural correlates of fluency (T), complexity (MLU), semantic variety (TTR) and WPM each entered separately (Fig. 2A–D). The results for T revealed involvement of the superior-posterior insula and portions of the frontal lobe including the pre-central gyrus and middle frontal gyrus. The status of underlying white matter was also correlated. The analysis for MLU revealed an overlapping albeit broader region, extending throughout the left inferior frontal gyrus (IFG), insula and putamen, along the SMA, motor cortex and the underlying white matter.
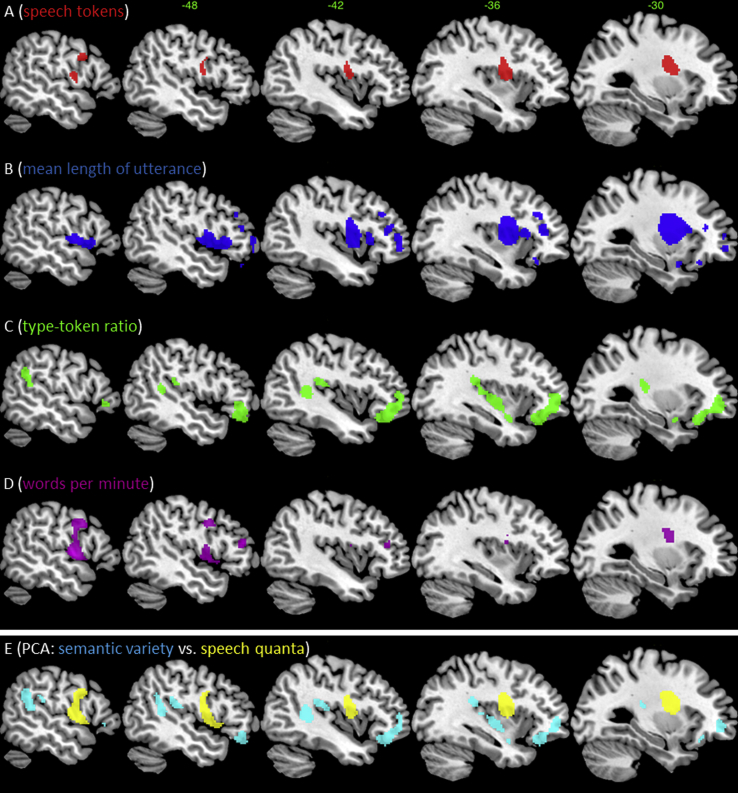
Fig. 2.
VBCM analysis using each speech production measure as the dependent measure [each t-map (scale 3) is thresholded at p < .005, cluster corrected at FWE of p < .001; without lesion volume correction]. Note each result reflects the raw (non-unique) correlation with each speech fluency measure (see text). (A) Lesion correlates for T. (B) Lesion correlates for mean length per utterance, (C) TTR. (D) Lesion correlates for WPM (uncorrected threshold p = .01, voxel-extent 50). (E) Lesion correlates of the unique PCA factors: F1 reflects speech quanta (yellow) and F2 reflects semantic variety (cyan). Note – no significant cluster remains for any factor after lesion volume correction (see text for further details & Fig. 1B).
The results for TTR, contrasted with those for T and MLU, in revealing a region that extended from supermarginal gyrus (SMG) and pMTG/STG through to aSTG as well as a portion of the anterior/orbito frontal gyrus. Peak voxels were in the IFG/frontal pole, SMG and posterior superior temporal gyrus (pSTG). Finally, the analysis for WPM revealed no significant correlations with lesion areas, although at an uncorrected voxel height of p = .01 (cluster extent 50 voxels) the results were very similar to those obtained for T.
3.3. Effect of lesion size
Given that some regions are more likely than others to be damaged after MCA stroke (Phan et al., 2005), we explored the intrinsic relationship between lesion location and size, and then controlled for this factor in the subsequent lesion–symptom analyses. Each participant's volume was calculated from the lesion identified by the modified segmentation–normalisation procedure. Lesion volume itself was correlated with the integrity of various regions with the MCA territory (Fig. 1B), representing the outer belt of the lesion overlap map (Fig. 1A). Crucially, including lesion size in the VBCM analysis for each speech measure reduced the significance across all measures and the underlying neural correlates (see Fig. 2A–D). The TTR and T measures did not correlate with any regions after accounting for lesion volume (even at a reduced image threshold of p < .01, FWE-cluster corrected p < .05). MLU was the only measure to reveal significant relationships with the left superior insula, putamen and underlying white matter after accounting for lesion volume.
3.4. Identifying principal speech-production components
The speech production measures were subjected to a rotated PCA and produced a two independent factor solution, which accounted for 84.65% of variance in participants' performance (F1 = 59.28%; F2 = 25.35%). The factor loading of each production measure is given in Table 3. The measures that tapped into overall quantity of speech (e.g., number of words and WPM) loaded heavily on factor 1; hence we refer to this factor as ‘speech quanta’. Factor 2 was interpreted as ‘semantic variety’, as the TTR measurement loaded heavily on it (e.g., proportion of unique words spoken). The MLU loaded heavily on factor 1, while loading to a lesser degree on factor 2. Lesion analysis for each factor, entered simultaneously, is displayed in Fig. 2E. The speech quanta measure (F1) was uniquely correlated with the left pre-central gyrus, superior insula and putamen, and the underlying white matter (including the superior longitudinal fasciculus, caudate–premotor tracts and the frontal ‘aslant’ tract (FAT) that connects the medial-superior portion of the frontal lobe to the inferior-lateral frontal region) (Basilakos et al., 2014, Catani et al., 2012, Rojkova et al., 2015). The semantic variety factor (F2) was uniquely correlated with the SMG and pMTG/STG plus the anterior/orbito frontal gyrus. Weaker correlation is observed along the STG to anterior lateral portions. After adding a covariate for lesion volume, no significant clusters remained for F1 or F2.
Table 3.
Factor loadings for PCA of speech production measures.
| Measure | F1 | F2 |
|---|---|---|
| TTR | .082 | .973 |
| MLU | .803 | .488 |
| T | .904 | −.157 |
| WPM | .800 | .258 |
Footnote: Factor loadings exceeding .5 are marked in bold.
3.5. Identifying principal speech, language and executive behavioural factors
In order to determine the relationship between speech production measures and a range of language and cognitive skills, the factor scores from the speech PCA were correlated with the factor scores for phonology, semantics and executive abilities, computed in our previous study (Butler et al., 2014). The speech quanta factor only correlated with the first (phonology) factor (r = .404, p = .01). The semantic variety speech factor only correlated with the second (semantics) factor (r = .452, p < .001). To understand the relationship between speech fluency and various language and cognitive measures more thoroughly, an omnibus PCA was conducted. This resulted in a four factor solution including phonological skills (54.8% variance), semantic ability (11.6% variance) and cognitive–executive function (8.3% variance). We note here that a variety of different tasks over-and-above the executive tests loaded on this third factor. These include measures associated with auditory–phonological processing (e.g., minimal pairs) or semantic memory (e.g., CCT), although in both cases there was additional high loadings on the component one would typically associate with these tests. This outcome probably reflects the fact that the PCA process ‘decomposes’ the components of each task (reflecting the fact that no task is a pure measure of a single underpinning cognitive function). In this case, all the tasks that load onto the third factor require a high degree of additional ‘problem-solving’ and executively-loaded working memory.
In this omnibus PCA, the ‘semantic variety’ characteristics of speech production were subsumed into the general semantics factor and a new additional fourth factor emerged based entirely on ‘speech quanta’ (6.3% variance) (see Table 4 for factor loadings and Table 5 for the patients' factors scores). The VBCM analysis for each independent factor, entered simultaneously, is shown in Fig. 3A and peak MNI co-ordinates are shown in Table 6. Each cluster shows where tissue concentration covaries uniquely with a given factor score, after accounting for lesion volume (the results without the lesion volume covariate were very similar). Results are thresholded at p < .005 voxel-level, p ≤ .001 family-wise error (FWE)-corrected cluster-level.
Table 4.
Factor loadings from the omnibus PCA.
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Non-word repetition immediate | .822 | .108 | .220 | .326 |
| Non-word repetition delayed | .912 | .106 | .126 | .175 |
| Word repetition immediate | .780 | .230 | .122 | .430 |
| Word repetition delayed | .783 | .315 | .168 | .411 |
| Forward digit span | .841 | .239 | .058 | .198 |
| Backward digit span | .774 | .191 | .166 | .070 |
| Non-word minimal pairs | .584 | .203 | .683 | .167 |
| Word minimal pairs | .586 | .421 | .505 | .237 |
| CAT – sentence | .726 | .388 | .374 | −.050 |
| Cambridge 64-item naming | .712 | .600 | .096 | .168 |
| BNT | .659 | .629 | .013 | .209 |
| 96-Synonym judgement | .353 | .714 | .347 | .244 |
| Spoken word-to-picture matching | .311 | .722 | .378 | −.190 |
| Written word-to-picture matching | .156 | .699 | .578 | .022 |
| Camel and Cactus – pictures | −.078 | .461 | .702 | .276 |
| Raven's Coloured Progressive | .091 | .034 | .918 | .079 |
| Brixton spatial anticipation test | .391 | .232 | .629 | .018 |
| TTR | .235 | .749 | .055 | .160 |
| WPM | .310 | .103 | .013 | .716 |
| Mean length per utterance | .294 | .397 | .000 | .814 |
| T | .154 | −.152 | .350 | .818 |
Footnote: Factor loadings exceeding .5 are marked in bold.
Table 5.
PCA factor scores from Butler et al. (2014), fluency-only PCA and the omnibus PCA.
|
Butler et al. (2014) |
Speech PCA |
All scores combined |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| F1-phon | F2-sem | F3-exe | F1-flu | F2-var | F1 | F2 | F3 | F4 | |
| DBb | −.332 | −2.321 | −2.018 | .290 | −.499 | −.775 | −1.600 | −2.606 | 1.043 |
| ES | −1.668 | −.266 | −.331 | −1.024 | .797 | −1.842 | .384 | −.564 | −.256 |
| ESb | −1.078 | −.701 | −1.911 | −1.360 | −3.030 | −.300 | −1.773 | −1.290 | −1.923 |
| KW | −1.211 | −.202 | .972 | −1.853 | .540 | −.832 | .387 | .700 | −1.929 |
| BS | −1.935 | .267 | .678 | −.136 | −.217 | −1.952 | .249 | .604 | .120 |
| KL | −1.810 | −.072 | .972 | −1.046 | −1.185 | −1.391 | −.516 | 1.193 | −1.175 |
| LM | −.873 | −1.682 | −.765 | −1.171 | −1.072 | −.843 | −1.445 | −.946 | −.606 |
| DB | .315 | −2.295 | .594 | 1.151 | −2.058 | −.149 | −2.555 | .658 | 1.491 |
| PE | −1.067 | .358 | .459 | 1.961 | −.980 | −1.426 | −.090 | .706 | 1.723 |
| KS | 1.726 | −2.395 | .718 | 1.209 | .616 | 1.274 | −1.524 | −.015 | 1.274 |
| KK | −1.207 | .161 | 1.319 | −.164 | −.642 | −1.129 | −.158 | 1.504 | −.149 |
| WM | −.036 | −.525 | −1.344 | −1.364 | −.297 | .363 | −.567 | −1.198 | −1.458 |
| GL | .618 | −.361 | .583 | −.284 | −1.181 | .971 | −1.148 | 1.107 | −.715 |
| DCS | .208 | .045 | 1.680 | −.642 | 1.080 | .346 | .564 | 1.351 | −.819 |
| JSa | −.321 | .309 | .233 | −.453 | .148 | −.240 | .358 | .221 | −.398 |
| JSc | .416 | .356 | −.256 | −.643 | .947 | .461 | .657 | −.344 | −.500 |
| JA | −.124 | .593 | .308 | −.099 | .279 | .009 | .377 | .417 | −.170 |
| JJ | .236 | 1.429 | −2.197 | .307 | .706 | .131 | 1.420 | −2.027 | .348 |
| JM | .993 | −.053 | .863 | 1.328 | .227 | .998 | −.210 | .805 | .683 |
| JSb | .472 | .006 | .120 | −.456 | .854 | .537 | .269 | .003 | −.409 |
| ER | −.241 | 1.337 | −1.092 | .677 | .612 | −.622 | 1.273 | −.957 | 1.189 |
| HN | −.089 | .712 | −.692 | .253 | .909 | −.534 | 1.048 | −.754 | .927 |
| BH | 1.204 | .216 | −.296 | −.214 | .392 | 1.422 | .090 | −.198 | −.522 |
| EB | .760 | .174 | 1.150 | 2.256 | −.526 | .451 | −.204 | 1.215 | 1.739 |
| DM | −.607 | 1.269 | .420 | −.566 | .525 | −.727 | 1.239 | .551 | −.013 |
| DS | .128 | 1.045 | .041 | −.199 | 1.760 | −.051 | 1.653 | −.282 | .129 |
| AG | 1.419 | .210 | −.064 | −.759 | .451 | 1.810 | .244 | −.018 | −1.321 |
| LH | .704 | .354 | .807 | .830 | −.240 | .765 | .101 | .885 | .234 |
| JMf | .811 | .737 | .110 | .624 | −.131 | .755 | .391 | .294 | .467 |
| AL | 1.446 | .262 | .228 | 1.285 | .842 | 1.353 | .365 | .000 | .834 |
| TJ | 1.141 | 1.029 | −1.289 | .265 | .373 | 1.165 | .718 | −1.013 | .161 |
Table 6.
Neural correlates for omnibus PCA factors after accounting for lesion volume.
| Principal component | Location | Extent (voxels) | Z | MNI co-ordinates |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Factor 1 (phonology) | Left temporal lobe | 4387 | ||||
| Planum polare | 4.24 | −48 | −16 | 0 | ||
| Posterior supramarginal gyrus | 4.23 | −48 | −50 | 14 | ||
| Posterior middle temporal gyrus | 4.06 | −62 | −18 | −14 | ||
| Factor 2 (semantic) | Left temporal lobe | 1030 | ||||
| Anterior middle temporal gyrus | 3.68 | −60 | −6 | −26 | ||
| Anterior temporal fusiform cortex | 3.63 | −38 | −6 | −28 | ||
| Posterior ITG | 2.77 | −70 | −24 | −30 | ||
| Factor 4 (speech quanta) | Left prefrontal lobe | 2164 | ||||
| Corticospinal tract | 3.85 | −24 | −4 | 32 | ||
| Pre-central gyrus | 3.80 | −30 | −4 | 28 | ||
| Pre-central gyrus | 3.58 | −54 | 8 | 32 | ||
Performance on the phonological factor was uniquely correlated with voxels across a number of left hemisphere regions: primary auditory cortex (Brodmann area 41 and 42); mid to posterior middle and superior temporal gyri (MTG and STG); superior temporal sulcus (STS); and posterior portions of the insula, Heschl's gyrus and the planum temporale. The phonological cluster also overlapped with white matter regions, encompassing part of the arcuate fasciculus, a key aspect of the dorsal language pathway (Catani and ffytche, 2005, Catani et al., 2005, Duffau et al., 2005, Parker et al., 2005, Saur et al., 2008).
Performance on the semantic factor was uniquely related to a cluster in the left hemisphere anterior temporal lobe (ATL; see Fig. 3A). The cluster overlapped with the anterior-MTG and the temporal stem (including the dorsal edges of the inferior temporal gyrus – ITG and FG). Thus, with regards to white matter, the cluster included part of the ventral language route, overlapping with the inferior longitudinal fasciculus, inferior fronto-occipital fasciculus and uncinate fasciculus (Duffau et al., 2009, Mummery et al., 1999, Parker et al., 2005, Saur et al., 2008).
Performance on the speech quanta factor was uniquely related to a cluster in the left hemisphere pre-central gyrus, superior insula and putamen, extending medially to the caudate nucleus. With regards to white matter, the cluster included an area corresponding to the anterior part of the dorsal language route (superior longitudinal fasciculus) as well as the caudate–premotor tracts and the FAT (Rojkova et al., 2015). It is notable that the effect of lesion size on the speech quanta factor when calculated using all neuropsychological scores is negligible which was not the case when using the speech production measures alone (see above, Figs. 2E and 3A).
In contrast to the phonological, semantic and fluency factors, there were no clusters that correlated uniquely with the executive factor score which survived correction for multiple comparisons. This factor did, however, correlate with lesion volume in a simple correlation [r(31) = −.373, p = .039], suggesting that larger lesions result in poorer executive ability.
3.6. Individual cases
The relationship of individual patients to the group-level analyses was explored for two reasons. First, to test clinical utility, it is important to explore how individual results relate to the group-level maps for each language–cognitive factor. Secondly, exemplar cases help interpretation of the behavioural factors and their neural correlates (given that PCA, by design, generates scores that are one step removed from raw clinical measures: see Lambon Ralph et al., 2003). To achieve this aim, the group was divided into two using a median split based on executive factor scores to cover a range of patient types. Two participants were selected from each split to provide contrasting pairs who scored high or low on the speech quanta factor (see Fig. 3B and C). In the high executive group, patient KW (Broca's aphasia) had the lowest speech quanta score (orange outline) and EB (anomic) had the highest (yellow outline). In the low executive group, patient ESb (global) had the lowest speech quanta score (purple outline) and KS (transcortical sensory aphasia) the highest (pink outline). It is clear and encouraging from these examples that the individual patient lesions and associated behavioural profiles both align with the group-level VBCM results and are stable regardless of overall executive functioning (as should be expected given that the PCA approach extracts statistically-independent factors).
4. Discussion
Mirroring the principal features of natural language, speech fluency, phonological and semantic deficits are common following left MCA stroke. In most aphasia classification systems, patients are first divided by speech fluency followed by the relative balance of the patients' phonological and semantic abilities (Goodglass and Kaplan, 1983, Kertesz, 1982). By measuring and applying novel analyses to the overall rate, complexity and variety of naturalistic speech simultaneously with other measures of language performance, we were able to provide insights into their neural substrates. The results provide novel insights not only about the core features and neural bases of naturalistic language, but also how these underpin the varieties and nature of post-stroke aphasia.
In the first analysis, we largely confirmed the basic speech fluency results from Borovsky et al. (2007), by showing that the rate of T and MLU in the patients' speech correlated with the insula, SMA and underlying white matter. In contrast, TTR correlated with the SMG, pMTG, aSTG, and anterior/orbito frontal gyrus. Whilst these results are interesting and support previous observations that speech fluency is a multi-faceted aphasic feature (Ackermann and Riecker, 2004, Basilakos et al., 2014), their interpretation is complicated by the fact that these behaviour–lesion correlations were not significant when lesion volume was controlled and no account is taken of the patients' other aphasic features – thus the picture of the lesion–symptom mappings is incomplete.
We addressed these limitations by utilising the PCA-VBCM methodology in the two subsequent stages. We initially undertook a PCA of the speech production measures alone. This PCA suggested that the clinical concept of ‘fluency’ reflects two more general and independent factors: ‘speech quanta’ (the quantity of speech produced) and ‘variety’ (the range of different words/token irrespective of the quantity produced). Scores from the speech quanta factor correlated uniquely with tissue damage in the left pre-central gyrus, superior insula and putamen, and the neighbouring white matter (including the superior longitudinal fasciculus, caudate–premotor tracts and the FAT that connects the medial-superior portion of the frontal lobe to the inferior-lateral frontal region) (Rojkova et al., 2015). In contrast, the speech variety factor was uniquely related to the SMG and pMTG/STG plus the anterior/orbito frontal gyrus. However, again the results for these speech production-only data were largely confounded with lesion volume.
These twin challenges of inter-correlation and severity were overcome in the final step in which we completed an omnibus PCA containing all the detailed behavioural data and the speech production measures, which also allowed us to explore the relationship between speech fluency and other aphasic features (Basilakos et al., 2014). This analysis revealed four unique factors (phonology, executive processing, semantic function which included the speech variety measure, and speech quanta). The phonological factor uniquely correlated with damage to left perisylvian areas, including left mid to posterior STG, MTG, STS and Heschl's gyrus (HG), as well as the underlying white matter (arcuate fasciculus portion of the dorsal language pathway). The semantic factor correlated uniquely with left anterior-MTG and the underlying temporal stem. The speech quanta factor correlated uniquely with left hemisphere pre-central gyrus, superior insula and putamen, extending medially to the caudate nucleus. With regards to white matter, the cluster included an area corresponding to the anterior part of the dorsal language route (superior longitudinal fasciculus) as well as the caudate–premotor tracts and the FAT. The executive factor did not covary, uniquely, with any brain regions, as found previously (Butler et al., 2014).
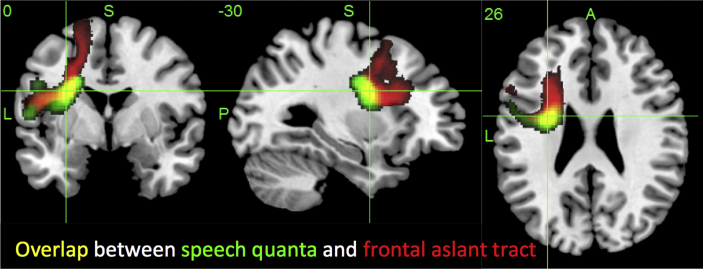
The speech quanta-related region included key cortical and subcortical areas associated with deficits of articulatory planning (Bates et al., 2003, Borovsky et al., 2007, Dronkers, 1996) and motor coordination of speech related movements (Ackermann & Riecker, 2004). Likewise, direct stimulation of ventral PMC and pars opercularis leads to speech inhibition (Kinoshita et al., 2014). The underlying white matter damage might also be crucial and, indeed, the core of the identified speech quanta-related region overlaps with the FAT (Rojkova et al., 2015; see Fig. 4). The integrity of this tract has been shown to correlate with non-fluent speech in primary progressive aphasia (Catani et al., 2013, Mandelli et al., 2014) and, when combined with the integrity of the anterior section of the arcuate fasciculus, to correlate with clinician-rated fluency in post-stroke aphasia (Basilakos et al., 2014). In keeping with our finding of a separate principal component for speech quanta over and above phonology and semantic skills, Catani et al. (2013) did not find a relationship between FAT integrity and repetition or syntactic abilities in primary progressive aphasia. Additional convergent evidence has been derived from neurosurgical investigations: direct electrical stimulation of FAT inhibits speech and subsequent surgical resection leads to transient postoperative inhibition (Kinoshita et al., 2014).
Fig. 4.
Overlap (yellow) between the speech quanta-related lesion correlate (green) and the FAT (red). The speech quanta image is thresholded at p < .005, FWE of p < .01 [image scale (t) 3–5], and the FAT is a probabilistic map (image scale .5–1).
The results for the phonological, semantic and executive factors reproduced those observed in our previous study and related investigations (Butler et al., 2014, Mirman et al., 2015a, Mirman et al., 2015b). It is interesting to note that speech ‘variety’ seems to be inherently linked to the patients' degree of remaining semantic abilities (factor 2) presumably because the use of a wide vocabulary is underpinned by fine semantic distinctions. More generally, the observed neural correlates of the phonological and semantic factors are highly consistent with data from other methods. Phonological processing was associated with left HG, mid to posterior MTG, STG and STS, which have all been identified in various previous reviews of phonological processing (Hickok and Poeppel, 2004, Hickok and Poeppel, 2007, Price, 2010, Price, 2012, Vigneau et al., 2006, Wise et al., 2001) and repetitive transcranial magnetic stimulation (rTMS) to pSTG has been shown to increase error rates in the speech production of neurologically-intact participants (Acheson, Hamidi, Binder, & Postle, 2011). The neural substrate for semantic performance (left anterior temporal regions) again converges with findings from large-scale functional magnetic resonance imaging (fMRI) reviews (Binder et al., 2009, Price, 2010, Vigneau et al., 2006, Visser et al., 2009), data from semantic dementia (Mion et al., 2010), rTMS (Pobric, Jefferies, & Lambon Ralph, 2007), direct electrical stimulation of inferior fronto-occipital fasciculus (IFOF) (Duffau, Gatignol, Mandonnet, Capelle, & Taillandier, 2008) and neuroanatomically-constrained models (Ueno, Saito, Rogers, & Lambon Ralph, 2011).
One thing that must be kept in mind when using PCA is that the factor solution is always dependent on the tests included in the decomposition. Thus, if an analysis fails to include behavioural measures that reflect a core feature of the target clinical populations then, by definition, the resultant PCA model will not derive a principal dimension for this clinically-relevant feature. Our current PCA model contains measures of phonological, semantic, speech fluency and executive skill. Whilst this model captures some of the core aspects of post-stroke aphasia, future studies will need to augment the range of assessments in order to capture other key features of this clinical disorder including syntactic processing, speech errors, written language processing, etc.
To conclude, in addition to revealing specific behavioural and neuroanatomical information about the nature of chronic post-stroke aphasia, the current study serves as a worked example for the utility of our PCA-VBCM method. Rather than adopting either a classical group-study or single-case investigation, the PCA data-driven approach not only generates a single unified model for the group as a whole (expressed in terms of the four emergent principal components) but is also able to capture the individual differences between patients (in terms of their relative positions along the principal behavioural axes). This method not only preserves the individual-level data (as per single-case and case-series methods) but is also able to place it within the broader context of the whole group (akin to group studies). Thus rather than ignoring or averaging across individual variations, the methodology actively embraces individual differences and extracts coherent variations. We also note here that by utilising varimax rotation, the emergent factors become relatively straightforward to interpret from a cognitive perspective and the fact that they are statistically-independent makes them ideal for the neuroimaging analyses (which call for orthogonal predictors rather than the strong inter-correlations that are found between the raw test results). Whilst we have applied this PCA-VBCM approach to post-stroke chronic aphasia in the current investigation, this methodological approach should be applicable and beneficial across a range of acute and progressive neurological conditions.
Acknowledgements
We are grateful to all the patients and carers for their continued support of our research programme. We thank Dr. Rebecca Butler for collection of the speech data analysed in this study and to Prof. Marco Catani and Dr. Michel Thiebaut de Schotten for providing the anatomical overlay for the FAT. This research was support by funding from the Rosetrees Trust, an NIHR (UK) senior investigator award and a Medical Research Council (UK) programme grant to MALR (MR/J004146/1).
Reviewed 10 March 2016
Contributor Information
Ajay D. Halai, Email: ajay.halai@manchester.ac.uk.
Matthew A. Lambon Ralph, Email: matt.lambon-ralph@manchester.ac.uk.
References
- Acheson D.J., Hamidi M., Binder J.R., Postle B.R. A common neural substrate for language production and verbal working memory. Journal of Cognitive Neuroscience. 2011;23(6):1358–1367. doi: 10.1162/jocn.2010.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann H., Riecker A. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain and Language. 2004;89(2):320–328. doi: 10.1016/S0093-934X(03)00347-X. http://dx.doi.org/10.1016/S0093-934X(03)00347-X [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. http://dx.doi.org/10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Basilakos A., Fillmore P.T., Rorden C., Guo D., Bonilha L., Fridriksson J. Regional white matter damage predicts speech fluency in chronic post-stroke aphasia. Frontiers in Human Neuroscience. 2014;8:845. doi: 10.3389/fnhum.2014.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso A., Lecours A.R., Moraschini S., Vanier M. Anatomoclinical correlations of the aphasias as defined through computerized tomography: exceptions. Brain and Language. 1985;26(2):201–229. doi: 10.1016/0093-934x(85)90039-2. http://dx.doi.org/10.1016/0093-934X(85)90039-2 [DOI] [PubMed] [Google Scholar]
- Bates E., Wilson S.M., Saygin A.P., Dick F., Sereno M.I., Knight R.T. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6(5):448–450. doi: 10.1038/nn1050. http://dx.doi.org/10.1038/nn1050 [DOI] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. http://dx.doi.org/10.1093/cercor/bhp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank C., Scott S.K., Murphy K., Warburton E.A., Wise R.J.S. Speech production: Wernicke, Broca and beyond. Brain. 2002;125(8):1829–1838. doi: 10.1093/brain/awf191. http://dx.doi.org/10.1093/brain/awf191 [DOI] [PubMed] [Google Scholar]
- Borovsky A., Saygin A.P., Bates E., Dronkers N. Lesion correlates of conversational speech production deficits. Neuropsychologia. 2007;45(11):2525–2533. doi: 10.1016/j.neuropsychologia.2007.03.023. http://dx.doi.org/10.1016/j.neuropsychologia.2007.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeat S., Lambon Ralph M.A., Patterson K., Garrard P., Hodges J.R. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38(9):1207–1215. doi: 10.1016/s0028-3932(00)00034-8. http://dx.doi.org/10.1016/S0028-3932(00)00034-8 [DOI] [PubMed] [Google Scholar]
- Broca P. Remarques sur le siege de la faculte du language articule suivies d'une observation d'aphemie. Bulletins de la Société Anatomique de Paris. 1861;6:330–357. [Google Scholar]
- Burgess P.W., Shallice T. Thames Valley Test Company; Thurston, Suffolk: 1997. The Hayling and Brixton tests. [Google Scholar]
- Butler R.A., Lambon Ralph M.A., Woollams A.M. Capturing multidimensionality in stroke aphasia: mapping principal behavioural components to neural structures. Brain. 2014;137(12):3248–3266. doi: 10.1093/brain/awu286. [Journal article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A., McCloskey M. The case for single-patient studies. Cognitive Neuropsychology. 1988;5(5):517–527. Special issue: methodological problems in cognitive neuropsychology. [Google Scholar]
- Catani M., Dell'Acqua F., Vergani F., Malik F., Hodge H., Roy P. Short frontal lobe connections of the human brain. Cortex. 2012;48(2):273–291. doi: 10.1016/j.cortex.2011.12.001. http://dx.doi.org/10.1016/j.cortex.2011.12.001 [DOI] [PubMed] [Google Scholar]
- Catani M., ffytche D.H. The rises and falls of disconnection syndromes. Brain. 2005;128(10):2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Catani M., Jones D.K., ffytche D.H. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M., Mesulam M.M., Jakobsen E., Malik F., Martersteck A., Wieneke C. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136(8):2619–2628. doi: 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase A. Stroke: improved lesion-symptom mapping in poststroke aphasia. Nature Reviews. Neurology. 2014;10(12):674. doi: 10.1038/nrneurol.2014.217. [DOI] [PubMed] [Google Scholar]
- Damasio A.R. Aphasia. New England Journal of Medicine. 1992;326(8):531–539. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- Dronkers N.F. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Duffau H., Gatignol P., Mandonnet E., Capelle L., Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. Journal of Neurosurgery. 2008;109(3):461–471. doi: 10.3171/JNS/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- Duffau H., Gatignol P., Mandonnet E., Peruzzi P., Tzourio-Mazoyer N., Capelle L. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128(4):797–810. doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Duffau H., Gatignol P., Moritz-Gasser S., Mandonnet E. Is the left uncinate fasciculus essential for language? Journal of Neurology. 2009;256(3):382–389. doi: 10.1007/s00415-009-0053-9. [DOI] [PubMed] [Google Scholar]
- Goodglass H. Academic Press; San Diego: 1993. Understanding aphasia. [Google Scholar]
- Goodglass H., Kaplan E. 2nd ed. Lea & Febiger; Philadelphia: 1983. The assessment of aphasia and related disorders. [Google Scholar]
- Hickok G., Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nature Reviews. Neuroscience. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hodges J.R., Patterson K., Oxbury S., Funnell E. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Jefferies E., Lambon Ralph M.A. Semantic impairment in stroke aphasia vs. semantic dementia: a case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jefferies E., Patterson K., Jones R.W., Lambon Ralph M.A. Comprehension of concrete and abstract words in semantic dementia. Neuropsychology. 2009;23(4):492–499. doi: 10.1037/a0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., Weintraub S. Lea & Febiger; Philadelphia: 1983. Boston naming test. [Google Scholar]
- Kay J., Lesser R., Coltheart M. Lawrence Erlbaum Associates Ltd; Hove, UK: 1992. Psycholinguistic assessments of language processing in aphasia. [Google Scholar]
- Kertesz A. Grune & Stratton; New York: 1982. Western aphasia battery. [Google Scholar]
- Kinoshita M., de Champfleur N., Deverdun J., Moritz-Gasser S., Herbet G., Duffau H. Role of fronto-striatal tract and frontal aslant tract in movement and speech: an axonal mapping study. Brain Structure and Function. 2014:1–14. doi: 10.1007/s00429-014-0863-0. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph M.A., Lowe C., Rogers T.T. Neural basis of category-specific semantic deficits for living things: evidence from semantic dementia, HSVE and a neural network model. Brain. 2007;130:1127–1137. doi: 10.1093/brain/awm025. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph M.A., McClelland J.L., Patterson K., Galton C.J., Hodges J.R. No right to speak? The relationship between object naming and semantic impairment: neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13:341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph M.A., Moriarty L., Sage K. Anomia is simply a reflection of semantic and phonological impairments: evidence from a case-series study. Aphasiology. 2002;16(1–2):56–82. [Google Scholar]
- Lambon Ralph M.A., Patterson K., Graham N., Dawson K., Hodges J.R. Homogeneity and heterogeneity in mild cognitive impairment and Alzheimer's disease: a cross-sectional and longitudinal study of 55 cases. Brain. 2003;126(11):2350–2362. doi: 10.1093/brain/awg236. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph M.A., Patterson K., Plaut D.C. Finite case series or infinite single-case studies? Comments on ‘Case series investigations in cognitive neuropsychology’ by Schwartz and Dell (2010) Cognitive Neuropsychology. 2011;28(7):466–474. doi: 10.1080/02643294.2012.671765. [DOI] [PubMed] [Google Scholar]
- Lee L.L. Northwestern University Press; Chicago, IL: 1974. Developmental sentence analysis. [Google Scholar]
- Mandelli M.L., Caverzasi E., Binney R.J., Henry M.L., Lobach I., Block N. Frontal white matter tracts sustaining speech production in primary progressive aphasia. The Journal of Neuroscience. 2014;34(29):9754–9767. doi: 10.1523/JNEUROSCI.3464-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion M., Patterson K., Acosta-Cabronero J., Pengas G., Izquierdo-Garcia D., Hong Y.T., Fryer T.D., Williams G.B., Hodges J.R., Nestor P.J. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133(11):3256–3268. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- Mirman D., Chen Q., Zhang Y., Wang Z., Faseyitan O.K., Coslett H.B. Neural organization of spoken language revealed by lesion-symptom mapping. Nature Communications. 2015;6 doi: 10.1038/ncomms7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D., Zhang Y., Wang Z., Coslett H.B., Schwartz M.F. The ins and outs of meaning: behavioral and neuroanatomical dissociation of semantically-driven word retrieval and multimodal semantic recognition in aphasia. Neuropsychologia. 2015 doi: 10.1016/j.neuropsychologia.2015.02.014. http://dx.doi.org/10.1016/j.neuropsychologia.2015.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr J., Pessin M., Finkelstein S., Funkenstein H., Duncan G., Davis K. Broca aphasia: pathologic and clinical. Neurology. 1978;28(4):311–324. doi: 10.1212/wnl.28.4.311. [DOI] [PubMed] [Google Scholar]
- Mummery C.J., Ashburner J., Scott S.K., Wise R.J.S. Functional neuroimaging of speech perception in six normal and two aphasic subjects. The Journal of the Acoustical Society of America. 1999;106(1):449–457. doi: 10.1121/1.427068. [DOI] [PubMed] [Google Scholar]
- Parker G.J.M., Luzzi S., Alexander D.C., Wheeler-Kingshott C.A.M., Ciccarelli O., Lambon Ralph M.A. Lateralization of ventral and dorsal auditory-language pathways in the human brain. NeuroImage. 2005;24(3):656–666. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Phan T.G., Donnan G.A., Wright P.M., Reutens D.C. A digital map of middle cerebral artery infarcts associated with middle cerebral artery trunk and branch occlusion. Stroke. 2005;36(5):986–991. doi: 10.1161/01.STR.0000163087.66828.e9. [DOI] [PubMed] [Google Scholar]
- Pobric G., Jefferies E., Lambon Ralph M.A. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(50):20137–20141. doi: 10.1073/pnas.0707383104. http://dx.doi.org/10.1073/pnas.0707383104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J. The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191(1):62–88. doi: 10.1111/j.1749-6632.2010.05444.x. http://dx.doi.org/10.1111/j.1749-6632.2010.05444.x [DOI] [PubMed] [Google Scholar]
- Price C.J. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. http://dx.doi.org/10.1016/j.neuroimage.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J.C. H. K. Lewis; London: 1962. Advanced progressive matrices, set II. [Google Scholar]
- Rojkova K., Volle E., Urbanski M., Humbert F., Dell'Acqua F., Thiebaut de Schotten M. Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Structure and Function. 2015;221(3):1751–1766. doi: 10.1007/s00429-015-1001-3. [DOI] [PubMed] [Google Scholar]
- Saur D., Kreher B.W., Schnell S., Kummerer D., Kellmeyer P., Vry M.-S. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. http://dx.doi.org/10.1073/pnas.0805234105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.F., Dell G.S. Case series investigations in cognitive neuropsychology. Cognitive Neuropsychology. 2011;27(6):477–494. doi: 10.1080/02643294.2011.574111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.F., Dell G.S., Martin N., Gahl S., Sobel P. A case-series test of the interactive two-step model of lexical access: evidence from picture naming. Journal of Memory and Language. 2006;54(2):228–264. doi: 10.1016/j.jml.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L., Ramlackhansingh A., Crinion J.T., Leff A.P., Price C. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. NeuroImage. 2008;41:1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Cambridge University Press; Cambridge: 1988. From neuropsychology to mental structure. [Google Scholar]
- Swinburn K., Baker G., Howard D. Psychology Press; New York: 2005. CAT: the comprehensive aphasia test. [Google Scholar]
- Thompson C.K., Cho S., Hsu C.-J., Wieneke C., Rademaker A., Weitner B.B. Dissociations between fluency and agrammatism in primary progressive aphasia. Aphasiology. 2012;26(1):20–43. doi: 10.1080/02687038.2011.584691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler L.K., Marslen-Wilson W., Stamatakis E.A. Dissociating neuro-cognitive component processes: voxel-based correlational methodology. Neuropsychologia. 2005;43(5):771–778. doi: 10.1016/j.neuropsychologia.2004.07.020. http://dx.doi.org/10.1016/j.neuropsychologia.2004.07.020 [DOI] [PubMed] [Google Scholar]
- Ueno T., Saito S., Rogers T.T., Lambon Ralph M.A. Lichtheim 2: synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal–ventral language pathways. Neuron. 2011;72(2):385–396. doi: 10.1016/j.neuron.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Vigneau M., Beaucousin V., Hervé P.Y., Duffau H., Crivello F., Houdé O. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Visser M., Jefferies E., Lambon Ralph M.A. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. Journal of Cognitive Neuroscience. 2009;22(6):1083–1094. doi: 10.1162/jocn.2009.21309. http://dx.doi.org/10.1162/jocn.2009.21309 [DOI] [PubMed] [Google Scholar]
- Wechsler D.A. Psychological Corporation; New York: 1987. Wechsler memory scale—revised manual. [Google Scholar]
- Wilke M., de Haan B., Juenger H., Karnath H.-O. Manual, semi-automated, and automated delineation of chronic brain lesions: a comparison of methods. NeuroImage. 2011;56(4):2038–2046. doi: 10.1016/j.neuroimage.2011.04.014. http://dx.doi.org/10.1016/j.neuroimage.2011.04.014 [DOI] [PubMed] [Google Scholar]
- Willmes K., Poeck K. To what extent can aphasic syndromes be localized? Brain. 1993;116(6):1527–1540. doi: 10.1093/brain/116.6.1527. [DOI] [PubMed] [Google Scholar]
- Wise R.J.S., Greene J., Büchel C., Scott S.K. Brain regions involved in articulation. The Lancet. 1999;353(9158):1057–1061. doi: 10.1016/s0140-6736(98)07491-1. [DOI] [PubMed] [Google Scholar]
- Wise R.J.S., Scott S.K., Blank C., Mummery C.J., Murphy K., Warburton E.A. Separate neural subsystems within ‘Wernicke's area’. Brain. 2001;124(1):83–95. doi: 10.1093/brain/124.1.83. http://dx.doi.org/10.1093/brain/124.1.83 [DOI] [PubMed] [Google Scholar]
- Woollams A.M., Lambon Ralph M.A., Plaut D.C., Patterson K. SD-squared: on the association between semantic dementia and surface dyslexia. Psychological Review. 2007;114(2):316–339. doi: 10.1037/0033-295X.114.2.316. [DOI] [PubMed] [Google Scholar]